Graphical abstract

Keywords: Hydrazonoyl halides, Dithiocarboxylates, Dithiocarbazates, Thiourea, Carbonothioic dihydrazide
Abstract
This review article presents a survey of the utility of a new synthetic strategy for 1,3,4-thiadiazole derivatives based on reactions of nitrilimines with various functionalized sulfur dipolarophiles which proceed via tandem in situ 1,3-dipolar cycloaddition and β-elimination of simple molecule from the initially formed cycloadduct. The biological activities of some of the compounds prepared by such a strategy are pointed out. Only the literature reports within the period from 2000 to mid 2012 are covered.
Introduction and scope of the review
A survey of the literature revealed that differently substituted 1,3,4-thiadiazoles and annelated 1,3,4-thiadiazoles have wide range of pharmacological activities such as antibacterial, antifungal, antituberculosis, antihepatitis B viral, antileishmanial, anti-inflammatory, analgesic, CNS depressant, anticancer, antioxidant, antidiabetic, molluscicidal, antihypertensive, diuretic, analgesic, antimicrobial, antitubercular, and anticonvulsant activities [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11]. These important biological activities encouraged several research groups to find out different methods for synthesis of new thiadiazoles using different synthones, such as thiosemicarbazides, thiocarbazides, dithiocarbazates, thioacylhydrazines, acylhydrazines, and bithioureas [4], [7], [8], [9], [10], [11].
We would like to report in this review the recent developments of a new synthetic strategy for the synthesis of 1,3,4-thiadiazoles. This strategy is based on an in situ 1,3-dipolar cycloaddition of nitrilimines A to functionalized sulfur dipolarophiles B, followed by β-elimination of simple molecule from the initially formed cycloadducts C (Scheme 1). This strategy proved useful and convenient for synthesis of various functionalized 1,3,4-thiadiazole derivatives D (Scheme 1). Such a target has not been covered hitherto in the foregoing review articles surveying the chemistry of both 1,3,4-thiadiazoles [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11] and nitrilimines as well as their precursors [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24].
Scheme 1.

Regarding the 1,3-dipolar cycloaddition, it is a process in which two reactants, namely a 1,3-dipole and a dipolarophile combine together to form a five membered ring via the formation of two new sigma bonds without loss of any small fragment (Fig. 1). The 1,3-dipole is basically a system of three atoms amongst which are distributed four π electrons, whereas the dipolarophile is usually an unsaturated system having two π electrons. It is usually a system with either a double or a triple bond. The 1,3-dipolar cycloaddition reactions are usually referred to either as (4π + 2π)cycloadditions or (3 + 2)cycloadditions on the basis of the number of electrons or the number of atoms in the two reactants, respectively.
Fig. 1.

1,3-Dipolar cycloaddition.
Several types of 1,3-dipoles are found in the literature. One class of such 1,3-dipolar species is the so-called nitrilimines of the general formula E. Such nitrilimines are 1,3-dipoles readily generated in situ from stable precursors. They are usually generated in the presence of an appropriate dipolarophile. If they are generated in the absence of suitable dipolarophile, they undergo head-to-tail dimerization to afford the corresponding cycloadduct, namely the corresponding 1,3,4,6-tetrasubstituted-1,2,4,5-tetrazine F as given below.
Several convenient methods have been reported for the generation of nitrilimines [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39]. These include (i) thermolysis of either 2,5-disubstituted tetrazoles 1, 1,3,4-oxadiazol-5-ones 2 or 1,2,3,4-oxathiadiazol-2-oxides 3 (ii) base treatment of either hydrazonoyl halides 4, α-nitroaldehyde hydrazones 5 or N-hydrazonoyl pyridinium salts 6 (iii) oxidation of aldehyde hydrazones 7 with lead tetracetate or ferric chloride and (iv) treatment of acid hydrazides 8 with triphenylphosphine in acetonitrile (Chart 1).
Chart 1.
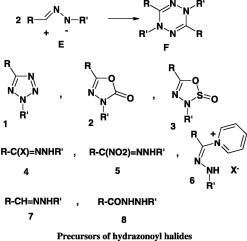
The present review covers only the papers dealing with the synthesis of 1,3,4-thiadiazoles via nitrilimines, generated by base-catalyzed dehydrohalogenation of hydrazonoyl halides. The various types of hydrazonoyl halides used in this synthesis are listed below in order of their citations in this review.
| I | Ar—C(X) = NNHAr′ |
| II | Het—COC(X) = NNHAr |
| III | R—C(X) = NNH—Het |
| IV | R—[C(X) = NNH—Ar]2 |
| V | ROOC—C(X) = NNH—Het |
| VI | MeCO—C(X) = NNH—Het |
| VII | ROOC—C(X) = NNH—Ar |
| VIII | Het—C(X) = NNHAr |
| IX | MeCOC(X) = NNHAr |
| X | ROOC—C(X) = NNH—Ar |
| XI | PhNHOC—C(X) = NNH—Ar |
| XII | ArCOC(X) = NNHAr |
| XIII | Het—C(X) = NNH—Het |
Regarding the dipolarophile, it can be almost any molecule having a double or triple bond of the following types (Chart 2). In this review, only cycloaddition reactions of nitrilimines to compounds having the C S double bond as dipolarophilic site are surveyed.
Chart 2.

Reactions
Reaction with alkyl dithiocarboxylates
Several reports on the reactions of alkyl dithiocarboxylates with nitrilimines have been published. In all cases, such reactions were carried out by stirring a mixture of the appropriate ester and hydrazonoyl halide in ethanol at room temperature in the presence of triethylamine [40], [41], [42], [43], [44], [45]. For example, Abdelhamid et al. [40], [41] reported that reaction of methyl 2-cyano-2-(benzoazol-2-yl)dithioacetates 9 with each of nitrilimines, derived from the corresponding hydrazonoyl halides I under such conditions, afforded the corresponding 1,3,4-thiadiazole derivatives 10 in 64–92% yield (Scheme 2).
Scheme 2.
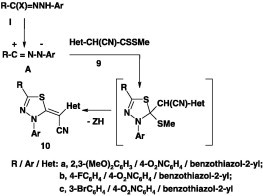
Likewise, the reactions of methyl 2-cyano-2-(benzothiazol-2-yl)dithiocarboxylates 9 with hydrazonoyl chlorides II [42] and VII(IX) [43] yielded the corresponding 1,3,4-thiadiazole derivatives 11 in 92% and 12 in 52–59% yields, respectively (Scheme 3).
Scheme 3.
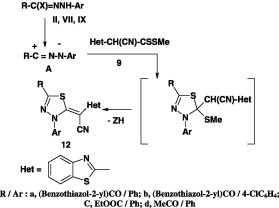
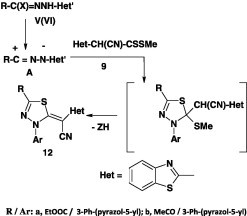
In another report [44], it was indicated that 1,3,4-thiadiazole derivatives 12 were formed in 55–68% yield when N-hetaryl-hydrazonoyl halides V(VI) were treated with methyl 2-cyano-2-(benzothiazol-2-yl)dithiocarboxylates 9 (Scheme 4).
Scheme 4.
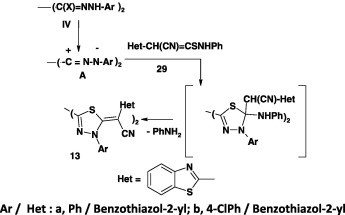
Reaction of bis-nitrilimines, derived from the bis-hydrazonoyl chlorides IV with methyl 2-cyano-2-(hetaryl)dithiocarboxylates 9, gave the corresponding bis-2,2′-(1,3,4-thiadiazole) derivatives 13 in 83–90% yield (Scheme 5) [45].
Scheme 5.
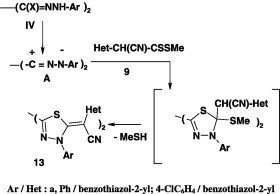
Ar/Het: a, Ph/benzothiazol-2-yl; 4-ClC6H4/benzothiazol-2-yl.
Also, the 1,3,4-thiadiazole derivatives 15 were furnished in 70–75% yield by reaction of methyl pyrazole-4-dithiocarboxylates 14 with hydrazonoyl halides I, V, and VI (Scheme 6) [41], [44].
Scheme 6.
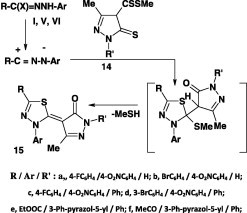
Reaction with thioamides
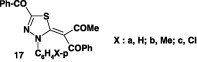
Many reactions of thioamides with nitrilimines were carried out by refluxing a mixture of the appropriate hydrazonoyl halide and thioamide in ethanol in the presence of triethylamine [49], [50], [54], [55], [56], [57]. For example, when N-phenyl 2-benzoyl-3-oxo-thiobutanamide 16 was reacted with hydrazonoyl bromide XII under such reaction conditions, it afforded the corresponding 3-benzoyl-4-aryl-5-[(acetyl,benzoyl)methylene]-1,3,4-thiadiazoles 17a–c (Scheme 7) [49].
Scheme 7.
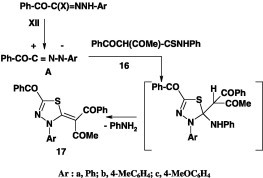
Also, treatment of the thioanilide 18 with each of the hydrazonoyl chlorides I and II under the same reaction conditions afforded the corresponding 1,3,4-thiadiazole derivatives 19a–c (Scheme 8) [50].
Scheme 8.

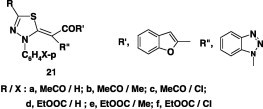
In a similar manner, the thioacetanilide 20 reacted with the hydrazonoyl halides I, VII, and IX gave also the corresponding 1,3,4-thiadiazole derivatives 21 in 62–68% yields (Scheme 9) [54].
Scheme 9.
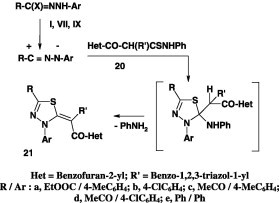
Treatment of the thioanilides 22 with the hydrazonoyl chlorides furnished also the corresponding thiadiazole derivatives 23 (Scheme 10) [55].
Scheme 10.
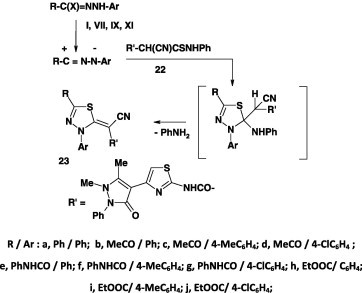
Likewise, the hydrazonoyl chlorides I, VII, IX, and XI were reported to react with the thioanilide 24 under the same reaction conditions and yielded the corresponding 1,3,4-thiadiazole derivatives 25 in 82–90% yields (Scheme 11) [56].
Scheme 11.
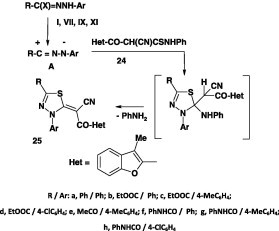
The reaction of the hydrazonoyl chlorides I, II, VII, IX, or XI with the thioanilide 26 in refluxing ethanol in the presence of triethylamine afforded the corresponding 1,3,4-thiadiazole derivatives 27 in 73–80% yield (Scheme 12) [57].
Scheme 12.
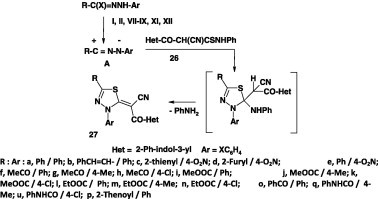
In some other reports, several 1,3,4-thiadiazole derivatives were also obtained by reaction of hydrazonoyl halides with the appropriate thioamides in ethanol containing triethylamine at room temperature. For example, the 1,3,4-thiadiazole derivatives 10 have been prepared in 64–92% yield by reaction of 2-hetaryl-cyanothioacetanilides 28 with the hydrazonoyl halides I and VIII under such reaction conditions [42] or in refluxing chloroform in the presence of triethylamine [40] (Scheme 13).
Scheme 13.
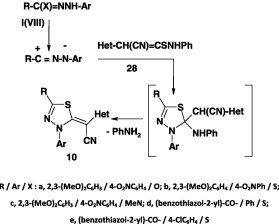
Similar treatment of each of the hydrazonoyl chlorides VII and IX with the N-methylthioacetamide derivative 29 in ethanol containing triethylamine at room temperature afforded the corresponding 1,3,4-thiadiazole derivatives 30 in 57–60% yield (Scheme 14) [59].
Scheme 14.
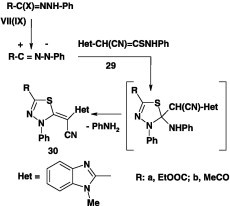
Bis-2,2′-(1,3,4-thiadiazole) derivatives 13 have been obtained in 83–90% yield by reaction of the bis-nitrilimines, derived from the respective bis-hydrazonoyl chlorides IV, with N-phenyl 2-cyano-2-(benzothiazol-2-yl)thioamide 29 under the same reaction conditions (Scheme 15) [45].
Scheme 15.
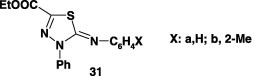
Only in one report [51], reaction of N-aryl-cyanothioformamides 30 with nitrilimines from hydrazonoyl halides VII and IX, when carried out in refluxing ethanol in the presence of sodium ethoxide, it afforded the corresponding 3-phenyl-5-substituted-2-N-(arylimino)-1,3,4-thiadiazoles 31 (Scheme 16) [51].
Scheme 16.

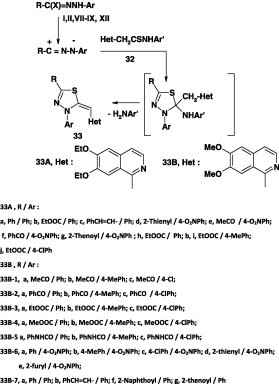
Abunada [46], Hassaneen et al. [47], and Abdallah et al. [48] demonstrated that treatment of the thioanilides 32A(B) each with hydrazonoyl halides I, II, VII, IX, and XII in refluxing chloroform in the presence of triethylamine yielded thethiadiazole derivatives 33 (Scheme 17).
Scheme 17.
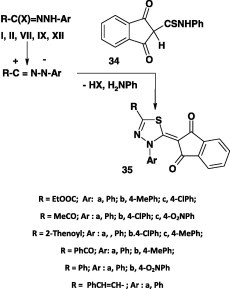
Similarly, the reaction of thioacetanilide 34 with various nitrilimines derived from the respective hydrazonoyl halides I, VII, IX, and XII in refluxing chloroform in the presence of triethylamine afforded the corresponding 1,3,4-thiadiazole derivatives 35 in 74–80% yield (Scheme 18) [52].
Scheme 18.
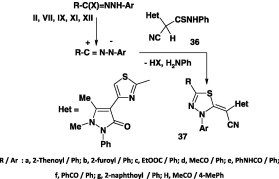
Several thioamides reacted with hydrazonoyl halides in dimethyl formamide in the presence of potassium hydroxide at room temperature to give the corresponding 1,3,4-thiadiazole derivatives [53], [58], [60], [61], [62]. For example, the interaction of 2-hetaryl-2-cyanothioacetanilide 36 with various nitrilimines derived from the respective hydrazonoyl halides II, VII, IX, XI, and XII under such reaction conditions gave mainly the 1,3,4-thiadiazole derivatives 37 in 53–60% yield (Scheme 19) [53].
Scheme 19.
Likewise, reaction of the thioanilide 38 with the hydrazonoyl chlorides I, VII, and XI was reported to furnish the corresponding thiadiazole derivatives 39 (Scheme 20) [58].
Scheme 20.

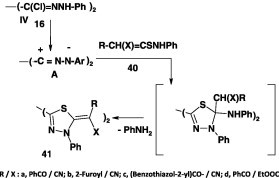
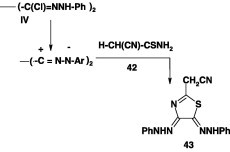
In another report [60], it was indicated that similar reaction of the thioanilides 40 with the bis-hydrazonoyl chlorides IV furnished the corresponding bis-2,2′-(1,3,4-thiadiazole) derivatives 41 in 66–70% yield (Scheme 21). In contrast to this finding, it was indicated that reaction of the same bis-hydrazonoyl chloride with cyanothioacetamide 42 in boiling ethanol in the presence of triethylamine yielded the thiazole derivative 43 (Scheme 22) [60].
Scheme 21.
Scheme 22.
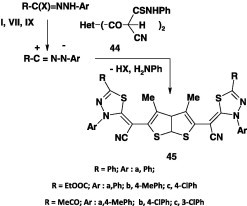
Recently, it was found that reaction of each of the hydrazonoyl halides I, VII and IX with the bis-thioanilide 44 inDMF containing KOH furnished the bis-1,3,4-thidiazole derivatives 45 in 49–66% yield (Scheme 23) [61].
Scheme 23.
Hassaneen et al. reported that treatment of (2-phenylimino-3-phenyl-4-oxothiazolidin-5-yl)thiocarboxanilide 46 with each of hydrazonoyl halides I, VII, VIII, IX, XI, and XII inDMF containing KOH afforded the corresponding thiadiazoline derivatives 47 (Scheme 24) [62]. Similar reaction of 1,3-diphenyl-2-thioxo-5-oxo-4-thiocarboxanilide 48 with the aforementioned hydrazonoyl halides under the same reaction conditions yielded also the corresponding 1,3,4-thiadiazole derivatives 49 (Scheme 25) [62].
Scheme 24.
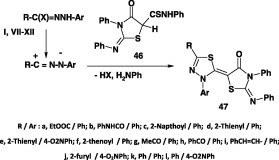
Scheme 25.
Reaction with alkyl dithiocarbamates
A number of papers have been published on reactions of alkyl dithiocarbamates with nitrilimines. In all of these papers, the reactions were carried out by stirring a mixture of the appropriate hydrazonoyl halide and the dithiocarbamate in ethanol in the presence of triethylamine at room temperature [44], [63], [64], [65], [66], [67], [68], [69]. For example, 1,3,4-thiadiazole derivatives 51 were readily obtained in 68–85% yield by reaction of diarylnitrilimines derived from the respective hydrazonoyl halides I, VII, IX, XI, and XII under such conditions with methyl N-aryldithiocarbamates 50 (Scheme 26) [63], [64].
Scheme 26.

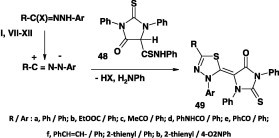
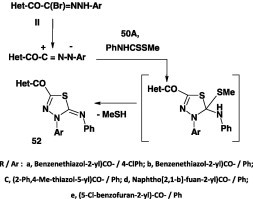
In like manner, the related 1,3,4-thiadiazole derivatives of type 52 were obtained in 55–87% yield by reaction of N-aryl-C-heteroyl hydrazonoyl halides II with methyl N-phenyldithiocarbamate 50A (Scheme 27) [42], [66], [67], [68].
Scheme 27.
Also, reaction of hydrazonoyl chlorides V(VI) with methyl N-phenyldithiocarbamate 50A in ethanolic triethylamine at room temperature furnished the corresponding 5-phenylimino-1,3,4-thiadiazole derivatives 53 in 55–65% yield (Scheme 28) [44].
Scheme 28.

Abdelhamid and Abdel-Wahab [64] and Abdelhamid et al. [69] investigated the reaction of methyl N-hetaryldithiocarbamate 54 with nitrilimines derived from the hydrazonoyl chlorides I, II, VII, IX, XI, and XII in ethanolic triethylamine at room temperature and characterized the products as 5-hetarylimino-1,3,4-thiadiazole derivatives 55. The latter products were obtained in 50–82% yield (Scheme 29).
Scheme 29.

2-Phenylimino-1,3,4-thidiazole derivative 56 were also produced in good yield by reaction of methyl N-aryldithiocarbamates 50A with the hydrazonoyl halide II in ethanolic triethylamine (Scheme 30) [66].
Scheme 30.

Reaction with thiourea and its derivatives
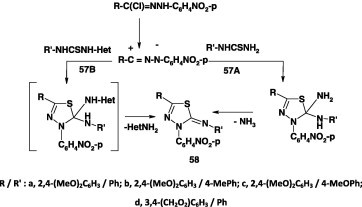
Direct synthesis of 5-phenylimino-1,3,4-thiadiazole derivatives 58 in 65–82% yield from C,N-diarylnitrilimines, derived by base-catalyzed dehydrohalogenation of the respective hydrazonoyl halides I in refluxing ethanol in the presence of triethylamine, and mono-substituted-thiourea and its N,N′-disubstituted derivatives 57A,B were recently reported (Scheme 31) [40], [63], [64].
Scheme 31.
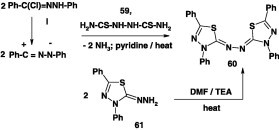
Also, it was reported that N-phenyl benzenecarbohydrazonoyl bromide reacted with bis-thiourea 59 in refluxing pyridine and yielded the azine derivative 60 (Scheme 32) [86]. The latter was also produced by heating 2-hydrazono-3,5-diphenyl-2,3-dihydro-1,3,4-thiadiazole 61 in DMF containing triethylamine as catalyst (Scheme 32) [86].
Scheme 32.
Reactions with alkyl dithiocarbazates
Unsubstituted dithiocarbazates
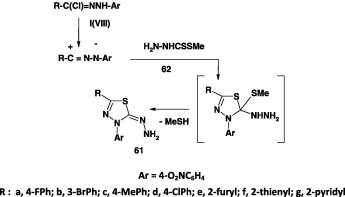
The thiadiazole derivatives 61a–d were readily produced from methyl dithiocarbazate 62 through its reaction with each of the hydrazonoyl bromides I in ethanol in the presence of triethylamine at room temperature [41]. However, the thiadiazole derivatives 61e–g were produced by refluxing a mixture of each halide VIII and alkyl dithiocarbazate 62 in ethanol [70] (Scheme 33). The yields of the compounds prepared were not pointed out, however.
Scheme 33.
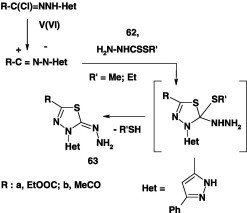
Likewise, reaction of N-hetaryl hydrzonoyl chlorides V and VI each with alkyl dithiocarbazates 62 in ethanol in the presence of triethylamine at room temperature furnished the corresponding thiadiazole derivatives 63 in 65% yield (Scheme 34) [40].
Scheme 34.
Alkyl N-aryldithiocarbazates
Reaction of methyl N-phenyldithiocarbazate 64 with the nitrilimines, generated from the N-arylC-hetaroylhydrazonoyl bromides II in ethanol in the presence of triethylamine, furnished the thiadiazole derivative 65 (Scheme 35) [66].
Scheme 35.

Alkyl N-acyldithiocarbazates
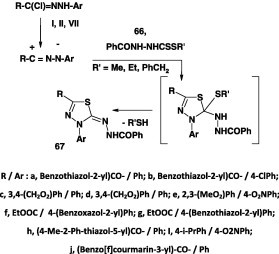
Several publications concerning reaction of alkyl N-acyldithiocarbazates with nitrilimines have been reported. Most of the reactions were studied by stirring a mixture of the appropriate hydrazonoyl halide and the alkyl N-acyldithiocarbazate in ethanol in the presence of Triethylamine at room temperature [42], [63], [64], [65], [71], [72]. Only in one report [66], the reaction between the hydrazonoyl halide and alkyl N-acyldithiocarbazate was carried out in refluxing chloroform containing triethylamine. Thus, reaction of alkyl N-benzoyldithiocarbazate 66 with nitrilimines generated from various hydrazonoyl halides I [63], [64], [71], II [65],and X [42], [66], [72] afforded the corresponding thiadiazole derivatives 67 in 71–85% yields (Scheme 36).
Scheme 36.
Furthermore, N-[5-acetyl-3-(aryl)-1,3,4-thiadiazol-2(3H)-ylidene]-5-(1H-indol-3-yl)-1-phenyl-1H-pyrazole-3-carbohydrazides 69 was prepared in 43–50% by direct heating the potassium salt of dithiocarbazate 68A with hydrazonoyl chlorides IXin ethanol (Scheme 37) [73].
Scheme 37.
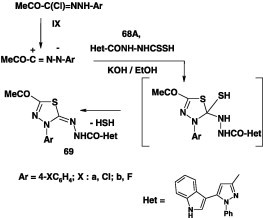
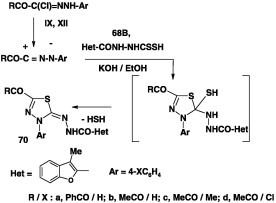
Likewise, reaction of the hydrazonoyl halides IX and XII each with potassium salt of the dithiocarbamate 68B in refluxing ethanol yielded the corresponding 1,3,4-thidiazole derivatives 70 in 72–76% yields (Scheme 38) [74].
Scheme 38.
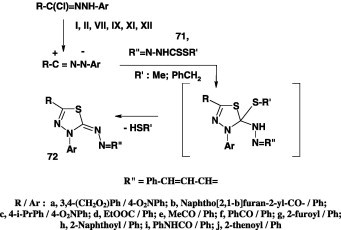
Alkyl N-cinnamylidene dithiocarbazates
The azine derivatives 72 were reported to be obtained in 65–90% in yields from the interaction of alkyl styrylmethylidenedithiocarbazate 71 with various nitrilimines derived from hydrazonoyl chlorides I, II, VII, IX, XI, and XII in ethanol in the presence of triethylamine at room temperature (Scheme 39). The formation of the latter products 72 was considered to result from initial cycloaddition of nitrilimines to the C S to form the cycloadducts which in turn underwent elimination of methanethiol [63], [67], [71], [75].
Scheme 39.
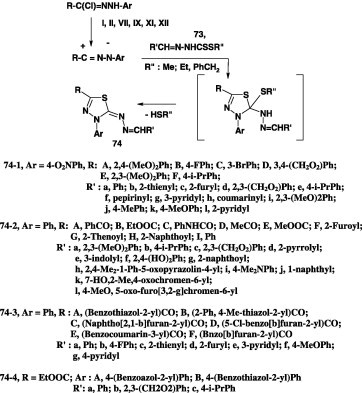
Alkyl N-arylmethylene dithiocarbazates
Several publications covering reactions of nitrilimines with alkyl N-alkylidene dithiocarbazates have been reported. In all cases examined, the reactions were carried out by stirring a mixture of the appropriate hydrazonoyl halide and alkyl N-alkylidene dithiocarbazate in ethanol containing triethylamine at room temperature. For example, reactions of the dithiocarbazates 73 with nitrilimines derived from various hydrazonoyl halides II, VII, IX, XI, and XII [40], [41], [42], [53], [63], [64], [65], [66], [67], [68], [71], [72], [75], [76], [78], [79] under such conditions furnished the corresponding azine derivatives 74 (Scheme 40).
Scheme 40.
Also, the thidiazole derivatives 75 have also been prepared by reaction of N-aryl 2-hetaryl-2-oxoethanehydrazonoyl chlorides II with alkyl N-arylidenedithiocarbazate 73 in ethanolic triethylamine at room temperature (Scheme 41) [71], [80].
Scheme 41.
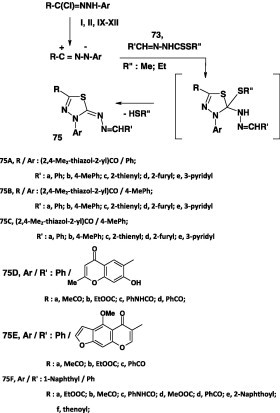
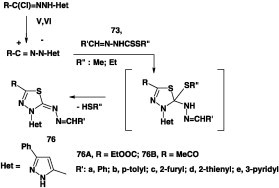
Similarly, reaction of N-hetarylhydrazonoyl chlorides V, VI, IX, and X each with alkyl N-arylidenedithiocarbazate 73 under the same reaction conditions was reported to furnish the corresponding thidiazole derivatives 76 in 60–80% yields (Scheme 42) [44].
Scheme 42.
Alkyl N-(1-aryl)ethylidene dithiocarbazates
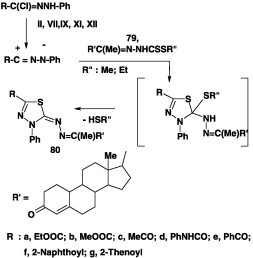
Numerous azine derivatives 78 were prepared in 56–90% yield by reactions of alkyl 1-substituted-ethylidene-dithiocarbazate 77 with various nitrilimines, generated from hydrazonoyl bromides I, V, VII, and VIII [40], [41], [63], [71], [65], [66], [68], [71], [72], [77], [78], [81], [83] in ethanol in the presence of triethylamine at room temperature (Scheme 43).
Scheme 43.

Likewise, 2,3-dihydro-1,3,4-thiadiazolyl steroids 80 were analogously prepared in 60–68% yields by reaction of alkyl dithiocarbazate 79 with various hydrazonoyl halides II, VII, IX, XI, and XII under the same reaction conditions (Scheme 44) [82].
Scheme 44.
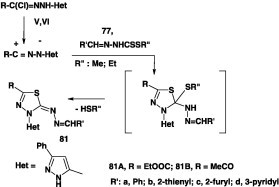
Reactions of alkyl 1-substituted-ethylidene-dithiocarbazates 77 with each of N-hetaryl hydrazonoyl chlorides V and VI in ethanolic at room temperature yielded the corresponding azine derivatives 81 in 60–85% yield (Scheme 45) [44].
Scheme 45.
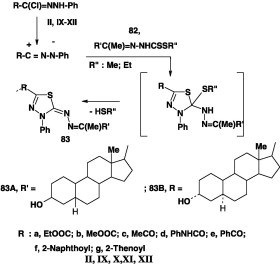
The steroidal dithiocarbazates 82A,B were also reported to undergo similar reaction with hydrazonoyl halides II, VII, IX, XI, and XII and gave the respective 1,3,4-thiadiazoles 83A,B (Scheme 46) [82].
Scheme 46.
Alkyl N-cycloakylidene dithiocarbazates
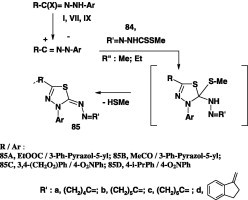
Reactions of nitrilimines with alkyl cycloalkylidene dithiocarbazates have been studied by several authors. In most of these cases, the reactions were carried out by stirring the appropriate nitrilimine precursor, namely the hydrazonoyl halide, and the dithiocarbazate ester in ethanol in the presence of triethylamine at room temperature. Thus, reactions of methyl cycloalkylidene dithiocarbazates 84 with the hydrazonoyl halides VII(IX) [44] and I [63], [71] under such conditions have been reported to yield the corresponding azine derivatives 85 in 50–78% yield (Scheme 47).
Scheme 47.
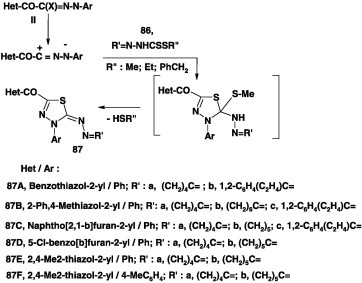
Also, the 1,3,4-thiadiazole derivatives 87 have been obtained in 64–88% yields by reactions of nitrilimines derived from the C-heteroyl-hydrazonoyl halides II with alkyl carbodithioates 86 under the same reaction conditions (Scheme 48) [43], [66], [67], [68], [80], [83].
Scheme 48.
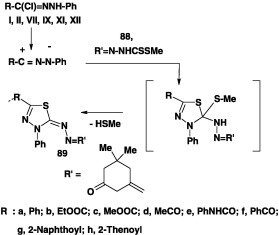
Rateb [84] reported that methyl dithiocarbazate 88 reacted with nitrilimines, derived from the hydrazonoyl halides I, II, VII and IX-XII in dimethyl formamide in the presence of potassium hydroxide at room temperature, and furnished the corresponding azine derivatives 89 in 55–67% yield (Scheme 49).
Scheme 49.
Similarly, other research groups [40], [41], [44], [65], [68] described the preparation of the thiadiazole derivatives 91 by reactions of alkyl dithiocarbazates 90 with various hydrazonoyl halides I, II, VII, and IX in ethanolic triethylamine at room temperature (Scheme 50).
Scheme 50.

Alkyl N-hetarylidene diothiocarbazates
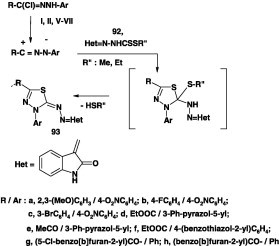
Several reports covering reactions of alkyl N-hetarylidene dithiocarbazates with nitrilimines have been published [40], [41], [44], [65], [67], [68], [69], [79]. In these reports, it was indicated that reaction of alkyl dithiocarbazates 92 with various hydrazonoyl halides I, II, VII, IX, and XI in ethanol in the presence of triethylamine afforded the corresponding thiadiazole derivatives 93 in 65–85% yield (Scheme 51).
Scheme 51.
Reactions with carbonothioic dihydrazide
Different results were reported concerning reactions of carbonothioic dihydrazide 94 with hydrazonoyl halides. For example, Sayed [86] reported that treatment of 94 with hydrazonoyl chloride IX in boiling DMF gave the corresponding thiadiazine derivatives 95 in 64% yield (Scheme 52) [86]. In contrast, the same reaction of 94 with N-aryl arenecarbohydrazonoyl halides I and VIII in refluxing ethanol was reported earlier by the same author to afford the thiadiazole derivatives 96 [70]. No rationalization was given for such difference.
Scheme 52.
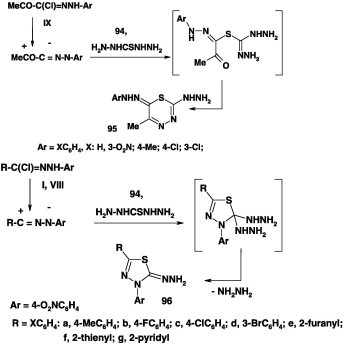
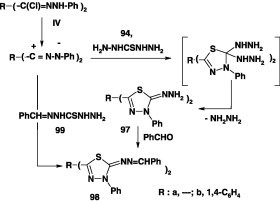
Similarly, reactions of carbonothioic dihydrazide 94 with the bis-hydrazonoyl chlorides IV in DMF in the presence of triethylamine furnished the corresponding 2,2′-bis(1,3,4-thiadiazole) derivatives 97 in about 60% yield (Scheme 53) [70], [86]. Compound 97 reacted with benzaldehyde to give the bis-hydrazone 98. The latter was also obtained by reaction of the bis-hydrazonoyl chloride IV with 2-(phenylmethylene)carbonothioic dihydrazide 99 in ethanolic triethylamine [70].
Scheme 53.
Reactions with heterocyclic thiones
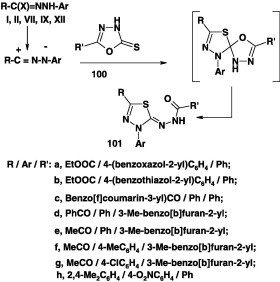
In a recent review by Shawali and Farghaly [22] on reactions of hydrazonoyl halides with many heterocyclic thiones having no α-hydrogen, it has been indicated that such reactions afford only the corresponding spiro(heterocycle[n,2′]-3H-1,3,4-thiadiazole). More recently, several research groups have reported that reactions of N-arylhydrazonoyl halides I and X with 5-substituted-1,3,4-oxadiazol-5(4H)thione 100 in boiling ethanol [65], [72], [74] or chloroform [64] in the presence of triethylamine furnished in all cases examined, products that were identified as the corresponding thiadiazole derivatives 101 in 72–76% yield. The latter products were assumed to be formed via the ring opening of the initially formed spirocycloadducts (Scheme 54). The latter products 101 were also obtained by reaction of the same hydrazonoyl halides each with alkyl N-benzoyldithiocarbazate (Scheme 54) [65], [72], [74].
Scheme 54.
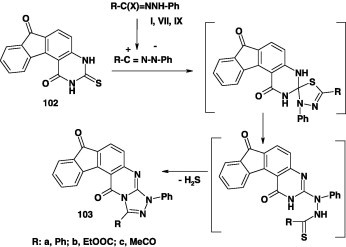
In contrast, it was reported that reactions of heterocyclic thione 102 with hydrazonoyl chlorides I, VII and X in chloroform in the presence of triethylamine were reported to afford the corresponding fused [1,2,4]triazoles 103 in 89–91% yields [85]. To account for the formation of the latter, it was suggested that the initially formed spiro-1,3,4-thiadiazole cycloadducts underwent tandem in situ rearrangement and elimination of hydrogen sulfide to give the respective fused [1,2,4]triazoles 103 as end products (Scheme 55) [85].
Scheme 55.
Biological activity
Many of the thiadiazole derivatives that have been prepared by the foregoing reactions, proved to possess wide range of pharmaceutical activities like antimicrobial, antivirus, anticancer, and molluscicidal effectiveness. In the following, a brief coverage of such activities is outlined.
Antimicrobial activities
Abdelhamid et al. [41] reported that certain 2,3-dihydro-1,3,4-thiadiazole derivatives 10a,b; 15a,b; 74a,b; 78a,b; 91 and 93 (Chart 3) possess high inhibitory activity against some strains of Gram positive bacteria, namely Staphylococcus albus, Staphylococcus faecalis and Bacillus subtilis and Gram negative bacteria Escherichia coli. Also compounds 74A [76] and 74B and 74C [78], 74D, 74E and 74F [79] and 74G, 74H, 74I (Chart 4, Chart 5, Chart 6, Chart 7) [79] exhibited a high inhibition toward Candida albicans and Asperills flavus fungi. Also, 2-N-arylimino-1,3,4-thidiazole derivatives 31a and 31b (Chart 8) were reported to exhibit moderate activity against Candida albicans [51].
Chart 3.
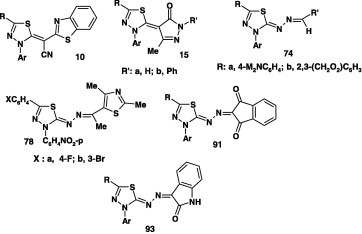
Chart 4.
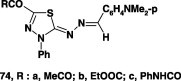
Chart 5.

Chart 6.

Chart 7.

Chart 8.
Antiviral activities
The thidiazoline derivatives 69a,b (Chart 9) were reported to show no antiviral activity [73].
Chart 9.
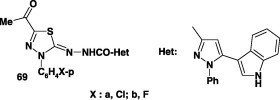
Anticancer and cytotoxic activity
Twelve of the azine derivatives 91 (Chart 10) were evaluated for their anticancer activity. The results showed that some of these compounds possess cytotoxicity against Ehrlich ascites carcinoma [65].
Chart 10.
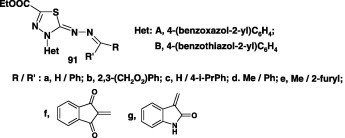
Anticonvulsant and anti-inflammatory activities
Some of the compounds 21 (Chart 11) [54] were screened for anticonvulsant activity. The results showed that 21a and 21d were found active in ScMet, whereas compound 21c was active in MES. Also, compounds 21 were found to exhibit antinociceptive effect and the order of their activity is in the order 21f > 21c > 21a > 21b > 21d. In addition, compounds 21 were screened for their anti-inflammatory activity. The results showed that 21a exhibited the most potent anti-inflammatory activity and from structure–activity relationship (SAR) the order is 21a > 21b > 21c.
Chart 11.
Molluscicidal activity
The toxicity of compounds 17a–c to Biomphalaria alexandrinai snails was screened, and the results showed that compound 17b showed the highest activity (Chart 12) [49].
Chart 12.
Conclusion
From the foregoing survey, it seems that tandem 1,3-dipolar cycloaddition of nitrilimines to functionalized sulfur dipolarophilic compounds followed by β-elimination of simple molecule such as alkanethiol from the initially formed cycloadducts provides a useful and convenient strategy for synthesis of numerous 1,3,4-thiadiazole derivatives. The subject of such reactions is still ongoing and undoubtedly will provide new fused functionalized 1,3,4-thiadiazoles of both industrial and biological interests.
Conflict of interest
The author has declared no conflict of interest.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Siddiqui N., Ahuja P., Ahsan W., Pandeya S.N., Alam M.S. Thiadiazoles: progress report on biological activities. J Chem Pharm Res. 2009;1:19–30. [Google Scholar]
- 2.Singh A.K., Mishra G., Jyoti K. Review on biological activities of 1,3,4-thiadiazole derivatives. J Appl Pharm Sci. 2009;1:44–49. [Google Scholar]
- 3.Kamal M., Shakya A.K., Jawaid T. 1,3,4-Thiadiazole as antimicrobial agent: a review. Int J Biomed Res. 2011;2:41–61. [Google Scholar]
- 4.Mishra G., Singh A.K., Jyoti K. Review article on 1,3,4-thiadiazole derivatives and its pharmacological activities. Int J Chem Tech Res. 2011;3:1380–1393. [Google Scholar]
- 5.Kamal M., Shakya A.K., Jawaid T. 1,3,4-Thiadiazole as antimicrobial agent: a review. Int J Biomed Res. 2011;2:1–4. [Google Scholar]
- 6.Kushwaha N., Kushwaha S.K.S., Rai A.K. Biologial activities of thiadiazole derivatives: a review. Int J Chem Tech Res. 2011;4:517–531. [Google Scholar]
- 7.Gupta J.K., Dudhey R., Sharma P.K. Synthesis and pharmacological activity of substituted 1,3,4-thiadiazole derivatives. Medichemonline. 2010;1:1–9. [Google Scholar]
- 8.Singh A.K., Mishra G., Jyoti K. Review on biological activities of 1,3,4-thiadiazolederivatives. J Appl Pharm Sci. 2011;1:44–49. [Google Scholar]
- 9.Ahmad T., Singh A.K., Jaiswal N., Singh D. Synthesis and pharmacological activity of 1,3,4-thiadiazole derivatives: a review. Int Res J Pharm. 2012;3:70–82. [Google Scholar]
- 10.Bhuva H., Sahu D., Shah B., Modi D.C., Patel M.B. Biological profile of thiadiazole. Pharmacol Online. 2011;1:528–545. [Google Scholar]
- 11.Kushwaha N., Kushwaha S.K.S., Rai A.K. Biologial activities of thiadiazole derivatives: a review. Int J Chem Tech Res. 2012;4:517–531. [Google Scholar]
- 12.Butler R.N., Scott F.L. Versatile reactive intermediates: hydrazidic halides. Chem Ind (Lond) 1970:1216–1221. [Google Scholar]
- 13.Shawali A.S., Parkanyi C. Hydrazonoyl halides in the synthesis of hterocycles. J Heterocycl Chem. 1980;17:833–854. [Google Scholar]
- 14.Shawali A.S. Reactions of hydrazonoyl halides with sulfur compounds. Heterocycles. 1983;20:2239–2285. [Google Scholar]
- 15.Shawali A.S. Reactions of heterocyclic compounds with nitrilimines and their precursors. Chem Rev. 1993;93:2731–2777. [Google Scholar]
- 16.Shawali A.S., Abdallah M.A. The chemistry of heterocyclic hydrazonoyl halides. Adv Heterocycl Chem. 1995;63:277–338. [Google Scholar]
- 17.Shawali A.S., Elsheikh S.M. Annelated [1,2,4,5]tetrazines. J Heterocycl Chem. 2001;38:541–559. [Google Scholar]
- 18.Shawali A.S., Mosselhi M.A.N. Hydrazonoyl halides: useful building blocks for synthesis of arylazoheterocycles. J Heterocycl Chem. 2003;40:725–746. [Google Scholar]
- 19.Shawali A.S., Mosselhi M.A.N. The chemistry of thiohydrazonates and their utility in organic synthesis. J Sulf Chem. 2005;26:267–303. [Google Scholar]
- 20.Shawali A.S., Edrees M.M. Reactions of nitrilimines with heterocyclic amines and enamines. Convenient methodology for synthesis and annulation of heterocycles. Arkivoc. 2006;ix:292–365. [Google Scholar]
- 21.Shawali A.S., Sherif S.M. The chemistry of hydrazonates. Curr Org Chem. 2007;11:773–799. [Google Scholar]
- 22.Shawali A.S., Farghaly T.A. Reactions of hydrazonoyl halides with heterocyclic thiones. Convenient methodology for heteroannulation, synthesis of spiroheterocycles and heterocyclic ring transformation. Arkivoc. 2008;i:18–64. [Google Scholar]
- 23.Shawali A.S., Samy N.A. Hydrazonoyl halides: their versatile biological activities. Open Bioactive Compds J. 2009;2:8–16. [Google Scholar]
- 24.Shawali A.S., Abdelhamid A.O. Synthesis of spiro-heterocycles via 1,3-dipolar cycloadditions of nitrilimines to exoheterocyclic enones. Site-, region- and stereo-selectivities overview. Curr Org Chem. 2012;16:2623–2639. [Google Scholar]
- 25.Huisgen R., Seidel M., Sauer J., Mefarland J.W., Wallbillich G. The formation of nitrile imines in the thermal breakdown of 2,5-disubstituted tetrazoles. J Org Chem. 1959;24:892–893. [Google Scholar]
- 26.Huisgen R., Seidel M., Wallbillich G., Knupfer H. Diphenyl-nitrilimin und seine 1.3-dipolaren additionen an alkene und alkine. Tetrahedron. 1962;17:3–29. [Google Scholar]
- 27.Sauer J., Mayer K.K. Thermolyse und photolyse von 3. 4-Diphenyl-Δ2-1,2,4-oxadia-zolinon-(5) und 2.4-diphenyl-Δ2-1.3.4-oxdiazolinon-(5) Tetrahedron Lett. 1968:325–339. [Google Scholar]
- 28.Grundmann C., Flory K. Uber dimesityl nitrilimin. Justus Leibigs Ann Chem. 1968;721:91–100. [Google Scholar]
- 29.Gladstone W.A.F., Aylward J.P., Norman R.O.C. Reactions of lead tetraacetate Part XVIII. Oxidation of aldehyde hydrazones: a new method for generation of nitrilimines. J Chem Soc (C) 1969:2587–2598. [Google Scholar]
- 30.Reimlinger H., Vandewalle J.J.M., King K.S.D., Linger W.R.F., Merenyi R. 1,5-dipolare cyclisierungen, III. Kondensierte Triazole aus konjugierten Nitriliminen. Struktur und Stabilität der Produkte aus N-acylierten cyclischen Amidrazonen und Thionylchlorid. Chem Ber. 1970;103:1918–1933. [Google Scholar]
- 31.Huseya Y., Chinune A., Ohta M. Photochemical reaction of 3,4-diphenyl-sydnone. Bull Chem Soc Jpn. 1971;44:1667–1668. [Google Scholar]
- 32.Angadiyavar C.S., George M.V. Photochemical cycloadditions of 1,3-dipolar systems. 1. Addition of N.C-diphenylsydnone and 2,5-diphenyltetrazole. J Org Chem. 1971;36:1589–1594. [Google Scholar]
- 33.Marky M., Hansen H.J., Schmid H. Photochemisches Verhalten von 3,4-Diarylsydnonen. Vorläufige Mitteilung. Helv Chem Acta. 1971;54:1257–1278. [Google Scholar]
- 34.Huisgen R., Grashey R., Gotthardt D.H., Schmidt R. 1,3-Dipolar addition of sydnones to alkynes. A new route into the pyrazole series. Angew Chem Int Ed Engl. 1962;1:48–49. [Google Scholar]
- 35.Lown J.W., Landberg B.E. Further synthetic applications of functionalized 1,3-dipoles, further synthetic applications of functionalized 1,3-dipoles. Can J Chem. 1975;53:3782–3790. [Google Scholar]
- 36.Marky M., Meier H., Wunderli A., Heimgatner H., Schmid H., Hansen H.J. Zum photochemischen Verhalten von Sydnonen und 1,3,4-Oxadiazolin-2-onen. 56. Mitteilung über Photoreaktionen. Helv Chim Acta. 1978;61:1477–1510. [Google Scholar]
- 37.Garanti L., Vigevani A., Zecchi G. Intramolecular carbine-type 1,1-cycloaddition by nitrile imines. Tetrahedron Lett. 1976;17:1527–1528. [Google Scholar]
- 38.Clovis J.S., Fliege W., Huisgen R. 1,3-Dipolare Cycloadditionen, 89. Neue Beiträge zur Chemie des Diphenylnitrilimins. Chem Ber. 1983;116:3062–3070. [Google Scholar]
- 39.Molteni G. Silver(I) salts as useful reagents in pyrazole synthesis. Arkivoc. 2007;ii:224–246. [Google Scholar]
- 40.Abdelhamid A.O., Abdel-Wahab B.A.M., Al-toom A.A. Reactions of hydrazonoyl halides 37: synthesis of triazolo[4,3-a]pyrimidines, 1,3,4-thiadiazoles and 1,3,4-selenadiazoles. Phosp Sulf Silicon Related Elem. 2004;179:601–613. [Google Scholar]
- 41.Abdelhamid A.O., Sayed A.R., Elzanate A.M. Reactions of hydrazonoyl halides 50: synthesis and antimicrobial activity of some new pyrimido[1,2-b]-[1,2,4,5]-tetrazin-6-one, tetrazino[3,2-b]quinazolin-6-one, triazolo[4,3-a]-pyrimidines and 2,3-dihydro-1,3,4-thiadiazoles. Chem J. 2005;2:287–292. [Google Scholar]
- 42.Abdelhamid A.O., Rateb N.M., Dawood K.M. Reactions with hydrazonoyl halides 30: synthesis of some 2,3-dihydro-1,3,4-thiadiazoles and unsymmetrical azines containing benzothiazole moiety. Phosp Sulf Silicon. 2000;167:251–258. [Google Scholar]
- 43.Abdelhamid A.O., Elghandour A.H., Rateb N.A., Awad A.M. Aconvenient synthesis of 1-amino-4-methyl-4H-3-thia-4,5a,10-triazacyclopenta[a]fluoren-5-ones and some new 2,3-dihydro-1,3,4-thiadiazoles. Phosp Sulf Silicon. 2006;181:1637–1646. [Google Scholar]
- 44.Abdelhamid A.O., Al-toom A. Reactions with hydrazonoyl halides 39: sythesis of some new triazoles and 2,3-dihydro-1,3,4-thiadiazoles. Phosp Sulf Silicon. 2004;179:2221–2233. [Google Scholar]
- 45.Dawood K.M., Raslan M.A., Farag A.M. Synthesis of 3,3′-bi-1,2,4-triazolo[4,5-a]-benzimidazole, 5,5′-bi-1,3,4-thiadiazole and thiazolo[3,2-a]-benzimidazole derivatives. Synth Commun. 2003;33:4079–4086. [Google Scholar]
- 46.Abunada N. Síntesis of 2-(isoquinolinemethylidene)-1,3,4-thiadiazole derivatives. J Al-Aqsa Univ. 2007;11:12–21. [Google Scholar]
- 47.Hassaneen H.M., Hassaneen H.M.E., Mohammed Y.S. Reactivity of 1-methyl-isoquinoline. Synthesis of pyrazoloyl triazoloisoquinoline and thiadiazolyl isoquinoline derivatives. Nat Sci. 2011;3:651–660. [Google Scholar]
- 48.Abdallah T.A., Abdelhadi H.A., Hassaneen H.M. Reactivity of 1-methyl-isoquinoline. Synthesis of 2-(1-isoquinolinemethylidene)-1,3,4-thiadiazole derivatives. Phosp Sulf Silicon. 2002;177:59–66. [Google Scholar]
- 49.Fadda A.A., Abdel-Latif E., El-Mekawy R. Synthesis and molluscicidal activity of some new thiophene, thiadiazole and pyrazole derivatives. Eur J Med Chem. 2009;44:1250–1256. doi: 10.1016/j.ejmech.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 50.Dawood K.M., Raslan M.A. Fused polyaza-heterocycles and 1,3,4-thiadiazoles via a tricyano synthon. J Heteocycl Chem. 2008;45:1–5. [Google Scholar]
- 51.Mahran A.M., Hassan N.A. A one-step synthesis and antimicrobial activities of new substituted dihydro-1,3,4-thiadiazoles. Arch Pharm Res. 2006;29:46–49. doi: 10.1007/BF02977467. [DOI] [PubMed] [Google Scholar]
- 52.Elwan N.M., Hassaneen H.M., Hassaneen H.M. Synthesis and reactions of indane-1,3-2-thiocarboxanilides with hydrazonoyl halides and active chloromethylene compounds. Heteroatom Chem. 2002;13:585–591. [Google Scholar]
- 53.Abdelhamid A.O., Afifi M.A.M. Reactions with hydrazonoyl halides 61: synthesis of 2,3-dihydro-1,3,4-thiadiazoles. Phosp Sulf Silicon Related Elem. 2008;183:2703–2713. [Google Scholar]
- 54.Dawood K.M., Abdel-Gawad H., Ragab E.A., Ellithey M., Mohamed H.A. Synthesis, anticonvulsant and anti-inflammatory evaluation of some new benzotriazole and benzofuran-based heterocycles. Bioorg Med Chem. 2006;14:3672–3680. doi: 10.1016/j.bmc.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 55.Kandeel Z.E., Dawood K.M., Ragab E.A., Farag A.M. Simple and convenient routes to new polyheterocycles incorporating pyrazole, thiazole, thiophene and 1,3,4-thiadiazole moieties. Heteroatom Chem. 2002;13:248. [Google Scholar]
- 56.Dawood K.M., Farag A.M., Abdel-Aziz H.A. Synthesis of some new benzofuran- based thiophene, 1,3-oxathiole and 1,3,4-oxa(thia)diazole derivatives. Heteroatom Chem. 2007;18:294–300. [Google Scholar]
- 57.Hassaneen H.M., Hassaneen H.M.E., Gomaa Z.Z.A. Reactivity of 3-cyanoacetyl-indole derivatives: synthesis of 3-hydrazonopyrazolyl and 3-thiadiazolyl indole derivatives. Int J Org Chem. 2011;1:97–104. [Google Scholar]
- 58.Dawood K.M., Ragab E.A., Farag A.M. Polyheterocyclic systems incorporating pyrazole, thiazole and thiadiazole moieties. J Chem Res. 2003 (S) 685-686, (M) 1151-1160. [Google Scholar]
- 59.Abdelhamid A.O., Elghandour A.H., Rateb N.A., Awad A.M. A convenient synthesis of1-amino-4-methyl-3-thia-4,5a,10-triazacyclopenta[a]-fluoren-5-ones and some new 2,3-dihydro-1,3,4-thiadiazoles. Phosp Sulf Silicon. 2006;181:1637–1646. [Google Scholar]
- 60.Dawood K.M., Elwan N.M. Synthesis of 3,3′-bi-1,3,4-thiadiazole and fused azole systems via bishydrazonoyl chlorides. J Chem Res. 2004:264–266. [Google Scholar]
- 61.Kheder N.A., Mabkhot Y.N. Synthesis and antimicrobial studies of some novel bis-[1,3,4]thiadiazole and bis-thiazole pendant to thieno[2,3-b]thiophene moiety. Int J Mol Sci. 2012;13:3661. doi: 10.3390/ijms13033661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hassaneen H.M., Miqdad O.A., Abunada N.M., Fares A.A. Reaction of thiocarboxanilide derivatives of 2-phenylimino-3-phenyl-4-thiazolidinone and 1,3-diphenyl-2-thioxo-4-imidazolone with hydrazonoyl halides and active chloromethylene compounds. Nat Sci. 2011;3:199–207. [Google Scholar]
- 63.Rateb N.M., Abdel-Riheem N.A., Al-Atoom A.A., Abdelhamid A.O. Reactions with hydrazonoyl halides 35: synthesis of some new 1,2,4-triazololino[4,3-a][yrimidines, 2,3-dihydro-1,3,4-thiadiazoles and 2,3-dihydro-1,3,4-selenadiazoles. Phosph Sulf Silicon. 2003;178:1101–1114. [Google Scholar]
- 64.Abdelhamid A.O., Abdel-Wahab B.A.M. Reactions with hydrazonoyl halides 38: synthesis of pyrrolo[3,4-c]pyrazoles, pyrazoles and 1,3,4-thiadiazoles. Affinidad. 2004;61:65–73. [Google Scholar]
- 65.Abdelall E.K.A., Mohamed M.A., Abdelhamid A.O. Reactions with hydrazonoyl halides 63: synthesis and anticancer activity of some new 1,3,4-thiadiazoles, 1,3,4-selenadiazoles and 1,2,4-triazolo[4,3-a]pyrimidines. Phosp Sulf Silicon. 2010;85:1862–2010. [Google Scholar]
- 66.Abdelhamid AO, Metwally NH, Bishai NS. Reactions with hydrazonoyl halides 29: synthesis of some new 1,2,4-triazolo4,3-a]-benzimidazoles, thiazolo[3,2-a]-benzimidazoles and unsymmetrical azine derivatives. J Chem Res 2000, (S)462–463, (M)1144–1154.
- 67.Abdelhamid A.O., Alkhodshi M.A.M. Reactions with hydrazonoyl halides 42: synthesis of some new 2,3-dihydro-1,3,4-thiadiazoles, 2,3-dihydro-1,3,4-selenadiazoles and triazolino[4,3-a]pyrimidines. Phosp Sulf Silicon. 2005;180:149–161. [Google Scholar]
- 68.Abdelhamid A.O., El-Ghandour A.H., Al-Reedy A.A.M. Reactions with hydrazonoyl halides 56: synthesis and reactions of 1-bromo-2-(5-chlorobenzo- furanyl)ethanedione-1-phenylhydrazone. Phosp Sulf Silicon. 2008;183:1272–1284. [Google Scholar]
- 69.Abdelhamid A.O., Abdelgawad S.M., El-Sharnoby S.F. Reactions with hydrazonoyl halides 33: synthesis of some new 2,3-dihydro-1,3,4-thiadiazoles containing pyrazol-3-yl, indolin-2-one-3-yl and indan-1,3-dione-2-yl moieties. Phosp Sulf Silicon. 2002;177:2699–2709. [Google Scholar]
- 70.Sayed A.R. Synthesis of novel thiadiazoles and bis-thiadiazoles from carbono-thioic dihydrazide. Tetrahedron Lett. 2010;51:4490–4493. [Google Scholar]
- 71.Abdelreheen N.A., Rateb N.M., Altoom A.A., Abdelhamid A.O. 1,3,4-Thia- and -selenadiazole and 1,2,4-triazolo[4,3-a]pyrimidine erivatives from hydrazonoyl halides. Heteroatom Chem. 2003;14:421–426. [Google Scholar]
- 72.Moustafa C., Abel-Reheem N.A., Abdelhamid A.O. Reactions with hydrazonoyl halides 40: synthesis of some new 1,3,4-thiadiazoles, pyrrolo-[3,4-c]pyrazoles, pyrazoles and pyrazolo[3,4-d]pyridazines. Synth Commun. 2005;35:249–261. [Google Scholar]
- 73.Abdelgawad H., Mohamed H.A., Dawood K.M., Badria F.A. Synthesis and antiviral activity of new indole-based heterocycles. Chem Pharm Bull. 2010;58:1529–1531. doi: 10.1248/cpb.58.1529. [DOI] [PubMed] [Google Scholar]
- 74.Dawood K.M., Farag A.M., Abdel-Aziz H.A. Synthesis an antimicrobial evaluation of some 1,2,4-triazole, 1,3,4-oxa(thia)diazole and 1,2,4-triazolo-[3,4-b]-1,3,4-thiadiazine derivatives. Heteroatom Chem. 2005;16:621–627. [Google Scholar]
- 75.Abu-Team O.S., Rateb N.M., Abdelhamid A.O. Reactions with hydrazonoyl halides 34: synthesis of some new 2,3-dihydro-1,3,4-thiadiazoles. Phosp Sulf Silicon. 2003;178:2363–2371. [Google Scholar]
- 76.Abdelhamid A.O., Sayed A.R., Zaki Y.H. Reactions with hydrazonoyl halides 51: a facile synthesis of 5-arylthiazoles and triazolo[4,3-a]pyrimidines as antimicrobial agents. Phosp Sulf Silicon. 2007;182:1447–1457. [Google Scholar]
- 77.Abdelhamid A.O., Ismail Z.H., Abdelaziem A.M. Reactions with hydrazonoyl halides 58: synthesis of 2,3-dihydro-1,3,4-thiadiazoles and 5-arylazothiazoles. Phosp Sulf Silicon. 2008;183:1735–1745. [Google Scholar]
- 78.Abdel-Gawad S.M., Elgendy M.S., Abdelhamid A.O. Reactions with hydrazonoyl halides 46: synthesis of some new 2,3-dihydro-1,3,4-thiadiazoles and triazolino[4,3-a]pyrimidines as antimicrobial agents. J Sulf Chem. 2005;25:21–31. [Google Scholar]
- 79.Abdelhamid A.O., Mohamed M.A., Zaki Y.H. Reactions with hydrazonoyl halides 59: synthesis and antimicrobial activity of 2,3-dihydro-1,3,4-thiadiazole, triazolino-[4,3-a]-pyrimidine and pyrimido[1,2-b]-[1,2,4,5]tetrazin-6-one containing benzofuran moiety. Phosp Sulf Silicon. 2008;183:1746–1754. [Google Scholar]
- 80.Abdelhamid A.O., El-Ghandour A.H., Hussein A.M., Zaki Y.H. Reactions with hydrazonoyl halides 44: synthesis of some new 1,3,4-thiadiazolines, 1,3,4-selenadiazo-lines and triazolino[4,3-a]pyrimidines. J Sulfur Chem. 2004;25:329–342. [Google Scholar]
- 81.Abdelhamid A.O., Alkhodshi M.A.M. Reactions with hydrazonoyl halides 43: synthesis of some new 2,3-dihydro-1,3,4-thiadiazoles, pyrazolo-[3,4-d]-pyridazines, thieno[2,3-b]-pyridines, pyrimidino[4″,5″:4,5]thieno-[2,3-b]pyridines and pyrrolo-[3,4-d]pyrazoles. J Heterocycl Chem. 2005;42:527–533. [Google Scholar]
- 82.Abdelhamid A.O., Baghos V.B., Doss S.H., Halim M.M. Reactions with hydrazonoyl halides 55: synthesis of 2,3-dihydro-1,3,4-thiadiazoles containing steroid moiety. Phosp Sulf Silicon. 2007;182:2843–2854. [Google Scholar]
- 83.Abdelhamid A.O., Zohdi H.F., Sallam M.M.M., Ahmed N.A. Reactions with hydrazonoyl halides 27: synthesis of some new triazolo[4,3-a]-benzimidazole and unsymmetrical azine erivatives. Phosp Sulf Silicon. 2000;164:181–188. [Google Scholar]
- 84.Rateb N.M. Synthesis of some new thiazole, thiophene, 2,3-dihydro-1,3,4-thiadiazole and pyrimidino[1,2-b]indazole derivatives. Phosp Sulf Silicon. 2005;180:2361–2372. [Google Scholar]
- 85.Hassaneen H.M., Abdallah T., Abdelhadi H.A., Hassaneen H.M.E., Pagni R.M. Polyfunctional fused heterocyclic compounds via indene-1,3-dione. Heteroatom Chem. 2003;14:491–497. [Google Scholar]
- 86.Sayed A.R. Synthesis of 1,3,4-thiadiazines, bis-1,3,4-thiadiazoles, [1,2,4]triazino-[3,4-b][1,3,4]thiadiazine, thiazolines from carbonothioic dihydrazide. Tetrahedron. 2012;68:2784–2789. [Google Scholar]



