Abstract
Since last few years, an impressive amount of data has been generated regarding the basic in vitro and in vivo biology of neural stem cells (NSCs) and there is much far hope for the success in cell replacement therapies for several human neurodegenerative diseases and stroke. The discovery of adult neurogenesis (the endogenous production of new neurons) in the mammalian brain more than 40 years ago has resulted in a wealth of knowledge about stem cells biology in neuroscience research. Various studies have done in search of a suitable source for NSCs which could be used in animal models to understand the basic and transplantation biology before treating to human. The difficulties in isolating pure population of NSCs limit the study of neural stem behavior and factors that regulate them. Several studies on human fetal brain and spinal cord derived NSCs in animal models have shown some interesting results for cell replacement therapies in many neurodegenerative diseases and stroke models. Also the methods and conditions used for in vitro culture of these cells provide an important base for their applicability and specificity in a definite target of the disease. Various important developments and modifications have been made in stem cells research which is needed to be more specified and enrolment in clinical studies using advanced approaches. This review explains about the current perspectives and suitable sources for NSCs isolation, characterization, in vitro proliferation and their use in cell replacement therapies for the treatment of various neurodegenerative diseases and strokes.
Keywords: Neural stem cells, Characterization, Neurodegenerative diseases, Stroke, Regeneration
Introduction
Neural stem cells (NSCs) are self-renewing, multipotent cells that generate the main phenotypes of the nervous system [1]. The hallmark characteristics of NSCs are its ability to proliferate and generate multiple cell lineages, such as neurons, astrocytes, and oligodendrocytes both in vitro and in vivo [2].
Grafting of neural stem cells into the mammalian central nervous system (CNS) has been performed for some decades now, both in basic research and clinical applications for neurological disorders such as Parkinson’s, disease, Huntington’s disease, stroke, and spinal cord injuries. Albeit the “proof of principle” status that neural grafts can reinstate functional deficits and rebuild damaged neuronal circuitries, many critical roadblocks have still to be overcome to reach clinical applications. Among these are the manifold immunological aspects that are encountered during the graft–host interaction in vivo. Different sources of stem cells have been proposed to the spontaneous recovery of most CNS injuries, but they mostly generate a restricted range of cell phenotypes. Induced pluripotent stem cells (iPS) have been recently proposed for autologous transplantation, but a major drawback of these genetically manipulated cells is the high risk of cancer formation, mainly due to the uncontrolled integration of retroviral vectors and recombination events. Therefore, the most feasible candidates to clinical neurological applications are currently the embryonic stem cells (ESCs) and adult somatic stem cells, particularly NSCs. On the contrary, NSC are mostly considered as the optimal cell type for cell mediated therapy of neural disorders because they share the same tissue origin of the damaged cells, meant to replenish and are amenable to local environmental cues able to commit their differentiation choice [3–5]. Accordingly, NSC have been shown to exert multiple therapeutic effects, such as secretion of neurotrophic factors and cytokines, scavenging of toxic molecules, immunomodulation of inflammatory milieu, where neural cell replacement plays only a minor role in the recovery of CNS damage [6–9].
During the last decade, an enormous amount of information has been generated regarding the basic in vitro and in vivo biology of NSCs. In 1989, Sally described multipotent, self-renewing progenitor and stem cells population in the subventricular zone (SVZ) of the mouse brain. In 1992, Reynolds and Weiss were the first to isolate neural progenitors and stem cells from the adult striatal tissue, including the SVZ [10]. Now it is well known that the continuous production of new neurons is facilitated by neural stem or progenitor cells (NSCs/NPCs). NSCs are self renewing, multipotent cells which possess the capability to differentiate into any neural cell type by symmetric and asymmetric cell division while progenitors are proliferative cells with a limited capacity for self-renewal and often considered as unipotent [11,12] (Fig. 1). As our current knowledge predicts that the production of new cells in the brain follows a multi-step process during which newborn cells are submitted to various regulatory factors and influence cell proliferation, maturation, fate determination and survival. Progenitor cells isolated from the forebrain can differentiate into neurons in vitro, as was demonstrated by Reynolds and Weiss in 1992. There are many types of cells present in the forebrain where NSCs are characterized by using multiple cell surface or intracellular markers such as nestin, musashi 1, sox-2, prominin-1 and intigrins either separately or in combination [13].
Fig. 1.
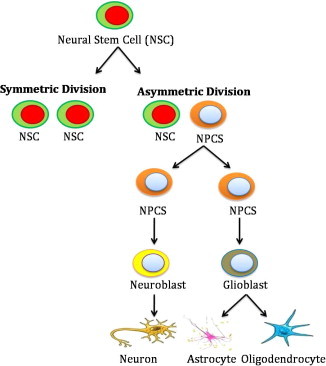
NSCs self-renewal and proliferation pathway.
Multiple studies have demonstrated that endogenous neurogenesis responds to insults such as ischemic stroke, multiple sclerosis or other neurodegenerative diseases and even brain tumors, supporting the existence of remarkable plasticity and significant regenerative potential in the mammalian brain. Today it is no more a far fetched hope, but a realistic goal, to claim that NSCs will be an inexhaustible source of neurons and glia for cell replacement therapies aimed for the treatment of disorders affecting the brain and spinal cord. Embryonic stem cells (ESCs) and stem cells from the fetal/adult central nervous system (CNS) or other tissues might all be suitable for the purpose of cell replacement therapy, since they all have shown the capacity to differentiate into multiple cell types of the adult CNS [14–16]. Researchers have succeeded in recovering brain function in adult animal models by transplantation of NSCs [17–19], indicating the existence of a regulatory mechanism for stem cell biology in the adult brain. Due to the enormous potential of NSCs in the treatment of many devastating hereditary and acquired neurological diseases extensive molecular profiling studies have performed in search of new markers and regulatory pathways.
Stem-cell-based therapies could potentially be beneficial by acting though several mechanisms: Cell replacement, where transplants of cells are given to directly replace those that are lost; trophic support, where the cells are used to promote survival of affected neurons and endogenous repair of the diseased brain areas; modulation of inflammation, which may be involved in the disease process. Any stem-cell-based approach for treating a neurodegenerative disorder must be proven to work through one or more of these mechanisms [20]. Here, we discuss the clinical translation of neural stem cells in the treatment of various neurodegenerative disorders: Parkinson’s disease, Alzheimer’s disease, Huntington’s disease, Amyotrophic lateral sclerosis, Spinal cord injury, Depression, brain tumors and Stroke.
Sources of neural stem cells
NSCs can be obtained from skin [21–23], ESCs [24], embryonic NSCs [25,26], bone marrow [27] and adipose-derived mesenchymal stem cells (MSCs) [28], induced pluripotent stem cells (iPSCs) [29,30], fetal [31] and adult nervous systems [32–35]. These all sources have been used to prove their potential for the treatment of several neurodegenerative diseases by generating the desired type of CNS cells; such as MSCs derived from different sources have been used for the production of dopamine neurons for the treatment of Parkinson’s disease [36]. Like this many other types of cell are also being used for their application in neurodegenerative diseases [37–39] and stroke [39,40]. But still there is need of getting more suitable source for in vitro and in vivo trans-differentiation into the correct phenotype (Table 1). In clinical applications the cultured/differentiated NSCs should be screened for bacterial, viral or fungal contaminations, complete media replacement with saline or PBS (Ca/Mg free), viability and integrity, etc.
Table 1.
Different sources of stem cells advantages and disadvantages for their applications in clinical practice.
| BMSCs | UCBSCs | ESCs | iPSCs | fNPCs | Spinal cord cells | Adipose MSCs | |
|---|---|---|---|---|---|---|---|
| Isolation | Challenging | Challenging | Challenging | Challenging | Challenging | Challenging | Easy |
| Ethical issues | Considerable | – | Significant | None | Significant | Considerable | Few |
| Pre-isolation storage | X | √ | X | X | X | X | ? |
| Post-isolation storage | √ | √ | √ | √ | √ | √ | √ |
| Tumorigenicity | X | X | √ | √ | X | X | X |
| Transfection | √ | √ | √ | √ | √ | √ | ? |
| Safety/risk | √ | √ | √ | ? | √ | √ | √ |
Isolation and in vitro expansion of NSCs
In vitro expansion of NSCs requires several growth factors such as EGF, FGF and LIF for their self-renewal, proliferation [41,42] and other stimulatory substances (FCS) for lineage differentiation [43,44]. Therefore, cell density, growth factor addition, medium supplementation, passaging techniques and timing are utter importance in the maintenance of culture conditions. Any small change in any of these factors in cultures of such heterogeneous cell populations can change the cells potential and possibly select for subpopulations of cells exhibiting similar properties to each other. NSCs are cultured mainly by two ways either as neurospheres or as monolayer.
In recent years, scientists have discovered a wide array of stem cells that have unique capabilities to self-renew, grow indefinitely, and differentiate/develop into multiple types of cells and tissues. Researchers now know that many different types of stem cells exist but they all are found in very small populations in the human body, in some cases 1 stem cell in 1,00,000 cells in circulating blood [45]. Several parts of the body has been identified as rich sources for stem cells such as SVZ in brain is a rich source of NSCs [33] (Fig. 2), bone marrow [46] and umbilical cord blood (UCB) [17] for MSCs. These sources are being used by researchers around the world for their proper isolation, characterization and differentiation potential both in vivo and in vitro. Many cell surface and intracellular markers have been identified for the characterization of NSCs isolated from various sources. But still there is need to search for more accurate, reliable and specific marker to identify NSCs and its lineages differentiated form NSCs, MSCs or Hematopoietic stem cells (HSCs), etc. Genetic and molecular biology techniques are extensively used to study how cells become specialized in the organism’s development. In doing so, researchers have identified genes and transcription factors (proteins found within cells that regulate a gene’s activity) that are unique in stem cells.
Fig. 2.
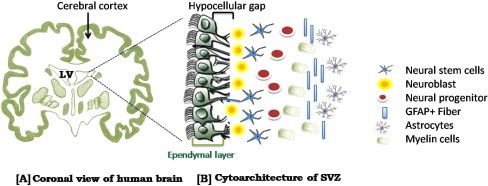
Subventricular zone of adult human brain. (A) Coronal view showing the lateral ventricles showing the cellular composition and cytoarchitecture of SVZ, consisting of ependymal cell layer, hypocellular gap, astrocytic ribbon containing astrocytes and migrating neuroblasts and transitional zone separating the SVZ from the striatum rich in neurons.
Neurosphere/Monolayer culture
In order to isolate and expand NSCs, Reynolds and Weiss developed the neurosphere assay [10] which is the most common way to expand human NSCs in vitro. A neurosphere is a free-floating, spherical cell aggregate potentially generated from one single cell responsive to epidermal growth factor (EGF) and/or basic-fibroblast growth factor (bFGF) to divide and generating daughter cells that are also responsive to these mitogens, forming a sphere in a controlled environment of 5% CO2 and 37 °C temperature [47]. Neurosphere cultures are considerably heterogeneous by nature. The neurosphere assay can be used to assess the stem cell characteristics of self-renewal and multipotency [48]. To test for self-renewal, clonally derived neurospheres are dissociated and then replated at clonal density, in order to determine the cells’ capacity to form new spheres, so called secondary sphere formation. To test for multipotency, clonally derived neurospheres are cultured under differentiating conditions, in order to monitor the ability of these cells to generate the three main cell types of the CNS, i.e. neurons, astrocytes and oligodendrocytes [49].
Human NSCs can also be expanded as attached monolayer cultures in the same environment of 37 °C temperature and 5% CO2 [50]. Clonogenic assays, to establish stem cell properties, are more difficult in attached monolayer cultures than for neurosphere cultures, but have been achieved by tagging individual cells with retroviral vectors [44,51]. Adherent NSCs from fetal and adult forebrain generate a quite homogenous population as assessed by both molecular and morphological methods. Monolayer cultures have until recently not been very successful for long-term culturing of human NSCs, unless the cells were immortalized. However, the addition of the mitogens EGF and FGF-2 to the defined and refined medium seems to reduce the rate of apoptosis and sustain the proliferative capacity of these cells for long-term [52].
Characterization of NSCs
It is essential to thoroughly characterize the NSCs before starting treatment, since isolated NSCs can become tumorigenic after serial passaging and transplantation [53,54]. Over the past few decades hematopoietic stem cells (HSCs) and progenitor cells have been identified by using monoclonal antibodies directed against their surface markers, which allows rare populations of cells to be enriched while remaining viable. In contrast to the detailed studies that have advanced our understanding of HSCs, the lack of effective methodologies for the prospective identification or purification of NSCs has slowed research into their biology to be defined experimentally as neurosphere-initiating cells [10].
Researchers have also applied a genetic engineering approach that uses fluorescence, but is not dependent on cell surface markers. The importance of this new technique is that it allows the tracking of stem cells as they differentiate or become specialized. Scientists have inserted into a stem cell a “reporter gene” called green fluorescent protein (GFP) [55]. The gene is only activated or “reports” when cells are undifferentiated and is turned off once they become specialized. Once activated, the gene directs the stem cells to produce a protein that fluorescence in a brilliant green color. This gene encodes a C2H2 zinc-finger protein. Its highly-specific expression in pluripotent stem cells has been confirmed in mouse and human ES cells [56], making it one of the most famous markers of pluripotency tested in various stem cells such as multipotent adult progenitor cells [57] and amniotic fluid cells [58]. Researchers are now coupling this reporting method with the fluorescence activated cell sorting (FACS) and microscopic methods described earlier to sort cells, identify them in tissues, and now, track them as they differentiate or become specialized.
Rapid advances in the stem cell biology have raised appealing possibilities of replacing damaged or lost neural cells by transplantation of in vitro-expanded stem cells and/or their neuronal progeny. However, sources of stem cells, large scale expansion, control of the differentiations, and tracking in vivo represent formidable challenges. The ability to identify hNSCs by brain imaging may have profound implications for diagnostic, prognostic, and therapeutic purposes. Currently, there are no clinical, high-resolution imaging techniques that enable investigations of the survival, migration, fate, and function of unlabeled NSC and their progeny. Noninvasive tracking methods that have been successfully used for the visualization of blood-derived progenitor cells include magnetic resonance imaging and radionuclide imaging using single-photon emission computed tomography (SPECT) and positron emission tomography (PET). The SPECT tracer In-111-oxine is suitable for stem cell labeling, but for studies in small animals, the higher sensitivity and facile quantification that can be obtained with PET are preferred [59].
These discovery tools are commonly used in research laboratories and clinics today, and will likely play important roles in advancing stem cell research. There are limitations, however. One of them is that single marker identifying pluripotent stem cells, those stem cells that can make any other cell, has yet to be found. As new types of stem cells are identified and research applications of them become increasingly complex, more sophisticated tools will be developed to meet investigators’ needs. For the foreseeable future, markers will continue to play a major role in the rapidly evolving world of stem cell biology.
The first example of immune-selection using a surface antigen was reported by Johansson et al. [60], who used an antibody to Notch1 to enrich NSCs from the adult mouse brain. Subsequently, Uchida et al. [61] succeeded in isolating a population enriched for human fetal NSCs by sorting CD133+, CD34-, CD45- cells. Neurospheres on repeated passages produce self-renewing, proliferating and differentiating cells, typically presenting prominin-1 cell surface antigen (CD133) and these cells are uniquely separated from the heterogenous cell population directly by magnetic beads conjugated with antibodies (MACS) or FACS by negative selection of CD34- and CD45- antigen markers cells (CD133+ CD34-CD45-). A list of positive and negative markers used to identify NSCs and its lineages is listed in Table 2.
Table 2.
NSCs, NPCs and its lineage specific markers.
| Type of cells | Positive markers | Negative markers |
|---|---|---|
| NSCs | Prominin-1 (CD133), CD56 (NCAM), Nestin, Sox-2, Oct-4, Notch-2, ABCB1, ABCG2, RBP1, RBP2, RBP7, HSPA4, HSPA9, HSPA14 | CD34, CD45 |
| Neuronal progenitors | PSNCAM, P75 Neurotrophin Receptor | – |
| Astrocytes progenitors | CD44, A2B5 | – |
| Olidodendrocyte progenitors | NG2, PDGFR-α, Olig-2 | – |
| Neurons | MAP-2, Doublecortin (DCX), β-tubulin III, RNA Binding Protein (HuC), Neuro D, Neu N | – |
| Astrocytes | GFAP | – |
| Oligodendrocytes | Olig-1, Olig-4, Galactocerebrocide (Gal C) | – |
NSCs have been identified to differentiate into neuronal and glial cell lineages. To evaluate the differentiation capacity of NSCs, normally the cells are exposed to differentiation signals coming from animal serum at varying concentration of 1–10% [44] or chemically defined compounds such as Poly-l-Ornithin, laminin or matrigel [43,49]. In some cases removal of growth factors in conjugation with an adherent substrate has been also added to promote NSCs differentiation [47]. Still there is need for improved differentiation and enrichment procedures to get the highly pure populations of NSCs, glia and neurons. One way to address this problem is to identify cell-surface signatures that enable the isolation of these cell types from heterogeneous cell populations by various techniques. Cell surface marker expression has been described for the identification and isolation of many neural cell types by FACS from embryonic and adult tissue from multiple species. The glycoprotein CD133 is a known stem/progenitor cell marker in many tissues and has been used to isolate NSCs from human brain [62]. In early 1970s neurobiologist kept their efforts to establish a battery of neural cell-specific markers which would serve studies of lineage and functional identification both in vivo and in vitro. Antibodies developed for intermediate filament proteins have been extensively used for cell identification such as neurons can be characterized by their associated neurofilament protein Tuj1 (β tubulin-III) [63,64], astrocytes by glial fibrillary acidic proteins (GFAP) [65] and oligodendrocytes by O4 [66]. In brief, advances in understanding the structure and role of cell-specific markers have greatly increased their usefulness in that they will allow functional aspects of the brain to be studied in its developments, differentiation and diseased states.
Major breakthroughs in stem cell research were made by the identification of proteins such as colony-stimulating factors (CSFs) and cell-surface CD molecules. Proteins are key players inside the cell. They have several diversified features that are not easily predictable from gene sequences or transcription levels. Therefore, proteome analysis is needed to analysis their properties. Both basic and clinically oriented stem cell research are confronted with many open questions that can be most efficiently answered by proteomics. For instance, the cell surface proteins and signaling cascades of stem cells and their differentiated progenies are largely unknown, as are the differentiation-specific proteins that can be used as biomarkers of the intermediate or terminal steps of cell differentiation, or discriminate tumorigenic cells from the pool [67]. However, as a corollary, great caution must be exercised in the interpretation of changes in the expression of such markers.
Role of human NSCs transplantation therapy in the treatment of neurodegenerative diseases and stroke
The nervous system, unlike many other tissues, has a limited capacity for self-repair; mature nerve cells lack the ability to regenerate, and NSCs, although they exist in the adult brain, have a limited ability to generate new functional neurons in response to injury. For this reason, there is great interest in the possibility of repairing the nervous system by transplanting new cells that can replace those lost through damage or disease. NSCs are extensively found in three areas of brain, the SVZ of the lateral ventricle, the external germinal layer of the cerebellum and the subgrannular zone of dentate gyrus [32,68,69]. These sources of progenitor population can be widely employed in the treatment of neurological disorders [70]. In one report, it has been estimated that ∼1.3 million people suffer from spinal cord injuries in USA [71]. As far as Indian sub continent is concerned, it is reported that every year India gets over 20,000 cases of spinal cord injury patients [72]. New insights into the biology of NSCs have raised significant use of these cells for the treatment of various neurological diseases, stroke and gliomas. Various reports and data in animal models of neurologic diseases suggest that transplanted NSCs may also attenuate deleterious inflammation, protect the CNS from degeneration, and enhance endogenous recovery processes.
Over the past few years, there has been continuous progress in developing approaches to generate the types of human-derived neurons and glial cells that are needed for cell replacement therapy based on pathology in the respective diseases. Patient’s specific cells that may be useful for transplantation can now be produced from iPSCs [73–75]. The objective for the grafted NSCs is either to stimulate and/or support the proliferation, survival, migration, and differentiation of endogenous cells, or, to replace the dying or dead endogenous cells. In the prospect of cell replacement therapy, the implanted NSCs must be able to survive and generate new neurons of the appropriate types that functionally integrate into the damaged host brain circuitry.
Stem cell-based approaches using umbilical cord blood, bone marrow- derived HSCs and MSCs have already been applied in patients with spinal cord injury (SCI), with claims of partial recovery [76]. Recent progresses in research on human fetal brain derived neural precursors clearly indicate that they may play a very potential role in cell transplantation therapy for neuronal regeneration in human brain and spinal cord. Intrastriatal transplantation of human fetal primary tissue, which is rich in post mitotic neurons and glia cells, has in clinical trials provided proof-of-principle that neuronal replacement can work in the human diseased brain [77] (Table 3).
Table 3.
Some recent clinical trials using human neural stem cells for treating neurological diseases.
| Trial title | Trial no. | Status | Duration | Country | Outcome measure |
|---|---|---|---|---|---|
| Human neural stem cell transplantation in amyotrophic lateral sclerosis (ALS) | NCT01640067 | Recruiting | 2012–2016 | Italy | To verify safety and tolerability of expanded human fetal neural stem cells |
| Human spinal cord derived neural stem cell transplantation for the treatment of Amyotrophic Lateral Sclerosis (ALS) | NCT01348451 | Active, not recruiting | 2009–2013 | USA | To determine the safety and measurement of incidence for adverse events in the ALS |
| The long-term safety and efficacy follow-up study of subjects who completed the phase I Clinical Trial of Neurostem®-AD | NCT01696591 | Recruiting | 2012–2013 | Republic of Korea | Changes in Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog) and Caregiver-administered Neuropsuchiatric Inventory |
| Molecular analysis of human neural stem cells | NCT01329926 | Enrolling by invitation | 2011–2014 | USA | Neuronal differentiation into dopaminergic neurons in Parkinson’s Diseased Brain |
A recent paradigm shift has emerged suggesting that the beneficial effects of stem cells may not be restricted to cell restoration alone, but also due to their transient paracrine actions. NSCs can secrete potent combinations of trophic factors that modulate the molecular composition of the environment to evoke responses from resident cells and have been implicated in repair and regeneration of the CNS injury. Recent directions in research on neurodegenerative diseases have aimed to elucidate the production of neurotrophic factors, and subsequent neuroprotective properties of neural stem cells. There is evidence suggesting that human neural stem cells (NSCs), human UCBs and murine BM-MSCs secrete glial cell- and brain-derived neurotrophic factors (GDNF and BDNF), IGF-1 and VEGF, which may protect dysfunctional motor neurons, thereby prolonging the lifespan of the animal into which they are transplanted in animal models of neurodegenerative diseases [78–81]. The secretion of GDNF, BDNF and NGF by NSCs has been implicated in increased dopaminergic neuron survival in in vitro and in vivo models of PD, and the release of anti-inflammatory molecules has been shown to attenuate microglia activation, thereby protecting dopaminergic neurons from death [82]. Based on this new insight, current research directions include efforts to elucidate, augment and harness NSCs paracrine mechanisms for CNS tissue regeneration (Table 4).
Table 4.
Summary of pathophysiology and NSCs based approaches for neurodegenerative diseases and stroke.
| S.no. | Disease | Cause | Symptoms | Available treatments | New strategies for treatment | ||
|---|---|---|---|---|---|---|---|
| 1 | Parkinson | Degeneration of dopaminergic neurons | Hypokinesia, Tremor, Rigidity, postural instability | DA Antagonists, Enzyme inhibitors, Deep brain stimulation, etc. | Transplantation of hNSCs or dopaminergic neurons into striatum or substantia nigra | ||
| 2 | Alzheimer | Impaired formation of hippocampal neurons in subgranular zone of the dentate gyrus | Memory impairement, cognitive decline, dementia | β-amyloid immunotherapy | Transplantation of hNSCs or basal fibroblast producing NGF or BDNF | ||
| 3 | Spinal cord injury | Loss of neurons and glia, scar formation, demyelination | Loss of movement, sensation and control below the injured spinal segment | No pharmacological treatment | Transplantation of OPCs, BMSCs and hNSCs | ||
| 4 | Huntington | Defective huntingtin protein, Progressive neurodegeneration in striatum and cortex | Loss of motor function, decline in mental abilities and behavioral and psychiatric problems | Fluoxetine, sertraline, nortriptyline | Transplantation of hNSCs producing GDNF into the striatum | ||
| 5 | ALS | Weakness of cerebral cortex and brain stem muscles | Muscle atrophy and fasciculations, muscle spasticity, dysarthria, dysphagia | Riluzole (Rilutek), trihexyphenidyl or amitriptyline | Delivery of motor neurons, hNSCs and hMSCs at multiple sites along the spinal cord | ||
| 6 | Multiple sclerosis | Demyelination of neurons | Hypoesthesia, paresthesia, ataxia, dysarthria | Fingolimod (Gilenya) | Transplantation of hNSCs at the site of injury | ||
| 7 | Brain tumor | Uncontrolled cell division in brain | Intracranial hypertension cognitive and behavioral impairment | Surgery radiotherapy chemotherapy | Modified NSCs to produce necessary cytokines | ||
| 8 | Stroke | Ischemic | Ebolic | Formation of embolus in any part of the body which travels in the blood vessel | Motor, sensory or cognitive impairments’, Loss of consciousness, headache, and vomiting | Tissue plasminogen activator (t-PA) and Aspirin | Cell replacement therapy using hNSCs or MSCs |
| Thrombolic | Formation of clot within the blood vessel | ||||||
| Hemorrhage | Intracerebral bleeding caused by the rupture of a vessel in the brain | ||||||
Parkinson’s disease (PD)
Degeneration of nigrostriatal dopaminergic neurons is the main pathology in PD, although other dopaminergic (DA) and non-dopaminergic systems are also affected. Rigidity, hypokinesia, tremor, and postural instability are the characteristic symptoms of PD. Although motor symptoms can be treated relatively well with l-3,4-dihydrophenylalanin (l-DOPA), DA agonists, enzyme inhibitors, and deep brain stimulation, effective therapies for non-motor symptoms, such as dementia, are lacking, and disease progression cannot be counteracted [83]. Cell transplantation to replace lost neurons is a new approach to the treatment of progressive neurodegenerative diseases (Fig. 3) [84]. Clinical trials with intrastriatal transplantation of human embryonic mesencephalic tissue, which is rich in postmitotic DA neuroblasts, have provided proof of principle that neuronal replacement can work in PD patients [85]. Replacement of dopaminergic neurons in patients with PD has spearheaded the development of this approach and was the first transplantation therapy to be tested in the clinic [86]. Experimental data from rodents and nonhuman primates demonstrated that dopaminergic neurons derived from fetal ventral mesencephalon formed synaptic contacts, released dopamine, and ameliorated PD-like symptoms when grafted intrastriatally. From these early studies many questions have raised regarding the adverse effect, graft rejection, optimal site of grafting and dose of cell delivery which need to be solved before cell therapy. Human fetal derived neural progenitors may provide first line treatment giving answers of these questions for repairing damaged neuronal circuitry and constitute a chance for patients who no longer derive benefit from pharmacological therapy. Fetal tissues transplantation have provided convincing evidence that midbrain dopaminergic can survive long term in patients with PD and can produce functionally relevant changes in dopaminergic functions [87]. The adult brain provides limited support for neuronal differentiation, migration, and synaptic integration, and the process of transplantation itself might reduce survival by the induction of an inflammatory response. In contrast to this fetal dopaminergic neurons transplanted into the neonatal substantia nigra have the ability to regrow axons into the striatum [88]. In the clinical perspectives some individuals who have received transplants of fetal dopaminergic neurons have shown clinical benefit-that is beyond any doubt [10,89].
Fig. 3.
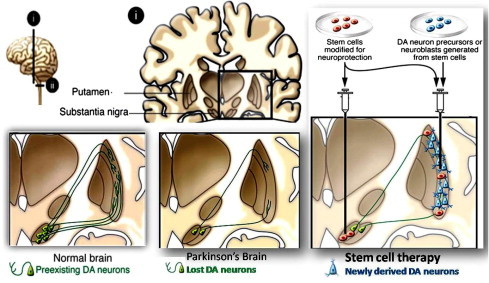
Pathology of Parkinson’s disease and NSCs based approach for cellular therapy.
Depression
In depression patient’s show a reduction in neurogenesis that may contribute to debilitating psychological symptoms such as low moods or impaired memory. It may occur only once in a person’s lifetime, but more often it recurs throughout the life. In preclinical work, Neuralstem’s lead pharmaceutical compound, NSI-189, demonstrated clear evidence of increased hippocampal volume in animals with a model of depression. Neuralstem believes NSI-189 has the potential to reverse the hippocampal atrophy associated with major depressive disorder and other related disorders, and to restore fundamental brain physiology (Fig. 4).
Fig. 4.
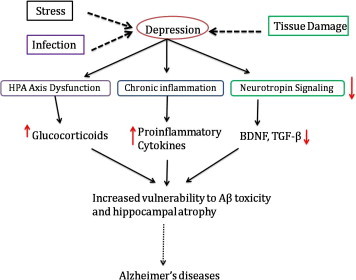
Pathology of Depression leading to the Alzheimer’s disease.
It is one of the most important causes of disability worldwide [90]. The high rate of inadequate treatment of the disorder remains a serious concern [91]. One of the cardinal features of depression is its recurrent nature. Some patients experience regular or periodic recurrence, whereas in other patients recurrence is aperiodic. It is tempting to speculate that such variation in mood might be attributable to the waning and waxing of some neural process in the brain.
Chronic stress, infections, tissue damage and adverse events of life has been subject of numerous investigations on the development of depression and the work has been influenced by studies of the somatic and endocrine consequences of stress in animals [92]. Life events preceding depression are variable and there is no clear cut difference in the presence of events provoking the onset of endogenous or non endogenous depression [93]. Epidemiological evidences for a genetic contribution, especially for bipolar disorders, and heritability is estimated to be as high as 80% in depression [94]. However, the inheritance does not follow the classical mendelian pattern, which suggests that a single major gene locus may not—or at least only in few families—account for the increased intra-familial risk for the disorder.
The first-generation anti-depressants such as tricyclic anti-depressants (TCAs) and MAO inhibitors (MAOIs) have been shown to be effective in alleviating the symptoms of depression. Although both types of drugs have been used with great success for many years, there are several undesirable side effects that limit their application. Therefore various attempts are being made towards stem cell therapy to overcome the side-effects of pharmacological treatment regimes and other complications.
Alzheimer disease (AD)
Alzheimer’s disease (AD) is the most common cause of dementia in the elderly. The disease usually becomes clinically apparent as insidious impairment of higher intellectual function with alteration in mood and behavior. Later, progressive disorientation, memory loss, and aphasia become manifested, indicating sever cortical disfunction. While pathogenic examination of the brain tissue remains necessary for the definitive diagnosis of Alzheimer’s disease, the combination of clinical assessment and modern radiological methods allows accurate diagnosis in 80–90% of cases. The neuropathological hallmarks of AD include “positive” lesions such as amyloid plaques and cerebral amyloid angiopathy, neurofibrillary tangles and glial responses, and “negative” lesions such as neuronal and synaptic loss [95]. The disease symptoms in AD could partly be due to impaired formation of new hippocampal neurons from endogenous NSCs in the subgranular zone of the dentate gyrus, which is believed to contribute to mood regulation, learning, and memory [96]. Memory impairment, cognitive decline, dementia, neuronal and synaptic loss, neurofibrillary tangles, and deposits of β-amyloid protein in senile plaques involve the basal forebrain cholinergic system, amygdala, hippocampus, and cortical areas in AD patients. The situation for neuronal replacement aiming at functional restoration in AD is extremely complex because the NSCs would have to be predifferentiated in vitro to many different types of neuroblasts for subsequent implantation in a large number of brain areas. However, to give long-lasting symptomatic benefit, a cholinergic cell replacement approach would require intact target cells, host neurons that the new cholinergic neurons can act on, and they are probably damaged in AD (Fig. 5).
Fig. 5.
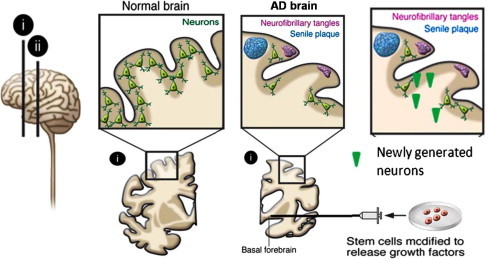
Pathology of Alzheimer’s disease and NSCs based approach for cellular therapy.
Approaches to enhance neurogenesis and/or maturation could be considered potential NSCs–based therapies for AD. Clearance of brain β-amyloid has been proposed to be of value in halting disease progression in AD. Active β-amyloid vaccination in young AD mice, using as antigen a sequence of the β-amyloid peptide, decreased β-amyloid burden and increased hippocampal neurogenesis [97]. The findings indicate that AD disturbs hippocampal neurogenesis, which may contribute to the cognitive deficits experienced by patients, suggesting that normalization of the formation and maturation of new hippocampal neurons, for example, by active or passive β-amyloid immunotherapy, could have therapeutic potential.
Stem cell–based gene therapy could deliver factors modifying the course of AD and may be advantageous because of the capacity of stem cells to migrate and reach large areas of the brain [9,98]. Preclinical studies that provide a rationale for this approach include one demonstrating that basal forebrain grafts of fibroblasts producing nerve growth factor (NGF), which counteracts cholinergic neuronal death, stimulate cell function and improve memory in animal models of AD [99]. Stem cells could also be engineered to carry other genes, such as that encoding BDNF, which has substantial neuroprotective effects in AD models [100].
Spinal cord injury (SCI) repair
The spinal cord has been an attractive target for cell-based therapy, in part because of the dearth of available treatment options for spinal cord injury (SCI), but also because of the rapid pace of advance in our understanding of how to effect axonal regeneration in the injured cord [101–103], without which cellular replacement alone would be of limited benefit. Pathological changes after spinal cord injury are complex and include interruption of ascending and descending pathways, loss of neurons and glial cells, inflammation, scar formation, and demyelination. Loss of movement, sensation, and autonomic control below the level of the injured spinal segment. Pharmacological treatments are not effective in the treatment. A wide array of stem cell transplantation strategies has been tested for the potential to promote recovery in animal models of SCI. These include, but are not limited to, oligodendrocytes progenitor cells (OPCs) [104,105], bone marrow stromal cells [106], Schwann cells [107], genetically-modified fibroblasts [108], embryonic-derived neural/glial stem cells [109], and fetal/adult NSCs [110]. While cell transplantation in many of these studies has been reported to result in improved recovery of function, engraftment and survival of the transplanted population have been, in most cases, either partially investigated or not addressed at all.
Different types of stem cells have been transplanted in injured spinal cord and improved functional outcome in animal models [76,111] probably through secretion of neutrotrophic factors, remyelination of spared axons, or modulation of inflammation. In clinical perspective, transplanted hNSCs should give rise to matured neurons and oligodendrocytes to promote functional recovery. Before neuronal replacement strategies can be applied in patients with spinal cord injury, it must be determined proliferation, differentiation into specific types of neurons that can be directed to form appropriate synaptic contacts (Fig. 6). High-purity OPCs generated from human embryonic stem cells in vitro have been shown to differentiate into oligodendrocytes and give rise to remyelination after transplantation into the demyelinated mouse spinal cord [112]. Recent studies on hNSCs transplantation into injured mouse spinal cord have provided significant improvement to generate neurons and oligodendrocytes and induced locomotor recovery [113]. In another study human hNSCs transplanted into the injured rat spinal cord were found to differentiate into neurons that found exons and synapses and establish contacts with post motor neurons [114]. Stem cell-based approaches using umbilical cord blood, bone marrow-derived HSCs, and NSCs have already been applied in patients with a spinal cord injury, with claims of partial recovery [76]. How such approaches can be scaled up from rodents to humans and adapted to optimize the functional efficacy of hNSCs transplanted must also be determined prior to application in patients.
Fig. 6.
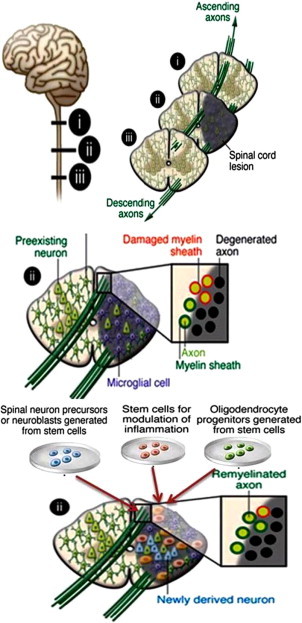
NSCs based approach of cellular therapy for Spinal cord injury repair.
Huntington’s disease (HD)
Huntington’s disease is an inherited fatal disorder in which the abnormal processing of the defective huntingtin protein results in progressive neurodegeneration, particularly in striatum and cortex. Many of the symptoms of HD result from the loss of inhibitory connections from the striatum to other structures such as the globus pallidus. Multiple animal models of HD have been developed to evaluate hNSCs therapy as a potential treatment for the disease [115]. The commonly used R6/1 and -2 mouse models express exon 1 of huntingtin with different CAG repeats, providing a close reproduction of HD [116].
The generation of these models involves the injection of glutamic acid analogs (e.g., kainic, ibotenic, and quinolinic acids) into the animal’s striatum to cause the excitotoxic cell death of neurons in this structure and thus produce characteristics similar to those of HD [18]. Human NSCs are then injected intravenously and home to the injury site, where they reduce striatal atrophy, differentiate into neurons, and improve functional outcome [117]. In a rat model of HD, involving the stereotactic transplantation of human fetal NSCs (identified by neurosphere formation) into the striatum, motor function improved [118].
Many clinical trails using intrastriatal implantation of fetal striatal tissues have done for the treatment of HD [119,120]. Recently researchers have identified that genetically modified hNSCs that produce glial cell derived neurotrophic factor (GDNF) protect striatal neurons from degeneration [121].
Amyotrophic lateral sclerosis (ALS)
Amyotrophy refers to the atrophy of muscle fibers, which are denervated as their corresponding anterior horn cells degenerate. Lateral sclerosis refers to hardening of the anterior and lateral columns of the spinal cord as motor neurons in these areas degenerate and is replaced by fibrous astrocytes. Current research has focused on excitotoxicity into the mechanisms resulting in sporadic and familial types of ALS. This may occur secondary to overactivation of glutamate receptors, autoimmunity to calcium ion channels, oxidative stress linked to free radical formation, or even cytoskeleton abnormalities such as intracellular accumulation of neurofilaments. Apoptosis has emerged as a significant pathogenic factor, and evidence suggests that insufficient vascular endothelial growth factor may also be a risk factor for ALS in humans. However, no direct mechanism has been identified and most researchers and clinicians agree that various factors, possibly a combination of some or all of the above processes, may lead to development of ALS.
Due to abnormal function and degeneration of motor neurons in the spinal cord, cerebral cortex, and brain stem muscle weakness and death occurs within a few years in ALS. Yet there is no effective treatment for ALS. Motor neurons have been generated in vitro from stem cells from various sources, including mouse and human embryonic stem (ES) cells [122], NSCs derived from fetal rat spinal cord [123], human fetal forebrain and human iPS cells [73]. For stem cell-based therapies in ALS patients it must be shown that the cells can be delivered at multiple sites along the spinal cord, integration of stem-cell derived motor neurons into existing spinal cord neural circuitries, receive appropriate regulatory input, and able to extend there axons long distances to reinnarvate muscles in humans. It also must be established that the differentiation of the spinal motor neurons can be directed to the correct cervical, thoracic, or lumber phenotype and that the final cell population projects to axial or limb muscles. For the treatment of ALS using motor neurons such as carticospinal neurons, which degenerate in ALS, also should be replaced for effective and life saving restoration of function (Fig. 7). In several reports glial cells carrying an ALS-causing genetic mutation impair the survival of human ES cell-derived motor neurons in culture [124]. Human fetal NSCs transplanted into the spinal cord in a rat model of ALS have been found to protect motor neurons and delay disease on set [80], probably has a result of their neuronal progeny releasing GDNF and brain derived neurotrophic factor (BDNF), dampening excitotoxicity, or both. In another report cortical, GDNF-secreting hNSCs have been shown to survive implantation into the spinal cord in a rat modal of ALS, migrate into degenerating areas, and increase motor neuron survival although they did not improve limb function due to a lack of continued innervations of muscle end plates [125,126]. Compared with direct gene transfer, an advantage of cell based therapy is that production of the trophic factor continues even if the disease process destroys the endogenous cells. HSCs transplantation or delivery of MSCs in order to alter the inflammation environment has already reached the clinic. Several studies clearly demonstrate the improved therapy in ALS using MSCs as well as hNSCs but more preclinical studies are needed prior to further patient application.
Fig. 7.
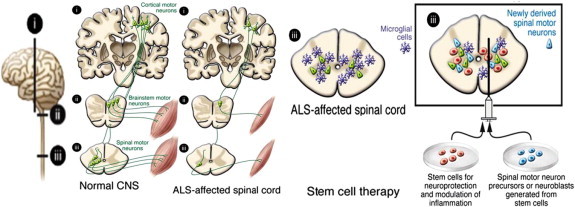
NSCs based therapy for Amyotrophic lateral sclerosis (ALS).
Multiple sclerosis (MS)
Multiple Sclerosis (MS) is an inflammatory autoimmune and demyelinating disease of the CNS. Demyelization causes messages to and from the brain to be slowed, distorted or stopped altogether leading to the MS symptoms. There are two approaches that might be able to correct this damage. One is to give drugs that make the NSCs already present work more effectively. The other is to transplant new cells that will repair the damage that the resident brain stem cells cannot. NSCs are likely to be believed that they can have an effect through immunomodulation and a direct effect on demyelization (Fig. 8). For the second approach various animal model studies has been provided good improvement in NSCs based therapies for MS.
Fig. 8.
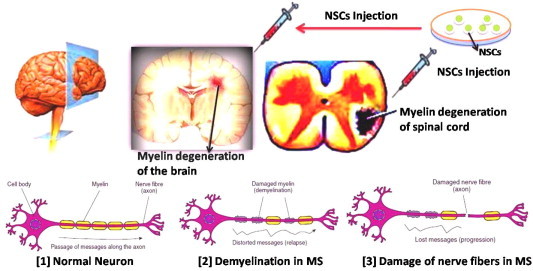
Pathology and NSCs based cell therapy for multiple sclerosis in brain and spinal cord.
One of the animal models of MS relies on the generation of lesions in white matter tracts of the brain of rodents using lysolecithin injections [127]. This model has been used to study the survival and migration of transplanted progenitor cells to the site of lesion and has shown that these cells survive and migrate to injured areas in the brain, differentiate into oligodendrocytes, and start remyelination at the site of disease [128]. Another widely used animal model of MS is the experimental autoimmune encephalomyelitis (EAE), in which inflammatory demyelination is generated after the injection of an encephalitogenic peptide [129]. In a rat model that uses X-ray irradiation and Ethidium bromide exposure of the rat spinal cord, transplanteation of human SVZ-derived NSCs of patients with glioblastoma multiforme led to remyelination of axons and recovery in the conduction velocities [130]. In a landmark study of Pluchino et al. (2003) has demonstrated that adult neural precursor cells promote multifocal remyelination and functional recovery after intravenous or intrathecal injection in a chronic model of multiple sclerosis [131]. This study revealed the functional impairment caused by EAE was almost abolished in transplanted mice, both clinically and neurophysiologically. These results with MS animal models show that transplanted hNSCs can improve disease progression and integrate into injured tissues to aid in regeneration.
Brain tumors
Endogenous NSCs home to sites of tumor cell implantation in a murine model of glioblastoma; this accumulation of NSCs around the tumor correlates with the formation of smaller tumors and longer survival [123]. Gliomas are the most frequent type of brain tumor and have a miserable prognosis. Depending upon the presence of malignant features such as high cellularity, nuclear atypia, mitosis, necrosis and endothelial proliferation, gliomas have been classified into four grades: I PA (pilocytic astrocytomas), II (low grade), III (anaplastic) and IV GBM (glioblastoma). The prognosis of gliomas varies with histological subtypes and grades therefore complete surgical resection of gliomas is virtually impossible due to their invasive growth pattern. In adults glioblastoma multiforme (GBM) is the most common and malignant primary brain tumor, representing up to 50% of all primary brain gliomas. The poor prognosis for glioma patients has not improved significantly over decades and need to develop new strategies for the treatment in order to achieve maximal efficacy with minimal toxicity or side effects.
In the era of therapies designed to target specific molecular lesions, a classification based on histopathology does not provide sufficient insight for patient stratification. Therefore, great efforts have been made to incorporate new information about the molecular landscape of gliomas into novel classifications that may potentially guide the treatment strategies. The cancer stem-cell hypothesis proposes that malignant tumors are likely to encompass a cellular hierarchy that parallels normal tissue and may be responsible for the maintenance and recurrence of glioblastoma multiforme (GBM) in patients. Identification and characterization of these cells may provide better understanding for glioma formation and their treatment strategies. Glass and colleagues (2005) also suggested an antitumorigenic property of NSCs by inducing apoptosis of tumor cells [132]. Exogenous NSCs migrate to gliomas whether implanted in the brain or administered intravenously [133].
NSCs can be modified to deliver different therapeutic molecules [121]. In the case of brain tumors, NSCs have been engineered to express cytosine deaminase [133] and thymidine kinase [134], two prodrug-converting enzymes; these studies showed that administration of engineered NSCs and the prodrug can decrease tumor growth. These studies provide important evidence of another application for NSC-based therapy of neurologic disease.
Stroke
Stroke is the leading cause of disability, and third leading cause of death, in the industrialized world after cardiovascular disease and cancer [135]. The pathology in stroke is very dynamic with initially necrotic that gradually is replaced by apoptosis as well as the inflammatory response following the insult. Stroke leads to motor, sensory or cognitive impairments. There are no effective treatments in the sub-acute phase after a stroke and, therefore, any kind of treatment beneficial for recovery is valuable. Stroke can be divided into two major types depending on the cause: ischemic and hemorrhagic. Hemorrhagic stroke results from intracerebral bleeding caused by a rupture of a vessel in the brain, which can cause physical damage to the brain due to the build-up of pressure. Ischemic stroke can in turn further be subdivided into embolic and thrombotic. Thrombotic stroke is due to a clot gradually forming within a vessel, while embolic stroke is results from an embolus formed somewhere in the body that travels through the blood stream, and blocks a vessel within the brain.
The brain has the highest demand of glucose and oxygen in the body and is therefore particularly sensitive to reduced blood flow. Due to the deprivation of glucose and oxygen caused by the stroke, a chain of detrimental events, including cell respiration failure, uncontrolled glutamate release, cellular edema, accumulation of free radical species, takes place, leading to cell death [136]. Within the ischemic core, cells quickly die through necrosis, which does not require any energy, is uncontrolled and usually involves several cells simultaneously [137]. In the tissue peripherally surrounding the ischemic core, the ischemic penumbra, cells gradually die through apoptosis, which is an individual cell’s execution of an internal suicide program. Apoptosis is either intrinsically or extrinsically activated and is ongoing for at least several days after the insult in parallel to inflammation [138].
Current treatments for stroke are very limited; focusing on removal of the clot in the acute phase, either by thrombolysis alone or in combination with mechanical removal of the clot [139] and not a single treatment has been successful in reversing the effects of the chronic stroke. Physical therapies are often used to promote functional recovery in long-term stroke patients, but recovery is still incomplete. Therefore, reversal of symptoms after a chronic stroke is a daunting problem that requires the improvement of the patient’s lost function achieved by the replacement of lost neurons and glia in the injured region, as well as the establishment of new functional connections. These requirements call for bold new treatments that induce new neural cells to differentiate and integrate into the circuitry that was damaged by the stroke, the cell replacement therapy (Fig. 9).
Fig. 9.
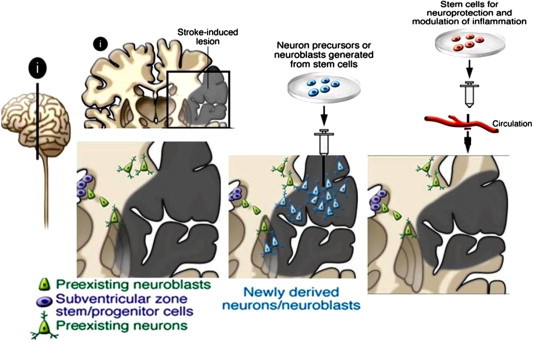
Pathology of stroke in brain and NSCs based cellular therapy.
Over the past few years large amount of data has been acquired on NSCs treatment in animal models of stroke that confirms that NSCs are present throughout life and that thousands of neurons are born on a daily basis in specific zones of the brain, the SVZ, Dentate gyrus, hippocampus, etc. [96]. In general, two broad approaches are needed to be considered simultaneously in the development of cell replacement therapy in stroke: the recruitment of endogenous NSCs and exogenous stem cells transplanted into the affected area. There are a lot of growth factors secreted by NSCs that has the potential to be implemented as a therapeutic tool in the recovery process after a stroke event, exploiting their neuroprotection and neurogenesis features [140]. In several studies NSCs derived from fetal brain tissue have been transplanted showing some recovery in animal models of stroke [141–143].
A variety of cell types have been tested in stroke models such as human bone marrow cells, human umbilical cord blood cells [144–146], rat trophic factor-secreting kidney cells [146], and immortalized cell lines such as the human neuron-like NT2N (hNT) cells [147] and MHP36, an embryonic murine immortalized neuroepithelial cell line [148]. Amongst all these, NSCs have been proposed as a potential source of new cells to replace those lost due to stroke, as well as a source of trophic molecules to minimize damage and promote recovery in clinical trials [147,149].
Expert commentary
Current status of the field and recommendations for existing as well as new paradigm choices
As the sources of NSCs are limited, they need to be maintained in vitro for a long time to get the maximum number of cells with same proliferative and differentiation capacity at the clinical level. SVZ derived NSCs have vast regenerative potential providing the strong possibilities for clinical application with less ethical issues. In vitro culture methods and conditions provide an important base for NSCs applicability and specificity in a definite target of the disease. Neurosphere culture method provides a better option for this purpose and to characterize these cells and their lineages requires a better understanding before transplantation. A cocktail of CD133+, CD34-/CD45- has been proved as a better marker for identifying the NSCs both in vitro and in vivo. The paracrine effect of NSCs along with the growth factors provides more disciplinary advantage for its proliferation, engraftment and homing at the site of injury. However, a complete understanding of the components, architectures and interaction in normal as well as pathological neurogenic niche is paramount for the creation of optimal condition in vitro to expand and culture NSCs and use them in the treatment of multiple neurodegenerative diseases.
Neurodegenerative disorders share a common pathological mechanism involving aggregation and diposition of misfolded proteins, leading to progressive CNS disorders. Although type of aggregated proteins and their distribution for deposition vary from disease to disease. Understanding the biology of major neurological disorders is a challenging scientific problem with enormous sociological and clinical relevance. The discovery of anti-depressant drugs and the investigation of their mechanism of action have revolutionized our understanding of neuronal functioning and the possible mechanisms. In spite of all the progress that has been achieved in the last decades, we must be aware that there are still today considerable problems in understanding and treating severe neurological disorders, and knowing the cause of treatment-resistant.
Although NSCs cell therapy seems to be a promising development for a number on neurological diseases, transplantation of NSC into the CNS is from an immunological viewpoint a true challenge both for the host as well as for the donor cells. Moreover more experiments are necessary to fully elucidate the mechanisms underlining the interactions between stem cells graft and host immune system.
Stem cell therapy and cell regenerative approaches to neurological diseases can be divided into a number of categories depending upon the target neurological disease. These diseases include those caused by acute injury, chronic neurodegenerative disorders, chronic inflammatory and immunologically mediated conditions, and genetic diseases that present in childhood. One of the reason for failure or neuroprotective treatments in an acute injury has been the need to start treatment early, where cell-based therapeutic approaches provide a better option. NSCs from various sources have shown effectiveness in improving motor function in several neurodegenerative diseases and stroke in animal experiments and clinical trial studies. However, factors that control the differentiation, survival, and maturation of stem cells in the context of a host degenerative brain must be more thoroughly understood before stem cell therapy will prove to be a robust and safe strategy that can be transferred to the clinic. Furthermore, long-term and large scale multicenter clinical studies are required to determine further the precise therapeutic effect of stem cell transplantation however; various ongoing clinical trails using hNSCs provide us better hope towards development of cellular therapies for neurological diseases.
The types of nervous system diseases that represent the best targets for stem cell-based therapies are those that would be improved by the transplant or induced replacement of a limited number of cell types. Parkinson’s disease, sensory disorders, and glial diseases fall into this category and could potentially be cured by a cell-replacement therapy. Motor system disorders and spinal cord injuries are more complex, but given their severity and lack of current treatment options, it can be argued that any improvement in function would be of great benefit using NSCs. There are nervous system disorders that do not currently make good targets for cell replacement therapies because the associated neurodegeneration is too widespread and diffuse. Alzheimer’s disease is one example, and it is unlikely to be ameliorated by adding more cells to the system. However, Alzheimer’s research could benefit enormously from disease-specific cells derived from NSCs that could be used to study the degeneration of neurons in vitro. The prospect of using stem cells to intervene in neurodegenerative disease is promising, but given the complexities of the nervous system, progress will likely continue in measured steps. To move the research toward useful therapies, it is critical to access the efficacy of any experiment involving human or animal subjects to be performed using a double-blind placebo-controlled method.
Although there is currently no stem cell–based treatment for diseases of the nervous system, the field has moved much closer to making this goal a reality. Progress has been driven by major jumps in our understanding of how neuronal subtypes and glia develop in vivo and in vitro. When there is a lack of rigorous controls, it is easy to falsely attribute an observed improvement to an incorrect cause. Finally, it is important to have scientific meetings to communicate both positive and negative results, as well as successes and cautionary tales. Despite the molecular differences between neurodegenerative diseases, their eventual stem cell therapies will likely share many features; Information gleaned in one field can drive forward progress in the others.
Concluding remarks
All together, NSCs research is one of the most exciting fields of modern neuroscience and there is still a long way to go before applying these cells in safety and efficacy to cure various neurodegerative diseases and stroke. It is also very important to understand the real impact of NSCs integration, engraftment and survival in normal as well as diseased brain. To this regard, the future development of more appropriate cellular therapeutic strategies will be needed to efficiently recruit endogenous or exogenous NSCs; one of the most important future challenges for basic and clinical research.
Conflict of interest
The authors have declared no conflict of interest.
Biographies

Dr. Sandeep Kumar Vishwakarma, Ph.D (Genetics,) from Osmania University, Hyderabad, Andhra Pradesh, India specializes in Stem Cell biology and Molecular Genetics. He has got excellence in gene cloning, expression, protein purification, cell culture, Neo-organogenesis, Bioinformatics and a variety of basic and advanced tools and techniques applied in biological research. With more than five years of research experience, he has spear headed neural stem cell work and published one book chapter and more than 30 articles in various reputed National and International Journals. Presently he is involved in bridging the gap between basic sciences to application of neural stem cell research and investigating new therapeutic targets in a variety of neurodegerative diseases. He has got a number of honor’s and best paper awards at both National and International levels. He is the member of several esteemed societies including “The Cytometry Society” and is involved in a variety of high quality research projects as leading investigator.

Dr. Avinash Bardia, Ph.D (Genetics) from Osmania University, Hyderabad, experiences in Molecular Biology and Microbial Genetics. He has got brilliancy in genetic analysis of microbes and animals and a variety of basic and advanced applied tools and techniques in biological research. With more than five years of research experience in basic and clinical sciences, he has published more than 20 articles in various reputed National and International Journals. He has been a recipient of several awards and honors both at National and International levels. He is the member of several esteemed societies including “The Cytometry Society” and is involved in a variety of leading research projects.

Dr. Santosh Tiwari, a doctorate in Genetics from Osmania University, Hyderabad specializes in the area of Microbial Genetics and Immunology. After spending a brief time at Cancer Genetics & Research Facility in University of Florida, USA, he made his way back to India. His passion towards writing paved way to one of the world’s leading pharmaceutical company. With more than 10 years research and a over 3 years experience as a professional scientific writer, he has more than 60 International publications to his credit. He has been a recipient of several awards and laurels both at National and International levels. He is the member of several esteemed societies including the Society for Translational and Regenerative Medicine. He has certifications from NIH, USA and Adis Wolters Kluwer, New Zealand in Clinical Research and Scientific Writing.

Dr. Syed Ameer Basha Paspala, a neurosurgeon and leading scientist in the area of neural stem cell biology has done his doctorate from JNTU, Hyderabad, Andhra Pradesh, India. He is involved in developing new therapeutic targets and stem cell therapy approach for the treatment of neurodegenerative diseases. He has published more than 20 articles in various national and international reputed journals. Presently, he is involved in bridging gap between basic and clinical research in neural stem cell biology and regeneration of brain. He has got a number of honor’s and best paper awards at both National and International levels. He is a great asset to the scientific society and man of tribute to the research perception.

Dr. Aleem Ahmed Khan, a leading scientist in hepatic stem cell biology has done his doctorate from Osmania University, Hyderabad, Andhra Pradesh, India in the area of Cell Biology and Transplantation Immunology. He has pioneered the hepatic stem cell technology for the treatment of liver cirrhosis. Recently he investigated trans-differentiation of hepatic stem cells into insulin producing pancreatic β-cells without any genetic manipulation. He has published more than 100 articles in various national and international reputed journals and has supervised more than 15 Ph.D scholars. Presently, he is involved in area of stem cell biology and neural regeneration research.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Anderson D.J. Stem cells and pattern formation in the nervous system: the possible versus the actual. Neuron. 2001;30:9–35. doi: 10.1016/s0896-6273(01)00260-4. [DOI] [PubMed] [Google Scholar]
- 2.Laywell E.D., Steindler D.A., Silver D.J. Astrocytic stem cells in the adult brain. Neurosurgery Clin North America. 2007;18:21–30. doi: 10.1016/j.nec.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Cao Q.L., Zhang Y.P., Howard R.M., Walters W.M., Tsoulfas P., Whittemore S.R. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp Neurol. 2001;167(1):48–58. doi: 10.1006/exnr.2000.7536. [DOI] [PubMed] [Google Scholar]
- 4.Shihabuddin L.S., Horner P.J., Ray J., Gage F.H. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci. 2000;20(23):8727–8735. doi: 10.1523/JNEUROSCI.20-23-08727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suhonen J.O., Peterson D.A., Ray J., Gage F.H. Differentiation of adult hippocampusderived progenitors into olfactory neurons in vivo. Nature. 1996;383(6601):624–627. doi: 10.1038/383624a0. [DOI] [PubMed] [Google Scholar]
- 6.Bacigaluppi M., Pluchino S., Peruzzotti J.L., Kilic E., Kilic U., Salani G. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain. 2009;132:2239–2251. doi: 10.1093/brain/awp174. [DOI] [PubMed] [Google Scholar]
- 7.Behrstock S., Ebert A., McHugh J., Vosberg S., Moore J., Schneider B. Human neural progenitors deliver glial cell line derived neurotrophic factor to parkinsonian rodents and aged primates. Gene Ther. 2006;13(5):379–388. doi: 10.1038/sj.gt.3302679. [DOI] [PubMed] [Google Scholar]
- 8.Ebert A.D., Beres A.J., Barber A.E., Svendsen C.N. Human neural progenitor cells overexpressing IGF-1 protect dopamine neurons and restore function in a rat model of Parkinson’s disease. Exp Neurol. 2008;209(1):213–223. doi: 10.1016/j.expneurol.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 9.Lindvall O., Kokaia Z. Stem cells in human neurodegenerative disorders-Time for clinical translation? J Clin Invt. 2010;120:29–40. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reynolds B., Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Sci. 1992;5052:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 11.Potten C.S., Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties, lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 12.Weiss S., Reynolds B.A., Vescovi A.L., Morshead C., Craig C.G., van der Kooy D. Is there a neural stem cell in the mammalian forebrain? Trends Neurosci. 1996;19:387–393. doi: 10.1016/s0166-2236(96)10035-7. [DOI] [PubMed] [Google Scholar]
- 13.Zipori D. The nature of stem cells: state rather than entity. Nat Rev Genet. 2004;11:873–878. doi: 10.1038/nrg1475. [DOI] [PubMed] [Google Scholar]
- 14.Akerud P., Holm P.C., Castelo-Branco G., Sousa K., Rodriguez F.J., Arenas E. Persephinoverexpressing neural stem cells regulate the function of nigral dopaminerg neurons and prevent their degeneration in a model of Parkinson’s disease. Mol Cell Neurosci. 2002;21:205–222. doi: 10.1006/mcne.2002.1171. [DOI] [PubMed] [Google Scholar]
- 15.Marchetto M.C., Winner B., Gage F.H. Pluripotent stem cells in neurodegenerative andneurodevelopmental diseases. Hum Mol Genet. 2010;19:R71–R76. doi: 10.1093/hmg/ddq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steiner B., Wolf S., Kempermann G. Adult neurogenesis and neurodegenerative disease. Regen Med. 2006;1:15–28. doi: 10.2217/17460751.1.1.15. [DOI] [PubMed] [Google Scholar]
- 17.Buzanska L., Jurga M., Stachowiak E.K., Stachowiak M.K., Domanska J.K. Neural stem-like cell line derived from nonhematopoietic population of human umbilical cord blood. Stem Cell Dev. 2006;15:391–406. doi: 10.1089/scd.2006.15.391. [DOI] [PubMed] [Google Scholar]
- 18.Wang J.M., Singh C., Liu L., Irwin R.W., Chen S., Chung E.J. Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2010;107:6498–6503. doi: 10.1073/pnas.1001422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan J., Xu L., Welsh A.M., Chen D., Hazel T., Johe K. Combinedimmunosuppressive agents or CD4 antibodies prolong survival of human neural stem cell grafts and improve disease outcomes in amyotrophic lateral sclerosis transgenic mice. Stem Cells. 2006;24:1976–1985. doi: 10.1634/stemcells.2005-0518. [DOI] [PubMed] [Google Scholar]
- 20.Lindvall O., Barker A.B., Brustle O., Isacson O., Svendsen C.N. Clinical translation of stem cells in neurodegenerative disorders. Cell Stem Cell. 2012;10:151–155. doi: 10.1016/j.stem.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joannides A., Gaughwin P., Schwiening C., Majed H., Sterling J., Compston A. Efficient generation of neural precursors from adult human skin: astrocytes promote neurogenesis from skin-derived stem cells. Lancet. 2004;364:172–178. doi: 10.1016/S0140-6736(04)16630-0. [DOI] [PubMed] [Google Scholar]
- 22.Toma J.G., Akhavan M., Fernandes K.J., Barnabe-Heider F., Sadikot A., Kaplan D.R. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 23.Valenzuela M.J., Dean S.K., Sachdev P., Tuch B.E., Sidhu K.S. Neural precursors from canine skin: a new direction for testing autologous cell replacement in the brain. Stem Cells Develop. 2008;17:1087–1094. doi: 10.1089/scd.2008.0008. [DOI] [PubMed] [Google Scholar]
- 24.Erceg S., Ronaghi M., Stojkovic M. Human embryonic stem cell differentiation toward regional specific neural precursors. Stem Cells. 2008;27:78–87. doi: 10.1634/stemcells.2008-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vescovi A.L., Gritti A., Galli R., Parati E.A. Isolation and intracerebral grafting of nontransformed multipotential embryonic human CNS stem cells. J Neurot. 1999;16:689–693. doi: 10.1089/neu.1999.16.689. [DOI] [PubMed] [Google Scholar]
- 26.Zhang P., Li J., Liu Y., Chen X., Kang Q. Transplanted human embryonic neural stem cells survive, migrate, differentiate and increase endogenous nestin expression in adult rat cortical peri-infarction zone. Neuropath. 2009;29:410–421. doi: 10.1111/j.1440-1789.2008.00993.x. [DOI] [PubMed] [Google Scholar]
- 27.Fu L., Zhu L., Huang Y., Lee T.D., Forman S.J., Shih C.C. Derivation of neural stem cells from mesenchymal stem cells: evidence for a bipotential stem cell population. Stem Cells Developm. 2008;17:1109–1121. doi: 10.1089/scd.2008.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jang S., Cho H., Cho Y.B., Park J.S., Jeong H.S. Functional neural differentiation of human adipose tissue-derived stem cells using bFGF and forskolin. BMC Cell Biol. 2010;11:25. doi: 10.1186/1471-2121-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amabile G., Meissner A. Induced pluripotent stem cells: current progress and potential for regenerative medicine. Trend Mol Med. 2009;15:59–68. doi: 10.1016/j.molmed.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Chambers S.M., Fasano C.A., Papapetrou E.P., Tomishima M., Sadelain M., Studer L. Highly efficient neural conversion of human ES and IPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogawa D., Okada Y., Nakamura M., Kanemura Y., Okano H.J., Matsuzaki Y. Evaluation of human fetal neural stem/progenitor cells as a source for cell replacement therapy for neurological disorders: properties and tumorigenicity after long-term in vitro maintenance. J Neurosci Res. 2009;2:307–317. doi: 10.1002/jnr.21843. [DOI] [PubMed] [Google Scholar]
- 32.Alvarez-Buylla A., Garcia-Verdugo J.M. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alvarez-Buylla A., Lim D.A. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 34.Feldmann R.E., Mattern R. The human brain and its neural stem cells postmortem: from dead brains to live therapy. Int J Leg Med. 2006;120:201–211. doi: 10.1007/s00414-005-0037-y. [DOI] [PubMed] [Google Scholar]
- 35.Sanai N., Berger M.S., Garcia-Verdugo J.M., Alvarez-Buylla A. Comment on “Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension”. Science. 2007:318–393. doi: 10.1126/science.318.5849.393a. [DOI] [PubMed] [Google Scholar]
- 36.Trzaska K.A., Kuzhikandathil Eldo V., Pranela R. Specification of a dopaminergic phenotype from adult human mesenchymal stem cells. Stem Cells. 2007;25:2797–2808. doi: 10.1634/stemcells.2007-0212. [DOI] [PubMed] [Google Scholar]
- 37.Constantin G., Marconi S., Rossi B., Angiari S., Calderan L., Anghileri E. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells. 2009;10:2624–2635. doi: 10.1002/stem.194. [DOI] [PubMed] [Google Scholar]
- 38.Fu Y.S., Cheng Y.C., Lin M.Y., Cheng H., Chu P.M., Chou S.C. Conversion of human umbilical cord mesenchymal stem cells in Wharton’s jelly to dopaminergic neurons in vitro: potential therapeutic application for Parkinsonism. Stem Cells. 2006;1:115–124. doi: 10.1634/stemcells.2005-0053. [DOI] [PubMed] [Google Scholar]
- 39.Kim S., Chang K.A., Park H.G., Ra J.C., Kim H.S., Suh Y.H. The preventive and therapeutic effects of intravenous human adipose-derived stem cells in Alzheimer’s disease mice. PLoS ONE. 2012;9:e45757. doi: 10.1371/journal.pone.0045757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jung Y.L., Chang H.J., Jin A.J., Seong M.K., Chung H.R., Yun H. Therapeutic effects of human umbilical cord blood-derived mesenchymal stem cells after intrathecal administration by lumbar puncture in a rat model of cerebral ischemia. Stem Cell Res Ther. 2011;2:38. doi: 10.1186/scrt79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Svendsen C.N., Borg M.G., Armstrong R.J., Rosser A.E., Chandran S., Ostenfeld T. A new method for the rapid and long term growth of human neural precursor cells. J Neurosci Meth. 1998;2:141–152. doi: 10.1016/s0165-0270(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 42.Yayon A., Klagsbrun M., Esko J.D., Leder P., Ornitz D.M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991;4:841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- 43.Ciccolini F., Svendsen C.N. Fibroblast growth factor 2 (FGF-2) promotes acquisition of epidermal growth factor (EGF) responsiveness in mouse striatal precursor cells: identification of neural precursors responding to both EGF and FGF-2. J Neurosci. 1998;19:7869–7880. doi: 10.1523/JNEUROSCI.18-19-07869.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palmer T.D., Takahashi J., Gage F.H. The adult rat hippocampus contains primordial neural stem cells. Mol Cellul Neurosci. 1997;8:389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- 45.Adams G.B., Scadden D.T. The hematopoietic stem cell in its place. Nat Immunol. 2006;7:333–337. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- 46.Hermann A., Gastl R., Liebau S., Popa M.O., Fiedler J., Boehm B.O. Efficient generation of neural stem cell-like cells from adult human bone marrow stromal cells. J Cell Sci. 2004;117:4411–4422. doi: 10.1242/jcs.01307. [DOI] [PubMed] [Google Scholar]
- 47.Reynolds B.A., Rietze R.L. Neural stem cells and neurospheres–re-evaluating the relationship. Nat Meth. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- 48.Seaberg R.M., Vander K.D. Stem and progenitor cells: the premature desertion of rigorous definitions. Trend Neurosci. 2003;26:125–131. doi: 10.1016/S0166-2236(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 49.Reynolds B.A., Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Develop Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- 50.Skogh C., Eriksson C., Kokaia M., Meijer X.C., Wahlberg L.U., Wictorin K. Generation of regionally specified neurons in expanded glial cultures derived from the mouse and human lateral ganglionic eminence. Mol Cellul Neurosci. 2001;17:811–820. doi: 10.1006/mcne.2001.0973. [DOI] [PubMed] [Google Scholar]
- 51.Palmer T.D., Ray J., Gage F.H. FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci. 1995;5:474–486. doi: 10.1006/mcne.1995.1035. [DOI] [PubMed] [Google Scholar]
- 52.Conti L., Pollard S.M., Gorba T., Reitano E., Toselli M., Biella G. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 2005;3:e283. doi: 10.1371/journal.pbio.0030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shiras A., Chettiar S.T., Shepal V., Rajendran G., Prasad G.R., Shastry P. Spontaneous transformation of human adult nontumorigenic stem cells to cancer stem cells is driven by genomic instability in a human model of glioblastoma. Stem Cells. 2007;25:1478–1489. doi: 10.1634/stemcells.2006-0585. [DOI] [PubMed] [Google Scholar]
- 54.Siebzehnrubl F.A., Jeske I., Muller D., Buslei R., Coras R., Hahnen E. Spontaneous in vitro transformation of adult neural precursors into stem-like cancer cells. Brain Pathol. 2008;19:399–408. doi: 10.1111/j.1750-3639.2008.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eiges R., Schuldiner M., Drukker M., Yanuka O., Itskovitz-Eldor J., Benvenisty N. Establishment of human embryonic stem cell-transduced clones carrying a marker of undifferentiated cells. Curr Biol. 2001;11:514–518. doi: 10.1016/s0960-9822(01)00144-0. [DOI] [PubMed] [Google Scholar]
- 56.Rogers M.B., Hosler B.A., Gudas L.J. Specific expression of a retinoic acid-regulated, zinc-finger gene, Rex-1, in preimplantation embryos, trophoblast and spermatocytes. Development. 1991;113:815–824. doi: 10.1242/dev.113.3.815. [DOI] [PubMed] [Google Scholar]
- 57.Jiang Y., Jahagirdar B.N., Reinhardt R.L., Schwartz R.E., Keene C.D., Ortiz-Gonzalez X.R. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 58.Hosler B.A., Rogers M.B., Kozak C.A., Gudas L.J. An octamer motif contributes to the expression of the retinoic acid-regulated zinc finger gene Rex-1 (Zfp-42) in F9 teratocarcinoma cells. Mol Cell Biol. 1993;13:2919–2928. doi: 10.1128/mcb.13.5.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katica S., Erik F.J., Dick H., Aren V.W., Rudi A.D., Inge S. [18F]FDG Labeling of neural stem cells for in vivo cell tracking with positron emission tomography: inhibition of tracer release by phloretin. Mol Imag. 2012;11(1):1–12. [PubMed] [Google Scholar]
- 60.Johansson C.B., Momma S., Clarke D.L., Risling M., Lendahl U., Frisén J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;1:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 61.Uchida N., Buck D.W., He D., Reitsma M.J., Masek M., Phan T.V. Direct isolation of human central nervous system stem cells. Proceed Nat Acad Sci USA. 2000;26:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwartz P.H., Bryant P.J., Fuja T.J., Su H., O’Dowd D.K., Klassen H. Isolation and characterization of neural progenitor cells from post-mortem human cortex. J Neurosci Res. 2003;74:838–851. doi: 10.1002/jnr.10854. [DOI] [PubMed] [Google Scholar]
- 63.Hyndman A.G., Lemmon V. Neurons and glia in purified retinal cultures identified by monoclonal antibodies to intermediate filaments. Neurosci Lett. 1987;75:121–126. doi: 10.1016/0304-3940(87)90284-9. [DOI] [PubMed] [Google Scholar]
- 64.Katsetos C.D., Legido A., Perentes E., Mork S.J. Class III beta-tubulin isotype: a key cytoskeletal protein at the crossroads of developmental neurobiology and tumor neuropathology. J Child Neurol. 2003;18:851–866. doi: 10.1177/088307380301801205. [DOI] [PubMed] [Google Scholar]
- 65.Bignami A., Eng L.F., Dahl D. Uyeda. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972;43:429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- 66.Sommer I., Schachner M. Monoclonal antibodies (01–04) to oligodendrocyte cell surfaces: an immunoctological study in the central nervous system. Dev Biol. 1981;83:311–327. doi: 10.1016/0012-1606(81)90477-2. [DOI] [PubMed] [Google Scholar]
- 67.Krijgsveld J., Whetton A.D., Lee B., Lemischka I., Oh S., Pera M. Proteome biology of stem cells: a new joint HUPO and ISSCR initiative. Mol Cell Proteom. 2008;7:204–205. doi: 10.1074/mcp.H800001-MCP200. [DOI] [PubMed] [Google Scholar]
- 68.Gage F.H., Kempermann G., Palmer T.D., Peterson D.A., Ray J. Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 69.Quiñones-Hinojosa A., Chaichana K. The human subventricular zone: a source of new cells and a potential source of brain tumors. Exp Neurol. 2007;205:313–324. doi: 10.1016/j.expneurol.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 70.Zhao Z.M., Li H.J., Liu H.Y., Lu S.H., Yang R.C., Zhu H.F. Transplantation of CD34+human umbilical cord blood stem cells after spinal cord hemisection injury improves function recovery in adult rats. Cell Transplant. 2004;13:113–122. doi: 10.3727/000000004773301780. [DOI] [PubMed] [Google Scholar]
- 71.Samadikuchaksaraei A. An overview of tissue engineering approaches for management of spinal cord injuries. J Neur Engin Rehabilitat. 2007;4:15. doi: 10.1186/1743-0003-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chhabra H.S., Lima C., Sachdeva S., Mittal A., Nigam V., Chaturvedi D. Autologous mucosal transplant in chronic spinal cord injury: an Indian Pilot Study. Spinal Cord. 2009;47:887–895. doi: 10.1038/sc.2009.54. [DOI] [PubMed] [Google Scholar]
- 73.Karumbayaram S., Novitch B.G., Patterson M., Umbach J.A., Richter L., Lindgren A. Directed differentiation of human induced pluripotent stem cells generates active motor neurons. Stem Cells. 2009;4:806–811. doi: 10.1002/stem.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soldner F., Hockemeyer D., Beard C., Gao Q., Bell G.W., Cook E.G. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;5:964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wernig M., Zhao J.P., Pruszak J., Hedlund E., Fu D., Soldner F. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc Nat Acad Sci USA. 2008;5:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jain K.K. Cell therapy for CNS trauma. Mol Biotechnol. 2009;3:367–376. doi: 10.1007/s12033-009-9166-8. [DOI] [PubMed] [Google Scholar]
- 77.Lindvall O., Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441:1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- 78.Crigler L., Robey R.C., Asawachaicharn A., Gaupp D., Phinney D.G. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol. 2006;1:54–64. doi: 10.1016/j.expneurol.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 79.Lu P., Jones L.L., Snyder E.Y., Tuszynski M.H. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;2:115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 80.Xu L., Yan J., Chen D., Welsh A.M., Hazel T., Johe K. Human neural stem cell grafts ameliorate motor neuron disease in SOD-1 transgenic rats. Transplantation. 2006;7:865–875. doi: 10.1097/01.tp.0000235532.00920.7a. [DOI] [PubMed] [Google Scholar]
- 81.Xu L., Yan J., Chen D., Welsh A.M., Hazel T., Johe K. Human neural stem cell grafts ameliorate motor neuron disease in SOD-1 transgenic rats. Transplant. 2006;82:865–875. doi: 10.1097/01.tp.0000235532.00920.7a. [DOI] [PubMed] [Google Scholar]
- 82.Kim Y.J., Park H.J., Lee G., Bang O.Y., Ahn Y.H., Joe E. Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia. 2009;1:13–23. doi: 10.1002/glia.20731. [DOI] [PubMed] [Google Scholar]
- 83.David J.B. Optimizing levodopa therapy for Parkinson’s disease with levodopa/carbidopa/entacapone: implications from a clinical and patient perspective. Neuropsychiatr Dis Treat. 2008;4(1):39–47. doi: 10.2147/ndt.s1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wakeman D.R., Dodiya H.B., Kordower J.H. Cell transplantation and gene therapy in Parkinson’s disease. Mt Sinai J Med. 2011;78(1):126–158. doi: 10.1002/msj.20233. [DOI] [PubMed] [Google Scholar]
- 85.Lindvall O., Kokaia Z., Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders—how to make it work. Nat Med. 2004;S42:S50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- 86.Zhongling F., Feng G. Stem cell challenges in the treatment of neurodegenerative disease. CNS Neurosci Therap. 2012;18:142–148. doi: 10.1111/j.1755-5949.2011.00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Piccini P., Brooks D.J., Bjorklund A., Gunn R.N., Grasby P.M., Rimoldi O. Dopamine release from nigral transplants visualized in vivo in a Parkinson’s patient. Nat Neurosci. 1999;2:1137–1140. doi: 10.1038/16060. [DOI] [PubMed] [Google Scholar]
- 88.Nikkhah G., Cunningham M.G., Cenci M.A., McKay R.D., Björklund A. Dopaminergic microtransplants into the substantia nigra of neonatal rats with bilateral 6-OHDA lesions, I: evidence for anatomical reconstruction of the nigrostriatal pathway. J Neurosci. 1995;15:3548–3561. doi: 10.1523/JNEUROSCI.15-05-03548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Defer G.L., Geny C., Ricolfi F., Fenelon G., Monfort J.C., Remy P. Long-term outcome of unilaterally transplanted Parkinsonian patients, 1: clinical approach. Brain. 1996;119:41–50. doi: 10.1093/brain/119.1.41. [DOI] [PubMed] [Google Scholar]
- 90.Lopez A.D., Mathers C.D., Ezzati M., Jamison D.T., Murray C.J. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 91.Kessler R.C., Berglund P., Demler O., Jin R., Koretz D., Merikangas K.R. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 92.Paykel E.S. The evolution of life events research in psychiatry. J Affect Disord. 2001;62:141–149. doi: 10.1016/s0165-0327(00)00174-9. [DOI] [PubMed] [Google Scholar]
- 93.Brown G.W., Harris T.O., Hepworth C. Life events and endogenous depression. A puzzle reexamined. Arch Gen Psychiatry. 1994;51:525–534. doi: 10.1001/archpsyc.1994.03950070017006. [DOI] [PubMed] [Google Scholar]
- 94.Berrettini W. Molecular linkage studies in bipolar disorder. Dialogues Clin Neurosci. 1999;1:12–21. doi: 10.31887/DCNS.1999.1.1/wberrettini. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kumer V., Abbas A., Fausto N., Aster J. Saunders Elseverier; 2010. “Degenerativer diseases affecting the cerebral cortex” in robbins and cotran pathological basis of disease. 1313–7. [Google Scholar]
- 96.Zhao C., Deng W., Gage F.H. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;4:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 97.Becker M., Lavie V., Solomon B. Stimulation of endogenous neurogenesis by anti-EFRH immunization in a transgenic mouse model of Alzheimer’s disease. Proc Nat Acad Sci USA. 2007;5:1691–1696. doi: 10.1073/pnas.0610180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhongling F., Gang Z., Lei Y. Neural stem cells and Alzheimer’s disease: challenges and hope. Am J Alzheimer’s Disease. 2009;15:241–254. doi: 10.1177/1533317508327587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tuszynski M.H., Thal L., Pay M., Salmon D.P., Sang H., Bakay R. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;5:551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- 100.Nagahara A.H., Merrill D.A., Coppola G., Tsukada S., Schroeder B.E., Shaked G.M. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat Med. 2009;3:331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gao Y., Deng K., Hou J., Bryson J.B., Barco A., Nikulina E. Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron. 2004;44:609–621. doi: 10.1016/j.neuron.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 102.Hu F., Strittmatter S.M. Regulating axon growth within the postnatal central nervous system. Sem Perinatol. 2004;28:371–378. doi: 10.1053/j.semperi.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 103.Ramer L.M., Au E., Richter M.W., Liu J., Tetzlaff W., Roskams A.J. Peripheral olfactory ensheathing cells reduce scar and cavity formation and promote regeneration after spinal cord injury. J Comparat Neurol. 2004;473:1–15. doi: 10.1002/cne.20049. [DOI] [PubMed] [Google Scholar]
- 104.Sasaki M., Hains B.C., Lankford K.L., Waxman S.G., Kocsis J.D. Protection of corticospinal tract neurons after dorsal spinal cord transection and engraftment of olfactory ensheathing cells. Glia. 2006;53:352–359. doi: 10.1002/glia.20285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nikulina E., Tidwell J.L., Dai H.N., Bregman B.S., Filbin M.T. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Nat Acad Sci USA. 2004;101:8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Swanger S.A., Neuhuber B., Himes B.T., Bakshi A., Fischer I. Analysis of allogeneic and syngeneic bone marrow stromal cell graft survival in the spinal cord. Cell Transplant. 2005;14:775–786. doi: 10.3727/000000005783982594. [DOI] [PubMed] [Google Scholar]
- 107.Hill C.E., Moon L.D., Wood P.M., Bunge M.B. Labeled Schwann cell transplantation: cell loss, host Schwann cell replacement, and strategies to enhance survival. Glia. 2006;53:338–343. doi: 10.1002/glia.20287. [DOI] [PubMed] [Google Scholar]
- 108.Shumsky J.S., Tobias C.A., Tumolo M., Long W.D., Giszter S.F., Murray M. Delayed transplantation of fibroblasts genetically modified to secrete BDNF and NT-3 into a spinal cord injury site is associated with limited recovery of function. Exp Neurol. 2003;1:114–130. doi: 10.1016/s0014-4886(03)00398-4. [DOI] [PubMed] [Google Scholar]
- 109.Keirstead H.S., Nistor G., Bernal G., Totoiu M., Cloutier F., Sharp K. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tarasenko Y.I., Gao J., Nie L., Johnson K.M., Grady J.J., Hulsebosch C.E. Human fetal neural stem cells grafted into contusion-injured rat spinal cords improve behavior. J Neurosci Res. 2007;85:47–57. doi: 10.1002/jnr.21098. [DOI] [PubMed] [Google Scholar]
- 111.Louro J., Pearse D.D. Stem and progenitor cell therapies: recent progress for spinal cord injury repair. Neurol Res. 2008;1:5–16. doi: 10.1179/174313208X284070. [DOI] [PubMed] [Google Scholar]
- 112.Nistor G.I., Totoiu M.O., Haque N., Carpenter M.K., Keirstead H.S. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinated after spinal cord transplantation. Glia. 2005;3:385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- 113.Cummings B.J. Human neural stem cells differentiate and promote locomotor recovery in spinal cord injured mice. Proceed Nat Acad Sci USA. 2005;39:14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yan J., Xu L., Welsh A.M., Hatfield G., Hazel T., Johe K. Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Med. 2007;2:e39. doi: 10.1371/journal.pmed.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim M., Lee S.T., Chu K., Kim S.U. Stem cell-based cell therapy for Huntington disease: A review. Neuropath. 2008;28:1–9. doi: 10.1111/j.1440-1789.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 116.Li J.Y., Popovic N., Brundin P. The use of the r6 transgenic mouse models of Huntington’s disease in attempts to develop novel therapeutic strategies. NeuroRx. 2005;2:447–464. doi: 10.1602/neurorx.2.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee S.T., Chu K., Park J.E., Lee K., Kang L., Kim S.U. Intravenous administration of human neural stem cells induces functional recovery in Huntington’s disease rat model. Neurosci Res. 2005;52:243–249. doi: 10.1016/j.neures.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 118.McBride J.L., Behrstock S.P., Chen E.Y., Jakel R.J., Siegel I., Svendsen C.N. Human neural stem cell transplants improve motor function in a rat model of Huntington’s disease. J Comparat Neurol. 2004;475:211–219. doi: 10.1002/cne.20176. [DOI] [PubMed] [Google Scholar]
- 119.Bachoud-Levi A.C. Safety and tolerability assessment of intrastriatal neural allografts in five patients with Huntington’s disease. Exp Neurol. 2000;161:194–202. doi: 10.1006/exnr.1999.7239. [DOI] [PubMed] [Google Scholar]
- 120.Philpott L.M., Kopyov O.V., Lee A.J., Jacques S., Duma C.M., Caine S. Neuropsychological functioning following fetal striatal transplantation in Huntington«s chorea: Three case presentations. Cell Transplant. 1997;6:203–212. doi: 10.1177/096368979700600303. [DOI] [PubMed] [Google Scholar]
- 121.Pineda J.R., Rubio N., Akerud P., Urban N., Badimon L., Arenas E. Neuroprotection by GDNF-secreting stem cells in a Huntington’s disease model: Optical neuroimage tracking of brain-grafted cells. Gene Ther. 2007;14:118–128. doi: 10.1038/sj.gt.3302847. [DOI] [PubMed] [Google Scholar]
- 122.Wichterle H., Lieberam I., Porter J.A., Jessell T.M. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;3:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 123.Glass J.D., Boulis N.M., Johe K., Rutkove S.B., Federici T., Polak M. Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: results of a phase I trial in 12 patients. Stem Cells. 2012;6:1144–1151. doi: 10.1002/stem.1079. [DOI] [PubMed] [Google Scholar]
- 124.Marchetto M.C., Muotri A.R., Mu Y., Smith A.M., Cezar G.G., Gage F.H. Non-cell-autonomous effect of human SOD1 G37r astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:649–657. doi: 10.1016/j.stem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 125.Klein S.M., Behrstock S., McHugh J., Hoffmann K., Wallace K., Suzuki M. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum Gene Ther. 2005;16:509–521. doi: 10.1089/hum.2005.16.509. [DOI] [PubMed] [Google Scholar]
- 126.Suzuki M., McHugh J., Tork C., Shelley B., Klein S.M., Aebischer P. GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS ONE. 2007;2:e689. doi: 10.1371/journal.pone.0000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Arenella L.S., Herndon R.M. Mature oligodendrocytes: division following experimental demyelination in adult animals. Arch Neurol. 1984;41:1162–1165. doi: 10.1001/archneur.1984.04050220060015. [DOI] [PubMed] [Google Scholar]
- 128.Windrem M.S., Roy N.S., Wang J., Nunes M., Benraiss A., Goodman R. Progenitor cells derived from the adult human subcortical white matter disperse and differentiate as oligodendrocytes within demyelinated lesions of the rat brain. J Neurosci Res. 2002;69:966–975. doi: 10.1002/jnr.10397. [DOI] [PubMed] [Google Scholar]
- 129.Mix E., Meyer-Rienecker H., Zettl U.K. Animal models of multiple sclerosis for the development and validation of novel therapies: Potential and limitations. J Neurol. 2008;6:7–14. doi: 10.1007/s00415-008-6003-0. [DOI] [PubMed] [Google Scholar]
- 130.Akiyama Y., Honmou O., Kato T., Uede T., Hashi K., Kocsis J.D. Transplantation of clonal neural precursor cells derived from adult human brain establishes functional peripheral myelin in the rat spinal cord. Exp Neurol. 2001;167:27–39. doi: 10.1006/exnr.2000.7539. [DOI] [PubMed] [Google Scholar]
- 131.Pluchino S., Quattrini A., Brambilla E., Gritti A., Salani G., Dina G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;6933:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- 132.Glass R., Synowitz M., Kronenberg G., Walzlein J.H., Markovic D.S., Wang L.P. Glioblastomainduced attraction of endogenous neural precursor cells is associated with improved survival. J Neurosci. 2005;25:2637–2646. doi: 10.1523/JNEUROSCI.5118-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Aboody K.S., Brown A., Rainov N.G., Bower K.A., Liu S., Yang W. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Nat Acad Sci USA. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li S., Gao Y., Tokuyama T., Yamamoto J., Yokota N., Yamamoto S. Genetically engineered neural stem cells migrate and suppress glioma cell growth at distant intracranial sites. Cancer Let. 2007;251:220–227. doi: 10.1016/j.canlet.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 135.Murray C.J., Lopez A.D. Global mortality, disability, and the contribution of risk factors: global burden of disease study. Lancet. 1997;349:1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 136.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 137.Yuan J., Lipinski M., Degterev A. Diversity in the mechanisms of neuronal cell death. Neuron. 2003;40:401–413. doi: 10.1016/s0896-6273(03)00601-9. [DOI] [PubMed] [Google Scholar]
- 138.Dirnagl U., Iadecola C., Moskowitz M.A. Pathobiology of ischaemic stroke: an integrated view. Trend Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 139.Smith W.S., Sung G., Starkman S., Saver J.L., Kidwell C.S., Gobin Y.P. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke: A j Cereb Circulat. 2005;36:1432–1438. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 140.Greenberg D.A., Jin K. Growth factors and stroke. NeuroRx. 2006;3:458–465. doi: 10.1016/j.nurx.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mattsson B., Sorensen J.C., Zimmer J., Johansson B.B. Neural grafting to experimental neocortical infarcts improves behavioral outcome and reduces thalamic atrophy in rats housed in enriched but not in standard environments. Stroke. 1997;28:1225–1231. doi: 10.1161/01.str.28.6.1225. [DOI] [PubMed] [Google Scholar]
- 142.Nishino H., Borlongan C.V. Restoration of function by neural transplantation in the ischemic brain. Prog Brain Res. 2000;127:461–476. doi: 10.1016/s0079-6123(00)27022-2. [DOI] [PubMed] [Google Scholar]
- 143.Riolobos A.S., Heredia M., Fuente J.A., Criado J.M., Yajeya J., Campos J. Functional recovery of skilled forelimb use in rats obliged to use the impaired limb after grafting of the frontal cortex lesion with homotopic fetal cortex. Neurobiol Learn Mem. 2001;75:274–292. doi: 10.1006/nlme.2000.3979. [DOI] [PubMed] [Google Scholar]
- 144.Chen J., Li Y., Wang L., Lu M., Zhang X., Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- 145.Chen J., Sanberg P.R., Li Y., Wang L., Lu M., Willing A.E. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 146.Savitz S.I., Rosenbaum D.M., Dinsmore J.H., Weschler L.R., Caplan L.R. Cell transplantation for stroke. Ann Neurol. 2002;53:266–275. doi: 10.1002/ana.60000. [DOI] [PubMed] [Google Scholar]
- 147.Borlongan C.V., Tajima Y., Trojanowski J.Q., Lee V.M., Sanberg P.R. Transplantation of cryopreserved human embryonal carcinoma-derived neurons (NT2N cells) promotes functional recovery in ischemic rats. Exp Neurol. 1998;149:310–321. doi: 10.1006/exnr.1997.6730. [DOI] [PubMed] [Google Scholar]
- 148.Modo M., Stroemer R.P., Tang E., Patel S., Hodges H. Effects of implantation site of stem cell grafts on behavioral recovery from stroke damage. Stroke. 2002;33:2270–2278. doi: 10.1161/01.str.0000027693.50675.c5. [DOI] [PubMed] [Google Scholar]
- 149.Saporta S., Borlongan C.V., Sanberg P.R. Neural transplantation of human neuroteratocarcinoma (hNT) neurons into ischemic rats. A quantitative doseresponse analysis of cell survival and behavioral recovery. Neuroscience. 1999;91:519–525. doi: 10.1016/s0306-4522(98)00610-1. [DOI] [PubMed] [Google Scholar]



