Evaluation of food chemicals is essential to make appropriate feeding decisions. The molecular genetic analysis of Gustatory receptor (Gr) genes and the characterization of the neural circuits they engage has led to a broad understanding of taste perception in adult Drosophila [1, 2]. For example, eight relatively highly conserved members (Gr5a, Gr61a, and Gr64a-f) of the Gr gene family, referred to as sugar Gr genes, are thought to be involved in sugar taste in adult flies [3–8], while the majority of the remaining Gr genes are likely to encode bitter taste receptors [9–11], albeit some function as pheromone [12–14] and carbon dioxide [15, 16] receptors. In contrast to the adult fly, relatively little is known about the cellular and molecular basis of taste perception in larvae. Here, we identify Gr43a, which was recently shown to function as a hemolymph fructose sensor in adult flies [17], as the major larval sugar receptor. We show that it is expressed in taste neurons, proventricular neurons, as well as sensory neurons of the brain. Larvae lacking Gr43a fail to sense sugars, while larvae mutant for all eight sugar Gr genes exhibit no obvious defect. Finally, we show that brain neurons are necessary and sufficient for sensing all main dietary sugars, which likely involves a postingestive mechanism of converting carbohydrates into fructose.
RESULTS
The larval taste system is compartmentalized, and taste neurons are found in five major anatomical sites (Figure 1A) [18, 19]. Two of these sites, the terminal and ventral organ (TO and VO; Figure 1A), sample soluble chemicals externally. Three internal taste organs, the dorsal, ventral and posterior pharyngeal sense organs (DPS, VPS and PPS) monitor chemicals as food passes through the pharynx (Figure 1A). About 40 Gr genes are expressed in neurons of both the external taste organs, as well as in internal pharyngeal sense organs; however, none of the eight sugar Gr genes is expressed in larval chemosensory neurons [18], even though larvae can sense most sugars [20].
Figure 1. GR43a is expressed in the larval taste organs, as well as the brain and the gastrointestinal system.
(A) Diagram of larval taste organs, adapted from Stocker [19]: Structures associated with taste sensing and processing are shown in gray. The cell bodies of the taste neurons are arrayed in ganglia, extend dendrites into the taste organ and axons into the subesophageal ganglion. Olfactory structures are shown in blue. Note that only three brain structures - subesophageal ganglion, the antennal lobe and the mushroom bodies - are specifically indicated. The pharynx is shown in green. Abbreviations: AL, antennal lobe; BR, brain; MB, mushroom bodies; SOG, subesophageal ganglion; DO/TO/VO, dorsal/terminal/ventral organ; DPS/VPS/PPS, dorsal/ ventral/posterior pharyngeal sense organ.
(B) Overall view of Gr43a expression in larvae. Gr43aGAL4 drives UAS-mCD8GFP expression in neurons located in chemosensory organs, the DPS/VPS and the PPS, and the proventricular ganglion. The live expression of mCD8GFP is at the left, the phase-contrast image at the center, the overlaid image is at the right.
(C and D) Expression analyses of Gr43aGAL4 UAS-mCD8RFP (red) and Gr66a-GFP-IRES-GFP-IRES-GFP (green) in chemosensory organs (C) and their projections to the SOG (D). Gr43a is expressed only in the DPS/VPS neurons, but it is not expressed in the same neurons as Gr66a. Note that Gr66a is also expressed in neurons of the TO ganglion besides of the DPS/VPS neurons. Asterisk indicate DO and TO.
(E) Gr43aGAL4 drives mCD8GFP expression in the CNS. The images on the right are enlargement view of the dorsal protocerebrum. Gr43aGAL4 is expressed in one or two big neurons per hemisphere and in many Kenyon cells. Gr43aGAL4 expressing neurons are also located in the VNC. Neuropil was counterstained with nc82.
Larvae exhibit high sensitivity to fructose and sucrose
To identify larval sugar receptor(s), we adapted the two-choice taste assay developed by Schipanski and colleagues [20]. w1118 (i.e. Gr43a+ control) larvae show strong preference for fructose and sucrose over a wide concentration range (500 mM to 0.8 mM; Supplemental Figure 1). Larvae exhibit an immediate attraction (i.e. within two minutes) to both sugars at concentrations as low as 20 mM, while preference at even lower concentrations is delayed and not noticeable until four minutes or more. An immediate preference for glucose and trehalose is observed only at very high concentrations (1M and 500 mM), but a preference for both sugars is also noticeable at 100 mM concentrations after 16 minutes (Supplemental Figure 1). These observations confirm previous findings [20] and reveal that larvae exhibit a distinct attraction for sugar containing agar in a time- and concentration-dependent fashion.
Gr43a is expressed in larval taste neurons
While the sugar Gr genes appear not to be expressed in larvae, Gr43a was found in two internal taste neurons of larvae [18]. To investigate Gr43a expression in more detail, we employed Gr43aGAL4, an allele in which the coding sequence of Gr43a was replaced by that of Gal4 using homologous recombination [17]. Gr43aGAL4 is expressed in ~ 4 neurons in the pharyngeal taste clusters (DPS, VPS and PPS), while no expression is observed in the external taste organs (TO and VO) [18] (Figure 1B). Combining Gr43aGAL4 and Gr66a-GFP-IRES-GFP-IRES-GFP, a marker for bitter-sensing neurons, revealed no overlap in expression between these two genes (Figure 1C and 1D), which is consistent with a possible function of Gr43a in sugar sensing. We note that Gr43aGAL4 is also expressed in neurons associated with the proventriculus (Figure 1B, Supplemental Figure 2). Furthermore, immunostaining identified Gr43aGAL4 expression in the brain, including neurons in the protocerebrum, the mushroom body and the ventral nerve cord (VNC; Figure 1E, Supplemental Figure 2).
Gr43a is the main sugar receptor in larvae
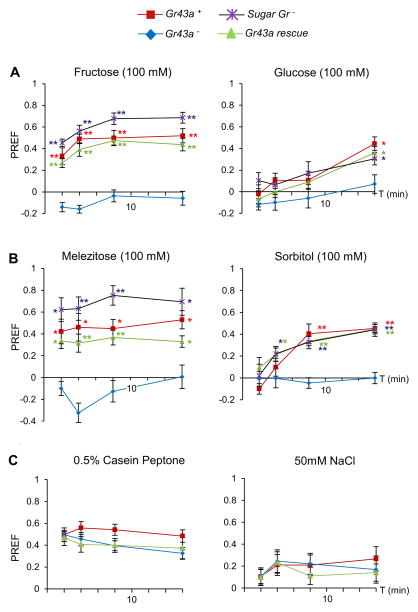
We next carried out behavioral two-choice preference assays for fructose and glucose using w1118 control larvae, larvae lacking all eight sugar Gr genes, Gr43aGAL4 mutant larvae and larvae in which the Gr43aGAL4 null mutation is rescued by a UAS-Gr43a transgene (Figure 2A). The preference for fructose was abolished in Gr43a mutants, but not in larvae lacking the sugar Gr genes. Importantly, Gr43a mutant larvae expressing a UAS-Gr43a transgene regained their preference for fructose (Figure 2A, Supplemental Figure 3A). When larvae were tested for glucose preference, all genotypes but the Gr43a mutants exhibited a pronounced attraction to this sugar (Figure 2A). However, establishing this preference required prolonged assay time (16 minutes). Regardless, these observations indicate that the preference for glucose and fructose is dependent on a single Gr protein, Gr43a, which is surprising since this receptor is narrowly tuned to fructose and does not respond to glucose [17, 21]. We therefore tested larvae with two additional sugars, melezitose and sorbitol (Figure 2B). Melezitose is a complex nutritious sugar containing a fructose moiety, and indeed was sensed immediately, just like fructose. Sorbitol, a tasteless sugar alcohol is sensed in a moderately delayed fashion (but faster than glucose) by wild type larvae, but not sensed at all in Gr43a mutants. Importantly, the phenotype of Gr43a mutants to both melezitose and sorbitol is rescued with the expected temporally displayed preference in the presence of a UAS-Gr43a transgene (Figure 2B). Lastly, lack of preference is specific to sugars because Gr43a mutant and control larvae showed similar preference for agar containing casein peptone (an amino acid/peptide mixture), a mixture of three amino acids or low concentration of sodium chloride (Figure 2C, Supplemental Figure 3B). Taken together, our analysis shows that in Drosophila larvae (i) fructose is the most stimulating sugar substrate, (ii) Gr43a is the main sugar receptor and acts independently of the sugar Gr proteins, (iii) the sugar Gr proteins play no significant role in sugar sensing and (iv) non-fructose containing sugars, such as glucose and trehalose, are likely sensed by a postprandial mechanism that relies on conversion of (a fraction of) these sugars into fructose ([22], see below).
Figure 2. Gr43a is required for fast and slow preference to fructose and non-fructose containing sugars.
Time-dependent taste preference of w1118 (Gr43a+), w; Gr43aGAL4/GAL4(Gr43a−), w R1 Gr5aLexA; R2/R2; Gr61a−ΔGr64/Gr61a− ΔGr64 (sugar Gr−); and w; Gr43aGal4/Gal4;UAS-Gr43a/+ (Gr43a rescue), to 100 mM sugars with and without a fructose moiety.
(A) Gr43a is necessary for both the immediate and late preference response to fructose and the late preference for glucose. 12≤ n ≤ 30, Mann-Whitney U-test was used to compare samples to Gr43a−; * P< 0.05, ** P< 0.001. Negative values for fructose at 2′ and 4′ and melezitose at 4′ (see B) by Gr43a mutant larvae (One sample sign test, P< 0.05) likely reflect avoidance of high osmolarity in the absence of any sugar taste capability.
(B) Response to melezitose and sorbitol recapitulate the response to fructose and glucose, both in Gr43a mutant and control larvae, implying a fructose moiety to be necessary for the immediate but not the late preference. 6≤ n ≤ 12, Mann-Whitney U test was used to compare to Gr43a−; * P< 0.05, ** P< 0.001.
(C) Larval preference for agar containing 2% casein peptone (mixture of amino acids and peptides) and 50 mM NaCl is not affected by the Gr43aGAL4 mutation. Mann-Whitney U-test was used to compare genotypes to w1118 (Gr43a+). 18≤ n≤ 27 for casein peptone, 12≤ n≤ 18 NaCl * P< 0.05, ** P< 0.001.
Sugar sensing by the larval brain
The two-choice feeding assay reveals a time-dependent component for establishing a food preference. This is evident when comparing the response for six different sugars vs. agar alone at 100 mM concentration over a 16 minute-assay period. Larvae show an immediate preference (i.e. within the first 2 minutes) for fructose and sugars containing a fructose moiety (sucrose, melezitose), while they fail to establish such a preference for glucose, trehalose or sorbitol (Figure 2A and 2B, Supplemental Figures 1 and 3A). Notably, after 16 minutes, wild type larvae, but not Gr43a mutant larvae, exhibit a robust, late preference for all these sugars as well (Figure 2A and 2B). We propose that the late preference for glucose, trehalose and sorbitol is mediated by internal Gr43a-expressing neurons, probably those in the brain. In adult flies, a fraction of ingested nutritious carbohydrates are converted into fructose, leading to transient fructose increase in the hemolymph, which in turn mediates satiation-dependent feeding activity [17].
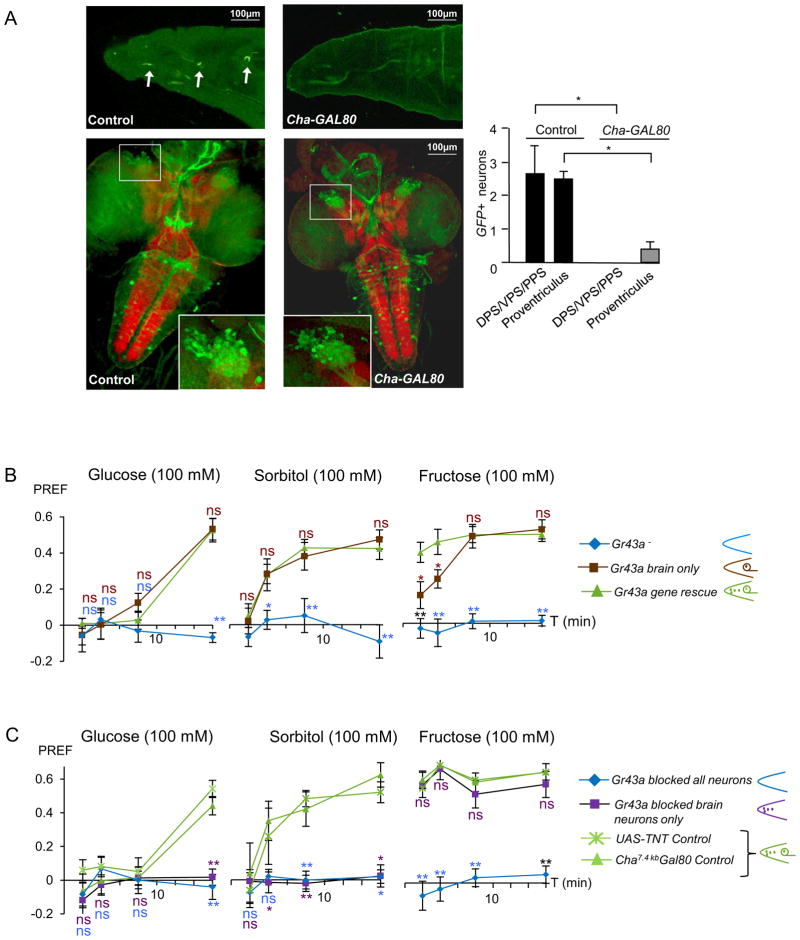
To address the role of the brain neurons in fructose sensing, we investigated the temporal preference dynamics, we generated Gr43aGAL4 mutant flies expressing a UAS-Gr43a transgene with and without Cha7.4kb-GAL80 (Figure 3). GAL80 binds to and suppresses transcriptional activity of GAL4 on UAS containing promoters [23]. Cha7.4kb-GAL80 suppresses GAL4 expression almost completely in the two internal taste clusters and the neurons associated with the proventriculus, but not the neurons in the brain (Figure 3A). As expected, “Gr43a brain-only-larvae” showed the same preference dynamics for glucose and sorbitol as control larvae; however, their immediate (2 minutes) preference to fructose was severely reduced, albeit not completely abolished (Figure 3A). This may be explained by residual expression of Gr43a in proventricular neurons of some larvae (Figure 3A), or by rapid uptake of fructose into the hemolymh. We then tested sugar preference in larvae in which Gr43a function was restricted to peripheral neurons by expressing tetanus toxin (TNT) in the brain (Gr43aGAL4/+; UAS-TNT/ Cha7.4kb-GAL80; TNT inhibits neural transmission [24]). Indeed, these larvae exhibited an immediate preference for fructose, but fail to generate a late preference for glucose and sorbitol (Figure 3C).
Figure 3. Gr43aGAL4 brain neurons are sufficient for sugar sensing.
(A) Gr43aGAL4 expression is repressed by Cha7.4kb-Gal80 in taste neurons DPS/VPS/PPS and neurons in the gastrointestinal tract, but not in the brain neurons. Image of the taste organs and quantification data was obtained from live GFP microscopy, and image of the brain is from immunostained preparations of third instar larvae using anti GFP antibody. Genotypes: w; Gr43aGAL4;UAS-mCD8GFP (control) and w; Gr43aGal4;UASmCD8GFP/Cha7.4kb-Gal80. Number of GFP expressing cells in taste organs and proventriculus was determined using live GFP microscopy. Weak expression in at most one proventricular neuron was observed in ~40% of larvae. Mann-Whitney U-test was used to compare genotypes.
(B) The slow response (16 minutes) to glucose and sorbitol is rescued in Gr43a mutant larvae in which Gr43a function is restricted to the brain. Immediate, but not slow response to fructose is reduced in these larvae. Genotypes are: w; Gr43aGAL4/GAL4; Cha7.4kb-Gal80/+ (Gr43a mutant control), w; Gr43aGal4/Gal4;UAS-Gr43a/+ (Gr43a rescue) and w; Gr43aGal4/Gal4;UAS-Gr43a/Cha7.4kb-Gal80 (Gr43a “brain only”). Mann-Whitney U test was used to compare genotypes to w; Gr43aGal4/Gal4;UAS-Gr43a/+ .12≤ n ≤ 18; *: P< 0.05, ** P< 0.001. ns = no statistical difference between Gr43a rescue and Gr43a “brain only” groups
C) Larvae in which the brain neurons are inactivated by expression of TNT show an immediate response to fructose (16 minutes), but their late response to glucose or sorbitol is completely abolished. Genotypes: w; Gr43GAL4/+; Cha7.4kb-Gal80/+ (Cha-GAL80 control), w; UAS-TNT/+ (UAS-TNT control), w; Gr43aGal4/UAS-TNT (all Gr43a neurons inactivated) and w; Gr43aGAL/UAS-TNT; Cha7.4kb-Gal80/+ (only brain Gr43a neurons inactivated). Mann-Whitney U-test was used to compare genotypes to controls. Note that significance at 4′ time point in sorbitol vs. agar is attained only when compared to Cha-GAL80 control; 6≤ n≤ 12; *: P< 0.05, ** P< 0.001. ns = no statistical difference between control groups and “only brain Gr43a neurons inactivated” group.
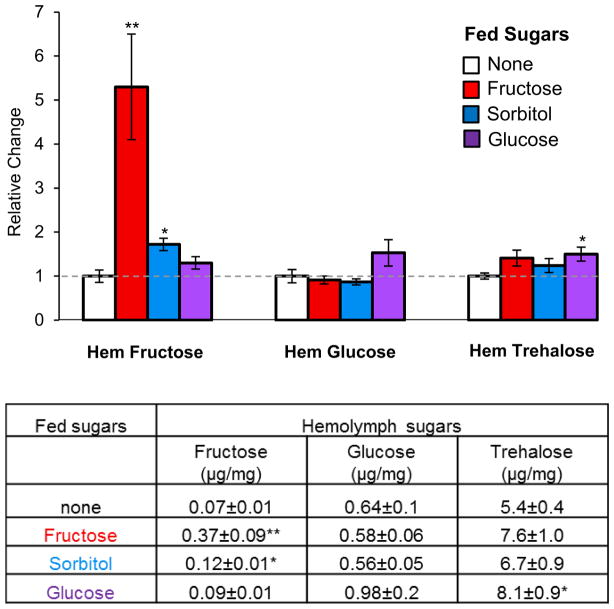
In adults, feeding of all nutritious sugars, such as fructose, sorbitol or glucose, leads to a significant increase of hemolymph fructose [17]. To examine the effects of dietary sugars on larval hemolymph fructose, larvae were let to feed on agarose only (control), or agarose containing 100mM sugars (fructose, sorbitol or glucose) before their hemolymph was collected and examined for the three major hemolymph sugars. As expected, hemolymph fructose levels increased significantly when fed sorbitol, while hemolymph glucose/trehalose levels did not change (Figure 4). When larvae were fed glucose, hemolymph glucose and trehalose levels increased, the latter significantly, while no change in hemolymph fructose was observed.
Figure 4. Concentration of hemolymph sugars after glucose, sorbitol or fructose feeding.
Larvae from the w1118 strain were fed on agarose containing 100 mM of each sugar and compared to larvae kept on plain agarose. The amount of each sugar (in μg) was normalized to larval weight (in mg). Data are presented as mean and error bars as ±SEM. Student’s t-test was used to compare sugar fed groups to agar fed group. 4≤ n≤ 8 for all groups; * P< 0.05, ** P< 0.001.
DISCUSSION
The sweet taste of sugars is crucial for providing animals a hedonic stimulus for feeding on nutritious carbohydrates. Here, we have shown that the sugar Gr genes mediating sweet taste in adult flies are dispensable for sugar sensing during the larval stage. Instead, Gr43a is the major sweet taste receptor in larvae. Gr43a is narrowly tuned to fructose and the fructose containing disaccharide sucrose [17, 21] found in most fruit. In contrast to adult flies, where Gr43a has only an auxiliary role in tasting dietary sugars and where its main role is to sense fructose in the brain hemolymph [17], larvae appear to rely almost exclusively on this receptor for carbohydrate sensing.
Why does Drosophila employ distinct sugar receptors during the different life stages? Larvae and adults face different sets of challenges to satisfy similar dietary needs of carbohydrates and proteins. Larvae are highly restricted in their choice of carbohydrates, which is determined by where they have been deposited as eggs. They consume a relatively homogenous source of sugar and protein, and nutrients associated with microorganisms (i.e. yeast) that grow on it. The major gustatory challenge, therefore, is not to locate and identify sugars, but to verify its presence and assure that it is void of hazardous chemicals that may be produced by microorganisms colonizing ripening and decaying fruit. Indeed, at least 40 putative bitter receptors are expressed in the larval taste sensory neurons [18], some of which may sense such harmful chemicals. In contrast, adults explore diverse habitats to locate appropriate food sources, find mates, escape predators and lay eggs. Thus, a set of Gr genes encoding different sugar receptors capable of detecting a variety of sugars is better suited to identify appropriate food sources in the complex environment of adult Drosophila.
Fructose as in internal nutrient signal
The two-choice feeding assay reveals two temporally distinct phases while acquiring a sugar preference, both of which are dependent on Gr43a. We propose that if a sugar can be sensed by taste neurons (such as fructose containing sugars), a preference is established within two minutes (Figure 2, Figure 3C and Supplemental Figure 1). The second phase in larval sugar perception is characterized by the establishment of a delayed preference, is dependent on Gr43a function in the brain (Figure 3), and most likely mediated by fructose in the hemolymph, converted from ingested sugar (Figure 4)[17]. The observation of a slow developing sugar preference is surprising, given the elapsed time from food intake and actual activation of brain neurons by fructose. Preliminary experiments suggest that crawl speed of larvae is dependent on the nutritious content of the agar, but more extensive behavioral analyses that considers turn frequency, digging activity etc will be necessary to uncover the rationale for the observed late sugar preference.
In adult flies, only about six brain neurons express Gr43a; they were shown to sense circulating fructose and regulate consumption of nutritious carbohydrate [17]. Larvae have several groups of Gr43aGAL4 expressing neurons, one likely corresponding to the neurons in the posterior superior lateral protocerebrum of adult flies, a group of mushroom body Keynon cells and about 20 to 30 neurons dispersed along the VNC. The Kenyon cells are unlikely to be involved in this process, as they are not necessary to mediate the late sugar preference (Supplemental Figure 4), but they may play a role in associative learning during feeding. Regardless, our analysis suggests that some of the brain neurons in the larvae function as nutrient sensors by detecting hemolymph fructose, derived from dietary fructose or fructose converted from other nutritious carbohydrates. Measurements of hemolymph fructose in flies show significant increases after glucose, sorbitol or fructose-based meals (3, 5 and 10 fold, respectively) [17]. These values are higher than those observed in larvae, where only feeding on fructose and sorbitol, but not on glucose, raises hemolymph fructose levels (Figure 4). Two scenarios that may account for this difference, both implying a role of the blood brain barrier (BBB): First, conversion of fructose may be restricted to the brain, or second, the conversion into fructose may be ubiquitous, but accumulation in the brain may be regulated by selective transporters for fructose across the BBB [25]. The specificity of Gr43a for fructose and the location of this sensor in the brain, therefore, invoke a postingestive mechanism requiring conversion of dietary sugars into fructose. Whatever the mechanism, the brain neurons are both necessary and sufficient to mediate the late sugar preference in larvae.
Gr43aGAL4 is also expressed in the larval proventriculus, reiterating the adult expression profile where this structure separates the foregut from the midgut [17]. Interestingly, the Gr43a orthologues of the cotton bollworm Helicoverpa armigera and the silkworm Bomyx mori are expressed in the gut, indicating an important role for this fructose sensor in the gastrointestinal tract [21, 26]. Sensing of dietary fructose may induce expression/secretion of carbohydrate modifying enzymes and/or regulate peristaltic movements of the midgut to aid in digestion. Future studies in Drosophila and other insects will illuminate the function of this unique gustatory receptor both in the gastrointestinal system and the brain.
Experimental Procedures
Genetics
For behavioral analysis, w1118 larvae were used as wild type controls, Gr43aGal4/Gal4 larvae as null mutants for Gr43a, R1 Gr5aLexA/R1 Gr5aLexA; R2/R2; Gr61a−ΔGr64/Gr61a− ΔGr64 larvae as triple mutant lacking all eight sweet Gr genes and Gr43aGal4/Gr43aGal4;UAS-Gr43a/+ larvae as Gr43a rescue controls. For larvae with Gr43aGAL4 expression restricted to the brain, we used Gr43aGal4/Gr43aGal4;UAS-Gr43a/Cha7.4kb-Gal80 larvae, while Gr43aGal4/Gr43aGal4; UAS-Gr43a/+ and Gr43aGal4/Gr43aGal4; Cha7.4kb-Gal80/+ were used as controls. Expression analysis was carried out on Gr43aGal4/+; UAS-mCD8GFP/+ and Gr43aGAL4 UAS-mCD8RFP/Gr66a-GFP-IRES-GFP-IRES-GFP. R1 and R2 are rescue transgenes containing non-Gr genes deleted in ΔGr64 [6].
Behavioral assays
2-choice preference assay: Flies were kept in mass culture under standard conditions. Third instar, feeding-stage larvae (4 days after egg laying) of the indicated strains were used for all assays. Larvae were collected from culture tubes and briefly washed in water to remove food particles. 15 larvae were used for each preference assay and placed on the midline separating the two agar media (one containing sugar and the other lacking sugar) on a 55 mm plastic petri dish. Images were taken after the indicated time points (2, 4, 8 and 16 minutes) to establish larval distribution and to calculate preference indices. Larvae that dug in the agarose or crawled onto the lid were excluded. A preference index (PREF) is then calculated as PREF = [#of larvae on Sugar/agar − #of larvae on agar only] / #Total. A positive PREF score indicates a preference for the sugar-containing agar, while a negative PREF score would indicate a preference for plain agar.
Preparation of media: 55 mm petri dishes are filled with a solution of 1 % agarose. After the agarose has solidified, the agar is split into two identical halves using a razor blade, and one half is discarded. The emptied half of the dish is then replaced with agar containing the sugar at the indicated concentration. Plates were used within an hour after preparation.
Hemolymph collection
Third instar larvae were kept on agar overnight, collected, rinsed in water, briefly dried and weighted in batches of five. A batch of larvae was then transferred on plates for 16 minutes of either plain agarose or agarose plates with 100mM glucose, sorbitol or fructose. Each batch was rinsed to remove food particles, briefly dried off and transferred to a clean dissection dish, filled with ~ 50 μl of distilled water. Larvae were carefully torn apart using forceps to drain hemolymph into the water, and 40 μl of the hemolymph containing solution was collected. Protein and cellular debris were removed by treatment with Barium Hydroxide and Zinc sulfate (0.3N).
Supplementary Material
Research Highlights.
Gr43a fructose receptor is the main sugar receptor necessary for larval sugar preference
Drosophila larvae do not require classic sugar receptors for sugar sensing
Immediate fructose preference is mediated by Gr43a-expressing taste sensory neurons in the pharynx
Late sugars preference is mediated by brain neurons expressing Gr43a
Footnotes
Author contribution: DM, AB and AY conducted all behavioral experiments, TM conducted the expression analysis and YHR and DHR carried out the MassSpec analysis. TM and DM collected larval hemolymph. JS provided reagents. DM, TM, YHR and HA conceived the experiments, and HA wrote the paper. This work was supported by grants 1RO1GMDC05606 and 1RO1DC009014 to HA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Dahanukar A, Foster K, van der Goes van Naters WM, Carlson JR. A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat Neurosci. 2001;4:1182–1186. doi: 10.1038/nn765. [DOI] [PubMed] [Google Scholar]
- 4.Miyamoto T, Chen Y, Slone J, Amrein H. Identification of a Drosophila glucose receptor using ca(2+) imaging of single chemosensory neurons. PLoS One. 2013;8:e56304. doi: 10.1371/journal.pone.0056304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ueno K, Ohta M, Morita H, Mikuni Y, Nakajima S, Yamamoto K, Isono K. Trehalose sensitivity in Drosophila correlates with mutations in and expression of the gustatory receptor gene Gr5a. Curr Biol. 2001;11:1451–1455. doi: 10.1016/s0960-9822(01)00450-x. [DOI] [PubMed] [Google Scholar]
- 6.Slone J, Daniels J, Amrein H. Sugar receptors in Drosophila. Curr Biol. 2007;17:1809–1816. doi: 10.1016/j.cub.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiao Y, Moon SJ, Montell C. A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mRNA tagging. Proc Natl Acad Sci U S A. 2007;104:14110–14115. doi: 10.1073/pnas.0702421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahanukar A, Lei YT, Kwon JY, Carlson JR. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56:503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee Y, Moon SJ, Montell C. Multiple gustatory receptors required for the caffeine response in Drosophila. Proc Natl Acad Sci U S A. 2009;106:4495–4500. doi: 10.1073/pnas.0811744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moon SJ, Kottgen M, Jiao Y, Xu H, Montell C. A taste receptor required for the caffeine response in vivo. Curr Biol. 2006;16:1812–1817. doi: 10.1016/j.cub.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Moon SJ, Lee Y, Jiao Y, Montell C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr Biol. 2009;19:1623–1627. doi: 10.1016/j.cub.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Han X, Mehren J, Hiroi M, Billeter JC, Miyamoto T, Amrein H, Levine JD, Anderson DJ. Hierarchical chemosensory regulation of male-male social interactions in Drosophila. Nat Neurosci. 2011;14:757–762. doi: 10.1038/nn.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyamoto T, Amrein H. Courtship Suppression by a Drosophila pheromone recpetor. Nature Neuroscience. 2008 doi: 10.1038/nn.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bray S, Amrein H. A putative Drosophila pheromone receptor expressed in male-specific taste neurons is required for efficient courtship. Neuron. 2003;39:1019–1029. doi: 10.1016/s0896-6273(03)00542-7. [DOI] [PubMed] [Google Scholar]
- 15.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci U S A. 2007;104:3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- 17.Miyamoto T, Slone J, Song X, Amrein H. A fructose receptor functions as a nutrient sensor in the Drosophila brain. Cell. 2012;151:1113–1125. doi: 10.1016/j.cell.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. Molecular and cellular organization of the taste system in the Drosophila larva. J Neurosci. 2011;31:15300–15309. doi: 10.1523/JNEUROSCI.3363-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stocker RF. Design of the larval chemosensory system. Adv Exp Med Biol. 2008;628:69–81. doi: 10.1007/978-0-387-78261-4_5. [DOI] [PubMed] [Google Scholar]
- 20.Schipanski A, Yarali A, Niewalda T, Gerber B. Behavioral analyses of sugar processing in choice, feeding, and learning in larval Drosophila. Chem Senses. 2008;33:563–573. doi: 10.1093/chemse/bjn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato K, Tanaka K, Touhara K. Sugar-regulated cation channel formed by an insect gustatory receptor. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1019622108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harvey RA, Ferrier DR, editors. Biochemistry (Lippincott’s Illustrated Reviews) 5. Philadelphia, PA: Lippincott, Williams and Wilkins; 2011. [Google Scholar]
- 23.Suster ML, Seugnet L, Bate M, Sokolowski MB. Refining GAL4-driven transgene expression in Drosophila with a GAL80 enhancer-trap. Genesis. 2004;39:240–245. doi: 10.1002/gene.20051. [DOI] [PubMed] [Google Scholar]
- 24.Martin JR, Keller A, Sweeney ST. Targeted expression of tetanus toxin: a new tool to study the neurobiology of behavior. Adv Genet. 2002;47:1–47. doi: 10.1016/s0065-2660(02)47001-0. [DOI] [PubMed] [Google Scholar]
- 25.Burant CF, Takeda J, Brot-Laroche E, Bell GI, Davidson NO. Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem. 1992;267:14523–14526. [PubMed] [Google Scholar]
- 26.Xu W, Zhang HJ, Anderson A. A Sugar Gustatory Receptor Identified from the Foregut of Cotton Bollworm Helicoverpa armigera. J Chem Ecol. 2012;38:1513–1520. doi: 10.1007/s10886-012-0221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.