Abstract
An efficient four-component synthesis of 1,2,4,5-tetrasubstituted imidazoles is described by one-step condensation of an aldehyde, benzil, ammonium acetate and primary aromatic amine with nanocrystalline magnesium aluminate in ethanol under ultrasonic irradiation. High yields, short reaction times, mild conditions, simplicity of operation and easy work-up are some advantages of this protocol.
Keywords: Four-component reaction, One-pot synthesis, Ultrasonic irradiation, Imidazole
Introduction
Imidazoles are an important group of five-membered nitrogen heterocycles that have attracted much attention because of the participation in the structure of biological active molecules [1]. Compounds bearing imidazole nucleus are known to show antiedema and anti-inflammatory [2], [3], analgesic [4], anthelmintic [5], anti-bacterial [6], antitubercular [7], anti-fungal [8], antitumor [9] and antiviral activities [10]. In addition, many of the substituted diaryl imidazoles are known as potential inhibitors of the p38 MAP kinase [11]. This versatile applicability highlights the importance of access to efficient synthetic routes to well benign highly substituted imidazole derivatives. These compounds are generally synthesized in a four-component condensation of aldehydes, 1,2-diketones, amines, and ammonium acetate in the presence of various catalysts such as silica gel or HY zeolite [12], silica gel/NaHSO4 [13], K5CoW12O40·3H2O [14], molecular iodine [15], HCLO4–SiO2 [16], heteropolyacids [17], InCl3·3H2O [18], FeCl3·6H2O [19], BF3–SiO2, AlCl3, MgCl2 [20], alumina [21], [22], copper acetate [23], 1,4-diazabicyclo[2,2,2]octane (DABCO) [24], ionic liquid [25], Zr(acac)4 [26], PPA–SiO2 [27], nano-TiCl4·SiO2 [28], nanocrystalline sulfated zirconia (SZ) [29], and silica-bonded propylpiperazine N-sulfamic acid (SBPPSA) [30], under microwave-irradiated, solvent-free or classical conditions. However, some of these synthetic methods have limitations such as harsh reaction conditions, use of hazardous chemicals with often expensive acid catalysts, complex working and purification procedures, significant amounts of waste materials, long reaction times, and moderate yields. Therefore, the development of simple, efficient, clean, high-yielding, and environmentally friendly approaches using new catalysts for the synthesis of highly substituted imidazoles is an important task for organic chemists.
Nanocrystalline magnesium aluminate spinel, MgAl2O4 possesses a variety of interesting electrical, magnetic and optical properties. The compound and its derivatives have so far attracted a great deal of interest of both researchers and engineers due to their remarkable physical and chemical properties such as high melting point, high mechanical strength, high resistance to chemical attack, and low electrical losses [31]. It can be potentially used as a new laser material, refractory ceramics, electrical and irradiation resistance materials, replacement of quartz glass, and as a catalyst or a catalyst support in petroleum industry [32].
Recently, organic synthesis is employing greener approach, due to advantages compared with conventional methods in terms of high selectivity, ease of manipulation, cleaner reaction profiles and relatively benign conditions. Greener synthesis technique involves mainly solvent-free reaction, ultrasound irradiation and solid phase synthesis using a catalyst and microwave irradiation. Ultrasound irradiation assisted organic synthesis has become an important method for organic and medicinal chemists in rapid organic synthesis avoiding by-product formation [33], [34]. Herein we wish to report an efficient, mild and simple method for preparation of tetrasubstituted imidazole derivatives under ultrasound irradiation using nanocrystalline magnesium aluminate as an efficient catalyst (Scheme 1).
Scheme 1.

Synthesis of tetrasubstituted imidazole derivatives under ultrasound irradiation.
Experimental
Chemical and apparatus
Chemical reagents were purchased from the Merck Chemical Company in high purity. All materials were of commercial reagent grade. Melting points were determined in open capillaries using an Electro thermal MK3 apparatus, Infrared (IR) spectra were recorded using a Perkin–Elmer FT-IR 550 Spectrometer. 1H NMR and 13C NMR spectra were recorded with a Bruker DRX-400 spectrometer at 400 and 100 MHz respectively. NMR spectra were obtained in DMSO-d6 solutions. The element analyses (C, H, N) were obtained from a Carlo ERBA Model EA 1108 analyzer or a Perkin–Elmer 240c analyzer. Ultrasonication was performed in a EUROSONIC® 4D ultrasound cleaner with a frequency of 50 kHz and an output power of 200 W. The reaction occurred at the maximum energy area in the cleaner, where the surface of reactants in the reaction vessel was slightly lower than the level of the water and the temperature of the water bath was controlled at 60 °C.
Preparation of 1,2,4,5-tetrasubstituted imidazoles by use of nanocrystalline MgAl2O4
Nanocrystalline magnesium aluminate spinel with high surface area and mesoporous structure was synthesized by a facile method with the addition of N-Cetyl-N,N,N-trimethylammonium Bromide (CTAB) as surfactant. The crystalline sizes are determined by XRD between 4 and 12 nm. The pore volume and pore size were also calculated from the N2 adsorption/desorption isotherm giving approximately 1.10 cm3 g−1 [35]. Then, for synthesis of tetrasubstituted imidazoles a 50 mL flask was charged with 1,2-diketone (1 mmol), aldehyde (1 mmol), ammonium acetate (4 mmol), and primary aromatic amine (4 mmol) in presence of nanocrystalline magnesium aluminate (0.05 g) and ethanol (2 mL). The mixture was sonicated under silent conditions by ultrasound (50 kHz) at 60 °C for the appropriate time, as shown in Table 3. The temperature of reaction mixture was controlled by a water batch. After the completion of the reaction (monitored by TLC), the reaction was allowed to cool, the solvent was evaporated, then the solid residue was recrystallized from acetone–water mixture to afford the pure 1,2,4,5-tetrasubstituted imidazole derivatives as colorless crystals.
Table 3.
Sonochemical synthesis of tetraarylimidazoles catalyzed by 0.035 mol% nanocrystalline MgAl2O4 at 60 °C a.
| Entry | Ar | Time (min) | Product | Yield (%) | M.p. (°C) |
|---|---|---|---|---|---|
| 1 | C6H5 | 15 | 5a | 91 | 216–218 [8] |
| 2 | p-Me C6H4 | 17 | 5b | 95 | 186–188 [8] |
| 3 | p-MeO C6H4 | 18 | 5c | 93 | 253–254 [8] |
| 4 | 3,4-(OMe)2 C6H3 | 20 | 5d | 90 | 178–180 |
| 5 | p-Cl C6H4 | 12 | 5e | 96 | 152–154 [8] |
| 6 | p-Br C6H4 | 12 | 5f | 94 | 165–168 |
| 7 | 2-F C6H4 | 15 | 5g | 93 | 165–168 |
| 8 | 2-OH C6H4 | 18 | 5h | 90 | 253–255 [8] |
| 9 | 3,5-(OMe)2 C6H3 | 14 | 5i | 97 | 163–165 |
| 10 | p-OH C6H4 | 25 | 5j | 89 | 282–285 [20] |
Conditions: 1 mmol benzil 1,1 mmol aldehyde 2, 4 mmol amine 3, 4 mmol ammonium acetate 4.
1,2,4,5-Tetraphenyl-1H-imidazole (5a)
White powder; Rf (petroleum ether:ethylacetate): 7:3 (v/v) = 0.71; IR (KBr) νmax: 3055 (C—H aromatic), 1599 (C C aromatic), 1496 (C N) cm−1; UV (CH3OH) λmax: 286 nm; 1H NMR (400 MHz, DMSO-d6): δH 7.16–7.49 (m, 20H, H—Ar) ppm; 13C NMR (100 MHz, DMSO-d6): δC 128.70, 128.63, 130.05, 130.85, 131.02, 131.55, 132.53, 132.67, 132.92, 133.87, 134.26, 134.81, 135.41, 136.23, 137.11, 138.40, 139.54 ppm; Anal. Calcd. for C27H20N2: C 87.07, H 5.41, N 7.52. Found: C 87.09, H 5.40, N 6.51%.
2-(4-Methylphenyl)-1,4,5-triphenyl-1H-imidazole (5b)
Yellow needle solid; Rf (petroleum ether:ethylacetate): 7:3 (v/v) = 0.8; IR (KBr) νmax: 3065 (C—H aromatic), 1590 (C C aromatic), 1491 (C N) cm−1; UV (CH3OH) λmax: 274 nm; 1H NMR (400 MHz, DMSO-d6): δH 2.25 (s, 3H, CH3), 7.07 (d, J = 8 Hz, 2H, H—Ar), 7.08–7.45 (m, 15H, H—Ar), 7.46 (d, J = 8 Hz, 2H, H—Ar) ppm; 13C NMR (100 MHz, DMSO-d6): δC 21.20, 126.83, 126.84, 128.03, 128.61, 128.83, 128.90, 129.13, 129.59, 130.92, 131.54, 131.59, 134.92, 137.19, 138.29, 146.61 ppm; Anal. Calcd. for C28H22N2: C 87.02, H 5.72, N 7.27. Found: C 87.01, H 5.74, N 7.25%.
2-(4-Methoxyphenyl)-1,4,5-triphenyl-1H-imidazole (5c)
Milky crystal; Rf (petroleum ether:ethylacetate): 7:3 (v/v) = 0.63; IR (KBr) νmax: 3058 (C—H aromatic), 1601 (C C aromatic), 1505 (C N), 1065 (C—O—Ar) cm−1; UV (CH3OH) λmax: 289 nm; 1H NMR (400 MHz, DMSO-d6): δH 3.24 (s, 3H, CH3), 6.83 (d, J = 7.4 Hz, 2H, H—Ar), 7.23–7.41 (m, 15H, H—Ar), 7.47 (d, J = 7.4 Hz, 2H, H—Ar) ppm; 13C NMR (100 MHz, DMSO-d6): δC 55.57, 114.07, 123.30, 126.83, 128.60, 128.77, 128.89, 129.12, 129.16, 129.24, 130.12, 131.10, 131.29, 131.59, 135.0, 137.07, 137.27, 146.49, 160.0 ppm; Anal. Calcd. for C28H22N2O: C 87.30, H 5.16, N 7.54. Found: C 87.33, H 5.15, N 7.52%.
2-(3,4-Dimethoxyphenyl)-1,4,5-triphenyl-1H-imidazole (5d)
White powder; Rf (petroleum ether:ethylacetate): 7:3 (v/v) = 0.62; IR (KBr) νmax: 3045 (C—H aromatic), 1617 (C C aromatic), 1578 (C N), 1154 (C—O—Ar) cm−1; UV (CH3OH) λmax: 293 nm; 1H NMR (400 MHz, DMSO-d6): δH 3.6 (s, 6H, 2CH3), 6.85 (d, J = 8.8 Hz, 2H, H—Ar), 7.15–7.33 (m, 15H, H—Ar), 7.48 (d, J = 7.2 Hz, 1H, H—Ar) ppm; 13C NMR (100 MHz, DMSO-d6): δC 55.57, 55.60, 115.18, 124.55, 127.18, 128.53, 128.61, 129.09, 129.19, 129.20, 129.36, 130.10, 131.15, 132.30, 132.48, 136.50, 136.55, 136.61, 140.49, 145.29 ppm; Anal. Calcd. for C29H24N2O2: C 80.53, H 5.60, N 6.48. Found: C 80.52, H 5.59, N 6.47%.
2-(4-Chlorophenyl)-1,4,5-triphenyl-1H-imidazole (5e)
Cream crystal; Rf (petroleum ether:ethylacetate): 7:3 (v/v) = 0.57; IR (KBr) νmax: 3050 (C—H aromatic), 1603 (C C aromatic), 1505 (C N), 1065 (C—Cl) cm−1; UV (CH3OH) λmax: 296 nm; 1H NMR (400 MHz, DMSO-d6): δH 7.15–7.36 (m, 17H, H—Ar), 7.47 (d, J = 7.4 Hz, 2H, H—Ar) ppm; 13C NMR (100 MHz, DMSO-d6): δC 127.30, 127.50, 127.70, 128.0, 128.20, 129.31, 129.70, 129.85, 130.10, 131.54, 132.69, 133.60, 133.68, 145.0, 149.72 ppm; Anal. Calcd. for C27H19ClN2: C 79.70, H 4.71, N 6.88. Found: C 79.72, H 4.70, N 6.87%.
2-(4-Bromophenyl)-1,4,5-triphenyl-1H-imidazole (5f)
White powder; Rf (petroleum ether:ethylacetate): 7:3 (v/v) = 0.71; IR (KBr) νmax: 3045 (C—H aromatic), 1604 (C C aromatic), 1588 (C N), 1072 (C—Br) cm−1; UV (CH3OH) λmax: 292 nm; 1H NMR (400 MHz, DMSO-d6): δH 7.15–7.40 (m, 17H, H—Ar), 7.50 (d, J = 7.2 Hz, 2H, H—Ar) ppm; 13C NMR (100 MHz, DMSO-d6): δC 128.22, 128.35, 128.49, 129.50, 129.58, 129.67, 132.19, 132.43, 133.50, 135.68, 137.38, 137.58, 139.50, 142.16, 145.92,147.30 ppm; Anal. Calcd. for C27H19BrN2: C 71.85, H 4.25, N 6.20. Found: C 71.84, H 4.24, N 6.21%.
2-(4-Flurophenyl)-1,4,5-triphenyl-1H-imidazole (5g)
White crystal; Rf (petroleum ether:ethylacetate): 7:3 (v/v) = 0.55; IR (KBr) νmax: 3050 (C—H aromatic), 1509 (C C aromatic), 1095 (C N), 1095 (C—F) cm−1; UV (CH3OH) λmax: 284 nm; 1H NMR (400 MHz, DMSO-d6): δH 7.11–7.30 (m, 15H, H—Ar), 7.41 (d, J = 8.0 Hz, 1H, H—Ar), 7.46 (d, J = 8.0 Hz, 2H, H—Ar), 7.53 (t, J = 8.0 Hz, 1H, H—Ar) ppm; 13C NMR (100 MHz, DMSO-d6): δC 129.82, 129.94, 130.51, 130.55, 130.64, 131.60, 132.75, 132.81, 133.65, 136.61, 136.82, 138.50, 140.50, 143.13, 144.90,148.02 ppm; Anal. Calcd for C27H19FN2: C 83.06, H 4.9, N 7.17. Found: C 83.5, H 4.93, N 7018%.
2-(1,4,5-Triphenyl-1H-imidazol-2-yl)phenyl (5h)
White powder; Rf (petroleum ether:ethylacetate): 7:3 (v/v) = 0.91; IR (KBr) νmax: 3448 (OH), 3061(C—H aromatic), 1590 (C C aromatic), 1485 (C N), 1254 (Ar—O) cm−1; UV (CH3OH) λmax: 320 nm; 1H NMR (400 MHz, DMSO-d6): δH 6.54 (t, J = 8.0 1H, H—Ar), 6.65 (d, 1H, H—Ar), 6.93 (d, 1H, H—Ar), 7.16–7.43 (m, 16H, H—Ar), 12.57 (s, 1H, OH) ppm; 13C NMR (100 MHz, DMSO-d6): δC 110.30, 112.51, 114.61, 116.48, 118.92, 121.35, 122.90, 124.35, 125.47, 127.74, 128.65, 130.24, 135.61, 137.66, 146.82, 160.72 ppm; Anal. Calcd for C27H20N2O: C 83.48, H 5.19, N, 7.21. Found: C 83.46, H 5.20, N 7.22%.
2-(3,5-Dimethoxyphenyl)-1,4,5-triphenyl-1H-imidazole (5i)
White powder; Rf (petroleum ether:ethylacetate): 7:3 (v/v) = 0.62; IR (KBr) νmax: 3057 (C—H aromatic), 1597 (C C aromatic), 1494 (C N), 1157 (C—O—Ar) cm−1; UV (CH3OH) λmax: 293 nm; 1H NMR (400 MHz, DMSO-d6): δH 3.55 (s, 6H, 2CH3), 6.43 (s, 1H, H—Ar), 6.55 (d, 2H, H—Ar), 7.15–7.57 (m, 15H, H—Ar) ppm; 13C NMR (100 MHz, DMSO-d6): δC 56.67, 56.73, 114.15, 122.29, 125.38, 126.43, 128.65, 129.0, 129.53, 130.21, 130.65, 130.81, 132.30, 132.63, 133.80, 135.0, 135.35, 135.51, 138.49, 142.16 ppm; Anal. Calcd for C29H24N2O2: C 80.51, H 5.61, N 6.45. Found: C 80.53, H 5.59, N 6.48%.
4-(1,4,5-Triphenyl-1H-imidazol-2-yl)phenol (5j)
White powder; Rf (petroleum ether:ethylacetate): 7:3 (v/v) = 0.90; IR (KBr) νmax: 3452 (OH), 3057 ( CH aromatic), 1604 (C C aromatic), 1578 (C N), 1230 (Ar—O) cm−1; UV (CH3OH) λmax: 330 nm; 1H NMR (DMSO-d6, 400 MHz): δH 6.87–6.91 (d, J = 8 Hz, 2H), 7.15–7.49 (m, 15H), 7.61–7.65 (d, J = 8.2 Hz) ppm; 13C NMR (DMSO-d6, 100 MHz): δC 115.3, 119.8, 125.3, 126.0, 126.7, 127.9, 128.2, 128.5, 128.6, 1293.3, 131.6, 131.8, 135.3, 137.3, 146.6, 159.3 ppm; Anal. Calcd. for C27H20N2O: C 83.48, H 5.19, N 7.21. Found: C 83.44, H 5.11, N 7.09%.
Results and discussion
Since tetrasubstituted imidazoles have become increasingly useful and important in the pharmaceutical fields, the development of clean, high-yielding, and environmentally friendly synthetic approaches are still desirable and much in demand. Many recent papers are illustrating the use of nanocatalyst in organic reactions [36], [37]. Thus, nanocatalysts are potential catalysts due probably to their high catalytic activities, low costs and ease of handling. MgAl2O4 is an important acid catalyst which efficiently catalyzes the preparation of 1,2,4,5-tetrasubstituted imidazoles. It seems that the existence of MgAl2O4 as an acidic catalyst can accelerate this cyclocondensation reaction by increasing the reactivity of benzaldehyde derivatives and benzil. Magnesium aluminate spinel used as catalyst, shows a relatively large surface area, small crystalline size and special active sites, which can be controlled by its preparation method. The high activity of magnesium aluminate nanoparticles is not only because of their high effective surface. In other words, the high impact of these nanoparticles is due to the high concentration of areas with low coordination and structural deficiencies in their surface. When the particle size decreases to nanoscale, defect is made in coordination of constituent atoms. Most atoms have a partial capacity and remain on the levels. Therefore, the crystal magnesium aluminate nanoparticles act as a mild lewis acid in the synthesis of tetrasubstituted imidazoles.
A proposed mechanism for the reaction is outlined in Scheme 2. Based on this mechanism, it is highly probable that the carbonyl groups of benzil and aldehydes have to be activated which occurs when the carbonyl oxygen is coordinated by MgAl2O4. Therefore, it may be proposed that the MgAl2O4 catalyst facilitates the formation of diamine intermediate [A] by increasing the electrophilicity of the carbonyl group of the aldehyde. Then nucleophilic attack of the nitrogen of ammonia obtained from NH4OAc on the activated carbonyl group, resulted in formation of diamine intermediate [A], and it followed by the nucleophilic attack of the in situ generated diamine [A] to carbonyl of benzil, giving the intermediate [B]. Their subsequent intramolecular interaction leads to cyclizations and eventually to the formation of intermediate [C], which dehydrates to the tetrasubstituted imidazoles.
Scheme 2.
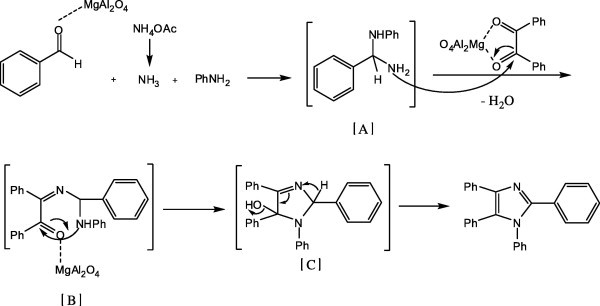
Postulated mechanism for imidazoles synthesis.
Effects of the catalyst under ultrasound irradiation
In an initial study, for examination of the catalytic activity of different catalysts such as AlCl3, SbCl3 and nanocrystalline MgAl2O4 in condensation reaction, benzaldehyde first reacted with aniline, benzil and ammonium acetate in ethanol (2 mL) for 15 min under ultrasound irradiation in the presence of each catalysts (0.035 mol%) separately. In this study, we found that nanocrystalline MgAl2O4 was the most effective catalyst in terms of yield of the tetraarylimidazole (90%) while other catalysts formed the product with the yields of 20–43%. In the absence of catalyst, the yield of the product was found to be very low. Therefore, we decided to use nanocrystalline MgAl2O4 with a high specific surface area as a catalyst with higher activity and better controlled selectivity. Herein, we report facile multi-component synthesis of 1,2,4,5-tetrasubstituted imidzoles by using nanocrystalline MgAl2O4 as a novel and efficient catalyst under ultrasound irradiation. To show the effect of ultrasound irradiation in these reactions, the synthesis of 2-(4-methoxyphenyl)-1,4,5-triphenylimidazole investigated as a model reaction in the presence of various amounts of nanocrystalline MgAl2O4 under ultrasound irradiation and reflux conditions (Table 1).
Table 1.
Comparison of the classical- and ultrasound irradiation methods for the synthesis of compound 5c using nanocrystalline MgAl2O4 as a catalystc.
| Entry | MgAl2O4 (mol%) | Yield (%)a | Yield (%)b |
|---|---|---|---|
| 1 | 0 | 15 | 10 |
| 2 | 0.007 | 20 | 15 |
| 3 | 0.014 | 37 | 28 |
| 4 | 0.020 | 56 | 40 |
| 5 | 0.028 | 78 | 65 |
| 6 | 0.035 | 95 | 90 |
| 7 | 0.042 | 88 | 79 |
Ultrasound irradiation.
Reflux conditions.
Conditions: temperature: 60 °C, time: 15 min.
In all cases, the results show that the reaction times are shorter and the yields of the products are higher under sonication. The best results were obtained using 0.035 mol% of the catalyst under both conditions.
Effects of reaction temperature and frequency under ultrasonic irradiation
Subsequent efforts were focused on optimizing conditions for formation of 1,2,4,5-tetrasubstituted imidazoles by using different temperatures and frequencies of ultrasonic irradiation to determine their effects on the above model reaction (Table 2). The maximum yield was obtained when the reaction was carried out under irradiation of 50 kHz at 60 °C for 15 min (Table 2, entry 6). Lower yield (89%) was observed when higher temperature than 60 °C was used.
Table 2.
The synthesis of 5c under ultrasound irradiation at different reaction conditions.
| Entry | Temperature (°C) | Frequency (kHz) | Time (min) | Yield (%) |
|---|---|---|---|---|
| 1 | 25 | 25 | 15 | 73 |
| 2 | 37 | 25 | 15 | 75 |
| 3 | 45 | 25 | 15 | 76 |
| 4 | 56 | 25 | 15 | 81 |
| 5 | 60 | 25 | 15 | 83 |
| 6 | 60 | 50 | 15 | 98 |
| 7 | 65 | 50 | 15 | 89 |
High efficiency synthesis by ultrasound irradiation
After optimizing conditions, the generality of this method was examined by the reaction of several aldehydes, benzil, ammonium acetate and primary aromatic amine with nanocrystalline magnesium aluminate in ethanol under ultrasonic irradiation. Interestingly, a variety of aldehydes participated well in this reaction (Table 3). Aldehydes bearing either electron-withdrawing or electron donating groups perform equally well in the reaction and imidazoles are obtained in high yields. Short reaction time, easy work up and high yields are several benefits of this method.
Conclusion
In summary, we described an efficient and convenient route to synthesize tetrasubstituted imidazoles. Nanocrystalline MgAl2O4 have been used as an new catalytic system for the promotion of the synthesis of 1,2,4,5-tetrasubstituted imidazole derivatives in the presence of solvent under ultrasonic irradiation. Good yields and easy availability of starting materials are valuable, noteworthy advantages of this method, which allows a privileged access to previously unattainable products. The improvement of the yield reveals the method reported as an attractive approach for the synthesis of many similar compounds.
Acknowledgement
We gratefully acknowledge the financial support from the Research Council of the University of Kashan (No. 159198/12).
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Laufer S.A., Zimmermann W., Ruff K.J. Tetrasubstituted imidazole inhibitors of cytokine release: probing substituents in the N − 1 position. J Med Chem. 2004;47(25):6311–6325. doi: 10.1021/jm0496584. [DOI] [PubMed] [Google Scholar]
- 2.Breslin H.J., Cai C., Miskowski T.A., Coutinho S.V., Zhang Sui-Po, Pamela H. Identification of potent phenyl imidazoles as opioid receptor agonists. Bioorg Med Chem Lett. 2006;16(9):2505–2508. doi: 10.1016/j.bmcl.2006.01.082. [DOI] [PubMed] [Google Scholar]
- 3.Yadav M.R., Puntambekar D.S., Sarathy K.P., Vengurlekar S., Giridhar R. Quantitative structure activity relationship studies of diarylimidazoles as selective COX-2 inhibitors. Indian J Chem. 2006;45:475–482. [Google Scholar]
- 4.Khabnadi-deh S., Rezaei Z., Khalafi-Nezhad A., Bahrinajafi R., Mohamadi R.F. Synthesis of N-alkylated derivatives of imidazole as antibacterial agents. Bioorg Med Chem Lett. 2003;13(17):2863–2865. doi: 10.1016/s0960-894x(03)00591-2. [DOI] [PubMed] [Google Scholar]
- 5.Kapoor V.K., Dubey S., Mahindroo N. Preparation, antiprotozoal and antibacterial evaluation and mutagenicity of some metronidazole derivatives. Indian J Chem. 2000;39(1):27–30. [Google Scholar]
- 6.Matysiak J., Niewiadomy A., Macik-Niewiadomy G., Krajewska-Kulak E. Synthesis of some 1-(2,4-dihydroxythiobenzoyl)imidazoles, -imidazolines and -tetrazoles and their potent activity against Candida species. Farmaco. 2003;58(6):455–461. doi: 10.1016/S0014-827X(03)00046-6. [DOI] [PubMed] [Google Scholar]
- 7.Narayanan S., Vangapandu S., Jain R. Regiospecific synthesis of 2,3-disubstituted-L-histidines and histamines. Bioorg Med Chem Lett. 2001;11(9):1133–1136. doi: 10.1016/s0960-894x(01)00154-8. [DOI] [PubMed] [Google Scholar]
- 8.Dahiya R. Synthesis, characterization and antimicrobial studies on some newer imidazole analogs. Sci Pharm. 2008;76(2):217–239. [Google Scholar]
- 9.Wang L., Woods K.W., Li Q., Barr K.J., McCroskey R.W., Hannick S.M., et al. Potent, orally active heterocycle-based combretastatin A-4 analogues: synthesis, structure–activity relationship, pharmacokinetics, and in vivo antitumor activity evaluation. J Med Chem. 2002;45(8):1697–1711. doi: 10.1021/jm010523x. [DOI] [PubMed] [Google Scholar]
- 10.Cheng J., Xie J.T., Luo X.J. Synthesis and antiviral activity against Coxsackie virus B3 of some novel benzimidazole derivatives. Bioorg Med Chem Lett. 2005;15(2):267–269. doi: 10.1016/j.bmcl.2004.10.087. [DOI] [PubMed] [Google Scholar]
- 11.Murry J.A. Synthetic methodology utilized to prepare substituted imidazole p38 MAP kinase inhibitors. Curr Opin Drug Discov Dev. 2003;6(6):945–965. [PubMed] [Google Scholar]
- 12.Balalaei S., Arabanian A. One-pot synthesis of tetrasubstituted imidazoles catalyzed by zeolite HY and silica gel under microwave irradiation. Green Chem. 2000;2(6):274–276. [Google Scholar]
- 13.Karimi A.R., Alimohammadi Z., Azizian J., Mohammadi A.A., Mohmmadizadeh M.R. Solvent-free synthesis of tetrasubstituted imidazoles on silica gel/NaHSO4 support. Catal Commun. 2006;7(9):728–732. [Google Scholar]
- 14.Nagarapu L., Apuri S., Kantevari S. Potassium dodecatugstocobaltate trihydrate (K5CoW12O40·3H2O): a mild and efficient reusable catalyst for the one-pot synthesis of 1,2,4,5-tetrasubstituted imidazoles under conventional heating and microwave irradiation. J Mol Catal A Chem. 2007;266(1–2):104–108. [Google Scholar]
- 15.Kidwai M., Mothsra P., Bansal V., Somvanshi R.K., Ethayathulla A.S., Dey S., et al. One-pot synthesis of highly substituted imidazoles using molecular iodine: a versatile catalyst. J Mol Catal A Chem. 2007;265(1–2):177–182. [Google Scholar]
- 16.Kantevari S., Vuppalapati S.V.N., Biradar D.O., Nagarapu L. Highly efficient, one-pot, solvent-free synthesis of tetrasubstituted imidazoles using HClO4–SiO2 as novel heterogeneous catalyst. J Mol Catal A Chem. 2007;266(1–2):109–113. [Google Scholar]
- 17.Heravi M.M., Derikvand F., Bamoharram F.F. Highly efficient, four-component, one-pot synthesis of tetrasubstituted imidazoles using Keggin-type heteropolyacids as green and reusable catalysts. J Mol Catal A Chem. 2007;263(1–2):112–114. [Google Scholar]
- 18.Sharma S.D., Hazarika P., Konwar D. An efficient and one-pot synthesis of 2,4,5- trisubstituted and 1,2,4,5-tetrasubstituted imidazoles catalyzed by InCl3·3H2O. Tetrahedron Lett. 2008;49(14):2216–2220. [Google Scholar]
- 19.Heravi M.M., Derikv F., Haghighi M. Highly efficient, four component, one-pot synthesis of tetrasubstituted imidazoles using a catalytic amount of FeCl3·6H2O. Monatsh Chem. 2008;139(1):31–33. [Google Scholar]
- 20.Sadeghi B., Mirjalili B.B.F., Hashemi M.M. BF3·SiO2: an efficient reagent system for the one-pot synthesis of 1,2,4,5-tetrasubstituted imidazoles. Tetrahedron Lett. 2008;49(16):2575–2577. [Google Scholar]
- 21.Usyatinsky A.Y., Khmelnitsky Y.L. Microwave-assisted synthesis of substituted imidazoles on a solid support under solvent-free conditions. Tetrahedron Lett. 2000;41(26):5031–5034. [Google Scholar]
- 22.Saberi A. Synthesis of novel highly potent antibacterial and antifungal agents. Asian J Mol Med. 2011;1(1):01–05. [Google Scholar]
- 23.Lipshutz B.H., Morey M.C. An approach to the cyclopeptide alkaloids (phencyclopeptines) via heterocyclic diamide/dipeptide equivalents. Preparation and N-alkylation studies of 2,4(5)-disubstituted imidazoles. J Org Chem. 1983;48(21):3745–3750. [Google Scholar]
- 24.Murthy S., Madhav B., Nageswar Y.V.D. DABCO as a mild and efficient catalyst system for the synthesis of highly substituted imidazoles via multicomponent condensation strategy. Tetrahedron Lett. 2010;51(40):5252–5257. [Google Scholar]
- 25.Zang H., Su Q., Mo Y., Cheng B.W., Jun S. Ionic liquid [EMIM]OAc under ultrasonic irradiation towards the first synthesis of trisubstituted imidazoles. Ultrason Sonochem. 2010;17(5):749–751. doi: 10.1016/j.ultsonch.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Hasaninejad A., Zare A., Shakouhy M. Catalyst-free one-pot four component synthesis of polysubstituted imidazoles in neutral ionic liquid 1-butyl-3-methylimidazolium bromide. J Comb Chem. 2010;12(6):844–849. doi: 10.1021/cc100097m. [DOI] [PubMed] [Google Scholar]
- 27.Montazeri N., Pourshamsian K., Khoddadi M., Kazem K. Poly phosphoric acid impregnated on silica gel (PPA–SiO2): a versatile and reusable catalyst for the synthesis of 1,2,4,5-Tetrasubstituted imidazoles under solvent-free and microwave irradiation conditions. Orient J Chem. 2011;27(3):1023–1027. [Google Scholar]
- 28.Mirjalili B.F., Bamoniri A.H., Zamani L. One-pot synthesis of 1,2,4,5-tetrasubstituted imidazoles promoted by nano-TiCl4·SiO2. Sci Iran. 2012;19(3):565–568. [Google Scholar]
- 29.Teimouri A., Chermahini A.N. An efficient and one-pot synthesis of 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazoles catalyzed via solid acid nano-catalyst. J Mol Catal A Chem. 2011;346(1–2):39–45. [Google Scholar]
- 30.Niknam K., Deris A., Naeimi F., Majleci F. Synthesis of 1,2,4,5-tetrasubstituted imidazoles using silica-bonded propylpiperazine N-sulfamic acid as a recyclable solid acid catalyst. Tetrahedron Lett. 2011;52(36):4642–4645. [Google Scholar]
- 31.Leger J.M., Haines J., Schmidt M., Petitet J.P., Pereira A.S., Da-Jornada J.A.H. Discovery of hardest known oxide. Nature. 1996;383:401. [Google Scholar]
- 32.Ahmad J., Mazhar M.E., Awan M.Q., Ashiq M.N. Effect of substitution of K+ ions on the structural and electrical properties of nanocrystalline MgAl2O4 spinel oxide. Physica B. 2011;406(18):3484–3488. [Google Scholar]
- 33.Mason T.J., Peters D. 2nd ed. EllisHorwood; London: 2002. Practical sonochemistry. [Google Scholar]
- 34.Zang H.J., Wang M.L., Cheng B.W., Song J. Ultrasound-promoted synthesis of oximes catalyzed by a basic ionic liquid [bmIm]OH. Ultrason Sonochem. 2009;16(3):301–303. doi: 10.1016/j.ultsonch.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Navaei Alver E., Rezaei M., Navaei Alver H. Synthesis of mosoporous nanocrystalline MgAl2O4 spinel via surfactant assisted precipitation route. Powder Technol. 2010;198(2):275–278. [Google Scholar]
- 36.Rostamizadeh S., Azad M., Shadjou N., Hasanzadeh M. (α-Fe2O3)-MCM-41-SO3H as a novel magnetic nanocatalyst for the synthesis of N-aryl-2-amino-1,6-naphthyridine derivatives. Catal Commun. 2012;25:83–91. [Google Scholar]
- 37.Kaboudin B., Abedi Y., Yokomatsu T. One-pot synthesis of 1,2,3-triazoles from boronic acids in water using Cu(ii)-β-cyclodextrin complex as a nanocatalyst. Org Biomol Chem. 2012;10(23):4543–4548. doi: 10.1039/c2ob25061f. [DOI] [PubMed] [Google Scholar]



