Abstract
Liver cancer grows silently with mild or no symptoms until advanced. In the absence of an effective treatment for advanced stage of hepatic cancer hope lies in early detection, and screening for high-risk population. Among Egyptians viral hepatitis is the most common risk factor for hepatocellular carcinoma (HCC). The current work was designed to determine the level of prothrombin induced by vitamin K absence-II (PIVKA-II) in sera of patients suffering from HCC and hepatitis C virus (HCV) patients being the most common predisposing factor for HCC. Our ultimate goal is diagnosis of HCC at its early stage. The current study was carried out on 83 individuals within three groups; Normal control, HCV and HCC groups. Patients were subdivided into cirrhotic and non-cirrhotic. Complete clinicopathological examination was carried out for each individual to confirm diagnosis. Individuals’ sera were subjected to quantitative determination of alpha-fetoprotein (AFP), PIVKA-II and other parameters. PIVKA-II proved to be superior to AFP for early detection of HCC patients being highly sensitive and specific. Furthermore it has the ability to discriminate between different histopathological grades of HCC and It has a powerful diagnostic validity to evaluate the thrombosis of portal vein and to differentiate between early and late stages of HCC. The direct relation between the level of PIVKA-II and the size of tumor makes it an attractive tool for early HCC diagnosis and surveillance. Using the best cut-off value of AFP (>28), showed a sensitivity of (44%) and specificity of (73.3%). While cut-off value of PIVKA-II (>53.7) showed 100% sensitivity and specificity.
Keywords: PIVKA-II, Hepatocelluler carcinoma, Early diagnosis
Introduction
Hepatocellular carcinoma (HCC) is an important cause of death worldwide [1,2]. It is the sixth most common cancer worldwide and the third cause of cancer death [3]. It kills more than 650,000 people around the world annually [4]. Incidence of HCC has risen over the last 5–8 years with no significant change in the survival rate in the last two decades [5].
In Egypt, Liver malignancies constitute 11.75% of the malignancies of the digestive organs and 1.6% of total malignancies. HCC ranks number one with an incidence rate of 70.48% [6].
The etiology of HCC differs according to geographic, economic, and health status. The most common causes are alcohol consumption [7], hepatitis C and B viruses [8] and chronic necro-inflammatory hepatic disease. Commonly cirrhosis is present in 60–80% of patients with HCC [9]. Among Egyptian patients HCV and HBV infections are the most common risk factors for HCC. About 10% – 20% of the general Egyptian population is infected with HCV [10]. Approximately 90% of Egyptian HCV isolates belong to subtype (4a) which responds less successfully to interferon therapy than other subtypes [11]. Most of the HCC occurs in cirrhotic patients associated with viral infection. However, 10–25% of cases develop in absence of cirrhosis. This is due to the direct oncogenic effect of HBV as HBV-DNA genome integrates in hepatocellular chromosomes [12]. In contrast HCV exerts its carcinogenic effect probably through production of cirrhosis [13]. Many studies showed that HCV has a direct oncogenic action through its core component [14].
All these facts made it essential to find sensitive markers for early diagnosis and monitoring of recurrence of HCC [15].
Ultrasound examination of the liver and detection of AFP level in serum are commonly used to screen for liver cancer [16]. Although detection of AFP level is easy and less expensive, but it shows less sensitivity [17], since elevation in AFP level is common in patients with chronic liver disease, pregnancy and germ cell tumors. AFP titers also rise with flares of active hepatitis, and may be persistently elevated in patients with cirrhosis [18]. Ultrasound is better, but is more expensive, operator dependent and less reliable in the presence of cirrhosis [19]. Thus, new markers with high sensitivity and specificity are required.
Prothrombin induced by vitamin K absence-II (PIVKA-II) is also known as Des-gamma carboxyprothrombin (DCP) is an abnormal prothrombin protein that is increased in the sera of patients with HCC. Generation of (PIVKA-II) is thought to be a result of an acquired defect in the post-translational carboxylation of the prothrombin precursor in malignant cells [20]. The validity of PIVKA-II as a tumor marker for HCC patients has been reported by many investigators [21–23]. None of the known markers are optimal, however when used together their sensitivity increases [24,25].
The present study was designed to investigate the potential role of PIVKA-II as a diagnostic, non-invasive marker for HCC at its early stages and to assess its sensitivity and specificity as compared with the usual recommended marker AFP.
Patients and methods
This study was conducted on 72 patients and 11 apparently healthy individuals as control. Patients were initially subjected to complete clinical examination and abdominal ultrasonography. Blood samples were collected for complete blood picture, liver and kidney function tests, Fasting blood sugar, serum potassium and sodium levels using the standard laboratory methods [26]. Hepatitis markers HBs Ag, HBs Ab, HBc Ag and HCV Ab were detected using ELISA technique, HCV-RNA by qualitative PCR. Diagnosis of HCC was confirmed by triphasic CT scan or liver biopsy guided by U/S. Serum was collected and stored at −70 °C until assayed. Level of serum AFP was detected using ELISA technique (RADIM SpA, Italy) and PIVKA-II level in the plasma using ELISA kit (Stago Diagnostic, France).
Patients with cholangiocarcinoma, hepatoblastoma, hemangioma, or any other hepatic tumor rather than HCC and metastasizing to the liver were excluded from the study. The diagnosis was confirmed by abdominal ultrasound, triphasic CT scan of the abdomen and tissue biopsies for histopathological examinations. HCC’s patients were classified according to Barcelona criteria [27], and patients with liver cirrhosis were classified according to Child- Pugh criteria [28].
The study was approved by the Ethical Committee of National Cancer Institute (NCI), Cairo University, which conforms to the code of ethics of the World Medical Association (Declarations of Helsinki). The study was explained to all individuals who were also informed with a written consent. Individuals were divided into three groups; group I (Control) consisted of 11 apparently healthy subjects, matched with patient’s age and sex. Group II included patients who had history of HCV infection that was confirmed by laboratory findings. This group consisted of 24 patients, 17 of them were males and seven were females. Their median age was 51.5 years (33–70), half of them with cirrhosis. Group III Consisted of 48 patients with HCC who attended NCI clinic, Cairo University during the years 2007 to 2009. Thirty four were males and 14 females. Their median age was 59.5 years (38–77) (Table 1). A 52.1% of them were cirrhotic, 10 patients were Child A (20.8%), 33 Child B (68.8%) and five were Child C (10.4%). Patients were classified according to the Barcelona Clinic Liver Cancer” (BCLC) system into18 patients stage A (37.5%), seven patients stage B (14.6%), 18 patients stage C (37.5%) and five patients stage D (10.4%) Table 5. Patients were also classified according to their clinic-pathological features including stage, grade and size of tumor.
Table 1.
Demographic characteristics of the three groups of patients.
| Parameter | Control | HCV | HCC |
|---|---|---|---|
| Sample size | 11 | 24 | 48 |
| Median age | 34 | 51.5 | 59.5 |
| Range | 29–52 | 33–70 | 38–77 |
| Sex | |||
| Males n (%) | 7 (63.63%) | 17 (70.83%) | 34 (70.8%) |
| Females n (%) | 4 (36.36%) | 7 (29.16%) | 14 (29.25) |
Values are expressed as medians (ranges) for age, and as number (percentage) for sex.
Table 5.
AFP and PIVKA-II level in different grades and stages of HCC patients.
| HCC subdivisions | Sample size | AFP | P-value | PIVKA-II | P-value |
|---|---|---|---|---|---|
| Grade I | 15 | 125.8 (2.7–248.9) | P = 0.088 | 39.6 (29.8–43.6) | P < 0.001 |
| Grade II | 14 | 350.65 (6.3–695) | 52.7 (42.3–89.2) | ||
| Grade III | 13 | 242.95 (5.9–480) | 145.8 (120.7–191.4) | ||
| Grade IV | 6 | 269.5 (120–419) | 191.9 (180.5–192.4) | ||
| Stage 1 | 8 | 11.65 (2.7–20.6) | P = 0.232 | 36.75 (29.8–39.6) | P2 = NS∗ |
| P3 < 0.001 | |||||
| P4 < 0.001 | |||||
| Stage 2 | 15 | 349.4 (3.8–695) | 43.6 (40–53.7) | P1 | |
| P3 = 0.002 | |||||
| P4 = 0.003 | |||||
| Stage 3 | 17 | 305.95 (5.9–606) | 130 (54.9–180.5) | P1 < 0.01 | |
| P2 < 0.01 | |||||
| P4 < 0.001 | |||||
| Stage 4 | 8 | 246.5 (13–480) | 191.4 (186.2–192.4) | P1 < 0.001 | |
| P2 < 0.001 | |||||
| P3 < 0.001 |
Results are expressed as median (range); P-value using is set at 0.05.
P1 stands for Stage 1.
P2 stands for Stage 2.
P3 stands for Stage 3.
P4 stands for Stage 4.
∗Not significant.
Statistical analysis
Continuous variables were expressed as median (range) and were compared by using nonparametric (Mann–Whitney test) for two groups comparison and Kruskall–Wallis test for multiple group comparison. The ROC (receiver operator characterizing) curve was drawn, to improve the specificity and sensitivity of the studied parameters. The analysis was performed using SPSS, version 14.
Results
This study was conducted on 72 patients and 11 apparently healthy individuals serving as control. Demographic characteristics, Clinic-pathological and laboratory investigations of individuals in different investigated groups are presented in Tables 1 and 2,
Table 2.
Comparison of laboratory investigations of individuals in the three groups.
| Parameter | Control (n = 11) | HCV (n = 24) | HCC (n = 48) |
|---|---|---|---|
| AST (up to 40 U/I) | 21 (17–25) | 55.5 (28–83) | 73.5 (8–322) |
| vs. control | 0.045 | <0.001 | |
| vs. HCV | 0.003 | ||
| ALT (up to 55 U/I) | 26 (22–29) | 50 (18–93) | 56 (10.2–170) |
| vs. control | NS⁎ | <0.001 | |
| vs. HCV | NS⁎ | ||
| Alk. phosphatase (61–190 U/l) | 120 (100–175) | 167 (125–230) | 252 (134–868) |
| vs. control | NS⁎ | <0.001 | |
| vs. HCV | <0.001 | ||
| direct bilirubin (up to 0.25 mg/dL) | 0.15 (0.1–0.19) | 1.8 (1.2–2.4) | 1.2 (0.7–2) |
| vs. control | <0.001 | <0.001 | |
| vs. HCV | <0.001 | ||
| Total bilirubin (up to 1 mg/dL) | 0.7 (0.42–0.8) | 2.8 (1.5–3.5) | 1.7 (0.4–7.7) |
| vs. control | <0.001 | <0.001 | |
| vs. HCV | NS⁎ | ||
| GGT (up to 55 IU/l) | 32 (18–39) | 53.7 (32.2–70.9) | 74.7 (59.5–89.6) |
| vs. control | <0.001 | <0.001 | |
| vs. HCV | <0.001 | ||
| Creatinine (0.5–1.4 mg/dL) | 0.8 (0.5–1.2) | 0.96 (0.7–1.6) | 0.7 (0.2–2.4) |
| vs. Control | NS⁎ | NS⁎ | |
| vs. HCV | NS | ||
| Urea g/dL (10–50 mg/dL) | 17 (13–21) | 39.7 (30.2–49.5) | 36 (27.8–49.5) |
| vs. Control | <0.001 | <0.001 | |
| vs. HCV | NS⁎ | ||
| Albumin (3.5–5 g/dL) | 4.1 (3.8–4.4) | 2.65 (1.5–4.1) | 3.05 (1.8–4.2) |
| vs. Control | <0.001 | <0.001 | |
| vs. HCV | 0.022 | ||
| Globulin g/dL (2.3–3.5 g/dL) | 3.5 (2.7–4.3) | 3.75 (2.3–5.2) | 4.6 (2.9–6.3) |
| vs. Control | NS⁎ | NS⁎ | |
| vs. HCV | NS⁎ | ||
| Total protein (6.4–8.2 g/dL) | 7.6 (6.9–8.2) | 6.9 (6.2–7.4) | 7.2 (6.5–8.2) |
| vs. Control | <0.001 | 0.01 | |
| vs. HCV | 0.001 | ||
| Albumin/Globulin ratio (1–1.5) | 1.22 (0.884–1.556) | 0.992 (0.288–1.696) | 0.724 (0.286–1.448) |
| vs. Control | <0.001 | 0.004 | |
| vs. HCV | NS⁎ | ||
| Prothrombin time (12–15 s) | 13.8 (13–14.7) | 16.6 (13–17.9) | 17.4 (14.9–22) |
| vs. control | <0.001 | <0.001 | |
| vs. HCV | <0.001 | ||
| Prothrmbin concentration (80–100%) | 85 (79–100) | 71.4 (62.5–100) | 65.5 (52.6–77) |
| vs. control | <0.001 | <0.001 | |
| vs. HCV | <0.001 | ||
| International Normalization Ratio (INR) (0.8–1.2) | 1.1 (1–1.2) | 1.4 (1–1.6) | 1.51 (1.24–1.9) |
| vs. control | <0.001 | <0.001 | |
| vs. HCV | <0.001 |
Results are expressed as medians (range); P-value is set at 0.05.
Statistical test; Mann–Whitney U test.
Not significant.
On comparing AFP and PIVKA-II levels in HCV patients to the control group there was no significant statistical difference. However, significant elevation was observed in HCC group when compared with control and HCV groups as illustrated in Table 3.
Table 3.
Comparison of Medians and ranges for level of AFP and PIVKA-II of individuals in different investigated groups.
| Control | HCV | HCC | |
|---|---|---|---|
| Sample size | 11 | 24 | 48 |
| Median AFP (ng/mL) | 2.3 | 14.6 | 64.6 |
| Range | 1–3.6 | 1.46–68.8 | 2.7–695 |
| vs. control | 0.734 | 0.002 | |
| vs. HCV | <0.001 | ||
| Median PIVKA-II (ng/mL) | 1 | 2.7 | 59.5 |
| Range | 0.6–1.5 | 1.2–3.5 | 29.8–192.4 |
| vs. control | 0.928 | <0.001 | |
| vs. HCV | <0.001 |
Values are expressed as median (range); P-value using is set at 0.05.
Statistical test: Mann–Whitney U for comparing HCC against HCV and control, and HCV against control.
AFP was significantly higher in patients with HCV associated with cirrhosis. However, in HCC patients AFP level was not significantly affected by presence of cirrhosis. While PIVKA-II level was not significantly affected with presence of cirrhosis in HCV and HCC patients, results are illustrated in Table 4.
Table 4.
Comparison of median Level of AFP and PIVKA-II in HCC and HCV patients based on cirrhosis.
| Group | AFP |
PIVKA-II |
||
|---|---|---|---|---|
| No cirrhosis | Cirrhosis | No cirrhosis | Cirrhosis | |
| HCV | ||||
| N | 12 | 12 | 12 | 12 |
| Median | 8.7 | 24.4 | 2.4 | 2.9 |
| Range | 3.2–68.8 | 1.5–29.9 | 2.7–3.5 | 1.2–2.6 |
| P-value | 0.005 | <0.001 | ||
| HCC | ||||
| N | 23 | 25 | 23 | 25 |
| Median | 120 | 34 | 130.1 | 53.7 |
| Range | 2.7–606 | 6.3–695 | 29.8–192.4 | 36–191.4 |
| P-value | 0.942 | 0.386 | ||
Results are expressed as median (range); P-value using is set at 0.05.
Statistical test: Mann–Whitney U.
AFP level showed no significant changes when measured in different grades and stages of HCC. On the other hands, PIVKA-II level showed gradual increase with grade and stage. This increase was statistically significant when comparing all grades and stages, except when comparing stage 1 with stage 2 as shown in Table 5.
PIVKA-II level showed significant increase with higher BCLC stages with a p-value less than 0.001, however this was not obvious with AFP level (p = 0.292), results are illustrated in Table 6.
Table 6.
Comparison of AFP and PIVKA-II level in different grades and stages of HCC patients.
| HCC subdivisions | Sample size | AFP (ng/mL) | P-value | PIVKA-II (ng/mL) | P-value |
|---|---|---|---|---|---|
| Grade I | 15 | 125.8 (2.7–248.9) | P = 0.088 | 39.6 (29.8–43.6) | P < 0.001 |
| Grade II | 14 | 350.65 (6.3–695) | 52.7 (42.3–89.2) | ||
| Grade III | 13 | 242.95 (5.9–480) | 145.8 (120.7–191.4) | ||
| Grade IV | 6 | 269.5 (120–419) | 191.9 (180.5–192.4) | ||
| Stage 1 | 8 | 11.65 (2.7–20.6) | P = 0.232 | 36.75 (29.8–39.6) | P2 = NS⁎ |
| P3 < 0.001 | |||||
| P4 < 0.001 | |||||
| Stage 2 | 15 | 349.4 (3.8–695) | 43.6 (40–53.7) | P1 | |
| P3 = 0.002 | |||||
| P4 = 0.003 | |||||
| Stage 3 | 17 | 305.95 (5.9–606) | 130 (54.9–180.5) | P1 < 0.01 | |
| P2 < 0.01 | |||||
| P4 < 0.001 | |||||
| Stage 4 | 8 | 246.5 (13–480) | 191.4 (186.2–192.4) | P1 < 0.001 | |
| P2 < 0.001 | |||||
| P3 < 0.001 | |||||
| BCLC A | 18 | 348.8 (2.7–695) | P = 0.292 | 41.75 (29.8–53.7) | P = 0.001 |
| B | 7 | 306.15 (6.3–303) | 110.25 (40–180.5) | ||
| C | 18 | 257.45 (5.9–509) | 112 (51.7–192.4) | ||
| D | 5 | 58.5 (12–105) | 93.5 (40.2–146.8) |
Results are expressed as median (range); P-value using is set at 0.05.
Statistical test: Kruskall–Walles for comparison of grades and BCLC stage.
Mann–Whitney-U for comparison of stage.
P1 stands for Stage 1.
P2 stands for Stage 2.
P3 stands for Stage 3.
P4 stands for Stage 4.
Not significant.
AFP level showed no significant changes between HCC subclasses with or without lymph nodes involvement, splenomegaly or portal vein thrombosis. However, PIVKA-II level was significantly higher in subgroups with any of those lesions Table 7.
Table 7.
Comparison of median level of AFP and PIVKA-II in HCC patients based on metastasis to lymph node, splenomegaly and portal vein thrombosis.
| HCC subdivisions | Number | AFP | P-value | PIVKA-II | P-value |
|---|---|---|---|---|---|
| Without lymph node metastasis | 36 | 348.85 (2.7–695) | NS⁎ | 45.4 (29.8–146.8) | P < 0.001 |
| With lymph node metastasis | 12 | 244 (8–480) | 188.2 (65.2–192.4) | ||
| Without splenomegaly | 14 | 348.85 (2.7–695) | NS⁎ | 47 (29.8–64.3) | P < 0.001 |
| With splenomegaly | 34 | 256.25 (3.5–509) | 112.65 (32.9–192.4) | ||
| Without portal vein thrombosis | 22 | 348.85 (2.7–695) | NS⁎ | 59.5 (29.8–89.2) | P < 0.001 |
| With portal vein thrombosis | 26 | 257.45 (5.9–509) | 1114.2 (36–192.4) |
Results are expressed as median (range); P-value using is set at 0.05.
Statistical test: Mann–Whitney U test.
Not significant.
AFP showed no significant changes in its level when measured in tumors with size less than 3 cm, size from 3 to 5 cm or tumors more than 5 cm. On the other hand, PIVKA-II showed gradual and significant increase correlating with the size of tumor Table 8.
Table 8.
Comparison of median level of AFP and PIVKA-II based on tumor size.
| Tumor size |
|||
|---|---|---|---|
| <3 cm | (3–5) cm | >5 cm | |
| Sample size | 8 | 15 | 25 |
| Median AFP | 104.35 | 349.4 | 305.95 |
| Range | 2.7–206 | 3.8–695 | 5.9–606 |
| p-value | NS⁎ | NS⁎ | |
| Median PIVKA-II | 34.7 | 46.85 | 123.6 |
| Range | 29.8–39.6 | 40–53.7 | 54.9–192.4 |
| p-value vs. <3 cm | NS⁎ | <0.001 | |
| vs. 3–5 cm | <0.001 | ||
Results are expressed as median (range); P-value using is set at 0.05.
Statistical test: Kruskall–Wallis test for comparison of three groups, and Mann–Whitney test for comparison between each two groups.
Not significant.
Multiple receiver operating characteristic curve (ROC) was drawn to evaluate validity of both AFP and PIVKA-II based on the distribution of HCC patients according to tumor size. As shown in Fig. 1A; comparison between HCC patients with tumor size <3 and 3–5 revealed sensitivity and specificity 73.3%, 75% respectively for AFP, and 100% for PIVKA-II with a cut-off value of AFP and PIVKA-II >22.31 and >39.6 ng/mL respectively. On the other hand, comparison between the HCC patients with tumor size 3–5 and >5 revealed sensitivity and specificity 44%, 73.30%, respectively for AFP, and 100% for PIVKA-II with a cut-off value of AFP and PIVKA-II >28 and >53.7 ng/mL respectively as shown in Fig. 1B.
Fig. 1.
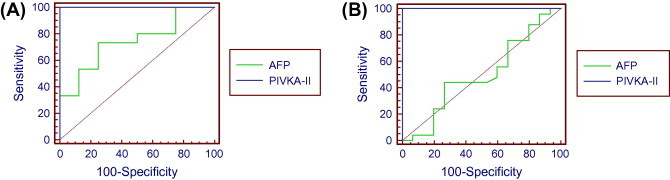
ROC curve statistics comparing AFP and PIVKA-II based on tumor size. (A) Comparison between tumor size <3 and 3–5. (B) Comparison between tumor size 3–5 and >5.
Discussion and conclusion
HCC is a leading cause of mortality among patients with cirrhosis [29]. Detection of HCC at early stages is critical for good clinical outcome as the prognosis of HCC patients is very poor when diagnosed at late stages [30,31].
Although serum AFP is the most established tumor marker in HCC and considered as the golden standard to which other markers are compared, it was found to be normal in about 30% of the patients, especially in early stages [30,31]. Elevated levels might be seen in patients with cirrhosis or exacerbation of chronic hepatitis [32,33]. Ultrasonography an important tool for diagnosis of HCC however it depends on the operator’s experience [34,35]. Accordingly the validity of other biomarkers in diagnosis of HCC including PIVKA-II needs to be investigated.
The present work was designed to study the impact of PIVKA-II on early diagnosis of HCC and to correlate it with different clinic-pathological features of the disease, as compared with AFP. The ultimate goal is to diagnose HCC at early stage.
The present study revealed significant male predominance; males represented 70.8% of all patients in HCC group, with 83.3% of patients over 50 years. These findings are consistent with Bressac et al. [36], Bosch et al. [13] and Parkin et al. [37]. Male predominance can be explained by more hepatitis carrier states, exposure to environmental toxins and hepatic effect of androgens [38].
Our results revealed a significant elevation of PIVKA-II and AFP levels in HCC group compared to control and HCV groups. PIVKA-II showed more increase than AFP level in malignant compared to benign liver diseases. These results could be explained in view of the findings of Okuda et al. [39] who demonstrated that there is excessive synthesis of prothrombin precursors by human HCC tissues, which might contribute to production of PIVKA-II, rendering the latter a useful marker for HCC with a very high specificity. These results were consistent with those of Marrero et al. [23] who reported that PIVKA-II is more sensitive than AFP for differentiating HCC from other benign liver diseases.
No significant changes were observed in serum level of AFP among different grades of HCC. On the other hand, plasma level of PIVKA-II showed significant gradual elevation correlating with progressive disease grade. AFP level was not affected by tumor size. However, plasma PIVKA-II level increased in correlation with tumor size to reach its maximum level for tumors with sizes more than 5 cm. These findings were consistent with those of Gotoh et al. [40], El-Assaly et al. [41] and Durazo et al. [42] who reported that PIVKA-II levels significantly correlate with histopathological grade of HCC and size of solitary tumors, being 25 times higher in tumors more than 2 cm, compared to those less than 2 cm. AFP showed no significant correlations with the stage of HCC. In contrary PIVKA-II showed gradual increase in its level with increase of disease stage. These findings were also consistent with those reported by Nagaoka et al. [43], and Kaibori et al. [44]. Beak et al. [45] demonstrated that PIVKA-II is more accurate than AFP for diagnosis of HCC. PIVKA-II was positive in 96%, 93% and 74% in patients with tumor size larger than 5, 3–5, and less than 3 cm while AFP was positive in 65%, 57% and 48% respectively.
In the present study, PIVKA-II levels showed significant elevation in HCC patients with portal vein thrombosis, while AFP level was not affected. These findings were consistent with those of El-Assaly et al. [40]. Gotoh et al. [41] also reported that PIVKA-II is better than AFP in reflecting invasive characteristics of HCC, especially invasion of extra and intrahepatic venous branches.
Same results were obtained in the present study concerning the levels of PIVKA-II and AFP for HCC patients with or without lymph node metastasis. These results were consistent with Baek et al. [45] who reported that PIVKA-II level increased with tumor burden and metastasis.
The present study revealed a significant elevated level of PIVKA-II in cases of HCC associated with hepatosplenomegaly 112.7 (95% CI; 51.83–173.57) compared to 43.24 (95% CI 34.9–51.58)in patients with no hepatosplenomegaly. These data are consistent with that of Bae et al. who reported that patients with a PIVKA-II production ⩾300 mAU/mL had a 2.7-fold (95% confidence interval; 1.5–4.8; P < 0.001) and 3.7-fold (95% confidence interval; 2.0–6.6; P < 0.001) increased risk for extrahepatic metastases after adjustment for stage, platelet count, alpha-fetoprotein ⩾400 ng/mL, and portal vein thrombosis according to the AJCC and BCLC staging systems, respectively [46].
ROC curve was drawn to compare between both markers depending on the tumor size and to determine the best cut-off value. The results revealed that, the comparison between tumor size <3 and 3–5 cm by using the best cut-off value of AFP (>22.31) shows sensitivity (73.3%) and specificity (75%). While for PIVKA-II (>39.6) it shows sensitivity (100%) and specificity (100%). Moreover, the pair wise comparison of both AFP and PIVKA-II show extremely high significance (<0.001). Different sensitivities and specificities from 62% to 95% and 53.3% to 98% were reported by other authors [23,47,48]. In a meta-analysis based on literature review of 20 publications, the overall sensitivity, specificity of DCP was 67% (95%CI, 58–74%), 92% (95%CI, 88–94%) respectively. [49] In a study conducted by Bertino et al. serum DCP was found to have a sensitivity ranging from 48% to 62%, a specificity of 81–98%. [50], these variations may be attributed to variation of the sample size, tumor size or number of masses in different studies.
In conclusion the present study reveals that PIVKA-II is superior to AFP in discrimination between HCC and other benign liver diseases. Furthermore, PIVKA-II can be used to differentiate between different histopathological stages and grades of HCC, and to evaluate portal vein thrombosis. The high sensitivity and specificity of PIVKA-II may give it value in screening high risk population and diagnose the disease at early stages when curative treatments are possible.
Footnotes
Peer review under responsibility of Cairo University.

References
- 1.Shibuya K., Yano E. Regression analysis of trends in mortality from hepatocellular carcinoma in Japan, 1972–2001. Int J Epidemiol. 2005;34:397–402. doi: 10.1093/ije/dyh358. [DOI] [PubMed] [Google Scholar]
- 2.Bosetti C., Levi F., Boffetta P., Lucchini F., Negri E., La Vecchia C. Trends in mortality from hepatocellular carcinoma in Europe, 1980–2004. Hepatology. 2008;48:137–145. doi: 10.1002/hep.22312. [DOI] [PubMed] [Google Scholar]
- 3.Parkin D.M., Bray F., Ferlay J., Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Mazzanti R., Gramantieri L., Bolondi L. Hepatocellular carcinoma: epidemiology and clinical aspects. Mol Aspects Med. 2008;29:130–143. doi: 10.1016/j.mam.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Bosch F.X., Ribes J., Diaz M., Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Mokhtar NM., Gouda I., Adel I. Cancer pathology registry (2003–2004) and time trend analysis. In: Mokhtar N.M., Gouda I., Adel I., editors. NCI, Cairo University; 2007. [Google Scholar]
- 7.Yu M.C., Yuan J.M., Lu S.C. Alcohol, cofactors and the genetics of hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23(Suppl 1):S92–97. doi: 10.1111/j.1440-1746.2007.05293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alacacioglu A., Somali I., Simsek I., Astarcioglu I., Ozkan M., Camci C. Epidemiology and survival of hepatocellular carcinoma in Turkey: outcome of multicenter study. Jpn J Clin Oncol. 2008;38:683–688. doi: 10.1093/jjco/hyn082. [DOI] [PubMed] [Google Scholar]
- 9.Sangiovanni A., Del Ninno E., Fasani P., De Fazio C., Ronchi G., Romeo R. Colombo, Increased survival of cirrhotic patients with hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004;126:1005–1014. doi: 10.1053/j.gastro.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 10.Habib M., Mohamed M.K., Abdel-Aziz F., Magder L.S., Abdel-Hamid M., Gami Fl. Hepatitis C virus infection in a community in the Nile Delta: risk factors for seropositivity. Hepatology. 2001;33:248–253. doi: 10.1053/jhep.2001.20797. [DOI] [PubMed] [Google Scholar]
- 11.Kamal S.M., Madwar M.A., Peters T., Fawzy R., Rasenack J. Interferon therapy in patients with chronic hepatitis C and schistosomiasis. J Hepatol. 2000;32:172–174. doi: 10.1016/s0168-8278(00)80207-x. [DOI] [PubMed] [Google Scholar]
- 12.El-Nady G.M., Ling R., Harrison T.J. Gene expression in HCV-associated hepatocellular carcinoma–upregulation of a gene encoding a protein related to the ubiquitin-conjugating enzyme. Liver Int. 2003;23:329–337. doi: 10.1034/j.1478-3231.2003.00862.x. [DOI] [PubMed] [Google Scholar]
- 13.Bosch F.X., Ribes J., Borras J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271–285. doi: 10.1055/s-2007-1007117. [DOI] [PubMed] [Google Scholar]
- 14.Szabo E., Paska C., Kaposi Novak P., Schaff Z., Kiss A. Similarities and differences in hepatitis B and C virus induced hepatocarcinogenesis. Pathol Oncol Res. 2004;10:5–11. doi: 10.1007/BF02893401. [DOI] [PubMed] [Google Scholar]
- 15.Yao D.F., Dong Z.Z., Yao M. Specific molecular markers in hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2007;6:241–247. [PubMed] [Google Scholar]
- 16.Daniele B., Bencivenga A., Megna A.S., Tinessa V. Alpha-fetoprotein and ultrasonography screening for hepatocellular carcinoma. Gastroenterology. 2004;127(Suppl):S108–S112. doi: 10.1053/j.gastro.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Bertino G., Ardiri A., Malaguarnera M., Malaguarnera G., Bertino N., Calvagno G.S. Hepatocellualar carcinoma serum markers. Semin Oncol. 2012;39(4):410–433. doi: 10.1053/j.seminoncol.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen M.H., Garcia R.T., Simpson P.W., Wright T.L., Keeffe E.B. Racial differences in effectiveness of alpha-fetoprotein for diagnosis of hepatocellular carcinoma in hepatitis C virus cirrhosis. Hepatology. 2002;36:410–417. doi: 10.1053/jhep.2002.34744. [DOI] [PubMed] [Google Scholar]
- 19.Sheu J.C., Sung J.L., Chen D.S., Lai M.Y., Wang T.H., Yu J.Y. Early detection of hepatocellular carcinoma by real-time ultrasonography, a prospective study. Cancer. 1985;56:660–666. doi: 10.1002/1097-0142(19850801)56:3<660::aid-cncr2820560338>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 20.Ono M., Ohta H., Ohhira M., Sekiya C., Namiki M. Measurement of immunoreactive prothrombin precursor and vitamin-K-dependent gamma-carboxylation in human hepatocellular carcinoma tissues: decreased carboxylation of prothrombin precursor as a cause of des-gamma-carboxyprothrombin synthesis. Tumour Biol. 1990;11:319–326. doi: 10.1159/000217667. [DOI] [PubMed] [Google Scholar]
- 21.Fujioka M., Nakashima Y., Nakashima O., Kojiro M. Immunohistologic study on the expressions of alpha-fetoprotein and protein induced by vitamin K absence or antagonist II in surgically resected small hepatocellular carcinoma. Hepatology. 2001;34:1128–1134. doi: 10.1053/jhep.2001.29202. [DOI] [PubMed] [Google Scholar]
- 22.Cui R., Wang B., Ding H., Shen H., Li Y., Chen X. Usefulness of determining a protein induced by vitamin K absence in detection of hepatocellular carcinoma. Chin Med J (Engl) 2002;115:42–45. [PubMed] [Google Scholar]
- 23.Marrero J.A., Su G.L., Wei W., Emick D., Conjeevaram H.S., Fontana R.J. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in American patients. Hepatology. 2003;37:1114–1121. doi: 10.1053/jhep.2003.50195. [DOI] [PubMed] [Google Scholar]
- 24.Malaguarnera G., Giordano M., Paladina I., Berretta M., Cappellani A., Malaguarnera M. Serum markers of hepatocelluler carcinoma. Dig Dis Sci. 2010;55(10):2744–2755. doi: 10.1007/s10620-010-1184-7. [DOI] [PubMed] [Google Scholar]
- 25.Bertino G., Neri S., Bruno C.M., Ardiri A.M., Calvagno G.S., Malaguarnera M. Diagnostic and prognostic value of alpha-fetoprotein, des-γ-carboxy prothrombin and squamous cell carcinoma antigen immunoglobulin M complexes in hepatocellular carcinoma. Minerva Med. 2011;102(5):363–371. [PubMed] [Google Scholar]
- 26.Thomas L. 1st ed. TH Books Verlagsgesellschaft; Frankfurt: 1998. Clinical laboratory diagnostics. p. 644–7. [Google Scholar]
- 27.Lovet J.M., Bru C., Bruix J. Prognosis of HCC: the BCLC staging classification. Seminars in Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 28.Pugh R.N., Murray-Lyon I.M., Dawson J.L. Transsection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 29.Zaman S.N., Melia W.M., Johnson R.D., Portmann B.C., Johnson P.J., Williams R. Risk factors in development of hepatocellular carcinoma in cirrhosis: prospective study of 613 patients. Lancet. 1985;1:1357–1360. doi: 10.1016/s0140-6736(85)91785-4. [DOI] [PubMed] [Google Scholar]
- 30.Kawano Y., Sasaki A., Kai S., Endo Y., Iwaki K., Uchida H. Short and long term outcomes after hepatic resection for hepatocellular carcinoma with concomitant esophageal varices in patients with cirrhosis. Ann Surg Oncol. 2008;15:1670–1676. doi: 10.1245/s10434-008-9880-7. [DOI] [PubMed] [Google Scholar]
- 31.Stefaniuk P., Cianciara J., Wiercinska-Drapalo A. Present and future possibilities for early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2010;16:418–424. doi: 10.3748/wjg.v16.i4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Libbrecht L., Severi T., Cassiman D., Vander Borght S., Pirenne J., Nevens F. Glypican-3 expression distinguishes small hepatocellular carcinomas from cirrhosis, dysplastic nodules, and focal nodular hyperplasia-like nodules. Am J Surg Pathol. 2006;30:1405–1411. doi: 10.1097/01.pas.0000213323.97294.9a. [DOI] [PubMed] [Google Scholar]
- 33.Zinkin N.T., Grall F., Bhaskar K., Otu H.H., Spentzos D., Kalmowitz B. Serum proteomics and biomarkers in hepatocellular carcinoma and chronic liver disease. Clin Cancer Res. 2008;14:470–477. doi: 10.1158/1078-0432.CCR-07-0586. [DOI] [PubMed] [Google Scholar]
- 34.Zhang B.H., Yang B.H., Tang Z.Y. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poon D., Anderson B.O., Chen L.T., Tanaka K., Lau W.Y., Van Cutsem E. Management of hepatocellular carcinoma in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10:1111–1118. doi: 10.1016/S1470-2045(09)70241-4. [DOI] [PubMed] [Google Scholar]
- 36.Bressac B., Kew M., Wands J., Ozturk M. Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature. 1991;350:429–431. doi: 10.1038/350429a0. [DOI] [PubMed] [Google Scholar]
- 37.Parkin D.M., Muir C.S., Whelan S.L. vol. 5. International Agency for Research on Cancer; IARC Lyon: 1997. (Cancer incidence in five continents). [Google Scholar]
- 38.Okuda K. Epidemiology of primary liver cancer. In: Tobe T., editor. Primary liver cancer in Japan. Springer-Verlag; Tokyo: 2003. p. 3. [Google Scholar]
- 39.Okuda H., Nakanishi T., Takatsu K., Saito A., Hayashi N., Takasaki K. Serum levels of des-gamma-carboxy prothrombin measured using the revised enzyme immunoassay kit with increased sensitivity in relation to clinicopathologic features of solitary hepatocellular carcinoma. Cancer. 2000;88:544–549. [PubMed] [Google Scholar]
- 40.Gotoh M., Nakatani T., Masuda T., Mizuguchi Y., Sakamoto M., Tsuchiya R. Prediction of invasive activities in hepatocellular carcinomas with special reference to alpha-fetoprotein and des-gamma-carboxyprothrombin. Jpn J Clin Oncol. 2003;33:522–526. doi: 10.1093/jjco/hyg096. [DOI] [PubMed] [Google Scholar]
- 41.El-Assaly N.M., El Ashry I.N., Mostafa I., El Ghannam M., Attia M. Serum chromogranin-A and serum PIVKA-II as useful complementary and diagnostic markers for HCC. Res J Med Med Sci. 2008;4(2):391–401. [Google Scholar]
- 42.Durazo F.A., Blatt L.M., Corey W.G., Lin J.H., Han S., Saab S. Des-gamma-carboxyprothrombin, alpha-fetoprotein and AFP-L3 in patients with chronic hepatitis, cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:1541–1548. doi: 10.1111/j.1440-1746.2008.05395.x. [DOI] [PubMed] [Google Scholar]
- 43.Nagaoka S., Yatsuhashi H., Hamada H., Yano K., Matsumoto T., Daikoku M. The des-gamma-carboxy prothrombin index is a new prognostic indicator for hepatocellular carcinoma. Cancer. 2003;98:2671–2677. doi: 10.1002/cncr.11839. [DOI] [PubMed] [Google Scholar]
- 44.Kaibori M., Matsui Y., Yanagida H., Yokoigawa N., Kwon A.H., Kamiyama Y. Positive status of alpha-fetoprotein and des-gamma-carboxy prothrombin: important prognostic factor for recurrent hepatocellular carcinoma. World J Surg. 2004;28:702–707. doi: 10.1007/s00268-004-7205-y. [DOI] [PubMed] [Google Scholar]
- 45.Baek Y.H., Lee J.H., Jang J.S., Lee S.W., Han J.Y., Jeong J.S. Diagnostic role and correlation with staging systems of PIVKA-II compared with AFP. Hepatogastroenterology. 2009;56:763–767. [PubMed] [Google Scholar]
- 46.Bae H.M., Lee J.H., Yoon J.H., Kim Y.J., Heo D.S., Lee H.S. Protein induced by vitamin K absence or antagonist-II production is a strong predictive marker for extrahepatic metastases in early hepatocellular carcinoma: a prospective evaluation. BMC Cancer. 2011;10:11–435. doi: 10.1186/1471-2407-11-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mita Y., Aoyagi Y., Yanagi M., Suda T., Suzuki Y., Asakura H. The usefulness of determining des-gamma-carboxy prothrombin by sensitive enzyme immunoassay in the early diagnosis of patients with hepatocellular carcinoma. Cancer. 1998;82:1643–1648. doi: 10.1002/(sici)1097-0142(19980501)82:9<1643::aid-cncr8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 48.Cui R., He J., Zhang F., Wang B., Ding H., Shen H. Diagnostic value of protein induced by vitamin K absence (PIVKAII) and hepatoma-specific band of serum gamma-glutamyl transferase (GGTII) as hepatocellular carcinoma markers complementary to alpha-fetoprotein. Br J Cancer. 2003;88:1878–1882. doi: 10.1038/sj.bjc.6601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao P., Li M., Tian Q.B., Liu D.W. Diagnostic performance of des-γ-carboxy prothrombin (DCP) for hepatocellular carcinoma: a bivariate meta-analysis. Neoplasma. 2012;59(2):150–159. doi: 10.4149/neo_2012_020. [DOI] [PubMed] [Google Scholar]
- 50.Bertino G., Ardiri A.M., Calvagno G.S., Bertino N., Boemi P.M. Prognostic and diagnostic value of des-γ-carboxy prothrombin in liver cancer. Drug News Perspect. 2010;23(8):498–508. doi: 10.1358/dnp.2010.23.8.1444236. [DOI] [PubMed] [Google Scholar]


