Abstract
The present investigation depicts the development of a simple and low cost method for the removal of color from textile dyeing and printing wastewater using ZnO as photocatalyst supported with porous activated carbon (AC). Photocatalytic degradation studies were carried out for water soluble toxic alizarin cyanin green (ACG) dye in aqueous suspension along with activated carbon (AC) as co-adsorbent. Different parameters like concentration of ACG dye, irradiation time, catalyst concentration and pH have also been studied. The pseudo first order kinetic equation was found to be applicable in the present dye-catalyst systems. It was observed that photocatalytic degradation by ZnO along with AC was a more effective and faster mode of removing ACG from aqueous solutions than the ZnO alone.
Keywords: Photocatalytic degradation, Alizarin cyanin green dye, ZnO, Activated carbon, Synergistic effect
Introduction
Many industries such as textile, plastics, paper and pulp generate streams of waste effluents which contain considerable amount of organic dyes [1–3]. When these compounds are discharged to the main water bodies without any prior treatment, they can cause havoc to the ecological balance in the environment as these molecules have carcinogenic and mutagenic properties towards aquatic organisms and thus pose threat to human life at the end of the food chain [4,5].
Heterogeneous photocatalysis has been considered as a cost-effective alternative as pre- or post-treatment of biological treatment process for the purification of dye-containing wastewater [6–10]. Among the available catalysts, ZnO finds wider application because of its availability, stability, low cost, and favorable band gap energy [11]. However, problems with the use of ZnO powders are also well recognized; specifically, (a) the difficulty in separating the powder from the solution after reaction is complete, (b) aggregation of particles in suspension, especially at high loadings, and (c) difficulty in application to continuous flow systems [12]. For these problems, various methods of photocatalyst particle support have been investigated such as alumina, zeolite, silica gel, fiber optic cable, glass beads, quartz, stainless steels, clays and activated carbon [13–16]. In particular, activated carbon (AC) has been extensively researched as a support for heterogeneous catalysis [17–24].
The aim of this work was to study the mineralization of ACG dye by photodegradation in aqueous solutions using ZnO as a catalyst supported with activated carbon, and to study the comprehend role of the activated carbon on photodegradation mechanism.
Experimental
Materials
Alizarin cyanin green (ACG) dye was purchased from E. Merck and the structure is given in Fig. 1. ZnO was supplied by May & Baker Ltd., Dagenham, England. AC was supplied by BDH, India. All the other chemicals and reagents were of AnalaR grade used as received. Deionized water was used for the preparation of all the solution and reagent.
Fig. 1.

Structure of ACG dye.
Preparation of AC–ZnO mixture
The zinc oxide–carbon composite was prepared by infiltration of a suspension in ethanol of commercial ZnO on the activated carbon in a rotary evaporator under vacuum for 45 min. After the rotation, the ethanol was evaporated out. Bare ZnO was also used as a standard for comparison purposes. Before each experiment, the ZnO–AC mixture is activated and dried at 110 °C overnight.
Equipment
High irradiation was performed with a UV-high pressure (“HEBER” photoreactor, model HIPR compact-MP-8/125/400) mercury lamp (max = 365 nm:400 W). The X-ray diffraction pattern of the AC–TiO2 film was taken with an analytical (Model PW 3040/60) X-ray diffractometer using Cu Kα radiation. Concentration of dye was determined with Spectrophotocolorimeter (Systronics-115). The pH of the dye solution was measured by using digital pen pH meter (Hanna instrument, Portugal). A magnetic stirrer is used for the constant stirring of the solution.
Results and discussion
Surface morphological studies
SEM studies provide useful information regarding the surface morphology of the materials. The SE micrographs of the pure ZnO, pure AC and AC–ZnO mixture is shown in Fig. 2. SE micrographs of the ZnO particles are shown in flake shape and the particles are agglomerated (Fig. 2a). SE micrographs photograph of the pure AC exhibit porous in nature with grain boundaries (Fig. 2b). Moreover, SE micrographs photographs of AC–ZnO mixture clearly reveal the surface texture and porosity nature. Besides, the AC–ZnO particles can be roughly as flake shapes and they appear to be quite uniform with internal pores (porous structures) or holes, which was observed at higher magnification (Fig. 2c). The immobilization of ZnO in the carbon matrix partially blocked the porosity of the carbon surface, although the composite still displays a porous character with a relatively large pore volume and surface area. This suggests that the ZnO did not enter the inner microporosity of the carbon during the immobilization, remaining on the outer surface and most accessible (large) pores. Consequently, the pores of smaller sizes remained unblocked. Besides, due to the synthetic route followed in the preparation of the AC–ZnO composite no chemical bonding is expected between ZnO and the carbon support, it seems that there exists a weak interaction (likely charge transfer). Similar observations have been reported in literature for AC–TiO2 mixture [24–26].
Fig. 2.

Surface morphology of the (a) pure ZnO; (b) pure AC and (c) AC–ZnO mixture. XRD spectrum of pure AC and AC–TiO2 mixture.
XRD measurement
To confirm the formation of AC–ZnO composite, XRD pattern has been observed for pristine CAC and 1:4 ratio of AC:ZnO mixture (Fig. 2d). The XRD pattern of the pure AC shows two broad diffraction peaks which can be indexed to (0 0 2) and (1 0 0) diffraction for typical graphite carbons. The clear and well-defined peaks at 31.6°, 34.2°, 36.2°, 47.4° and 56.6° (JCPDS 36-1451) are appeared in the nanocomposites which confirm the typical hexagonal wurtzite structure of ZnO particles in the XRD pattern of AC:ZnO mixture. Besides there was no AC peak in the XRD pattern of AC:ZnO mixture, this suggests that the crystal structure of ZnO particles has not modified due to the presence of AC [27–29].
Effect of initial concentration
Photo catalytic degradation studies on the extent of removal of ACG dye on ZnO at different initial concentrations in the presence of UV irradiation at room temperature (30 ± 1°C) are shown in Fig. 3. Similarly, the photo catalytic degradation of ACG dye was also carried out under same experimental conditions in the presence of AC. The extent of removal of the dye, in terms of the values of percentage removal of dye has been calculated using the following equation:
| (1) |
where Ci is the initial concentration of dye (ppm) and Ct is the final concentration of dye (ppm) at given time.
Fig. 3.
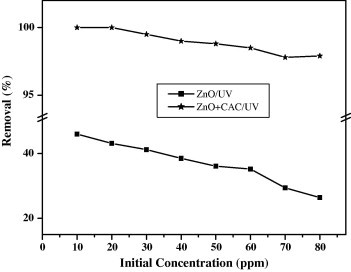
Effect of initial concentration of ACG dye in the presence and absence of AC (contact time: 90 min.; dose of the catalyst: 200 mg; dose of the CAC: 40 mg).
It was observed from the figure, that the percentage removal of dye both the presence and absence of AC decreases exponentially with the increase in the initial concentration of dye. This may be due to the immediate solute degradation, on the catalyst surface, compared to the relatively large number of active sites required for the high initial concentration of dye. This is also due to the fact that when dye molecules is increased the solution became more intense colored and the path length of photons entering the solution decreased thereby only fewer photons reached the catalyst surface. And therefore, the production of hydroxyl and super oxide radicals were limited. Hence, the percentage removal is decreased. At still higher concentration of the dye, the path length was further reduced due to coloration and the photo degradation was found to be negligible [25].
Effect of irradiation time and kinetics of photo degradation
The effect of irradiation time on the extent of removal of ACG dye was depicts in Fig. 4a. The degradation experiments by UV irradiation of ACG dye containing photo catalyst in presence and absence of AC follow pseudo-first-order kinetics with respect to the irradiation time (t). The following kinetic equation was used to study the kinetics of photo degradation of ACG dye.
| (2) |
where Co is the initial concentration of dye solution (in ppm), Ct the final concentration of dye solution of various time (in min.,) and kt is the first order rate constant for degradation of dye (in min−1).
Fig. 4.

(a) Effect of contact time and (b) kinetics of ACG dye in the presence and absence of AC (initial concentration of ACG: 30 ppm; dose of the catalyst: 200 mg; dose of the CAC: 40 mg).
The value of ln (Co/Ct) is plotted against time (in min) and the plots were found to be linear. From the slope, the rate constants were calculated for the degradation of dye ACG in presence and absence of AC [22–24].
The pseudo first order plot for the photodegradation of ACG in the absence and presence of AC by ZnO in UV light is shown in Fig. 4b. The pseudo first order rate constant (kt) (in min−1) for ZnO in the absence of AC is 0.0051 and in the presence of AC is 0.0465. The above data indicate that the photo degradation of dyes is more effective in the presence of AC.
Effect of dose of the catalyst
The effect of dose of the catalyst on the extent of removal of dye was shown in Fig. 5a. The percentage removal of dye increased exponentially with the increase in dose of the catalyst in the presence and absence of AC. This may be due to the increase in the availability of surface active sites. The effect of dose of the catalyst on the degradation rate was also studied and found that the rate of removal of ACG depends on the driving force per unit area, and in this case since, the initial concentration of dye (Ci) was kept constant an increase in the dose of the catalyst will result in the increase in the surface area for photo degradation and hence, the percentage removal increases [26–29].
Fig. 5.

(a) Effect of dose (Ci = ACG: 30 ppm; contact time: 90 min) and (b) Effect of pH (Ci: 30 ppm; dose of the catalyst: 200 mg; dose of the CAC: 40 mg; contact time: 90 min) of ACG dye in the presence and absence of AC.
Effect of pH
The percentage removal of dye linearly increases with the decrease in initial pH for photodegradation of ACG dye for both in the presence and absence of AC (Fig. 5b), which indicates that the acidic pH is found to be more suitable for the removal of ACG dye. This study showed that the degradation rate of ACG is strongly influenced by the solution pH. This may be due to the zero point charge of ZnO is known to be 6.25. Above pH 8.8, the surface charge of ZnO is negative and below 8.8, it is positive. ACG dye is an acidic dye that has negative charge in solution, which favors electrostatic interactions between the AC–ZnO surface and dye cation leading to the strong adsorption. These observations clearly demonstrates the significance of choosing the optimum degradation parameters to obtain high degradation rate [18,22–24].
Decolorization mechanism
In this study we have shown that nature of a porous carbon used as a adsorbent and a support for immobilization of ZnO plays an outstanding role in the mechanism of ACG dye photodegradation. Compared to pure ZnO, AC–ZnO composite promotes the photodegradation of ACG and the rate of the process is also largely accelerated. The performance mostly depends on the textural and chemical features of the carbon. Indeed, although ZnO immobilization on the carbon support is carried out by physical mixture, measurement of pHPZC suggests the occurrence of weak interactions between the carbon surface and the ZnO, which provokes the enhancement in the photodegradation of ACG [30–36].
The removal efficiency (that in a porous catalyst encompassing both adsorption and photodegradation) was significantly enhanced with respect to the immobilization of ZnO on the porous carbon support, which boosts the photoactivity of pure ZnO. The increase in the rate constant upon irradiation can be ascribed to the preferential adsorption and surface concentration of the pollutant onto the carbon porosity, followed by a spontaneous transfer from the support to the ZnO surface, where it is more rapidly decomposed due to the large concentration gradient between the two solid phases (Fig. 6). In such a case, there seems to exist a synergistic effect in the composite due to the combination of the adsorption capacity of the carbon and the photoactivity of zinc oxide. In the absence of AC, ACG dye molecules must collide with the ZnO by chance, and remain in contact for the photocatalysis to proceed. When this is not achieved, the reactants or intermediate products will pass back into solution and can only react further when they collide with ZnO again. Similar observations about the synergic effect of activated carbon as additive to ZnO in the photodegradation of organic pollutants have been described in literature [37–41].
Fig. 6.

Photodegradation mechanism of ACG dye in the presence and absence of AC.
Desorption studies
After 90 min of photodegradation experiment, the residue of CAC–ZnO composite was separated and immersed in 4 mL of ethanol under ultrasonication for 20 min. Then the filtrate was collected and analyzed by UV–Vis spectrophotometer. The UV–Vis spectrum (figure not shown) of filtrate in ethanol solution does not show any significant peak corresponds to ACG dye (disappearance absorption peak), which confirms that the removal of color is due to photodegradation and not for adsorption. This result clearly illustrates that molecules of ACG that have been adsorbed and accumulated on CAC during the initial photocatalytic degradation are able to be transferred to ZnO where they are decomposed under irradiation. Continuous migration and subsequent photocatalytic oxidation on the surface of ZnO accelerated ACG removal efficiency greatly. This transfer occurs through the CAC–ZnO interface with the concentration gradient as the driving force.
From the above studies we conclude that the decolourization of ACG dye is due to photodegradation process not by pure adsorption and the enhancement of photodegradation efficiency is due to synergistic or cooperative effect.
Conclusion
In this work, the photocatalytic degradation of ACG was studied. The findings can be summarized as below:
-
•
The ACG dye was successfully degraded by the UV/ZnO–AC and UV/ZnO system. The rate of degradation is high for the UV/ZnO–AC system than the UV/ZnO system, but no degradation was observed when the solution was exposed to UV radiation in the absence of ZnO.
-
•
The degradation rate for ACG under investigation is strongly influenced by the reaction pH. The degradation rate for the mineralization of dye was found to be lower at higher pH values and increases with reduced pH.
-
•
The UV/ZnO–AC system showed significant improvement in photoreactivity compared to UV/ZnO system. This is due the synergistic effect.
Acknowledgment
Authors thank the University Grants Commission (UGC), India for the financial support in form of Major Research Project (MRP).
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Ali S.M., Sabae S.Z., Fayez M., Monib M., Hegazi N.A. The influence of agro-industrial effluents on River Nile pollution. J Adv Res. 2011;2:85–95. [Google Scholar]
- 2.Ismail IM, Fawzy AS, Abdel-Monem NM, Mahmoud MH, El-Halwany MA. Combined coagulation flocculation pre-treatment unit for municipal wastewater. J Adv Res. 2012;3:331–336.
- 3.Rushdi M.M., El-Kilani, Belal M.H. Modelling an environmental pollutant transport from the stacks to and through the soil. J Adv Res. 2010;1:243–253. [Google Scholar]
- 4.Helmes C., Tucker C.I. Disperse blue 79. Environmental safety and human health effects of this commercially significant dye. Text Chem Colorist. 1993;25:15–17. [Google Scholar]
- 5.Alnuaimi M.M., Rauf M.A., Ashraf S.S. Comparative decoloration study of neutral red by different oxidative processes. Dyes Pigm. 2007;72:367–371. [Google Scholar]
- 6.Kaneko M., Okura I. Application to environmental cleaning. In: Kaneko M., Okura I., editors. Photocatalysis: Science and Technology. Kodansha, Springer; Tokyo, Berlin: 2002. pp. 109–184. [Google Scholar]
- 7.Chong M.N., Jin B., Chow C.W.K., Saint C. Recent developments in photocatalytic water treatment technology: a review. Water Res. 2010;44(10):2997–3027. doi: 10.1016/j.watres.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 8.Forgas E., Cserhati T., Oros G. Removal of synthetic dyes from wastewater: a review. Environ Int. 2004;30:953–971. doi: 10.1016/j.envint.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann M.R., Martin S.T., Choi W., Bahnemann D.W. Environmental applications of semiconductor photocatalysis. Chem Rev. 1995;95(1):69–96. [Google Scholar]
- 10.Hashimoto K., Irie H., Fujishima A. TiO2 photocatalysis: a historical overview and future prospects. Jpn J Appl Phys. 2005;44(12):8269–8285. [Google Scholar]
- 11.Velmurugan R., Swaminathan M. An efficient nanostructured ZnO for dye sensitized degradation of reactive red 120 dye under solar light. Solar Energy Mater Sol Cells. 2011;95:942–950. [Google Scholar]
- 12.El-Sheikh A.H., Newman A.P., Al-Daffaee H., Phull S., Cresswell N., York S. Deposition of anatase on the surface of activated carbon. Surf Coat Technol. 2004;187(2–3):284–292. [Google Scholar]
- 13.Fernandez A., Lassaletta G., Jimenez V.M., Justo A., Gonzalez-Elipe A.R., Herrmann J.M. Preparation and characterization of TiO2 photocatalysts supported on various rigid supports (glass, quartz and stainless steel): comparative studies of photocatalytic activity in water purification. Appl Catal B. 1995;7(1–2):49–63. [Google Scholar]
- 14.Chen X., Mao S.S. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem Rev. 2007;107(7):2891–2959. doi: 10.1021/cr0500535. [DOI] [PubMed] [Google Scholar]
- 15.Linsebigler A.L., Lu G., Yates J.T. Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results. Chem Rev. 1995;95(3):735–758. [Google Scholar]
- 16.Ni M., Leung M.K.H., Leung D.Y.C., Sumathy K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew Sust Energy Rev. 2007;11(3):401–425. [Google Scholar]
- 17.Subramani A., Byrappa K., Ananda S., Lokanatha Rai K., Lokanatha Rai K., Yoshimura M. Photocatalytic degradation of indigo carmine dye using TiO2 impregnated activated carbon. Bull Mater Sci. 2007;30(1):37–41. [Google Scholar]
- 18.Velasco L.F., Parra J.B., Ania C.O. Role of activated carbon features on the photocatalytic degradation of phenol. Appl Surf Sci. 2010;256(17):5254–5258. [Google Scholar]
- 19.Zhang X., Zhou M., Lei L. Preparation of photocatalytic TiO2 coatings of nanosized particles on activated carbon by APMOCVD. Carbon. 2005;43(8):1700–1708. [Google Scholar]
- 20.Zhang X., Zhou M., Lei L. TiO2 photocatalyst deposition by MOCVD on activated carbon. Carbon. 2006;44(2):325–333. [Google Scholar]
- 21.Leary R., Westwood A. Carbonaceous nanomaterials for the enhancement of TiO2 photocatalysis. Carbon. 2011;49:741–772. [Google Scholar]
- 22.Lee D.-K., Kim S.-C., Kim S.-J., Chung I.-S., Kim S.-W. Photocatalytic oxidation of microcystin-LR with TiO2-coated activated carbon. Chem Eng J. 2004;102(1):93–98. [Google Scholar]
- 23.Li Puma G., Bono A., Krishnaiah D., Collin J.G. Preparation of titanium dioxide photocatalyst loaded onto activated carbon support using chemical vapour deposition: a review paper. J Hazard Mater. 2008;157(2–3):209–219. doi: 10.1016/j.jhazmat.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 24.Rodrıguez-Reinoso F. The role of carbon materials in heterogeneous catalysis. Carbon. 1998;36:159–175. [Google Scholar]
- 25.Malato S., Fernandez-Ibanez P., Maldonado M.I., Blanco J., Gernjak W. Decontamination and disinfection of water by solar photocatalysis: recent overview and trends. Catal Today. 2009;147:1–59. [Google Scholar]
- 26.Manova E., Aranda P., Angeles Martın-Luengo M., Letaıef S., Ruiz-Hitzky E. New titania-clay nanostructured porous materials. Microporous Mesoporous Mater. 2010;131:252–260. [Google Scholar]
- 27.Patil B.N., Naik D.B., Shrivastava V.S. Photocatalytic degradation of hazardous Ponceau-S dye from industrial wastewater using nanosized niobium pentoxide with carbon. Desalination. 2011;269:276–283. [Google Scholar]
- 28.Peill N.J., Hoffmann M.R. Chemical and physical characterization of a TiO2-coated fiber optic cable reactor. Environ Sci Technol. 1996;30(9):2806–2812. doi: 10.1021/es00012a013. [DOI] [PubMed] [Google Scholar]
- 29.Pirkanniemi K., Sillanpaa M. Heterogeneous water phase catalysis as an environmental application: a review. Chemosphere. 2002;48(10):1047–1060. doi: 10.1016/s0045-6535(02)00168-6. [DOI] [PubMed] [Google Scholar]
- 30.Tryba B., Morawski A.W., Inagaki M. Application of TiO2-mounted activated carbon to the removal of phenol from water. Appl Catal B. 2003;41:427–433. [Google Scholar]
- 31.Velasco L.F., Parra J.B., Ania C.O. Role of activated carbon features on the photocatalytic degradation of phenol. Appl Surf Sci. 2010;256:5254–5258. [Google Scholar]
- 32.Li Y., Zhang S., Yu Q., Yin W. The effects of activated carbon supports on the structure and properties of TiO2 nanoparticles prepared by a sol–gel method. Appl Surf Sci. 2007;253:9254–9258. [Google Scholar]
- 33.Wang X., Hu Z., Chen Y., Zhao G., Liu Y., Wen Z. A novel approach towards high-performance composite photocatalyst of TiO2 deposited on activated carbon. Appl Surf Sci. 2009;255:3953–3958. [Google Scholar]
- 34.Zhang X., Zhou M., Lei L. Preparation of photocatalytic TiO2 coatings of nanosized particles on activated carbon by APMOCVD. Carbon. 2005;43:1700–1708. [Google Scholar]
- 35.Zhang X., Zhou M., Lei L. TiO2 photocatalyst deposition by MOCVD on activated carbon. Carbon. 2006;44:325–333. [Google Scholar]
- 36.Ao Y., Xu J., Fu D., Yuan C. A simple route for the preparation of anatase titania-coated magnetic porous carbons with enhanced photocatalytic activity. Carbon. 2008;46:596–603. [Google Scholar]
- 37.Matos J., Laine J., Herrmann J.M. Synergy effect in the photocatalytic degradation of phenol on a suspended mixture of titania and activated carbon. Appl Catal B. 1998;18:281–291. [Google Scholar]
- 38.Lee D.-K., Kim S.C., Kim S.J., Chung I.S., Kim S.W. Photocatalytic oxidation of microcystin-LR with TiO2-coated activated carbon. Chem Eng J. 2004;102:93–98. [Google Scholar]
- 39.Arana J., Dona-Rodrıguez JM., Tello Rendon E., Garrigai Cabo C., Gonzalez-Dıaz O., Herrera-Melian JA. TiO2 activation by using activated carbon as a support: part II. Photoreactivity and FTIR study. Appl Catal B. 2003;(44):153–160. [Google Scholar]
- 40.Ao Y., Xu J., Shen X., Fu D., Yuan C. Magnetically separable composite photocatalyst with enhanced photocatalytic activity. J Hazard Mater. 2008;160:295–300. doi: 10.1016/j.jhazmat.2008.02.114. [DOI] [PubMed] [Google Scholar]
- 41.Cordero T., Chovelon J.M., Duchamp C., Ferronato C., Matos J. Surface nano-aggregation and photocatalytic activity of TiO2 on H-type activated carbons. Appl Catal B. 2007;73:227–235. [Google Scholar]



