Abstract
Objectives
We hypothesized that administration of bevacizumab, a monoclonal antibody that neutralizes vascular endothelial growth factor, in combination with high-dose interferon-alpha2b (IFN-α2b), an inhibitor of basic fibroblast growth factor, would have clinical activity in patients with metastatic ocular melanoma.
Methods
Patients with metastatic ocular melanoma received bevacizumab (15 mg/kg intravenously every 2 weeks) plus IFN-α2b (5 MU/m2 subcutaneously 3 times weekly for 2 weeks followed by a dose of 10 MU/m2 subcutaneously thereafter). Patients exhibiting a clinical response or stabilization of disease were treated until disease progression.
Results
In this pilot study, 5 patients were treated (3 men, 2 women) with a mean age of 63.8 years (range, 53–71 years). Overall, the regimen was well-tolerated. The following adverse events were noted: grade 3 dyspnea (2 patients), grade 3 and 4 fatigue (2), grade 3 muscle weakness (1), grade 3 anorexia (1), grade 1 and 2 proteinuria (2), and grade 3 diarrhea (1). All adverse events resolved with a treatment holiday or dose reduction. One patient had reduction in tumor burden of 23% by Response Evaluation Criteria in Solid Tumors criteria and 2 patients had stabilization of disease lasting 28 and 36 weeks, respectively. Two patients failed to respond and progressed after 6 and 7 weeks of therapy.
Conclusion
Bevacizumab and IFN-α2b were well tolerated in this patient population, and clinical activity was observed. Further study of high-dose IFN-α2b in combination with bevacizumab in this setting is warranted.
Keywords: bevacizumab, vascular endothelial growth factor, angiogenic factor, interferon-alpha2b, uveal neoplasm
Ocular melanomas represent 5% of all melanomas; of these, 85% are uveal (ie, involving the iris, ciliary body, or choroid), making it the most common primary intraocular tumor.1,2 Uveal melanoma is more common in males and whites. However, unlike cutaneous melanoma, there is no clear evidence implicating sunlight as an etiologic factor.2 The relative 5-year survival rate for uveal melanoma (77%–84%) has not changed significantly in the past 25 years, regardless of whether enucleation or plaque radiotherapy is employed as treatment for the primary tumor.2
Angiogenesis with tumor neovascularization has been proven to be a critical factor in the progression of malignant melanoma.3 Vascular endothelial growth factor (VEGF) is known to be an important modulator of this process.4 Endothelial cells express a family of tyrosine receptor kinases which bind VEGF with high affinity.5 Binding of VEGF to its receptor activates several intracellular signaling pathways that induce endothelial cell mitosis and migration.6 VEGF promotes tumor metastasis via its ability to induce endothelial cell proliferation, migration, and survival.7 Inhibition of VEGF-induced angiogenesis slows tumor growth in murine models.8 Ocular melanoma may be especially sensitive to the inhibition of angiogenesis, since melanoma metastases are known to be highly vascular.9 Previous studies have demonstrated that ocular melanoma cell lines are proangiogenic, and that freshly isolated ocular melanoma cells elaborate proangiogenic factors, particularly VEGF.10–14
Bevacizumab (Avastin) is a recombinant humanized murine antihuman VEGF monoclonal antibody that recognizes all isoforms of VEGF with high affinity (Kd approx. 8 × 10−10 M). Several phase III clinical trials have shown that bevacizumab is effective in metastatic colon,15 lung,16 breast,17,18 and renal cancers.19 A randomized phase III trial demonstrated that the administration of bevacizumab with irinotecan, 5-fluorouracil, and leucovorin led to improved overall survival, progression-free survival, clinical response rate, and duration of response as compared with chemotherapy alone in patients with metastatic colorectal cancer.15 Hypertension, proteinuria, hemorrhage (primarily in patients with lung cancer), poor wound healing,20 and an increase in arterial thromboembolic events were the primary adverse events associated with bevacizumab administration.21 Although bevacizumab has not been used in clinical trials to treat ocular melanoma, intraocular administration of bevacizumab resulted in complete resolution of neovascularization in a patient with a cutaneous melanoma that had metastasized to the vitreous of the eye.22
In melanoma, interferon-alpha2b (IFN-α2b) is administered at high doses of 10 MU/m2 subcutaneously thrice weekly for 1 year as an adjunct to surgical resection of high-risk early stage tumors. Low dose IFN-α2b has antiproliferative activity and inhibits tumor-induced angiogenesis.23,24 In murine models, daily IFN-α2b therapy down-regulates the expression of basic fibroblast growth factor (bFGF), a critical factor for tumor neovascularization. 23 In addition, IFN-α2b has also been used at doses of up to 3 MU/m2 per day to treat life-threatening hemangiomas of infancy with remarkable success.25 Previous work in our laboratory has demonstrated that IFN-α2b inhibits the secretion of VEGF in several melanoma cell lines.26
We hypothesized that the combination of bevacizumab and high dose IFN-α2b would inhibit tumor angiogenesis and mediate regression of metastatic disease in patients with ocular melanoma. The primary objective of this pilot trial was to assess the tolerability and objective response rate in patients with metastatic ocular melanoma who received bevacizumab with IFN-α2b.
MATERIALS AND METHODS
Eligibility Criteria and Study Design
Following approval of the Ohio State University institutional review board, a National Cancer Institute-sponsored phase 2 trial of bevacizumab and high dose IFN-α2b was conducted at the Ohio State University Comprehensive Cancer Center beginning in July 2004. A cohort of patients with ocular melanoma were enrolled in this trial and examined separately as a pilot study. Patients included in this analysis had histologically or cytologically confirmed ocular melanoma, clinical evidence of metastatic disease, and met the following criteria: the ability to provide informed written consent, age ≥ 18 years, life expectancy >6 months, Eastern Cooperative Oncology Group (ECOG) performance status ≤2, normal organ and marrow function: leukocytes ≥3000/µL, absolute neutrophil count ≥1500/µL, platelets ≥100,000/µL, total bilirubin ≤2.0 mg/dL, AST and ALT ≤2.5 × upper limit of normal, and creatinine ≤1.5 mg/dL or creatinine clearance ≥60 mL/min/1.73 m2 for patients with creatinine > 1.5 mg/dL. Patients who had previously received cytokine therapy for metastatic disease (interleukin-2 or IFN-α2b) or adjuvant therapy with any antiangiogenic agent were excluded from participation. Prior adjuvant IFN-α2b was permitted as long as at least 4 weeks had passed since the last treatment. Patients were allowed to have had one prior chemotherapy regimen for metastatic disease. Patients were required to have a normal ratio of prothrombin time (PT) to partial thromboplastin time (PTT) and a normal international normalized ratio (INR). Patients with the factor V Leiden mutation, a history of arterial thromboembolic phenomena, or inadequately controlled hypertension were excluded. All patients underwent magnetic resonance imaging of the brain. Because of the potential for hemorrhagic complications, patients with intracranial lesions and pregnant women were excluded from the trial. Informed written consent was obtained from each patient prior to participation in the trial.
Treatment Regimen and Toxicity Assessment
Bevacizumab was administered intravenously at a dose of 15 mg/kg on day 1 of the 2 week cycle. IFN-α2b was administered subcutaneously at a dose of 5 MU/m2 3 times weekly for 2 weeks. Beginning with the second cycle, the dose of IFN-α2b was increased to 10 MU/m2 if the patient had tolerated the previous dose of IFN-α2b. Toxicity was assessed using the National Cancer Institute Common Toxicity Criteria Version 3.0. Patients exhibiting any clearly IFN-α2b-related or bevacizumab-related irreversible or Grade 4 nonhematological toxicity were removed from the trial. Patients who experienced bevacizumab-related, nonhematological grade 3 toxicity had treatment held for one cycle. If toxicity resolved to grade 1, bevacizumab therapy was resumed at a reduced dose of 7.5 mg/kg. IFN-α2b-related nonhematologic Grade 3 toxicity was managed by a rest period of 2 to 4 weeks followed by IFN-α2b administration at the original dose. Patients who were not able to tolerate IFN-α2b at 10 MU/m2 after dose escalation from 5 MU/m2 were dose reduced to 7.5 MU/m2. If Grade 3 toxicity recurred, the rest period was repeated once, followed by administration at a further reduced dose of 5 MU/m2. Grade 3 toxicity that recurred despite these measures mandated that the patient be removed from the trial. Blood chemistries, complete blood count, and urinalysis were obtained weekly. A coagulation profile consisting of PT, PTT, INR, antithrombin III, protein C, protein S, and factor V Leiden levels were obtained at baseline. Fibrinogen and D-dimer levels were assessed at baseline and monitored throughout the trial.
Assessment of Disease Response
Before enrollment, patients underwent computed tomography scans of the chest, abdomen, and pelvis, and other sites as appropriate. Patients were restaged after 6 cycles using the Response Evaluation Criteria in Solid Tumors (RECIST) criteria, and patients with progressive disease were removed from the trial. Those patients exhibiting a complete or partial response or stable disease were allowed to continue therapy until disease progression.
RESULTS
Patient Characteristics
Patient demographics are listed in Table 1. To be eligible for the trial patients must have had ocular melanoma with distant metastasis. A total of 5 patients (3 men, 2 women) with ocular melanoma were treated. The mean age was 63.8 years (range, 53–71). All patients had metastases to the liver (100%). In addition, 3 patients had pulmonary metastases (60%). Two patients had radioactive plaque therapy and 3 underwent surgical enucleation for their primary tumors. Prior to this study, 1 patient had received a combination of temozolomide and thalidomide, 1 patient underwent resection for hepatic and cardiac metastases, and 1 patient had a right hepatic lobectomy for an isolated metastatic lesion. No patients received adjuvant IFN-α2b therapy. Four patients had an ECOG performance status of zero and one had a performance status of 1.
TABLE 1.
Patient Demographics
| Characteristics | All Patients |
|---|---|
| No. patients | 5 |
| Sex | |
| Male | 3 |
| Female | 2 |
| Age | |
| Mean | 63.8 |
| Median | 64 |
| Range | 53–71 |
| Adjuvant therapy | |
| Yes | 0 |
| No | 5 |
| Metastatic classification of tumor | |
| M1 | 0 |
| M2 | 0 |
| M3 | 5 |
| Site of metastasis | |
| Liver | 5 |
| Lung | 3 |
| Previous chemotherapy regimens | |
| Yes | 1 |
| No | 4 |
| ECOG performance status | |
| 0 | 4 |
| 1 | 1 |
Toxicity
In general, this regimen was well-tolerated. Toxicities are summarized in Table 2. Grade 1 and 2 constitutional and gastrointestinal symptoms attributable to IFN-α2b were transient and did not require treatment delays. Grade 3 and 4 toxicities included dyspnea, fatigue, muscle weakness, anorexia, and diarrhea. They were attributed to IFN-α2b therapy and all resolved following a treatment break. Dyspnea in 1 patient required hospitalization and administration of furosemide. One patient had grade 1 hypertension and 2 patients had grade 1 or 2 proteinuria. The overall incidence of proteinuria and hypertension were 40% and 20%, respectively. Grade 3 toxicities were reversed by a drug-free period, dose reduction, and supportive therapy. No arterial thromboembolic events were observed. No patient was removed from the study due to drug toxicity.
TABLE 2.
Toxicities
| Toxicity | Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Total |
|---|---|---|---|---|---|
| Alkaline phosphatase | 2 | 0 | 0 | 0 | 2 |
| Anorexia | 1 | 2 | 1 | 0 | 4 |
| Constipation | 0 | 1 | 0 | 0 | 1 |
| Dehydration | 0 | 1 | 0 | 0 | 1 |
| Depression | 1 | 0 | 0 | 0 | 1 |
| Diaphoresis | 1 | 0 | 0 | 0 | 1 |
| Diarrhea | 1 | 0 | 1 | 0 | 2 |
| Dizziness | 1 | 0 | 0 | 0 | 1 |
| Dyspepsia | 1 | 0 | 0 | 0 | 1 |
| Dyspnea | 0 | 1 | 2 | 0 | 3 |
| Fatigue | 0 | 2 | 1 | 1 | 4 |
| Fever | 1 | 0 | 0 | 0 | 1 |
| Headache | 2 | 1 | 0 | 0 | 3 |
| Hypertension | 1 | 0 | 0 | 0 | 1 |
| Leucocytes | 1 | 2 | 0 | 0 | 3 |
| Lymphopenia | 0 | 1 | 0 | 0 | 1 |
| Mood alteration/anxiety | 1 | 0 | 0 | 0 | 1 |
| Muscle weakness | 0 | 0 | 1 | 0 | 1 |
| Nausea | 0 | 1 | 0 | 0 | 1 |
| Neutrophills/granulocytes | 1 | 1 | 0 | 0 | 2 |
| Proteinuria | 1 | 1 | 0 | 0 | 2 |
| Rigor/chills | 2 | 0 | 0 | 0 | 2 |
| Aspartate aminotransferase | 2 | 0 | 0 | 0 | 2 |
| Alanine Aminotrasnferase | 2 | 0 | 0 | 0 | 2 |
| Taste disturbance | 2 | 0 | 0 | 0 | 2 |
| Vomiting | 1 | 0 | 0 | 0 | 1 |
| Weight loss | 2 | 1 | 0 | 0 | 3 |
No patient had abnormal elevations of PT, INR, or protein S. One patient each had a mild elevation of PTT, antithrombin III, and protein C prior to starting therapy. The average maximum increase in fibrinogen over baseline was 29% (±22.78%) and the average maximum increase in D-dimer over baseline was 120% (±130%).
Response to Therapy
Bevacizumab (15 mg/kg) was administered to patients in combination with high-dose IFN-α2b. Two patients received IFN-α2b at 10 MU/m2 and 3 received a dose of 5 MU/m2 due to toxicity at higher dose level. One patient had a 23% reduction in tumor size on computed tomography (CT) scan by RECIST criteria, as shown in Figure 1. Two patients had stable disease for 28 and 36 weeks, respectively. Two patients failed to respond and progressed at 6 and 7 weeks. There was an average progression-free survival of 18 weeks and an average overall survival of 43 weeks (range, 7–91 weeks). Outcomes data are summarized in Table 3.
FIGURE 1.
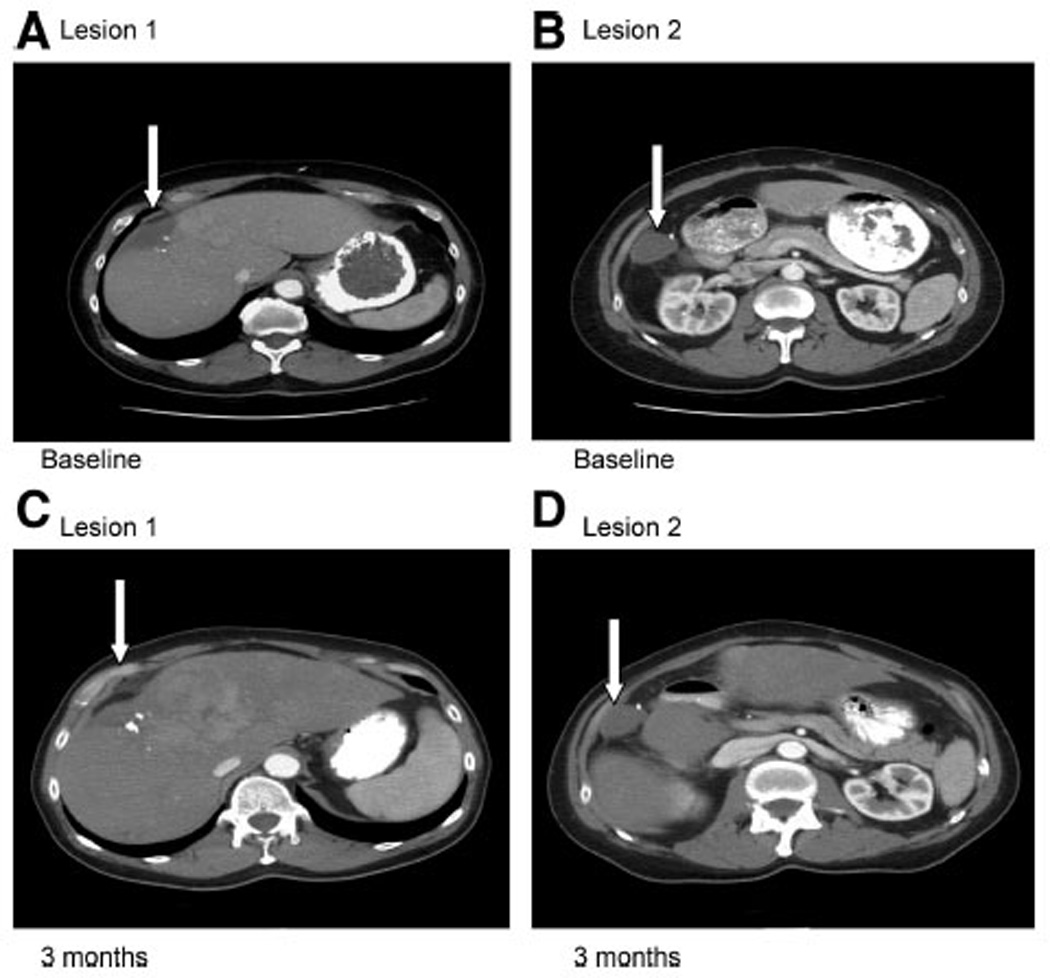
A CT scan showing target lesions of patient with 23% reduction in tumor size. The patient had 2 target lesions in the liver (indicated by arrows). Images 1 A and B are the patient’s baseline scan. Images C and D are the restaging scans performed after 6 cycles of therapy.
TABLE 3.
Outcome Data
| Best Response | Bevacizumab + IFN |
|---|---|
| Partial response (PR) | 0 |
| Stable disease (SD) | 3 |
| Progressive disease (PD) | 2 |
| Progression free survival (wk) | |
| Mean | 18 |
| Range | 6–36 |
| Overall survival (wk) | |
| Mean | 43 |
| Range | 7–91 |
Patient 1 initially presented with liver and lung metastases 10 years after surgical enucleation of a 9.3-mm tumor. She was placed on a regimen of temozolomide plus thalidomide but progressed after 2 months of treatment. She had stable disease on the present trial that lasted 36 weeks at which time she exhibited progression in the liver and lung. During therapy, she experienced grade 3 fatigue, dyspnea, anorexia, muscle weakness, and diarrhea that were felt to be due to IFN-α2b therapy. She survived for a total of 91 weeks after the initiation therapy.
Patient 2 was initially diagnosed with an 11-mm choroidal melanoma and treated with radioactive plaque therapy (87 Gy). Six years later, she developed liver metastases. Administration of bevacizumab and IFN-α2b led to stabilization of her disease for 28 weeks. She experienced only grade 1 and 2 toxicities. Bevacizumab was held twice due to grade 1 proteinuria. She died 63 weeks after the initiation of therapy.
Patient 3 had a 21-mm ocular melanoma that involved the choroid and extended focally to involve the ciliary body, which was treated with enucleation. Three years later, he was found to have liver metastases. During the trial, the IFN-α2b dose could not be escalated to 10 MU/m2 because of neutropenia. He also experienced grade 3 diarrhea and therefore the IFN-α2b dose was maintained at 5 MU/m2. After 6 weeks of therapy, the patient developed nausea and vomiting which was found to be due to progression of disease in the liver. He survived for a total of 31 weeks after the initiation of therapy.
Patient 4 was initially diagnosed with a choroidal melanoma that measured 9.7 mm in diameter and was treated with radioactive plaque therapy (86 Gy). Four years later, he was found to have an isolated right hepatic lobe metastasis that was treated with a right hepatic lobectomy. Five months after his surgery, he developed bilateral pulmonary metastases and metastases to the liver which prompted his enrollment to this clinical trial. He progressed 7 weeks after the initiation of therapy with bevacizumab and IFN-α2b.
Patient 5 was diagnosed with a 13-mm epitheliod and spindle cell ocular melanoma that was treated with enucleation. Three years later, he developed liver and cardiac septal metastases that were treated with sequential resection. Three months after surgery, he developed additional liver metastases. While on trial he developed grade 3 dyspnea and grade 4 fatigue, which required a drug holiday. He had a 23% reduction in tumor size by RECIST criteria at 11 weeks (Fig. 1). However, 3 weeks later, he had an elevation in liver function tests and a CT scan demonstrated that the liver metastases had increased in size. He survived a total of 24 weeks after starting the trial.
DISCUSSION
Bevacizumab (15 mg/kg intravenously) was administered in combination with high-dose IFN-α2b to 5 patients with hepatic metastases from ocular melanoma. This regimen was well tolerated and produced clinical benefit in 3 of the 5 patients, including one who had a 23% reduction in tumor size by RECIST criteria. The average progression-free survival was 18 weeks, and the average overall survival was 43 weeks. Toxicities were mild and easily reversed with dose reduction and/or drug-free period. The overall incidence of proteinuria was 40% and the incidence of hypertension was 20%. Despite baseline elevations of fibrinogen and D-dimer in 60% of patients, no arterial thromboembolic events were observed. No patient was removed from the study because of drug toxicity.
Although IFN-α2b has been used in ocular melanoma, it has had very low efficacy. Two separate studies initially showed a 15% to 20% response rate in patients with ocular melanoma using bleomycin, vincristine, lomustine, and dacarbazine (BOLD) plus either human leukocyte IFN or IFN-α2b.27,28 However in EORTC 88941, a multicenter phase 2 trial studying BOLD plus IFN-α2b in patients with metastatic ocular melanoma, there were no responses to therapy. The trial enrolled a total of 24 patients and achieved disease stabilization in 2 patients. However, no patients had a partial or complete response.29
Ocular melanomas have a higher incidence of liver metastasis than cutaneous melanoma (90% vs. 24%, respectively) and these metastases are often resistant to chemotherapy.30 VEGF and bFGF are expressed in ocular melanoma cell lines,13,14,31,32 although no correlation has been found with metastases.32–34 VEGF has also been shown to be increased in the serum of patients with ocular melanoma.35 In addition, an increase in microvascular density is correlated with a worse prognosis.36,37 Bevacizumab has been used successfully to treat neovascular ocular diseases, such as age-related macular degeneration and proliferative diabetic retinopathy, but its activity against ocular melanoma is not known.38 Yang et al showed in a murine model that the combination of IFN-α2b with angiostatin, a drug that inhibits migration and VEGF expression, significantly decreased liver metastases in mice that had ocular melanoma.39 Targeting angiogensis in ocular melanoma patients is an attractive option for the prevention and treatment of metastases.
Several targeted antiangiogenic agents are being investigated in the setting of metastatic ocular melanoma. Two trials have looked at the efficacy of imatinib in patients with metastatic uveal melanoma. One found a clinical response in 8 of 10 patients40 and the other reported one response in 10 patients.41 Two trials currently in follow-up are investigating the antitumor effects of lenalidomide, which is known to inhibit the secretion of angiogenic cytokines including bFGF. The first study is evaluating single agent lenalidomide and the other is investigating the combination of lenalidomide, sunitinib, and cycylophosphomide.38 In addition to these 2 studies, there is currently a trial that is studying the use of VEGF Trap, which binds free VEGF.38 Going forward, there are several ways in which antiangiogenic agents could be explored in the setting of ocular melanoma. One approach would be to combine these agents with active chemotherapy. However, ocular melanoma does not respond to current cytotoxic regimens. Alternatively, agents like bevacizumab could be combined with biologics as was attempted in the current study. Finally, the effects of anti-VEGF agents could be enhanced by the use of drugs that block other aspects of the angiogenic process such as the effects of FGF.
This trial demonstrated that the combination of bevacizumab and high-dose IFN-α2b may have activity in patients with stage IV ocular melanoma. This regimen may have been more effective in patients with lower tumor burden, or if bevacizumab was used in conjunction with other drugs. We are currently working to develop a fully powered phase II study of Bevacizumab and IFN-α2b in patients with metastatic ocular melanoma. We are also exploring the possible use of bevacizumab in combination with carboplatin and paclitaxel, a trial that would mirror a recently completed trial in cutaneous melanoma.42
Acknowledgments
Supported by National Institutes of Health Grants U01 CA76576, N01 CM62207, K24 CA093670, T32 CA009338, and CA093071.
REFERENCES
- 1.Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83:1664–1678. doi: 10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 2.Singh AD, Bergman L, Seregard S. Uveal melanoma: epidemiologic aspects. Ophthalmol Clin North Am. 2005;18:75–84. viii. doi: 10.1016/j.ohc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Srivastava A, Ralhan R, Kaur J. Angiogenesis in cutaneous melanoma: pathogenesis and clinical implications. Microsc Res Tech. 2003;60:208–224. doi: 10.1002/jemt.10259. [DOI] [PubMed] [Google Scholar]
- 4.Graeven U, Rodeck U, Karpinski S, et al. Modulation of angiogenesis and tumorigenicity of human melanocytic cells by vascular endothelial growth factor and basic fibroblast growth factor. Cancer Res. 2001;61:7282–7290. [PubMed] [Google Scholar]
- 5.Mustonen T, Alitalo K. Endothelial receptor tyrosine kinases involved in angiogenesis. J Cell Biol. 1995;129:895–898. doi: 10.1083/jcb.129.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 7.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parangi S, O’Reilly M, Christofori G, et al. Antiangiogenic therapy of transgenic mice impairs de novo tumor growth. Proc Natl Acad Sci U S A. 1996;93:2002–2007. doi: 10.1073/pnas.93.5.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danson S, Lorigan P. Improving outcomes in advanced malignant melanoma: update on systemic therapy. Drugs. 2005;65:733–743. doi: 10.2165/00003495-200565060-00002. [DOI] [PubMed] [Google Scholar]
- 10.Rosenblatt MI, Azar DT. Anti-angiogenic therapy: prospects for treatment of ocular tumors. Semin Ophthalmol. 2006;21:151–160. doi: 10.1080/08820530500350787. [DOI] [PubMed] [Google Scholar]
- 11.Tapper D, Langer R, Bellows AR, et al. Angiogenesis capacity as a diagnostic marker for human eye tumors. Surgery. 1979;86:36–40. [PubMed] [Google Scholar]
- 12.Boyd SR, Tan D, Bunce C, et al. Vascular endothelial growth factor is elevated in ocular fluids of eyes harbouring uveal melanoma: identification of a potential therapeutic window. Br J Ophthalmol. 2002;86:448–452. doi: 10.1136/bjo.86.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ijland SA, Jager MJ, Heijdra BM, et al. Expression of angiogenic and immunosuppressive factors by uveal melanoma cell lines. Melanoma Res. 1999;9:445–450. doi: 10.1097/00008390-199910000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Boyd SR, Tan DS, de Souza L, et al. Uveal melanomas express vascular endothelial growth factor and basic fibroblast growth factor and support endothelial cell growth. Br J Ophthalmol. 2002;86:440–447. doi: 10.1136/bjo.86.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 16.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 17.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 18.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 19.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scappaticci FA, Fehrenbacher L, Cartwright T, et al. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J Surg Oncol. 2005;91:173–180. doi: 10.1002/jso.20301. [DOI] [PubMed] [Google Scholar]
- 21.Gordon MS, Cunningham D. Managing patients treated with bevacizumab combination therapy. Oncology. 2005;69(suppl 3):25–33. doi: 10.1159/000088481. [DOI] [PubMed] [Google Scholar]
- 22.Jaissle GB, Ulmer A, Henke-Fahle S, et al. Suppression of melanomaassociated neoangiogenesis by bevacizumab. Arch Dermatol. 2008;144:525–527. doi: 10.1001/archdermatol.2007.38. [DOI] [PubMed] [Google Scholar]
- 23.Dinney CP, Bielenberg DR, Perrotte P, et al. Inhibition of basic fibroblast growth factor expression, angiogenesis, and growth of human bladder carcinoma in mice by systemic interferon-alpha administration. Cancer Res. 1998;58:808–814. [PubMed] [Google Scholar]
- 24.Sangfelt O, Erickson S, Castro J, et al. Molecular mechanisms underlying interferon-alpha-induced G0/G1 arrest: CKI-mediated regulation of G1 Cdkcomplexes and activation of pocket proteins. Oncogene. 1999;18:2798–2810. doi: 10.1038/sj.onc.1202609. [DOI] [PubMed] [Google Scholar]
- 25.Ezekowitz RA, Mulliken JB, Folkman J. Interferon alfa-2a therapy for life-threatening hemangiomas of infancy. N Engl J Med. 1992;326:1456–1463. doi: 10.1056/NEJM199205283262203. [DOI] [PubMed] [Google Scholar]
- 26.Raig ET, Jones NB, Varker KA, et al. VEGF secretion is inhibited by interferon-alpha in several melanoma cell lines. J Interferon Cytokine Res. 2008;28:553–561. doi: 10.1089/jir.2008.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pyrhonen S, Hahka-Kemppinen M, Muhonen T, et al. Chemoimmunotherapy with bleomycin, vincristine, lomustine, dacarbazine (BOLD), and human leukocyte interferon for metastatic uveal melanoma. Cancer. 2002;95:2366–2372. doi: 10.1002/cncr.10996. [DOI] [PubMed] [Google Scholar]
- 28.Nathan FE, Berd D, Sato T, et al. BOLD+interferon in the treatment of metastatic uveal melanoma: first report of active systemic therapy. J Exp Clin Cancer Res. 1997;16:201–208. [PubMed] [Google Scholar]
- 29.Kivela T, Suciu S, Hansson J, et al. Bleomycin, vincristine, lomustine and dacarbazine (BOLD) in combination with recombinant interferon alpha-2b for metastatic uveal melanoma. Eur J Cancer. 2003;39:1115–1120. doi: 10.1016/s0959-8049(03)00132-1. [DOI] [PubMed] [Google Scholar]
- 30.Albert DM, Ryan LM, Borden EC. Metastatic ocular and cutaneous melanoma: a comparison of patient characteristics and prognosis. Arch Ophthalmol. 1996;114:107–108. [PubMed] [Google Scholar]
- 31.Notting IC, Missotten GS, Sijmons B, et al. Angiogenic profile of uveal melanoma. Curr Eye Res. 2006;31:775–785. doi: 10.1080/02713680600865052. [DOI] [PubMed] [Google Scholar]
- 32.Sahin A, Kiratli H, Tezel GG, et al. Expression of vascular endothelial growth factor a, matrix metalloproteinase 9 and extravascular matrix patterns in iris and ciliary body melanomas. Ophthalmic Res. 2007;39:40–44. doi: 10.1159/000097905. [DOI] [PubMed] [Google Scholar]
- 33.Kvanta A, Steen B, Seregard S. Expression of vascular endothelial growth factor (VEGF) in retinoblastoma but not in posterior uveal melanoma. Exp Eye Res. 1996;63:511–518. doi: 10.1006/exer.1996.0141. [DOI] [PubMed] [Google Scholar]
- 34.Sheidow TG, Hooper PL, Crukley C, et al. Expression of vascular endothelial growth factor in uveal melanoma and its correlation with metastasis. Br J Ophthalmol. 2000;84:750–756. doi: 10.1136/bjo.84.7.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeMoraes ED, Terai M, Aoyama T, et al. Serum vascular endothelium growth factor (VEGF) levels in patients with metastatic uveal melanoma and effect caused by hepatic artery embolization (HAE); ASCO Annual Meeting; 2002. abstr 1384. [Google Scholar]
- 36.Foss AJ, Alexander RA, Jefferies LW, et al. Microvessel count predicts survival in uveal melanoma. Cancer Res. 1996;56:2900–2903. [PubMed] [Google Scholar]
- 37.Makitie T, Summanen P, Tarkkanen A, et al. Microvascular density in predicting survival of patients with choroidal and ciliary body melanoma. Invest Ophthalmol Vis Sci. 1999;40:2471–2480. [PubMed] [Google Scholar]
- 38.Triozzi PL, Eng C, Singh AD. Targeted therapy for uveal melanoma. Cancer Treat Rev. 2008;34:247–258. doi: 10.1016/j.ctrv.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Yang H, Grossniklaus HE. Combined immunologic and anti-angiogenic therapy reduces hepatic micrometastases in a murine ocular melanoma model. Curr Eye Res. 2006;31:557–562. doi: 10.1080/02713680600718962. [DOI] [PubMed] [Google Scholar]
- 40.Chan KR, Gundala S, Laudadio M, et al. A pilot study using sunitinib malate as therapy in patients with stage IV uveal melanoma. J Clin Oncol. 2008;26 Abstract 9047. [Google Scholar]
- 41.Mouriaux F, Delcambre C, Durando X, et al. A Canceropole Nord-Ouest multicenter phase II trial of high-dose imatinib mesylate in metastatic uveal melanoma. J Clin Oncol. 2008;26 doi: 10.1007/s10637-008-9143-2. Abstract 9061. [DOI] [PubMed] [Google Scholar]
- 42.O’Day SJ. BEAM: A randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paciltaxel in patients with previously untreated advanced melanoma. Paper presented at: ECCO/ESMO; September 23, 2009; Berlin, Germany. [DOI] [PMC free article] [PubMed] [Google Scholar]


