Abstract
Enzalutamide is an oral androgen receptor inhibitor that targets multiple steps in the androgen receptor signaling pathway. In the randomized phase III AFFIRM study, significant improvements in survival versus placebo were observed when enzalutamide was used as a treatment for patients with metastatic castration-resistant prostate cancer (mCRPC) following prior treatment with docetaxel. Additional benefits included significant delay in time to first skeletal-related event, and improvement in several measures of pain and health-related quality of life. Treatment effects were consistent across all prespecified subgroups. The phase III PREVAIL study evaluated enzalutamide versus placebo in patients with mCRPC who had not received chemotherapy. Enzalutamide significantly decreased the risk of radiographic progression and death. There were also significant improvements in all secondary and prespecified exploratory endpoints, including delayed initiation of chemotherapy, reduction in risk of first skeletal-related event and a high percentage of patients with objective response compared with placebo. Enzalutamide was also studied in hormone naïve patients (as monotherapy) in a small, open-label phase II study in patients with prostate cancer who were eligible for androgen-deprivation therapy. A prostate-specific antigen (PSA) response, defined as ⩾80% decline in PSA level from baseline at week 25, was achieved in 92.5% of patients. Long-term follow up is ongoing. Despite differences between these three trials, enzalutamide displayed a favorable safety profile in all three patient populations. Similar rates of adverse events between the enzalutamide and placebo groups were observed in AFFIRM and PREVAIL, with fatigue, diarrhea, back pain and hot flashes being more common with enzalutamide than with placebo. Hypertension was reported at a higher rate in the enzalutamide group than in the placebo group in PREVAIL. Breast-related disorders associated with enzalutamide treatment were also reported in the Monotherapy trial. Few seizures were reported in any trial. Enzalutamide is being studied in several early disease state populations.
Keywords: AFFIRM trial, efficacy, enzalutamide, PREVAIL trial, prostate cancer, safety
Introduction
Prostate cancer is the fifth leading cause of death from cancer in men globally, resulting in an estimated 307,000 deaths worldwide in 2012 [Ferlay et al. 2014]. There remains a high level of unmet need in the management of this disease. Recommended treatments vary depending upon the stage of disease. Patients with advanced prostate cancer are initially managed with testosterone suppression via surgical or medical castration, but most will subsequently progress to castration-resistant prostate cancer (CRPC), a highly morbid state of prostate cancer in which skeletal-related events (SREs) such as bone pain, fractures, spinal cord compression and vertebral collapse are common [Kirby et al. 2011]. Metastatic disease is present in >80% of patients with CRPC. Until recently, treatment options for metastatic CRPC (mCRPC) were limited to chemotherapy with the taxane docetaxel and symptomatic relief of pain associated with bone metastases. However, some patients may not receive docetaxel due to poor performance status, comorbidities, or tolerability concerns. Consequently, further options are needed as alternatives to docetaxel, and in the event that treatment with docetaxel fails.
It is now well established that the androgen receptor (AR) signaling pathway is a major driver of prostate cancer growth and remains a relevant target in patients with mCRPC [Merseburger et al. 2013b; Sternberg et al. 2014b]. Indeed, novel androgen blocking therapies, including enzalutamide and abiraterone acetate in combination with prednisone, are among several new treatments for mCRPC that have demonstrated improvements in survival in clinical trials and have been approved for use [Heidenreich et al. 2013; Merseburger et al. 2013a]. Others include immunotherapy with sipuleucel-T (in patients with asymptomatic or minimally symptomatic CRPC), the radioisotope radium-223 (in patients with symptomatic CRPC both pre- and post-docetaxel), and cabazitaxel (second-line chemotherapy after docetaxel failure).
Based on the results of the randomized phase III, double-blind, placebo-controlled, multinational AFFIRM trial [ClinicalTrials.gov identifier: NCT00974311] [Scher et al. 2012], enzalutamide was approved in the United States in 2012 for the treatment of patients with mCRPC who have previously received docetaxel, and by the European Commission in June 2013. As of 23 September 2014, enzalutamide is registered in 45 countries worldwide. Enzalutamide is an oral AR inhibitor with a long halflife (mean of 5.8 days after a single oral dose, halflife of active metabolite another 7.8–8.6 days) that allows once daily dosing, which can be taken with or without food, and may be administered with or without steroids [Astellas Pharma US Inc. and Medivation Inc., 2014a, 2014b]. In vitro experiments have shown that enzalutamide acts at multiple points in the AR pathway, where it binds to the AR, prevents AR nuclear translocation and DNA binding, and induces apoptosis [Tran et al. 2009]. Enzalutamide binds to the AR with a higher affinity than the nonsteroidal antiandrogen, bicalutamide, and has no agonist activity when AR is overexpressed [Tran et al. 2009].
The antitumor activity and safety of enzalutamide was first investigated clinically in a phase I/II dose escalation study of 140 patients with progressive mCRPC, with or without previous exposure to chemotherapy [Scher et al. 2010]. Once daily doses of 30–600 mg were evaluated and the maximum tolerated dose was identified as 240 mg. The most common grade 3/4 adverse event (AE) was dose-dependent fatigue, which generally resolved after dose reduction. Enzalutamide showed encouraging activity, with antitumor effects noted at all doses in patients both with and without prior chemotherapy. These results led to the initiation of trials in both the post- and prechemotherapy settings, with a once daily dose of 160 mg selected for phase III enzalutamide trials.
This review provides an overview of currently published enzalutamide studies, including the AFFIRM trial in the mCRPC postchemotherapy setting, and subsequent clinical investigations of enzalutamide at earlier stages of prostate cancer development, including in the prechemotherapy mCRPC setting (PREVAIL trial) [Beer et al. 2014] and an exploratory study in the hormone-naïve setting (Monotherapy trial) [Tombal et al. 2014]. We also reflect on the current role of enzalutamide in clinical practice and discuss future areas of investigation.
Enzalutamide in patients progressing post- docetaxel (AFFIRM)
AFFIRM is the key trial that supports the use of enzalutamide for the treatment of patients with mCRPC post-docetaxel, the first approved indication for enzalutamide [Scher et al. 2012]. The main findings of this trial have been well reported and hence are covered only briefly here [Saad, 2013; Scher et al. 2012]. More recently, prospective data on patient-reported outcomes and post hoc analysis results from patient subgroups have been published [Fizazi et al. 2014; Sternberg et al. 2014a].
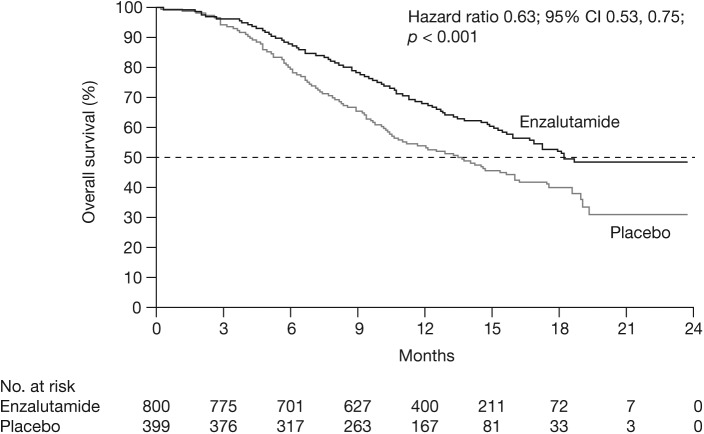
AFFIRM was a randomized phase III, double-blind, placebo-controlled, multinational trial that evaluated enzalutamide in men with mCRPC who had previously received docetaxel [Scher et al. 2012]. In this trial, 1199 men were randomized in a 2:1 ratio to receive enzalutamide 160 mg/day or placebo. Baseline characteristics were well matched between groups with regard to demographic characteristics, previous treatment history and extent of disease. At the planned analysis (at the time of 520 deaths), median time on treatment was 8.3 months with enzalutamide and 3.0 months with placebo. Enzalutamide demonstrated a significant improvement in the primary endpoint of overall survival (OS) with a hazard ratio (HR) of 0.63 (95% confidence interval [CI] 0.53, 0.75; p < 0.001); the median OS was 18.4 months in the enzalutamide group versus 13.6 months in the placebo group (Figure 1) [Scher et al. 2012]. The OS benefit was consistent across all subgroups including age, baseline pain intensity, geographic region and type of disease progression at study entry. The benefits of enzalutamide in terms of the secondary measures of response and time to progression in AFFIRM were consistent with the OS benefit (all p < 0.001 versus placebo) (Table 1) [Scher et al. 2012].
Figure 1.
Overall survival, AFFIRM.
CI, confidence interval.
From Scher et al. (2012). Copyright © 2012 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Table 1.
Summary of key secondary endpoints from AFFIRM.
| Endpoint | Enzalutamide (n = 800) | Placebo (n = 399) | Hazard ratio (95% CI) | p value |
|---|---|---|---|---|
| Confirmed PSA decline | ||||
| Patients with ⩾1 post baseline PSA assessment, n (%) | 731 (91) | 330 (83) | ||
| PSA decline of ⩾50% from baseline, n/total n (%) | 395/731 (54) | 5/330 (2) | <0.001 | |
| PSA decline of ⩾90% from baseline, n/total n (%) | 181/731 (25) | 3/330 (1) | <0.001 | |
| Soft tissue objective response | ||||
| Patients with measurable soft tissue disease, n (%) | 446 (56) | 208 (52) | ||
| Complete or partial objective response, n/total n (%) | 129/446 (29) | 8/208 (4) | <0.001 | |
| FACT-P quality of life response | ||||
| Patients with ⩾1 post baseline assessment, n (%) | 651 (81) | 257 (54) | <0.001 | |
| Quality of life response, n/total n (%) | 281/651 (43) | 47/257 (18) | ||
| Median time until PSA progression, months | 8.3 | 3.0 | 0.25 | <0.001 |
| (0.20, 0.30) | ||||
| Median radiographic progression-free survival, months | 8.3 | 2.9 | 0.40 | <0.001 |
| (0.35, 0.47) | ||||
| Median time until first skeletal-related event, months | 16.7 | 13.3 | 0.69 | <0.001 |
| (0.57, 0.84) | ||||
CI, confidence interval; FACT-P, Functional Assessment of Cancer Therapy-Prostate scale; PSA, prostate-specific antigen.
From Scher et al. (2012). Copyright © 2012 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Some of the most painful and functionally compromising aspects of mCRPC are SREs related to bone metastases, which adversely affect health-related quality of life (HRQoL). Delaying or preventing SREs is an unmet clinical need [Sieber, 2014]. Therefore, prospectively defined analyses of patient-related secondary outcomes such as SRE, pain and HRQoL were carried out in AFFIRM and highlighted that the benefits of enzalutamide extend beyond survival, with significant improvements in these outcomes [Fizazi et al. 2014]. Median time to first SRE was 16.7 months in the enzalutamide group versus 13.3 months in the placebo group (p = 0.0001) (Table 1). Enzalutamide provided consistent benefits across various measures of pain including pain severity and pain interference scores (as measured with the Brief Pain Inventory Short Form), and having pain and pain progression, as measured by Functional Assessment of Cancer Therapy-Prostate (FACT-P) scale, item 4. Significantly greater improvements in overall HRQoL (FACT-P total score) were reported by patients receiving enzalutamide compared with those receiving placebo. These findings suggest that significant improvements with enzalutamide versus placebo in patient-reported disease-related symptoms, such as pain, translate into HRQoL benefits.
AFFIRM subgroup analyses
Additional analyses of the AFFIRM trial have demonstrated that the benefits of enzalutamide are observed across different subgroups. Given that prostate cancer is predominantly a disease of older men and older patients are more likely to present with advanced disease, with many patients not receiving optimal treatment due to decisions based on age alone, it was of interest to determine if there were any differential age-related effects of enzalutamide treatment. In a post hoc analysis, enzalutamide treatment resulted in a similar survival benefit in patients ⩾75 years and <75 years [Sternberg et al. 2014a]. A significant improvement in OS was observed with enzalutamide versus placebo in both subgroups (patients <75 years: HR 0.63; 95% CI 0.52, 0.78; median not yet reached versus 13.6 months; and patients ⩾75 years: HR 0.61; 95% CI 0.43, 0.86; median 18.2 months versus 13.3 months). The superiority of enzalutamide over placebo was also shown for all secondary endpoints for both age groups, including radiographic progression-free survival (rPFS), time to prostate-specific antigen (PSA) progression and PSA response rate.
The majority of patients in the AFFIRM trial were recruited from Europe (EU) and North America (NA). These regions have different clinical management, diagnosis and treatment guidelines for prostate cancer across the disease spectrum, which may influence disease recurrence and progression patterns. Therefore, it was of interest to explore if there were any differences in outcomes between EU and NA patients through a post hoc analysis of the AFFIRM study. Enzalutamide significantly improved OS compared with placebo in both EU and NA patients [Merseburger et al. 2014]. Of note, the median OS in EU patients was longer than in NA patients in both treatment groups. However, the relative treatment effect, expressed as HR (and 95% CI), was similar in both regions: 0.64 (0.50, 0.82) for EU and 0.63 (0.47, 0.83) for NA. The benefit of enzalutamide over placebo was consistent across all secondary endpoints in patients in both regions, with significant improvements (p < 0.001) in PSA, soft tissue and quality of life responses, time to PSA progression, and rPFS.
PSA is a commonly used marker of prostate cancer disease burden and the relationship of baseline PSA to consequent treatment effect was the focus of another post hoc analysis of the AFFIRM study. Enzalutamide consistently improved OS, rPFS and time to PSA progression compared with placebo, regardless of baseline PSA level (subgroups divided by baseline PSA quartile) [Saad et al. 2014].
Enzalutamide in chemo-naïve patients (PREVAIL)
Although chemotherapy has been shown to improve OS in patients with mCRPC, not all patients are able to receive such therapy due to pre-existing medical conditions or concerns about toxicity. Therefore, there remains a need for effective, convenient and less toxic therapies. For this reason, and further to encouraging results in chemo-naïve patients with CRPC as part of the phase I/II trial [Scher et al. 2010], the multinational, double-blind, randomized, placebo-controlled, phase III PREVAIL study [ClinicalTrials.gov identifier: NCT01212991] evaluated enzalutamide in men with chemo-naïve mCRPC that had progressed despite the use of androgen deprivation therapy (ADT) (luteinizing hormone-releasing hormone analogue or orchiectomy) [Beer et al. 2014]. Patients were asymptomatic (score of 0 to 1 on the Brief Pain Inventory Short Form, question 3) or mildly symptomatic (score of 2–3) and had not received cytotoxic chemotherapy, ketoconazole or abiraterone acetate. From 207 global sites, 1717 patients were enrolled and randomized to oral enzalutamide 160 mg/day or placebo, with baseline demographic and disease characteristics well balanced between the two groups. The median time that patients received study drug was longer in the enzalutamide group than in the placebo group (16.6 months versus 4.6 months).
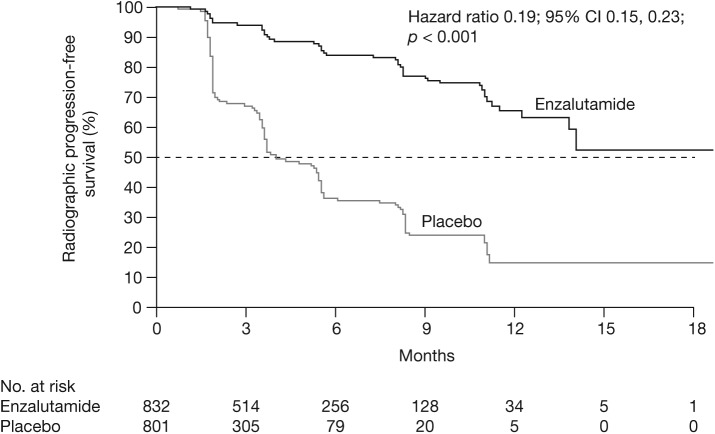
Based on the co-primary efficacy and safety results at the planned interim analysis, it was recommended by the independent data and safety monitoring committee that the study should be halted and enzalutamide offered to eligible patients from the placebo group [Beer et al. 2014]. The study met its coprimary endpoints, with significant improvements for enzalutamide versus placebo in both rPFS and OS. These benefits were seen soon after randomization (from 2 months for rPFS and 4 months for OS). At 12 months of follow up, the rate of rPFS was 65% for enzalutamide-treated patients versus 14% for patients receiving placebo (81% risk reduction; HR 0.19; 95% CI 0.15, 0.23; p < 0.001) (Figure 2). The median rPFS was not yet reached in the enzalutamide group versus 3.9 months in the placebo group. At the time of the planned interim analysis, the median duration of follow up for survival was approximately 22 months. Fewer deaths occurred in the enzalutamide group (241 out of 872 patients, 28%) than in the placebo group (299 out of 845 patients, 35%). This resulted in a 29% reduction in the risk of death with enzalutamide compared with placebo (HR 0.71; 95% CI 0.60, 0.84; p < 0.001). Median OS was estimated at 32.4 months and 30.2 months for enzalutamide and placebo, respectively. The treatment effect of enzalutamide on both rPFS and OS was consistent across all prespecified subgroups, including patients with visceral disease, and was unaffected by previous exposure to antiandrogens.
Figure 2.
Radiographic progression-free survival, PREVAIL.
CI, confidence interval.
From Beer et al. (2014). Copyright © 2014 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
In an updated analysis of OS, 82% of enzalutamide-treated patients and 73% of patients receiving placebo were alive at 18 months [Beer et al. 2014]. Estimated median OS was not yet reached for enzalutamide and 31.0 months for placebo (HR 0.73; 95% CI 0.63, 0.85; p < 0.001).
Fewer patients in the enzalutamide group received subsequent antineoplastic therapy than the placebo group (40% versus 70%, respectively); the most common being docetaxel (33% versus 57%, respectively) and abiraterone (21% versus 46%, respectively) [Beer et al. 2014]. The superiority of enzalutamide over placebo was shown with significant improvements in all secondary and prespecified exploratory endpoints, including a delay in the initiation of chemotherapy (28.0 months versus 10.8 months for enzalutamide and placebo, respectively; HR 0.35; 95% CI 0.30, 0.40; p < 0.001) (Table 2). There was a 28% reduction in risk of first SRE with enzalutamide (278 patients, 32%) versus placebo (309 patients, 37%) (HR 0.72; 95% CI 0.61, 0.84; p < 0.001). Objective responses in patients with measurable soft tissue disease were observed in 59% of patients in the enzalutamide group compared with 5% in the placebo group (p < 0.001). Other secondary endpoints included time to PSA progression and decline in PSA level of ⩾50% from baseline. Prespecified exploratory endpoints included quality of life (FACT-P scale) and a decline in PSA level of ⩾90% from baseline (Table 2).
Table 2.
Summary of key secondary and prespecified exploratory endpoints from PREVAIL.
| Endpoint | Enzalutamide (n = 872) | Placebo (n = 845) | Hazard ratio (95% CI) | p value |
|---|---|---|---|---|
| Median time until initiation of cytotoxic chemotherapy, months | 28.0 | 10.8 | 0.35 | <0.001 |
| (0.30, 0.40) | ||||
| Median time until decline in the FACT-P global score, months | 11.3 | 5.6 | 0.63 | <0.001 |
| (0.54, 0.72) | ||||
| Median time until first skeletal-related event, months | 31.1 | 31.3 | 0.72 | <0.001 |
| (0.61, 0.84) | ||||
| Median time until PSA progression, months | 11.2 | 2.8 | 0.17 | <0.001 |
| (0.15, 0.20) | ||||
| Confirmed change in PSA | ||||
| Patients with ⩾1 post baseline PSA assessment, n (%) | 854 (98) | 777 (92) | ||
| PSA decline ⩾50% from baseline, n/total n (%) | 666/854 (78) | 27/777 (3) | <0.001 | |
| PSA decline ⩾90% from baseline, n/total n (%) | 400/854 (47) | 9/777 (1) | <0.001 | |
| Patients with measurable soft tissue disease, n (%) | 396 (45) | 381 (45) | ||
| Objective response | 233 (59) | 19 (5) | <0.001 | |
| Complete response | 78 (20) | 4 (1) | ||
| Partial response | 155 (39) | 15 (4) | ||
CI, confidence interval; FACT-P, Functional Assessment of Cancer Therapy-Prostate scale; PSA, prostate-specific antigen.
From Beer et al. (2014). Copyright © 2014 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Enzalutamide in hormone-naïve patients (Monotherapy trial)
Current methods of ADT (surgery or luteinizing hormone-releasing hormone agonist) for the first-line treatment of advanced prostate cancer are associated with a range of side effects, including metabolic side effects, such as decreased bone mineral density. Nonsteroidal antiandrogens, such as bicalutamide, have a different side effect profile, but are associated with AR agonistic effects and only a minimal effect on survival. Consequently, there is a need for alternative treatments with an improved AE profile combined with clinical activity. Against this background, and given the good tolerability profile and antitumor activity of enzalutamide observed in early clinical trials, the first clinical trial of enzalutamide in patients with hormone-naïve prostate cancer assessed the potential of enzalutamide monotherapy in patients eligible for ADT [Tombal et al. 2014]. Given the exploratory nature of this analysis, the trial was an open-label, single-arm, phase II study of enzalutamide 160 mg/day in men with hormone-naïve prostate cancer for whom hormone therapy was indicated [ClinicalTrials.gov identifier: NCT01302041]. Of the 67 patients enrolled from 12 centers in Europe, 39% presented with metastases, 36% had previously undergone radical prostatectomy and 24% had received radiotherapy. A total of 63 patients (94%) completed the 24-week study period. Patients were allowed to continue enzalutamide at the discretion of the investigator, until disease progression or the occurrence of an unacceptable safety or tolerability issue.
The primary outcome of PSA response, defined as a ⩾80% decline in PSA level from baseline at week 25, was achieved in 62 patients (92.5%) (Figure 3). Secondary outcomes were PSA dynamics and kinetics, and changes from baseline in hormone levels. PSA was undetectable (⩽0.1 ng/ml) in 30 patients (45%) at week 25. Despite variations in disease severity at baseline, PSA outcomes were consistent between patients with and without metastases. As expected from a potent AR inhibitor, sex hormone levels were substantially increased from baseline, with the greatest changes observed for luteinizing hormone, testosterone and sex hormone-binding globulin. Luteinizing hormone and testosterone levels increased rapidly in weeks 1 to 5. Luteinizing hormone levels continued to increase slightly through to week 25, whereas testosterone levels plateaued around week 13.
Figure 3.
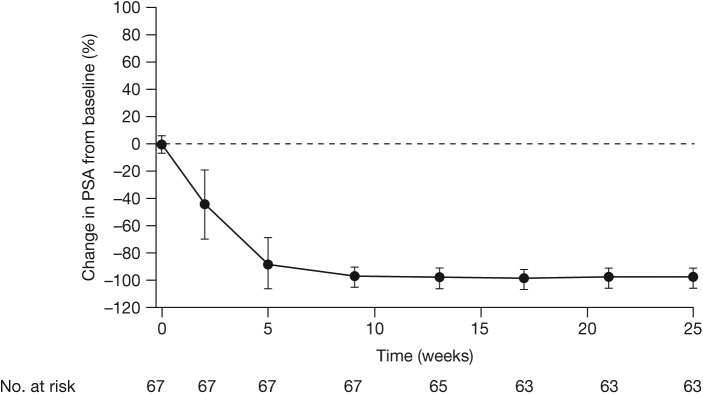
Overall PSA change from baseline to week 25 (Monotherapy study).
Datapoints are means and whiskers depict standard deviations. PSA, prostate-specific antigen.
From Tombal et al. (2014). Reprinted with permission from Elsevier.
Protocol-defined exploratory outcomes included objective disease response, body composition, bone turnover and metabolic outcomes. Of 16 patients with metastases and measurable disease at baseline, three patients (19%) had an objective complete response, five patients (31%) had a partial response and three patients (19%) had stable disease. The effects of enzalutamide on body composition (fat body mass and lean body mass) were small and similar to those previously reported for ADT. Bone mineral density remained stable, with only small changes noted during the study, similar to previously reported results with bicalutamide and in contrast to substantial decreases reported with leuprolide [Smith et al. 2004]. The authors suggest that maintenance of bone mineral density with enzalutamide could be attributed to increased estrogen levels and markers of bone resorption [Ohlsson et al. 2012; Argoud et al. 2014]. Patient-reported HRQoL was generally maintained with enzalutamide, although fatigue increased, as measured on the EORTC QLQ-C30. Extended 1-year and 2-year follow up of patients is still ongoing and the results will be reported in due course.
This study was undertaken to provide an initial profile of enzalutamide in this population and to inform potential future trial development. The short-term findings suggest that enzalutamide monotherapy results in substantial post-treatment decline of PSA and only small changes in body composition, similar to those previously reported for ADT. HRQoL was generally maintained over 24 weeks of treatment. These results provide a rationale for further investigation of enzalutamide use in men with hormone-naïve prostate cancer.
Safety profile of enzalutamide
Across the three key trials reported to date, enzalutamide was well tolerated and has demonstrated a consistent safety and tolerability profile. In the placebo-controlled AFFIRM and PREVAIL trials, the AE profile was generally comparable between the two treatment groups, with the exception of hot flash and fatigue, which was more common in those treated with enzalutamide [Beer et al. 2014; Scher et al. 2012]. In the AFFIRM trial, although the period of observation for patients receiving enzalutamide was more than twice that for those receiving placebo, the rates of AEs were similar in the two groups, with fewer AEs of grade 3 or above in the enzalutamide group (Table 3). The median time to an AE of grade 3 or above was 8.4 months longer in the enzalutamide group than in the placebo group. The most common AEs that occurred more frequently in the enzalutamide group included fatigue, diarrhea and hot flashes (Table 3). In the PREVAIL trial, AEs that occurred more frequently with enzalutamide than with placebo included fatigue and hot flash, and additionally, back pain, hypertension, asthenia and fall (Table 4) [Beer et al. 2014].
Table 3.
Summary of the adverse event profile from the AFFIRM study: adverse event summary – most frequent adverse events more common with enzalutamide than placebo.
| Adverse event, n (%) | Enzalutamide (n = 800) |
Placebo (n = 399) |
||
|---|---|---|---|---|
| Any grade | Grade ⩾3 | Any grade | Grade ⩾3 | |
| Any adverse event | 785 (98) | 362 (45) | 390 (98) | 212 (53) |
| Any serious adverse event | 268 (34) | 227 (28) | 154 (39) | 134 (34) |
| Any adverse event leading to treatment discontinuation | 61 (8) | 37 (5) | 39 (10) | 28 (7) |
| Any adverse event leading to death | 23 (3) | 23 (3) | 14 (4) | 14 (4) |
| Most common adverse events* | ||||
| Fatigue | 269 (34) | 50 (6) | 116 (29) | 29 (7) |
| Diarrhea | 171 (21) | 9 (1) | 70 (18) | 1 (<1) |
| Hot flash | 162 (20) | 0 | 41 (10) | 0 |
| Musculoskeletal pain | 109 (14) | 8 (1) | 40 (10) | 1 (<1) |
| Headache | 93 (12) | 6 (<1) | 22 (6) | 0 |
Included in this category are adverse events that were reported in at least 10% of patients in the enzalutamide group at a rate that was at least two percentage points higher than that in the placebo group.
From Scher et al. (2012). Copyright © 2012 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Table 4.
Summary of the adverse event profile from the PREVAIL study: adverse event summary – most frequent adverse events more common with enzalutamide than placebo.
| Adverse event, n (%) | Enzalutamide (n = 871) |
Placebo (n = 844) |
||
|---|---|---|---|---|
| Any grade | Grade ⩾3 | Any grade | Grade ⩾3 | |
| Any adverse event | 844 (97) | 374 (43) | 787 (93) | 313 (37) |
| Any serious adverse event | 279 (32) | NR | 226 (27) | NR |
| Any adverse event leading to treatment discontinuation | 49 (6) | NR | 51 (6) | NR |
| Any adverse event leading to death | 37 (4) | NR | 32 (4) | NR |
| Most common adverse events* | ||||
| Fatigue | 310 (36) | 16 (2) | 218 (26) | 16 (2) |
| Back pain | 235 (27) | 22 (3) | 187 (22) | 25 (3) |
| Constipation | 193 (22) | 4 (<1) | 145 (17) | 3 (<1) |
| Arthralgia | 177 (20) | 12 (1) | 135 (16) | 9 (1) |
| Decreased appetite | 158 (18) | 2 (<1) | 136 (16) | 6 (1) |
| Hot flash | 157 (18) | 1 (<1) | 65 (8) | 0 |
| Diarrhea | 142 (16) | 2 (<1) | 119 (14) | 3 (<1) |
| Hypertension | 117 (13) | 59 (7) | 35 (4) | 19 (2) |
| Asthenia | 113 (13) | 11 (1) | 67 (8) | 8 (1) |
| Fall | 101 (12) | 12 (1) | 45 (5) | 6 (1) |
| Weight loss | 100 (11) | 5 (1) | 71 (8) | 2 (<1) |
| Edema peripheral | 92 (11) | 2 (<1) | 69 (8) | 3 (<1) |
| Headache | 91 (10) | 2 (<1) | 59 (7) | 3 (<1) |
Included in this category are adverse events that were reported in at least 10% of patients in the enzalutamide group at a rate that was at least two percentage points higher than that in the placebo group. NR, not reported.
From Beer et al. (2014). Copyright © 2014 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Regarding specific AEs of interest, the rate of seizures was low in both trials. AFFIRM reported seizures in five out of 800 patients receiving enzalutamide, most of whom had predisposing conditions or concomitant treatments [Scher et al. 2012]. Seizure occurred also in one patient (0.1%) in each treatment group of PREVAIL, both of whom, it was later discovered, had a history of seizure [Beer et al. 2014]. Hepatotoxicity has previously been documented as a side effect of some antiandrogens [McLeod, 1997]. However, no evidence of hepatotoxicity was observed in the enzalutamide group in the AFFIRM, PREVAIL, or Monotherapy studies. In AFFIRM, fewer liver function abnormalities were reported with enzalutamide (1% of patients) than placebo (2% of patients). In PREVAIL, liver function abnormality (specifically elevations in alanine aminotransferase level) was comparable between the enzalutamide (1%) and placebo groups (1%).
In contrast to the AFFIRM and PREVAIL trials in mCRPC, the tolerability profile of enzalutamide in the study by Tombal and colleagues [Tombal et al. 2014] was slightly different, as it was performed in patients at an earlier disease stage, in the absence of castration, and using an uncontrolled open-label design. Enzalutamide was generally well tolerated, with gynecomastia and fatigue the most commonly reported AEs. Mild-to-moderate breast-related disorders may have been related to high testosterone levels, but no patients withdrew due to these breast-related events. There was one event of seizure (1.5%) in the Monotherapy trial [Tombal et al. 2014].
Other observations
The antiandrogen withdrawal syndrome is defined as a confirmed PSA decline (by ⩾50% from the last on-treatment PSA level) following complete cessation of antiandrogen treatment [Rodriguez-Vida et al. 2014]. It has previously been described for antiandrogens such as flutamide and bicalutamide, but information regarding enzalutamide withdrawal is currently limited [Rodriguez-Vida et al. 2014; von Klot et al. 2014a, 2014b]. Two recent reports, based on 30 patients and 31 patients with mCRPC, respectively, suggest that antiandrogen withdrawal syndrome with enzalutamide appears to be rare with the currently available follow up and withdrawal timeframes [Phillips, 2014; Rodriguez-Vida et al. 2014; von Klot et al. 2014a].
Discussion
The trials discussed in this review demonstrate that the role of enzalutamide is evolving. In addition to being associated with survival benefits in patients with mCRPC following docetaxel, enzalutamide improves HRQoL in this population and has shown consistent efficacy benefits across different subgroups, further supporting its broad use in this indication.
US and EU treatment guidelines have not yet incorporated data from PREVAIL [Cookson et al. 2013; Mottet et al. 2014; NCCN, 2014]. Current National Comprehensive Cancer Network (NCCN) guidelines include a 2A recommendation for the use of enzalutamide in the prechemotherapy mCRPC setting, in addition to a recommendation as a new standard of care following failure of docetaxel in mCRPC [NCCN, 2014]. American Urological Association guidelines refer to use of enzalutamide in patients with symptomatic mCRPC who have received docetaxel and have either good or poor performance status [Cookson et al. 2013]. European Association of Urology guidelines state that enzalutamide is effective in the management of progressive CRPC following docetaxel, but acknowledge that there is no definitive strategy regarding treatment choice [Mottet et al. 2014]. In addition, the guidelines note that second-line salvage hormonal treatment using enzalutamide might become a valid option in mCRPC.
Clinical trial experience of enzalutamide in the prechemotherapy setting has demonstrated significant improvements in rPFS and OS with enzalutamide versus placebo and has confirmed the safety profile. Results from the phase II enzalutamide Monotherapy study in patients with hormone-naïve prostate cancer are also encouraging, suggesting that it warrants further investigation as a potentially viable alternative to castration. Evidence derived from the published data for enzalutamide supports further investigation of this drug in an earlier disease setting, possibly even in an adjuvant setting in locally advanced disease, or in combination with radiation therapy in high-risk local disease. However, future trials will be required to shed more light on these hypotheses.
The therapeutic options for men with prostate cancer are expanding. Several agents are now reported to improve survival for patients with mCRPC that has progressed after ADT, including abiraterone acetate, radium-223, cabazitaxel and sipuleucel-T, in addition to enzalutamide [Merseburger et al. 2013a; Sternberg et al. 2014b]. Abiraterone acetate is a hormonal therapy that has been approved in combination with prednisone for use in patients with mCRPC who have failed one or two prior chemotherapy regimens (including docetaxel). This is based on results from the phase III COU-AA-301 trial, which demonstrated significantly longer OS with abiraterone [de Bono et al. 2011; Fizazi et al. 2012]. Abiraterone plus prednisone has also been approved in the prechemotherapy setting based on results from the phase III COU-AA-302 trial, which demonstrated improvement in median rPFS with abiraterone plus prednisone versus prednisone alone [Rathkopf et al. 2014].
With the recent approvals of abiraterone, enzalutamide and other agents for mCRPC, the optimal sequencing of treatments in patients post chemotherapy needs to be defined. Currently, the only available data on sequencing are from a number of small, often retrospective, analyses in heavily pretreated patients who took part in compassionate use programs. These analyses evaluated patients with mCRPC who received enzalutamide treatment after progressing on chemotherapy and abiraterone [Badrising et al. 2014; Bianchini et al. 2014; Brasso et al. 2014; Schmid et al. 2014; Schrader et al. 2014; Thomsen et al. 2014] and, conversely, those receiving abiraterone treatment after progressing on chemotherapy and enzalutamide [Loriot et al. 2013; Noonan et al. 2013]. Evaluation of PSA-based outcomes and survival criteria in these analyses showed modest clinical activity with both scenarios in heavily pretreated patients with mCRPC and highlighted the need for further systematic investigation of treatment sequencing, as well as clarification of the possible mechanism of cross-resistance between enzalutamide and abiraterone. Recent data from an open-label, prospective, phase II study of enzalutamide treatment in 60 patients with bone mCRPC who had bone marrow biopsy has assessed expression of molecular components of AR signaling and has provided some insight into mechanisms of resistance [Efstathiou et al. 2014]. The presence of the AR splice variant AR-V7 was associated with primary resistance to enzalutamide [Efstathiou et al. 2014; Nakazawa et al. 2014].
Some large, prospective studies of enzalutamide have recently been initiated and are designed to address questions such as sequencing in mCRPC, including a phase IV study of the use of enzalutamide after abiraterone in patients with mCRPC [ClinicalTrials.gov identifier: NCT02116582] and a phase IV trial in patients with chemonaïve mCRPC, evaluating continued enzalutamide treatment beyond progression (enzalutamide plus abiraterone/prednisone versus placebo plus abiraterone/prednisone), with a primary endpoint of PFS [PLATO, ClinicalTrials.gov identifier: NCT01995513]. The combination of enzalutamide with abiraterone/prednisone is also being evaluated in a phase III trial in patients with mCRPC [ClinicalTrials.gov identifier: NCT01949337].
Several trials of enzalutamide are ongoing in earlier disease settings, including phase II randomized, double-blind, head-to-head trials of enzalutamide versus bicalutamide, with primary endpoints of PFS in patients who progressed despite ADT [TERRAIN, ClinicalTrials.gov identifier: NCT01288911; STRIVE, ClinicalTrials.gov identifier: NCT01664923]. A phase III trial in nonmetastatic CRPC is also recruiting, with a primary endpoint of metastasis-free survival [PROSPER, ClinicalTrials.gov identifier: NCT02003924].
Other areas of active research interest in prostate cancer include the use of established agents earlier in the disease pathway, such as chemotherapy in hormone-sensitive disease. Sweeney and colleagues reported improved survival with upfront chemohormonal therapy (ADT plus docetaxel, 49.2 months) versus hormonal therapy alone (ADT, 32.2 months) for men with high-volume newly metastatic prostate cancer [Sweeney et al. 2014]. These data suggest the use of more aggressive, earlier treatment of metastatic prostate cancer.
Conclusion
Efficacy and safety of enzalutamide established in the AFFIRM trial led to approval in the United States and Europe for its use in patients with mCRPC after failure of docetaxel. The PREVAIL study data led to the recent approval of enzalutamide for use in chemo-naïve patients in the United States. Ongoing and planned trials will help further define the optimal use of enzalutamide in the treatment of prostate cancer.
Acknowledgments
The authors would like to thank Karen Brayshaw, PhD, at Complete HealthVizion, for assistance with writing and revising the draft manuscript, based on detailed discussion and feedback from all authors. Writing assistance was funded by Astellas Pharma, Inc and Medivation, Inc. Primary responsibility for opinions, conclusions, and interpretation of data lies with the authors. All authors read and approved the final version of this manuscript.
Footnotes
Conflict of interest statement: A.S.M. has received consulting fees from Medivation, Astellas, Janssen-Cilag, Bayer, Roche, Ipsen, Novartis, and Amgen, and payment for speaker bureau from Novartis, Bayer, Janssen-Cilag, Astellas, Ipsen, GlaxoSmithKline, Hexal and Amgen. G.P.H. is an employee of Astellas. C.-A.vK. has received consulting fees from Janssen-Cilag, Teva and Astellas.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Contributor Information
Axel S. Merseburger, Department of Urology and Urologic Oncology, Hannover Medical School (MHH), Carl Neuberg Str. 1, 30625 Hannover, Germany
Gabriel P. Haas, Astellas Global Medical Affairs, Inc., Northbrook, IL, USA
Christoph-A. von Klot, Hannover Medical School (MHH), Hannover, Germany
References
- Argoud T., Boutroy S., Claustrat B., Chapurlat R., Szulc P. (2014) Association between sex steroid levels and bone microarchitecture in men: the STRAMBO study. J Clin Endocrinol Metab 99: 1400–1410. [DOI] [PubMed] [Google Scholar]
- Astellas Pharma US Inc. and Medivation Inc. (2014a) XTANDI US prescribing information. Available at: http://www.astellas.us/docs/12A005-ENZ-WPI.pdf
- Astellas Pharma US Inc. and Medivation Inc. (2014b) XTANDI summary of product characteristics. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002639/WC500144996.pdf.
- Badrising S., van der Noort V., van Oort I., van den Berg H., Los M., Hamberg P., et al. (2014) Clinical activity and tolerability of enzalutamide (MDV3100) in patients with metastatic, castration-resistant prostate cancer who progress after docetaxel and abiraterone treatment. Cancer 120: 968–975. [DOI] [PubMed] [Google Scholar]
- Beer T., Armstrong A., Rathkopf D., Loriot Y., Sternberg C., Higano C., et al. (2014) Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 371: 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchini D., Lorente D., Rodriguez-Vida A., Omlin A., Pezaro C., Ferraldeschi R., et al. (2014) Antitumour activity of enzalutamide (MDV3100) in patients with metastatic castration-resistant prostate cancer (CRPC) pre-treated with docetaxel and abiraterone. Eur J Cancer 50: 78–84. [DOI] [PubMed] [Google Scholar]
- Brasso K., Thomsen F., Schrader A., Schmid S., Lorente D., Retz M., et al. (2014) Enzalutamide antitumour activity against metastatic castration-resistant prostate cancer previously treated with docetaxel and abiraterone: a multicentre analysis. Eur Urol [Epub ahead of print]. DOI: 10.1016/j.eururo.2014.07.028. [DOI] [PubMed] [Google Scholar]
- Cookson M., Roth B., Dahm P., Engstrom C., Freedland S., Hussain M., et al. (2013) Castration-resistant prostate cancer: AUA Guideline. J Urol 190: 429–438. [DOI] [PubMed] [Google Scholar]
- de Bono J., Logothetis C., Molina A., Fizazi K., North S., Chu L., et al. (2011) Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 364: 1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstathiou E., Titus M., Wen S., Hoang A., Karlou M., Ashe R., et al. (2014) Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur Urol [Epub ahead of print]. DOI: 10.1016/j.eururo.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J., Soerjomataram I., Ervik M., Dikshit R., Eser S., Mathers C., et al. (2014) GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11, International Agency for Research on Cancer, Lyon, France. Available at: http://globocan.iarc.fr.
- Fizazi K., Scher H., Miller K., Basch E., Sternberg C., Cella D., et al. (2014) Effect of enzalutamide on time to first skeletal-related event, pain, and quality of life in men with castration-resistant prostate cancer: results from the randomised, phase 3 AFFIRM trial. Lancet Oncol 15: 1147–1156. [DOI] [PubMed] [Google Scholar]
- Fizazi K., Scher H., Molina A., Logothetis C., Chi K., Jones R., et al. (2012) Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 13: 983–992. [DOI] [PubMed] [Google Scholar]
- Heidenreich A., Pfister D., Merseburger A., Bartsch G. (2013) Castration-resistant prostate cancer: where we stand in 2013 and what urologists should know. Eur Urol 64: 260–265. [DOI] [PubMed] [Google Scholar]
- Kirby M., Hirst C., Crawford E. (2011) Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract 65: 1180–1192. [DOI] [PubMed] [Google Scholar]
- Loriot Y., Bianchini D., Ileana E., Sandhu S., Patrikidou A., Pezaro C., et al. (2013) Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100). Ann Oncol 24: 1807–1812. [DOI] [PubMed] [Google Scholar]
- McLeod D. (1997) Tolerability of nonsteroidal antiandrogens in the treatment of advanced prostate cancer. Oncologist 2: 18–27. [PubMed] [Google Scholar]
- Merseburger A., Bellmunt J., Jenkins C., Parker C., Fitzpatrick J. (2013a) Perspectives on treatment of metastatic castration-resistant prostate cancer. Oncologist 18: 558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merseburger A., Kuczyk M., Wolff J. (2013b) [Pathophysiology and therapy of castration-resistant prostate cancer]. Urologe A 52: 219–225. [DOI] [PubMed] [Google Scholar]
- Merseburger A., Scher H., Bellmunt J., Miller K., Mulders P., Stenzl A., et al. (2014) Enzalutamide in European and North American men participating in the AFFIRM trial. BJU Int [Epub ahead of print]. DOI: 10.1111/bju.12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottet N., Bastian P., Bellmunt J., van den Berg R., Bolla M., van Casteren N., et al. (2014) EAU Guidelines on prostate cancer, European Association of Urology, Arnhem, The Netherlands: Available at: http://www.uroweb.org/gls/pdf/1607%20Prostate%20Cancer_LRV3.pdf. [Google Scholar]
- Nakazawa M., Antonarakis E., Luo J. (2014) Androgen receptor splice variants in the era of enzalutamide and abiraterone. Horm Cancer 5: 265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCCN (2014) NCCN guidelines for the treatment of prostate cancer 2014, National Comprehensive Cancer Network, Washington DC: Available at: http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. [Google Scholar]
- Noonan K., North S., Bitting R., Armstrong A., Ellard S., Chi K. (2013) Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann Oncol 24: 1802–1807. [DOI] [PubMed] [Google Scholar]
- Ohlsson C., Borjesson A., Vandenput L. (2012) Sex steroids and bone health in men. Bonekey Rep 1: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R. (2014) Prostate cancer: an enzalutamide antiandrogen withdrawal syndrome. Nat Rev Urol 11: 366. [DOI] [PubMed] [Google Scholar]
- Rathkopf D., Smith M., de Bono J., Logothetis C., Shore N., de S., et al. (2014) Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol [Epub ahead of print]. DOI: 10.1016/j.eururo.2014.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Vida A., Bianchini D., Van Hemelrijck M., Hughes S., Malik Z., Powles T., et al. (2014) Is there an anti-androgen withdrawal syndrome with enzalutamide? BJU Int [Epub ahead of print]. DOI: 10.1111/bju.12826. [DOI] [PubMed] [Google Scholar]
- Saad F. (2013) Evidence for the efficacy of enzalutamide in postchemotherapy metastatic castrate-resistant prostate cancer. Ther Adv Urol 5: 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad F., de Bono J., Shore N., Fizazi K., Loriot Y., Hirmand M., et al. (2014) Efficacy outcomes by baseline prostate-specific antigen (PSA) quartile in the AFFIRM trial. Eur Urol [Epub ahead of print]. DOI: 10.1016/j.eururo.2014.08.025. [DOI] [PubMed] [Google Scholar]
- Scher H., Beer T., Higano C., Anand A., Taplin M., Efstathiou E., et al. (2010) Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet 375: 1437–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher H., Fizazi K., Saad F., Taplin M., Sternberg C., Miller K., et al. (2012) Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 367: 1187–1197. [DOI] [PubMed] [Google Scholar]
- Schmid S., Geith A., Boker A., Tauber R., Seitz A., Kuczyk M., et al. (2014) Enzalutamide after docetaxel and abiraterone therapy in metastatic castration-resistant prostate cancer. Adv Ther 31: 234–241. [DOI] [PubMed] [Google Scholar]
- Schrader A., Boegemann M., Ohlmann C., Schnoeller T., Krabbe L., Hajili T., et al. (2014) Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur Urol 65: 30–36. [DOI] [PubMed] [Google Scholar]
- Sieber P. (2014) Emerging therapeutic for the treatment of skeletal-related events associated with metastatic castrate-resistant prostate cancer. Rev Urol 16: 10–20. [PMC free article] [PubMed] [Google Scholar]
- Smith M., Goode M., Zietman A., McGovern F., Lee H., Finkelstein J. (2004) Bicalutamide monotherapy versus leuprolide monotherapy for prostate cancer: effects on bone mineral density and body composition. J Clin Oncol 22: 2546–2553. [DOI] [PubMed] [Google Scholar]
- Sternberg C., de Bono J., Chi K., Fizazi K., Mulders P., Cerbone L., et al. (2014a) Improved outcomes in elderly patients with metastatic castration-resistant prostate cancer treated with the androgen receptor inhibitor enzalutamide: results from the phase III AFFIRM trial. Ann Oncol 25: 429–434. [DOI] [PubMed] [Google Scholar]
- Sternberg C., Petrylak D., Madan R., Parker C. (2014b) Progress in the treatment of advanced prostate cancer. Am Soc Clin Oncol Educ Book 117–131. [DOI] [PubMed] [Google Scholar]
- Sweeney C., Chen Y., Carducci M., Liu G., Jarrard D., Eisenberger M., et al. (2014) Impact on overall survival (OS) with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer (mPrCa): an ECOG-led phase III randomized trial. J Clin Oncol 32 (suppl): 5s (abstr LBA2). [Google Scholar]
- Thomsen F., Røder M., Rathenborg P., Brasso K., Borre M., Iversen P. (2014) Enzalutamide treatment in patients with metastatic castration-resistant prostate cancer progressing after chemotherapy and abiraterone acetate. Scand J Urol 48: 268–275. [DOI] [PubMed] [Google Scholar]
- Tombal B., Borre M., Rathenborg P., Werbrouck P., Van Poppel H., Heidenreich A., et al. (2014) Enzalutamide monotherapy in hormone-naive prostate cancer: primary analysis of an open-label, single-arm, phase 2 study. Lancet Oncol 15: 592–600. [DOI] [PubMed] [Google Scholar]
- Tran C., Ouk S., Clegg N., Chen Y., Watson P., Arora V., et al. (2009) Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324: 787–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Klot C., Kramer M., Böker A., Herrmann T., Peters I., Kuczyk M., et al. (2014a) Is there an anti-androgen withdrawal syndrome for enzalutamide? World J Urol 32: 1171–1176. [DOI] [PubMed] [Google Scholar]
- von Klot C., Kuczyk M., Merseburger A. (2014b) No androgen withdrawal syndrome for enzalutamide: a report of disease dynamics in the postchemotherapy setting. Eur Urol 65: 258–259. [DOI] [PubMed] [Google Scholar]