Abstract
Arsenic is a prevalent contaminant at a large number of US Superfund sites; establishing techniques that accelerate As remediation could benefit many sites. Hundreds of tons of As were released into the environment by the Vineland Chemical Co. in southern New Jersey during its manufacturing lifetime (1949–1994), resulting in extensive contamination of surface and subsurface soils and sediments, groundwater, and the downstream watershed. Despite substantial intervention at this Superfund site, sufficient aquifer cleanup could require many decades if based on traditional pump and treat technologies only. Laboratory column experiments have suggested that oxalic acid addition to contaminated aquifer solids could promote significant As release from the solid phase. To evaluate the potential of chemical additions to increase As release in situ and boost treatment efficiency, a forced gradient pilot scale study was conducted on the Vineland site. During spring/summer 2009, oxalic acid and bromide tracer were injected into a small portion (~50 m2) of the site for 3 months. Groundwater samples indicate that introduction of oxalic acid led to increased As release. Between 2.9 and 3.6 kg of As were removed from the sampled wells as a result of the oxalic acid treatment during the 3-month injection. A comparison of As concentrations on sediment cores collected before and after treatment and analyzed using X-ray fluorescence spectroscopy suggested reduction in As concentrations of ~36% (median difference) to 48% (mean difference). While further study is necessary, the addition of oxalic acid shows potential for accelerating treatment of a highly contaminated site and decreasing the As remediation time-scale.
1. Introduction
1.1 Background
Arsenic is a common contaminant in the environment, present at hundreds of US Superfund sites.[1, 2] One of the most frequently used and widely- accepted technologies at sites with contaminated groundwater is pump and treat (P&T) remediation.[3, 4] Of the approximately 78 As contaminated sites that were in the design phase or were actively being remediated as of October 2006, approximately 58% of the sites listed P&T remediation as part of their clean up plan.[5] However, P&T remediation can be costly. Additionally, remediation of As sites via P&T can require extended periods of treatment to reach As cleanup goals, at least in part, due to limitations on desorption of As from iron and aluminum (hydr)oxides in sediments.[4, 6] It may be possible to accelerate As release and improve remediation efficiency at sites using P&T technologies by making judicious chemical additions. Laboratory column experiments suggest that introducing a 10 mM oxalic acid solution can release ~88% of the As from the contaminated solids and similar release was seen at the grain scale in column experiments run in a synchrotron beamline while groundwater alone can release only 5% of the As.[7, 8] Here we extend these laboratory studies to a field setting and investigate the possibility of using oxalic acid to increase As mobilization and potentially accelerate remediation by P&T in a 3-month pilot scale study at the Vineland Chemical Co. Superfund site.
The US EPA (Environmental Protection Agency) estimates that there are over 700 P&T systems in operation at US Superfund sites.[5] However, a growing body of evidence indicates that P&T can have limited effectiveness for contaminant removal due to a process known as tailing [3, 4, 9, 10]. Pump and treat generally has two functions in a remediation plan: (1) to contain the contaminant plume by influencing the hydrology and (2) to remove contaminant from the aquifer and lower dissolved contaminant concentrations to acceptable levels [9, 10]. Through careful planning and well placement, the first objective can generally be reached. However, aquifer conditions and the geochemistry of the contaminant can lead to limitations for reaching the second objective. Arsenic mobility is often controlled by sorption-desorption processes, which can be influenced by pH, oxidation-reduction potential, presence of anions and ligands, microbial activity, and presence of binding sites on solid surfaces.[6, 11–16] At As contaminated sites using P&T, cleanup procedures may be prolonged by slow desorption of As from soil or sediment surface sites, resulting in decreases in contaminant removal over time (also known as tailing). Systems in which tailing is caused by chemical processes may be amenable to P&T enhancement by chemical additions.[4]
For As contaminated sites using P&T, additions of oxalic acid (C2H2O4·2H2O) have the potential to help accelerate As release from solids and thus improve P&T efficiency, defined as more As removed with each volume of groundwater pumped from the subsurface. Oxalic acid is a low molecular weight organic acid found in natural soil environments; typical concentrations in soil solutions are 0–50 μM, though concentrations up to 1 mM have been reported.[17–19] Oxalic acid is involved in the vertical transport of Al and Fe through soils; sources of organic acids (including oxalic acid) in soil solutions include decay of plant materials, exudation from plant roots, and microbial degradation.[19, 20] Studies suggest that dissolution of Al and Fe species by organic acids leads to As release or that there is competitive sorption between organic acids and As (or a combination of the two processes play a role).[8, 21–23] Oxalic acid is often included in sequential soil extraction schemes investigating As phases. [24–27] Additionally, studies have shown As release in the presence of oxalic acid [7, 8, 22, 28, 29] and inhibition of As sorption in the presence of oxalic acid under certain conditions.[21]
In this work, we examine the possibility of using oxalic acid in a field setting to increase release of As from contaminated aquifer solids and potentially improve P&T remediation. A small pilot scale study was conducted at the Vineland Chemical Co. Superfund site in which two different concentrations of oxalic acid were introduced to a small portion of the study site over the course of three months. Groundwater samples were taken throughout the pilot study to evaluate oxalate concentrations and As release at a monitoring well and P&T recovery well and to assess the potential for using chemical injection methods in a field setting.
1.2 Site Overview
The Vineland Chemical Co. Superfund site is located in Southern New Jersey, USA. The site is underlain by the unconsolidated sands of the Cohansey Formation. Between 1949 and 1994, Vineland Chemical manufactured As-based biocides, predominantly monosodium acid methanearsonate (MSMA) and disodium acid methanearsonate (DSMA), which are sodium salts of monomethyl arsonic acid (MMA).[30] Waste salts including up to 1–2% As were stored in open piles and in abandoned chicken coops on site prior to 1978. These poor chemical storage and disposal practices led to the release of hundreds of tons of As into the environment.[31] The groundwater and sandy subsurface materials of the site were contaminated with high levels of organic and inorganic As species. Groundwater concentrations of total As exceeded 10,000 μg/L before remediation began; the US drinking water standard is currently set at 10 μg/L.[32] Additionally, discharge of contaminated water into an adjacent stream, Blackwater Branch, resulted in impacts on the flood plain soils as well as bottom sediments of the Maurice River and Union Lake further downstream.[31, 33]
A large-scale cleanup operation onsite has included pump and treat (P&T) for managing groundwater contamination as well as a soil washing plant for the unsaturated zone sediments in the “hot” zone (most contaminated region of the site) and flood plain areas. The P&T system consists of 16 extraction wells situated around the site. Up to 2 million gallons (7.5 million liters) of contaminated water are extracted from the subsurface each day and cleaned at the treatment facility; treated water ([As]<10 μg/L) has been currently discharged to the Blackwater Branch.[34] Contaminated unsaturated zone sands in the former “hot” zone had a maximum of >500 mg As/kg; soil washing procedures decreased soil As concentrations to <20 mg/kg. Offsite samples suggest that a representative background As concentration of the aquifer sediments is less than 5 mg/kg (average As concentration = 0.7 mg/kg, standard deviation = 0.5 mg/kg, N=3) as measured by digestion and ICP MS analysis.[7] Additionally, only unsaturated zone sediments and the top layer of aquifer solids were excavated (maximum excavation depth of ~4.9–5.6 m below ground surface) and treated at the soil washing plant. Aquifer materials below this depth can still have elevated As (typically 20–250 mg/kg), making the aquifer solids a reservoir of As that can continue to contaminate the groundwater. Measurements made in 2010 at the P&T recovery wells showed groundwater As concentrations on site could still be several hundred μg/L. A Classification Exception Area-Well Restriction Area was established by the US EPA to prohibit groundwater well installation and protect human health.[35]
2. Methods
2.1 Hydrogeological modeling
Groundwater numerical modeling was performed prior to well installation and prior to the oxalic acid injection experiment to help determine optimal well placement, dilution factors, and other hydrological parameters.[36]
2.2 Field methods
2.2.1 Well installation
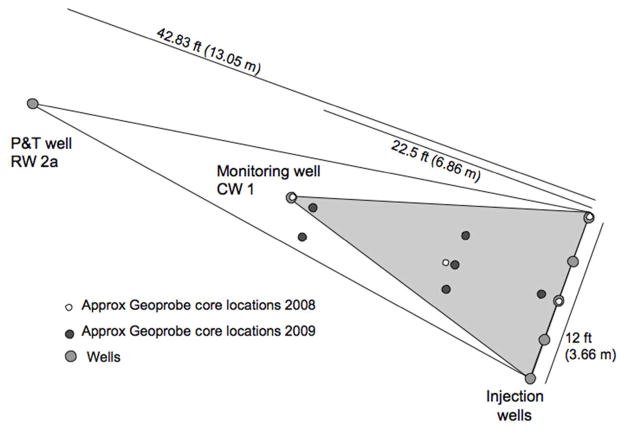
Injection and monitoring wells were installed within the cone of depression of an existing well (RW 2a) on the Vineland site (Figure 1a). There were two primary reasons for installing the wells within the cone of depression: the pump and treat (P&T) recovery well focuses water toward it creating a forced gradient, therefore, (1) simplifying the groundwater flow regime in that area and (2) ensuring that any chemicals injected and any As mobilized should be captured by the combination of the monitoring well and the high-volume P&T recovery well. The injection wells and monitoring wells were installed by SGS Environmental (West Creek, NJ) in June 2008. Prior to well installation sediment cores were obtained using direct push methods (Macro-Core© Soil Sampling tools with 2.25 inch [5.715 cm] OD sampler tube, Geoprobe, Salina, KS) with a maximum depth of 40 ft (12.2 m) below ground surface (bgs); cores taken at the end of the oxalic acid injection experiment were obtained by the same method and had maximum depths of 50 ft (15.2 m) bgs. Five injection well nests (CW 2-CW 6) with three 1 inch (2.54 cm) wells per nest were installed for a total of 15 injection wells. In each well nest, the wells were screened at 27–28 ft (8.2–8.5 m), 29–30 ft (8.8–9.1 m), and 31–32 ft (9.4–9.8 m) bgs; the water table is approximately 15 ft (4.6 m) bgs. One 2 inch (5.08 cm) monitoring well (referred to as CW 1 or monitoring well) was also installed; this well was screened at 27–40 ft (8.2–12.2 m) bgs. The nearby P&T recovery well (RW 2a) is screened 25–45 ft (7.6–13.7 m) bgs. The distance from injection wells to monitoring well (CW 1) was 22.5 ft (6.86 m) and from monitoring well to pump and treat well RW 2a was 20.33 ft (6.19 m) (Figure 1b). A 1.8 inch (4.57 cm) low pressure pneumatic well packer (Model 800, Solinst, Canada) was used to sample different parts of the monitoring well (CW 1) during initial tracer tests and the oxalic injection experiments.
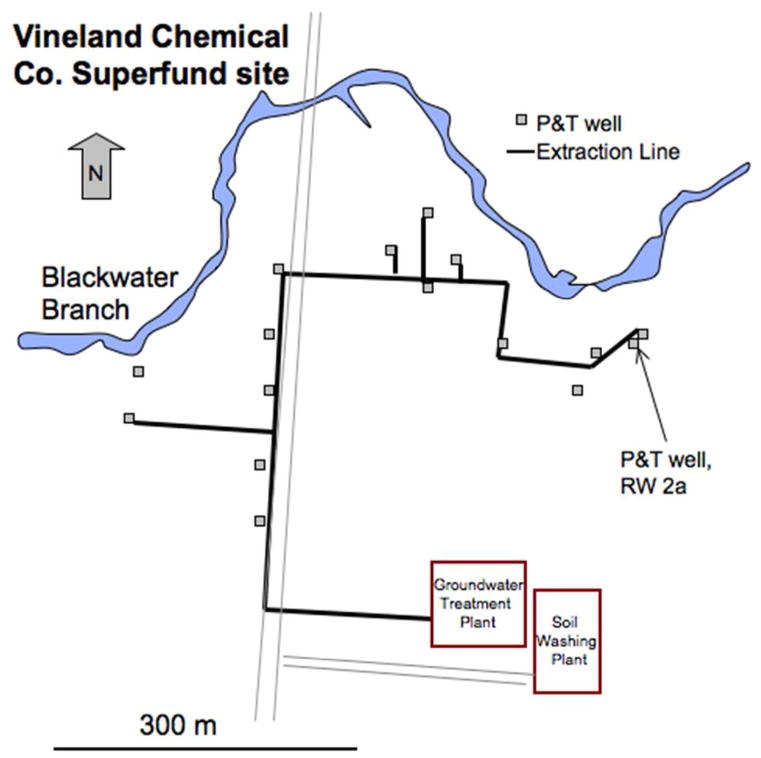
Figure 1.
Figure 1a. Simplified map of the Vineland Chemical Company site showing locations of pump and treat extraction wells and the extraction line as well as the groundwater treatment and soil washing facilities. The pump and treat well that was part the pilot study area, RW 2a, is noted on the map.
Figure 1b. The schematic shows a map view of the distances between wells and approximate Geoprobe locations for 2008 and 2009. The injection wells and monitoring well were installed in 2008.
2.2.2 Oxalic acid injection, April 3 – July 2, 2009
Prior to oxalic acid injection, a forced gradient tracer experiment using sodium bromide (NaBr) was performed in 2008 (June 24–July 2) to confirm that significant amounts of injected materials were captured in the monitoring well (CW 1) and to help calibrate the hydrological models.[36] The results of this experiment along with the associated hydrological modeling helped inform the design of the 2009 oxalic acid injection experiment.
Injection of oxalic acid and a bromide tracer was begun 4/4/09 and lasted 90 days and was performed in two phases; concentrations of influent solutions were approximately 100 mM for oxalic acid and 50 mg/L for bromide in Phase I (4/4–5/7/09, day 1–34) and approximately 350 mM for oxalic acid and 100 mg/L for bromide in Phase II (5/7–7/2/09, day 34–90) (Table 1). To make the influent solutions, oxalic acid and sodium bromide solids were mixed in 300-gallon (1136 L) polyethylene tanks (McMaster-Carr) with groundwater from a P&T recovery well (RW 2) outside of the pilot study area and screened deeper within the aquifer (54–74 ft or 16.5–22.6 m bgs) than the injection wells and monitoring well (CW 1) in the pilot area. The influent solutions were pumped from the tanks using a chemical metering pump (model C131-26S, LMI Milton Roy, Ivyland, PA) through an injection manifold system, which split the flow evenly to each of the 15 injection wells. During Phase I and Phase II total injection rates were 0.27 L/min and 0.56 L/min, respectively. Injection rates were small compared with the P&T extraction rates in the pilot area (the flow rate at P&T well RW 2a averaged 301 L/min). Details regarding the design and testing of the injection manifold system have been presented elsewhere.[37]
Table 1.
Influent concentrations and flow rates during the 2009 oxalic acid injection experiment
| BromideA Influent Concentration (mg/L)C | Oxalic AcidB Influent Concentration (mM)C | Injection flow rate (mL/min) | ||
|---|---|---|---|---|
|
| ||||
| Total | Per wellD | |||
| Phase I (4/4/09–5/7/09, day 1–34) | 48 ± 5 | 93 ± 11 | 270 | 18 |
| Phase II (5/7/09–7/2/09, day 34–90) | 92 ± 10 | 351 ± 30 | 560 | 37 |
Sodium bromide anhydrous, 99+%, Acros Organics
Oxalic acid dihydrate technical grade, 99.5%, Univar, Redmond, WA and Chemical Distributors, Inc., Buffalo, NY
Average and standard deviation of daily samples.
Total number of injection wells = 15
The monitoring well (CW 1) and P&T well (RW 2a) were sampled over the course of the 90 days of injection as well as 69 days after injection was stopped; samples were taken to monitor for tracer and oxalate breakthrough as well as changes in As, Fe, Al, and Mn concentrations and other groundwater parameters (pH, ORP, conductivity, etc – Supplemental Information Table S1). A packer was placed within the well screen for the duration of the experiment; the packer can be inflated to seal off portions of the well for targeted sampling. Samples at the monitoring well (CW 1) were taken above and below the inflatable packer to evaluate conditions both excluding the coarser layer and within the coarser layer (found at 36–38 ft or 11.0–11.6 m bgs at monitoring well CW 1). Water was continuously extracted from above the packer at the monitoring well (CW 1) while the oxalic and bromide influent solutions were being injected and for 13 days afterward; extraction was accomplished using a 12 V submersible plastic pump and low flow controller (Typhoon, Groundwater Essentials) and the extraction rate was kept as close to 2.5 L/min as possible. Samples taken beyond that time were obtained from the monitoring well (CW 1) after purging the well for at least 60 minutes at 2.5 L/min prior to sampling (more than 30 well volumes). A limited number of samples were taken below the packer to sample a coarser layer discovered at ~36–38 ft bgs (11.0–11.6 m) in the monitoring well (CW 1) and were obtained using a peristaltic pump (Master Flex Environmental Sampler, Cole Parmer) set to extract water at a rate of ~100 mL/min; the well was purged for at least 60 minutes before sampling (more than two well volumes below the packer). Flow rates at the P&T recovery well (RW 2a) averaged 301 L/min during the 2009 oxalic acid injection experiment.
Daily influent samples were taken and tested for oxalic acid, bromide, and As concentration. Periodic samples were also taken at one of the injection wells (CW 3.2) using the peristaltic pump (and dedicated tubing).
Samples to be analyzed by magnetic sector inductively coupled plasma-mass spectrometry (ICP MS) for metal concentrations were filtered through 0.45 μm PES or PP filters (Whatman) into acid washed HDPE bottles and acidified to 1% acid with trace metal grade or better nitric acid. Samples to be analyzed for oxalate and bromide concentration by ion chromatography were filtered into HDPE bottles and kept cold (4°C or frozen).
2.3 Analytical techniques
2.3.1 Analysis of dissolved As, Fe, Al and Mn, oxalate, and bromide
Water samples were analyzed for As, Fe, Al, and Mn content using ICP MS with a magnetic sector Single Collector instrument (Axiom, Thermo Elemental, Germany) using previously published methods.[7]
Water samples were analyzed for oxalate and bromide concentrations using a Dionex ICS-2000 (Sunnyvale, CA) ion chromatography system run in gradient mode with an IonPac AS-11 HC column. Four to five point calibration curves were used for quantification and were analyzed throughout each batch of samples; calibration curves used for analysis had R2>0.98. Replicate standards analyzed with each batch had %RSD of less than 10%. Field measurements for bromide were also made using a bromide specific electrode (Orion, Thermo Scientific, Waltham, MA).
2.3.2 Analysis of As, Fe, and Al on solids
Arsenic, Fe, and Al concentrations on the sediment core materials were determined by X-ray fluorescence (XRF) spectroscopy with a Spectro Xepos desktop instrument (Spectro Analytical Instruments, Germany). Samples were sieved to remove particles >2 mm (greater than sand sized) and powdered using an Angstrom TE-110 shatterbox (Angstrom, Inc; IL). Larger particles were removed because As likely exists as coatings on the solids and including larger particles could create a low As concentration bias in the results. A quality control sample was measured after every 11 unknown samples; percent relative standard deviation for these replicate analyses were 10% or less for all elements of interest and typically less than 5%. The limit of quantitation (LOQ) was estimated to be 0.7 ppm for As, 70 ppm for Al, and less than 50 ppm for Fe. Calibration curves specific to these powdered sands were created by performing complete digestion procedures on a subset of the powdered, sieved samples. Oven dried samples were digested using nitric, perchloric, and hydrofluoric acids and analyzed for total As, Fe, Al, and Mn concentration by ICP MS.[38] Total As concentrations were corrected for recovery of a standard reference material (18% difference between certified and calculated digest values for the reference material). These values were then used to update the factory supplied XRF calibration; calibrating the powdered Vineland samples this way corrects for matrix and particle size effects. The same calibration was used for samples collected before and after oxalic acid treatment.
2.4 Safety precautions
Oxalic acid exposure can lead to burns of the skin, respiratory system and eyes and can cause kidney damage.[39] Use of proper personal protective equipment is important when making up influent solutions. Arsine gas is a toxic gas that can form in the presence of As, acid, and a strong reductant.[40] We did not expect to see arsine gas under the conditions present in this experiment and indeed saw no indication of arsine gas formation using arsine sensitive test strips. Portable arsine gas sensors and dosimeter badges can also be used to monitor for arsine gas formation. Further information regarding the health effects of arsine gas can be found through the Agency for Toxic Substances and Disease Registry.[41]
3. Results and discussion
3.1 Overview
Oxalic acid treatment led to an increase in dissolved As concentration at both the monitoring well (CW 1) and the pump and treat well (RW 2a), and thus successfully increased arsenic mobilization. Arsenic mobilization increased with both levels of oxalic acid treatment (~100 mM and ~350 mM) at the monitoring well (CW 1) but only with the higher concentration treatment at the pump and treat well (RW 2a). Approximately 3 kg of As (conservatively) was removed from the pilot area as a result of the 3-month oxalic acid treatment. Based on XRF data from core samples before and after treatment, As concentrations decreased 36–48% on the sediments following the oxalic acid injection experiment, again showing that As was removed from the system as a result of the oxalic acid treatment. The data suggest that the bromide tracer was completely recovered by the combination of the monitoring (CW 1) and pump and treat (RW 2a) wells and only 60% of the oxalic acid was recovered.
3.2 Bromide and oxalic acid recovery during the 2009 oxalic acid injection experiment
Hydrological modeling had suggested approximately 14-fold dilution of a conservative tracer (like bromide) from the injection wells to monitoring well (CW 1).[36] During Phase I of the oxalic acid injection, this would translate to a concentration at the monitoring well (CW 1) of 3.4 mM bromide and 6.5 mM oxalic acid (if oxalic acid were to also behave conservatively, which is not expected due to the possibility of reaction, precipitation, adsorption, and/or degradation). Actual dilution was greater than expected. For instance, the Phase I plateau values represent a 35-fold and 44-fold dilution of the injected bromide and oxalic acid, respectively, at monitoring well CW 1 (Table 2, Figure S1). Dilutions at the pump and treat well (RW 2a) were significantly greater, with at least 900x dilution for oxalic acid and ~360-fold dilution for bromide during Phase II (Figure S2), however, flow rates at RW 2a were also significantly greater (301 L/min vs. 2.5 L/min at monitoring well CW 1). Furthermore, pump and treat well RW 2a is at the center of the cone of depression and receives water from all sides, not just the pilot area.
Table 2.
Summary of bromide and oxalic acid concentrations and recoveries during oxalic acid injection experiment. Data are presented graphically in Figures S1 and S2.
| Bromide (mg/L) | Oxalic Acid (mM) | |
|---|---|---|
| Monitoring well, CW 1A
| ||
| Start | ND | <0.02 |
| Phase I plateau | 1.4 (4/15–5/9/09, day 12–36)) | 2.1 (4/26–5/9/09, day 23–36) |
| Phase II plateau | No plateau reached | 11.9 (5/18–5/23/09, day 45–50) |
| End | ND | <0.02 |
|
| ||
| P&T recovery well, RW 2a
| ||
| Start | ND | <0.01 |
| Phase I plateau | <0.1B | 0.06 (4/23–5/8/09, day 20–35) |
| Phase II plateau | 0.15 (5/16–6/1/09, day 43–59) C | 0.38 (5/15–6/1/09, day 42–59) |
| 0.25 (6/15–7/10/09, day 73–98) | ||
| End | ND | <0.01 |
| % Recovery | Bromide (%) | Oxalic Acid (%) |
|---|---|---|
| Monitoring well, CW 1 | 15% | 13% |
| P&T recovery well, RW 2a | 90–125% D | 46% |
| Total | 105–140% | 59% |
ND=non detect
Monitoring well, CW 1 above packer
Concentrations at P&T well RW 2a <0.1 mg/L bromide during Phase I except for a few concentration spikes, max = 0.27 mg/L.
Two plateaus were observed. The pump and treat system was shutdown between 6/1/09 and 6/5/09 (day 59–63). This impacted the groundwater flow regime and thus the concentrations at the wells.
Recovery dependent on whether lower limit cut off value applied in calculations.
The data suggest that complete capture of bromide was likely through the combined efforts of the monitoring well (CW 1) and the P&T well (RW 2a) (Table 2). Approximately 15% of the injected bromide was captured at the monitoring well (CW 1) and approximately 101% was captured at the P&T well (RW 2a), for a total of 116% bromide recovery. The percentage bromide recovery for each well was estimated using trapezoidal integration to calculate the area under the concentration vs. volume curve (to equal mass bromide captured) and comparing with the mass of bromide injected. Volumes of water collected at each well, therefore, controls recoveries. Although bromide concentrations were lower at the P&T well (RW 2a), the volume of water collected was much greater and the recovery at the P&T well (RW 2a) was much higher than the monitoring well (CW 1). The excess bromide at P&T well RW 2a may be related to measurement uncertainty since the bromide concentrations at the P&T well tended to be at the very low end or below the calibrated range. Here we restricted the recovery calculation to exclude concentration values less than half of the lowest standard (i.e., <0.15 mg/L) which led to 116% recovery between the two wells.
While complete capture of bromide was likely, approximately 59% of the injected oxalic acid was recovered by the combination of the two wells (~13% at monitoring well CW 1 and ~46% was captured at P&T well RW 2a). It is possible, therefore, that the remaining oxalate was adsorbed to the aquifer solids, precipitated as insoluble oxalate salts (e.g., calcium oxalate) at the fringes of the oxalic acid plume, or was degraded by microbial activity. Concentrations of oxalate at the monitoring well (CW 1) decreased and remained below 0.05 mM by 7/20/09 and the pH increased to greater than 6 (oxalic acid injection was stopped 7/2/09, 18 days prior), indicating that adsorption and re-release of oxalate may be limited. Adsorption and degradation or just degradation by microbes seems to be the most plausible explanation, with the possibility of some salt precipitation as well. Both aerobic and anaerobic bacteria have been identified that can utilize oxalate for metabolic activities, and it is believed that biodegradation may play a role in removal of organic acids from soil solutions in various ecosystems.[20, 42]
3.3 Dissolved As, Fe, and Al concentrations during the 2009 oxalic acid injection experiment
3.3.1 Influent solutions
Daily influent samples were taken throughout the injection experiment (Table 3). Samples were obtained from the tank connected to the injection pump.
Table 3.
Average ± standard deviation for influent concentrations
| As (μg/L) | Fe (μg/L) | Al (μg/L) | |
|---|---|---|---|
| Influent | 358 ± 17 | 1240 ± 990 | <150 |
3.2.2 Monitoring well, CW 1
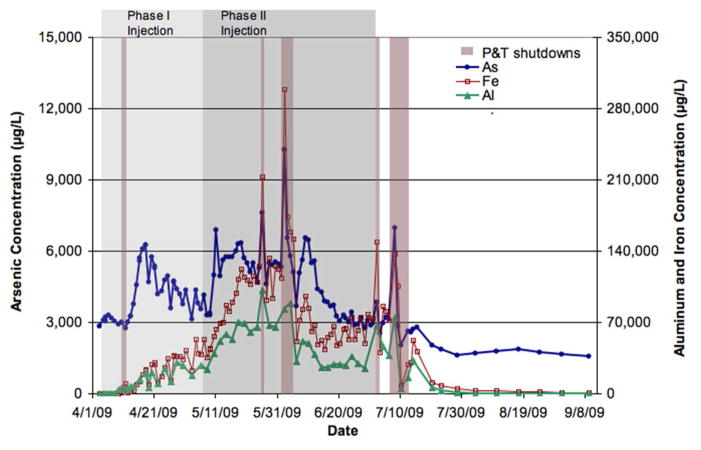
It was clear from the 2008 tracer test that the presence of bromide in the subsurface did not impact groundwater As, Fe, or Al concentrations as there was no systematic or significant change in those concentrations at any of the wells tested.[36] During the 2009 oxalic acid injection experiment, As concentrations began at 3080 ± 150 μg/L (mean ± 1 standard deviation; 4/4/09–4/9/09, days 1–5) and increased at the monitoring well (CW 1) after the introduction of oxalic acid, reaching a maximum of 6250 μg/L in the first phase of oxalic acid injection after which As decreased (Figure 2). Despite continued input of ~100 mM oxalic acid, As concentrations began to decrease. One explanation for the decrease could be related to the pH distribution within the pilot study area during the first phase of oxalic acid injection. The pH at the monitoring well (CW 1) decreased during Phase I of the oxalic acid injection but did not fall below 4.5 (Supplemental Information, Figure S3). Previous research has shown that the optimal pH for Fe release by oxalic acid is 2–3, at which point the dominant form of oxalic acid would be HC2O4−.[43, 44] If As mobilization is related to Fe release as suggested in several studies [22, 28, 45], then low pH would be needed for optimal As release as well. The pH in the aquifer near the injection wells may have been low enough for efficient As release in this portion of the pilot area. However, minor amounts of reactive components within the sandy sediments closer to the monitoring well (CW 1) may have continued to buffer the solution keeping the pH at the monitoring well 4.5 or greater during oxalic acid injection Phase I (Supplemental Information). Therefore, there may have been some re-precipitation or adsorption closer to the monitoring well (CW 1) because of decreased oxalate concentration (dilution along the flow path) and slightly higher pH (buffer capacity not exceeded in the sediments closer to the monitoring well). There was evidence of Fe precipitation in the pump tubing (red/orange staining and precipitate) during the first phase of oxalic acid injection; this precipitation of Fe could generate As sorption sites and result in further removal of As from solution.[14, 15, 46–48] Additionally, it is possible that the first phase of oxalic acid injection may have moved most or all of the As that is easily mobilized or available at that oxalic acid level from the low pH region. Since the pH later in the flow path was still not low enough for optimum release, overall As release, as monitored at CW 1, declined. Therefore, it is important to keep the pH low and the concentration of oxalate high enough in order to maximize As release and transport and minimize re-precipitation/sorption.
Figure 2.
Concentrations of Al, Fe, and As over the course of the oxalic acid injection experiment at the monitoring well, CW 1, above the packer (and therefore excluding the coarser layer). The injection concentrations for oxalic acid influent were ~100 mM in Phase I and ~350 mM in Phase II.
Approximately three days following introduction of the higher oxalic acid influent (~350 mM), As concentrations increased to 5000 μg/L (Figure 2) at which point the pH measured in the monitoring well (CW 1) had dropped to 3.3; the next day the As concentrations had increased further to 6900 μg/L and the pH fell to 2.5. Additionally, the pump tubing became clear of Fe precipitates coincident with the decreased pH and increased oxalate concentration. Arsenic increased concurrently with the pH decrease, supporting the idea that maintaining a low pH in the system is important for maintaining As release. However, we do not believe that the pH alone is responsible for As release, i.e., the release mechanisms are not solely proton-promoted. In batch laboratory extractions, simply acidifying the system with an inorganic acid such as hydrochloric or nitric acids resulted in less As mobilization than the same concentration and similar pH of oxalic acid;[36] for instance, depending on extraction time 1 mM HCl mobilized 5–6% of the As from aquifer solids vs. 41–56% with 1 mM oxalic acid and 10 mM HCl mobilized 11–45% of As vs. 80–93% with 10 mM oxalic acid (Supplemental Information, Sediment Extractions and Figure S5).
During the second phase of oxalic acid injection, there was a more sustained increase in As concentration than in the first phase, with concentrations averaging 5780 μg/L between 5/11/09 and 6/12/09 (days 38–70). There was some variation in As concentration in large part due to P&T plant shutdowns, which disrupted the forced gradient flow in the subsurface. However, even though the injection of oxalic acid was continued until 7/2/09 (day 90), the As concentrations began to decrease on 6/12/09 (day 70). This decrease could indicate that most or all of the As that could be mobilized with that level of oxalic acid had been removed from the system. This decrease was also seen in laboratory column studies with 10 mM oxalic acid treatment; a large pulse of As was released from the column with effluent As concentrations decreasing after reaching a peak of 100 mg/L and >40 % of the As had been removed from the solids.[7]
Fe and Al showed steady increases in concentration at the monitoring well (CW 1) during Phase I of the oxalic acid injection, despite evidence of Fe precipitation in the pump tubing (Figure 2). During Phase II of the injection, there was a sharper increase in Fe and Al concentrations due to the lower pH value and higher oxalate concentration. Similar to As, concentrations of Fe and Al decreased before the oxalic acid injection was stopped. The decline in Fe and Al concentrations indicates the possibility that most of the oxalate leachable Fe and Al had already been released. The Fe and Al concentrations remained above their background levels at the end of sampling (9/9/09, day 159), which could indicate greater retardation in Fe and Al transport than As.
At the monitoring well (CW 1), As concentrations averaged 3080 μg/L before oxalic acid treatment and concentrations showed an overall decrease of 45% after oxalic acid injections with an average of 1700 μg/L. This marked decrease in As concentration indicates that As was moved out of the system as a result of the oxalic acid treatment. Further decrease in As concentration following the oxalic acid treatment may have been prevented because upgradient water coming into the pilot study area already had elevated As concentrations, which would not be impacted by the oxalic acid injection. For instance, As concentrations at one of the injection wells could be >8000 μg/L. A total of 0.5 kg of As (above initial background levels) were captured at the monitoring well (CW 1) during the 2009 oxalic acid injection experiment.
3.3.3 Pump and treat well, RW 2a
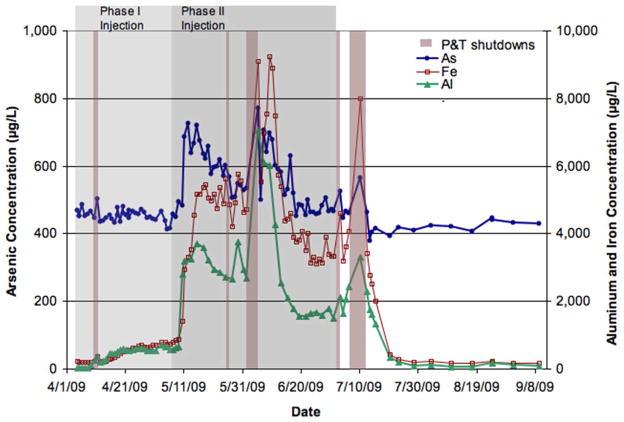
At the pump and treat well (RW 2a) As concentrations did not increase significantly or systematically in the first phase of oxalic acid injection; the As concentration averaged 454 ± 18 μg/L (mean ± 1 standard deviation) between 4/4/09–5/8/09 (day 1–35). The absence of change could be caused by a combination of dilution and Fe precipitation; dilution of oxalate would impact the ability to maintain Fe and As in solution.
In the second phase of oxalic acid injection, the As concentration at the P&T well (RW 2a) increased to a maximum of ~726 μg/L on 5/12/09, day 39 (Figure 3). As with the monitoring well (CW 1), the increase in As occurred rapidly following the introduction of the higher oxalic acid influent may be due to a relatively rapid drop in aquifer pH as the buffer capacity of the solids was partly spent during the first phase of oxalic acid injection. This drop in pH could allow for re-dissolution of precipitated As, Fe, and Al as well as release of previously undissolved species. Perhaps mobilization would have been higher (and opportunities for re-precipitation and re-adsorption lower) if the pH at the P&T well (RW 2a) had been lower; the pH at the well averaged 3.4 during Phase II of the oxalic acid injection. The pH increased once injection was stopped (7/2/09, day 90), reached pre-injection levels by 7/15/09 (day 103), and stabilized over the next several weeks of sampling (Supplemental Information, Figure S4).
Figure 3.
Concentrations of Al, Fe, and As at the pump and treat recovery well (RW 2a) over the course of the oxalic acid injection experiment. The injection concentrations for oxalic acid influent were ~100 mM in Phase I and ~350 mM in Phase II.
Similar to what was seen in the monitoring well (CW 1), As, Fe, and Al concentrations at the pump and treat well (RW 2a) decreased before oxalic acid injection was stopped. The general decline was interrupted by peaks in concentration around 6/5/09 (day 63) and 7/10/09 (day 98), which resulted from changes to the forced gradient flow by P&T plant shutdowns and restarts. Again the decrease in concentrations despite input of oxalic acid could indicate that all of the As, Fe, and Al that could be mobilized at that level of oxalic acid had been mobilized. Arsenic, Fe, and Al concentrations at RW 2a track together during this time (6/5/09–7/10/09, days 63–98). Concentrations decreased consistently after 7/12/09 (day 100); the oxalic acid injection had been stopped by that time.
By the end of sampling, As and Fe concentrations had decreased below their initial values at the pump and treat well (RW 2a) while Al concentrations remained slightly elevated. Ending As and Fe concentrations averaged ~8% and 20% lower than initial averages, respectively. Though the As concentrations showed a slight decrease from before to after oxalic acid treatment, it is likely due to a background decline in As concentration at that well since it was turned on in 2006 (based on historical site data). Based on the slope of decline in As concentration at P&T well RW 2a between Jan 2008 and March 2009, an As concentration of 409 μg/L would be expected on 9/9/09 (day 159); it was measured as 428 μg/L. Since the capture zone of P&T well RW 2a is much larger than just the pilot study area and the well has such a high extraction rate (301 L/min), it may be difficult to see an oxalic acid prompted decline in As concentration at that well after the injection experiment. A total of 2.4 kg of As (above initial background levels) were captured at P&T well RW 2a during the 2009 oxalic acid injection experiment, for a total of 2.9 kg captured between the combination of the monitoring well (CW 1) and P&T well RW 2a. However, this mass of As would represent a conservative estimate since the background correction is based on the average initial As concentration. Data from P&T well RW 2a from Jan 2008–March 2009 suggest a gradual decrease in As concentrations within that well, therefore, the background correction should account for this gradual decrease. To estimate the declining background at P&T well RW 2a, we assumed an initial As value of 454 μg/L (average concentration 4/4/09–5/8/09, days 1–35), an ending As value of 417 μg/L (average concentration 7/13/09–9/9/09, days 101–159), and interpolated linearly in between; the mass of As captured at P&T well RW 2a as a result of the oxalic acid treatment was then calculated to be 3.1 kg, an increase of approximately 30% compared to the conservative estimate. The total quantity of As captured between the two wells would be 3.6 kg using these estimates. Although the overall concentrations of As at the P&T recovery well (RW 2a) were lower than in the monitoring well (CW 1), the majority of the mass of As was captured there due to the high volume of water pumped. It is clear from this data that oxalic acid treatment lead to mobilization of As at both the monitoring well (CW 1) and the P&T recovery well (RW 2a).
3.4 Effects of P&T plant shutdowns during the 2009 oxalic acid injection
There were several P&T plant shutdowns during the oxalic acid injection experiment (4/10–4/11/09, days 7–8 ; 5/25–5/26/09, days 52–53; 6/1–6/5/09, days 59–63; and 7/6–7/12/09, days 93–100 which was after the injection phases). Since the groundwater flow under the site is controlled by the pumping at the P&T wells, pumping well shutdowns change the flow regime in the subsurface; groundwater velocity in the pilot area decreases and flow directions may also change. Much of the high frequency variability in concentrations seen during the 2009 oxalic acid injection experiment could likely be explained by P&T plant shutdowns (Figures 2 and 3).
Aquifer tests performed at the sandy site in 2002 and 2003 evaluated recovery of groundwater elevations when pump and treat wells were turned off; the study indicated that even after 9 days full recovery was not attained at monitoring wells and static equilibrium conditions were not achieved, though groundwater elevations started to recover soon after pump and treat wells were shutdown.[49] Therefore, it is unlikely that the aquifer system fully recovered to non-pumping groundwater elevations during the short shutdowns (maximum of 6 days) experienced during the 2009 oxalic acid injection experiment, although changes to groundwater flow directions and a decrease in groundwater velocities would occur. These changes could also increase contact time between oxalic acid and sediments and expose sediments to oxalic acid that were not impacted under pumping conditions.
3.5 Arsenic concentrations on the solids and percent As mobilization from the aquifer solids
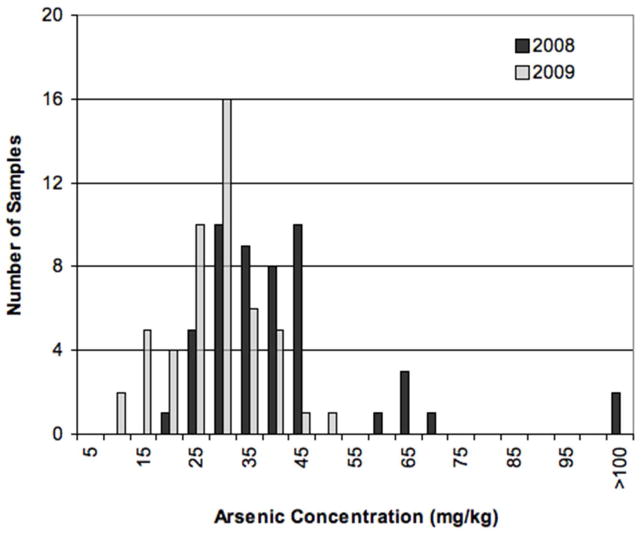
X-ray fluorescence spectroscopy (XRF) was used to evaluate As concentrations of sediment cores obtained in 2008 (prior to the oxalic acid injection) and in 2009 (at the end of the oxalic acid injection experiment). An XRF calibration specific to Vineland sands was created based on digestion of a subset of samples and As was adjusted for low recovery of a standard reference material. Pairs of data points (N=50 pairs) were compared for 2008 and 2009 sediments; pairs were matched for depth and location and covered depths of approximately 27–40 ft (8.2–12.2 m) bgs, the depth range of the screened region of the monitoring well (CW 1). Sediment samples were collected from three locations for these comparisons: at/near the injection wells, midway between injection and monitoring wells, and at/near the monitoring well (CW 1) in 5 ft (1.5 m) sections (see Figure 1). The sections were subsampled in roughly 20 cm increments. Following oxalic acid treatment, the solids over the 27–40 ft (8.2–12.2 m) depth range showed a decrease in As concentration by 28–36% depending on whether the median or mean values were used to make the comparison (Table 4, Figure 4). The two populations of samples, 2008 cores and 2009 cores, which were matched for similar location and depth, show a statistically significant decrease in As concentration (paired t-test, p<0.001) further verifying the efficacy of the oxalic acid treatment.
Table 4.
Arsenic concentrations and percent As removal.A Data is shown for matched pairs for the full depth range of the monitoring well (CW 1) or 27–40 ft (8.2–12.2 m) bgs, as well as depth sections 27–30 ft (8.2–9.1 m), 30–35 ft (9.1–10.7 m), 35–40 ft (10.7–12.2 m) and 30–40 ft (9.1–12.2 m).
| 2008 Cores (before oxalic acid treatment) | 2009 Cores (after oxalic acid treatment) | Percent Difference 2008 – 2009 (%)B | |
|---|---|---|---|
| 27–40 ft (8.2–12.2 m) bgs (N=50 pairs)C | |||
|
| |||
| Mean (mg/kg) | 40 | 26 | 36% |
| % RSD | 59% | 34% | |
| Median (mg/kg) | 35 | 25 | 28% |
|
| |||
| 27–30 ft (8.2–9.1 m) bgs (N=16 pairs)D | |||
|
| |||
| Mean (mg/kg) | 33 | 32 | 3% |
| % RSD | 18% | 23% | |
| Median (mg/kg) | 33 | 30 | 8% |
|
| |||
| 30–35 ft (9.1–10.7 m) bgs (N=15 pairs)E | |||
|
| |||
| Mean (mg/kg) | 52 | 25 | 52% |
| % RSD | 71% | 27% | |
| Median (mg/kg) | 42 | 25 | 40% |
|
| |||
| 35–40 ft (10.7–12.2 m) bgs (N=19 pairs)E | |||
|
| |||
| Mean (mg/kg) | 37 | 21 | 43% |
| % RSD | 40% | 39% | |
| Median (mg/kg) | 30 | 24 | 21% |
|
| |||
| 30–40 ft (9.1–12.2 m) bgs (N=34 pairs)F | |||
|
| |||
| Mean (mg/kg) | 44 | 23 | 48% |
| % RSD | 63% | 34% | |
| Median (mg/kg) | 38 | 24 | 36% |
These values are based on XRF data using a calibration specific to the Vineland sands and adjusted for low As recovery of a standard reference material in the digestion procedure.
Percent difference was calculated based on comparison between the average or median value for 2008 and 2009.
If all available data for the 27–40 ft (8.2–12.2 m) depth range are included, not just matched pairs, the mean and median values are nearly identical. For 2008 data, the mean and median values were 40 and 35 mg/kg, respectively (N=51); for 2009 data, the mean and median values were 25 and 25 mg/kg, respectively (N=80).
The matched pairs for this depth range are not statistically different (paired t-test, p>0.05).
The matched pairs for each of these depth ranges are statistically different (paired t-test, p<0.02).
The matched pairs for this depth range are statistically different (paired t-test, p<0.001).
Figure 4.
Histogram showing As concentrations on sediments in the pilot area in 2008 (before oxalic acid treatment) and 2009 (after oxalic acid treatment) in the depth range 27–40 ft (8.2–12.2 m) bgs. Only data with matched depth/location pairs for 2008–2009 are included. The x-axis displays the bins for each concentration; the bin marked 5 includes all values greater than 0 and up to 5, the bin marked 15 includes values greater than 10 and up to 15.
Additionally, the As data were grouped by depth; doing so indicates there was little As removal from the shallowest depths, 27–30 ft (8.2–9.1 m) bgs, compared with deeper samples, 30–35 ft (9.1–10.7 m) or 35–40 ft (10.7–12.2 m) bgs (Table 4). Combining data for the 30–40 ft depths (9.1–12.2 m), an As removal of 36–48% was calculated depending on whether the median or mean values, respectively, were used to make the comparison. Core samples deeper than 12.2 m or 40 ft (40–50 ft range) in 2009 had an average and median As concentration of 45 mg/kg and 37 mg/kg, respectively (N=45), values which are more similar to the average and median for 2008 samples (40 and 35 mg/kg) than 2009 samples (26 and 25 mg/kg) at 27–40 ft (8.2–12.2 m) bgs. These data may suggest limited oxalate exposure for sediments deeper than 40 ft (12.2 m). However, no direct comparison can be made to 2008 samples at the same depth range (40–50 ft or 12.2–15.2 m), as the maximum sampling depth in 2008 was 40 ft (12.2 m). X-ray absorption near edge structure (XANES) indicated that arsenate was the predominant form of As in the solids both before and after oxalic acid treatment (Supplemental Information, Table S2).
Aluminum and Fe concentrations were also examined by XRF. Following oxalic acid treatment, the solids over the 30–40 ft (9.1–12.2 m) depth range did not show a statistically significant change in Al (paired t-test, p>0.05). Fe concentrations decreased by 6–27% depending on whether the median or mean values, respectively, were used to make the comparison; this difference was statistically significant when the two populations of samples, 2008 cores and 2009 cores, were matched for similar location and depth and were compared (paired t-test, p<0.03). Extended X-ray absorption fine structure (EXAFS) data suggest that the Fe (III) oxides dominate, some of the oxides are removed with oxalic acid treatment, and there is no evidence for a major shift in Fe oxidation state (Supplemental Information, Figure S6).
Two methods can be used to quantify the percent As mobilized from the aquifer solids: (1) comparison of concentrations on the solids from cores taken before and after oxalic acid treatment in the zone thought to be impacted (as discussed above) and (2) comparison of mass of As removed with mass of As in the oxalic impacted aquifer (discussed below). Both methods ideally require knowledge of the volume of the aquifer impacted by the oxalic acid treatment. To help define the oxalic impacted area simple hydrological modeling was performed in preparation of the field experiment; these results suggested that the injected materials would be focused as they moved from injection wells to the monitoring well (CW 1) and P&T well (RW 2a) and that a pie-shaped geometry would result. [36] Models and methods like those used for delineating well head protection areas around a pumping well would have suggested a similar shape, something of a modified pie.[50] To define the oxalic impacted depth for this experiment, sediment core data was evaluated. Most of the As was mobilized from the 30–40 ft (9.1–12.2 m) depth range in this experiment (Table 4); therefore, the oxalic impacted depth was taken to be 10 ft (3 m). Using this impacted thickness, the dimensions of the pilot area between injection wells and P&T well RW 2a (12 ft × 42.83 ft or 3.7 m × 13.1 m), the bulk density (1.75 g/cm3), and the median starting As concentration on the solids of 35 mg/kg (27–40 ft or 8.2–12.2 m bgs) one can estimate the inventory of As in the targeted areas. The total mass of As in the pie wedge between the injection wells and P&T well RW 2a would be 4.4 kg. Therefore, using the conservative estimate of 2.9 kg total As mobilized by the oxalic acid treatment, 64% of the As in the aquifer solids of the pilot area would have been mobilized by the oxalic acid injection and captured by the monitoring well (CW 1) and the P&T well (RW 2a); 11% at the monitoring well (CW 1) and 53% at the P&T well (RW 2a). However, since the size and shape of the oxalic acid plume were not experimentally determined, alternate geometries should be considered. One could also imagine a rectangular section including aquifer solids between injection wells and the P&T well (RW 2a). Basing calculations for percent arsenic mobilized on these two shapes (pie and rectangle) should lead to upper and lower bounds for percent mobilization, respectively (Table 5). Between 33% (rectangular geometry) and 64% (pie geometry) of the As could have been mobilized from the aquifer solids and captured as a result of the oxalic acid injection using the conservative estimate. If instead we use the estimate for As mobilization at the P&T well (RW 2a) that accounts for the decreasing background concentration of As at that well (3.6 kg As mobilized and captured), the total percent As mobilized by the oxalic acid treatment would be calculated as 41–80% (Table 5).
Table 5.
Percent As mobilized during the 2009 oxalic acid injection experiment based on various geometries
| Conservative estimate for As mobilized | Estimate accounting for declining background [As] at RW 2a | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Mass As Captured (kg) | Mass As Captured (kg) | |||||
| Monitoring well, CW 1 | P&T well, RW 2a | Total | Monitoring well, CW 1 | P&T well, RW 2a | Total | |
|
|
||||||
| 0.5 | 2.4 | 2.9 | 0.5 | 3.1 | 3.6 | |
|
| ||||||
| GeometryA | Percent As Captured (%)B | Percent As Captured (%)B | ||||
| Monitoring well, CW 1 | P&T well, RW 2a | Total | Monitoring well, CW 1 | P&T well, RW 2a | Total | |
|
| ||||||
| Pie-shaped wedge | 11% | 53% | 64% | 11% | 69% | 80% |
| Rectangle | 6% | 27% | 33% | 6% | 35% | 41% |
Assumed 10 ft (3 m) depth section; both geometries include area between injection wells and P&T well RW 2a.
Starting concentration on soils taken as 35 mg/kg based on the median As value from 2008 sediment cores.
While As mobilization of 33–80% represents quite a large range, the lower value of 33% mobilization is still substantial considering the short time frame of the injection experiment (3 months). Additionally, 33% mobilization is consistent with comparisons of sediment concentrations on cores taken before and after the oxalic acid treatment (Table 4). The XRF data from the 2008 and 2009 Geoprobe cores suggested 36% (median) – 48% (mean) As removal (30–40 ft or 9.1–12.2 m bgs) (Table 4).
Similar As mobilization was also reported (~35–45%) for laboratory miniature column experiments (0.635 cm ID × ~6 cm long) using solids collected from one of the 2008 sediment cores and treated with oxalic acid in two phases with concentrations similar to those seen at the monitoring well (CW 1).[36] However, previous laboratory experiments involving larger columns (4.2 cm ID × ~23 cm) and aquifer sediment collected from the site in 2006 had shown 88% removal of As when treated with 10 mM oxalic acid.[7] One interpretation is that the Fe and Al in the 2008 sediment core and thus in the pilot area of the site may be more resistant to oxalic acid (and thus release less As) than in other areas of the site. However, the starting As concentration on sediments used for the larger column experiment was significantly higher (81 mg/kg) than for the miniature column (28 mg/kg). Arsenic removal of 35–45% brought the miniature column sediments down to 16–18 mg/kg; arsenic removal of 88% would decrease the large column sediments to ~10 mg/kg. Therefore, the final 10–20 mg/kg As may be difficult to remove from the sediments via oxalic acid treatment. It is clear, however, that the oxalic acid treatment was able to accelerate As release in each case.
To further assess the success of the 2009 oxalic acid injection experiment, one can compare the As removed as a result of the oxalic acid treatment with various estimates of As removed from the pilot area by the pump and treat system alone. For estimate 1, we compare the area of the full site with the area of the pilot study. Site managers had indicated that ~885 kg As were removed from the full site by the pump and treat plant per year. [51] The full site has an area of ~0.22 km2 [31] while the pilot experiment took place in an area of ~50 m2 (rectangular section between injection wells and pump and treat well RW 2a); the area of the pilot study is therefore 0.02% the area of the full site. If As removal is assumed to occur evenly across the site, then one would expect removal of approximately 0.2 kg As/yr or 0.05 kg over a three-month period from the pilot area. Introduction of oxalic acid to the pilot area, therefore, led to a significant increase in arsenic release, with 2.9–3.6 kg As mobilized by the injection experiment.
One can also approach this evaluation in another way. For estimate 2, we consider a simple circular capture zone area for pump and treat well RW 2a with a radius of ~40 ft (12.2 m), equivalent to the length between injection wells and pump and treat well RW 2a in our study. Within this circular capture zone (the zone from which all water will be captured by the pump and treat well), the pilot area with pie-shaped geometry would make up 4.5% of the RW 2a capture zone. (Due to the characteristics of the aquifer and the high pumping rate at P&T well RW 2a, this scenario would significantly underestimate the size of capture zone from which As is being mobilized and thus overestimates the fraction made up by the pilot area.) Based on the pump rate at P&T well RW 2a during the oxalic acid injection experiment (301 L/min), the average As concentration at that well prior to oxalic acid treatment (454 μg/L), and the fraction of the circular pump and treat well RW 2a capture zone represented by the pilot area (4.5%), one would estimate As removal of ~0.8 kg As in a three-month period from the pilot study area. The expected removal is much larger than the 0.05 kg calculated in estimate 1 above, both because As removal does not occur evenly across the site (as assumed in estimate 1) and we have overestimated the fraction of the pump and treat well (RW 2a) capture zone represented by the pilot area (in estimate 2). Since the 2009 oxalic acid injection experiment induced mobilization of ~3 kg (conservatively) from the pilot area with just three months of oxalic acid injection, the treatment represents a clear improvement in terms of As removal as compared to pump and treat alone.
3.6 Consideration of alternate flow regimes
Even though the subsurface sediments at this site appear to consist of fairly homogeneous medium sands it is important to consider unexpected flow paths due to local heterogeneities in physical and chemical properties of the solids; there may be plume sinkage, preferential flow paths, or other complicated flow regimes that do not conform to our simple geometries above and may limit the arsenic mobilization potential.
Since a coarser layer at 36–38 ft bgs (11.0–11.6 m) was discovered during installation of the monitoring well (CW 1) and another coarse section at 38–39 ft bgs (11.6–11.9 m) midway between injection and monitoring wells, samples were taken to evaluate the possibility for preferential flow through these more permeable regions. These coarser layers were deeper in the aquifer than injection wells (27–32 ft bgs or 8.2–9.8 m). The bromide data from the 2008 tracer test suggested that there was limited transport of injected materials through the coarser layer; average bromide concentrations from samples within the coarser layer were at least 7 times lower than samples excluding the coarser layer. However, samples obtained from the coarser layer during the 2009 oxalic acid injection experiment indicated that there was some transport of injected materials through this layer. Elevated oxalate and bromide were detected in the coarse layer; average concentrations were 11 mM and 4 mg/L, respectively, between 6/10/09 and 7/1/09 (days 68–89). These concentrations are similar to those seen in samples that excluded the coarser layer (average of 8 mM oxalate and 3 mg/L bromide for the same time frame). The transport of injected materials deeper into the aquifer during the 2009 injection experiment may have led to greater dilution of bromide and oxalic acid at the monitoring well (CW 1) than had been anticipated and thus lower concentrations of oxalic acid reaching target areas.
A number of factors may have influenced the discrepancy between 2008 tracer data and 2009 injection experiment data. Since the density of injected materials is greater than that of water, one might consider density induced plume sinkage.[52] However, this cannot be the primary explanation as the vertical change cannot be accounted for using realistic site values for porosity, hydraulic conductivity, groundwater transit time, and dilution factors. More important are differences in the duration and set up of the experiments. In the 2008 tracer test, tracers were introduced in a short (~3 h) pulse with sampling for ~9 days, while in the 2009 experiment the injection of oxalic acid and bromide influents and sampling took place over 3 months. The probability for alternate flow paths to become available increases with increasing experiment time. Additionally, injection rates were greater in 2009, but pumping rates at the monitoring well (CW 1) were lower, and the 2009 experiment was continued later into the summer season when heads may decline naturally; all of these could contribute to the differences.
Sediment cores obtained at the end of the 2009 oxalic acid injection experiment also suggest the possibility of preferential flow paths. Some cores show evidence for substantial oxalic acid leaching in small sections. In the most visually pronounced case, a sediment core appears “bleached” at 34 ft (10 m) bgs and has correspondingly low As concentrations, 2–7 mg/kg whereas the mean and median values for that core are 21 and 25 mg/kg, respectively. The lack of a uniform oxalic acid concentration front could have limited the mobilization potential in this pilot experiment.
Perhaps a different injection configuration could have helped overcome this issue of unexpected flow paths. For future oxalic acid injection studies, it will be important to better define the size, shape and extent of the oxalic acid plume using geophysical resistivity surveys both before and during oxalic acid injection. For large-scale use on a Superfund site, it is imperative to maximize the oxalic acid concentration to the desired areas while minimizing wasted oxalic acid. Therefore, chemical additions may be well suited to targeting small areas of the site where As concentrations are known to be high. Additionally, it would be important to do further pilot studies on any site (where different geochemical conditions could impact the way oxalic acid behaves in the subsurface) before large-scale implementation.
4. Conclusions
Oxalic acid solutions were injected into a small section (~50 m2) of an As contaminated Superfund site over the course of 3-months to evaluate its potential for As release in a field setting. The complete capture of co-injected bromide tracer was likely between the combination of the monitoring well (CW 1) and the pump and treat recovery well (RW 2a), however, only ~60% of the oxalic acid was recovered due to reaction, precipitation, adsorption to sediments and/or degradation by microbes in the subsurface. The oxalic acid treatment resulted in (1) increased As mobilization at the monitoring well (CW 1) during treatment with both concentrations of oxalic acid (~100 mM and ~350 mM), (2) increased As mobilization at the pump and treat well (RW 2a) during treatment with the higher concentration of oxalic acid, (3) a decrease in As concentration at the monitoring well following treatment, and (4) a conservative estimate of approximately 3 kg of As (33–64% of As inventory in the pilot area) removed from the system, which represents a substantial improvement as compared with pump and treat alone. Furthermore, after injections were stopped, pH values rebounded quickly suggesting no long term impact from oxalic acid injections. It was difficult to determine the shape and extent of the oxalic acid impacted portion of the aquifer, therefore, future studies should make use of geophysical measurements to help define the oxalic plume. While further study is certainly necessary if these methods are to be expanded at this or any other site, oxalic acid application shows promise for As release and therefore, potential for improving the efficiency of pump and treat remediation of As contaminated aquifers.
Supplementary Material
Environmental Context.
Arsenic is one of the most common contaminants at US Superfund sites; therefore, establishing techniques to accelerate As remediation could benefit many sites. In a pilot scale study, we determined that addition of oxalic acid to the subsurface has the potential to increase arsenic release from sediments and possibly improve remediation efficiency by pump and treat techniques. Since pump and treat remediation can require many decades to sufficiently decrease contaminant levels, methods for improving remediation could lead to substantial savings in time and resources.
Acknowledgments
The authors would like to thank the US EPA, the US Army Core of Engineers, and Sevenson Environmental at the Vineland Superfund Site for access to samples and for support on site. The authors also thank Alison Powell, Nathan Rollins, Patrick McNamara, Kamini Doobay and Bethany O’Shea for their assistance in the field and the lab. This work was supported by the Superfund Research Program (NIEHS Grant ES010349). Additional support was provided by NIEHS Grant ES0090890. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02-76SF00515. This is LDEO publication XXXX.
References
- 1.US Environmental Protection Agency. Arsenic treatment technologies for soil, waste, and waste, and water. 2002 http://www.clu-in.org/download/remed/542r02004/arsenic_report.pdf.
- 2.US Environmental Protection Agency. EPA Basic query for National Priorities List, 681 Superfund. US EPA; 2007. [Google Scholar]
- 3.Mackay DM, Cherry JA. Groundwater contamination: Pump-and-treat remediation. Environ Sci Technol. 1989;23(6):630–7. [Google Scholar]
- 4.Palmer CD, Fish W. Chemical enhancements to pump-and-treat remediation. EPA Ground Water Issue. 1992 http://www.epa.gov/ada/download/issue/chemen.pdf.
- 5.US Environmental Protection Agency. EPA basic query - Annual status report remediation database (data through Oct 2006) 2011 http://cfpub.epa.gov/asr/search.cfm.
- 6.Smedley PL, Kinniburgh DG. A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem. 2002;17:517–68. [Google Scholar]
- 7.Wovkulich K, Mailloux BJ, Lacko A, Keimowitz AR, Stute M, Simpson HJ, Chillrud SN. Chemical Treatments for Mobilizing Arsenic from Contaminated Aquifer Solids to Accelerate Remediation. Appl Geochem. 2010;25(10):1500–9. doi: 10.1016/j.apgeochem.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wovkulich K, Mailloux BJ, Bostick BC, Dong H, Bishop ME, Chillrud SN. Use of microfocused X-ray techniques to investigate mobilization of As by oxalic acid. Geochim Cosmochim Acta. 2012;91:254–70. doi: 10.1016/j.gca.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.EPA. Pump-and-Treat Ground-Water Remediation: A Guide for Decision Makers and Practitioners. Office of Research and Development; 1996. EPA/625/R-95/005. [Google Scholar]
- 10.Voudrias EA. Pump-and-treat remediation of groundwater contaminated by hazardous waste: Can it really be achieved? Global Nest Int J. 2001;3(1):1–10. [Google Scholar]
- 11.Ahmann D, Krumholz LR, Hemond HF, Lovley DR, Morel FMM. Microbial mobilization of arsenic from sediments of the Aberjona Watershed. Environ Sci Technol. 1997;31(10):2923–30. [Google Scholar]
- 12.Anawar HM, Akai J, Sakugawa H. Mobilization of arsenic from subsurface sediments by effect of bicarbonate ions in groundwater. Chemosphere. 2004;54(6):753–62. doi: 10.1016/j.chemosphere.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 13.Darland JE, Inskeep WP. Effects of pH and phosphate competition on the transport of arsenate. J Environ Qual. 1997;26(4):1133–9. [Google Scholar]
- 14.Dixit S, Hering JG. Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: Implications for arsenic mobility. Environ Sci Technol. 2003;37(18):4182–9. doi: 10.1021/es030309t. [DOI] [PubMed] [Google Scholar]
- 15.Jeong YR, Fan MH, Van Leeuwen J, Belczyk JF. Effect of competing solutes on arsenic(V) adsorption using iron and aluminum oxides. J Environ Sci China. 2007;19(8):910–9. doi: 10.1016/s1001-0742(07)60151-x. [DOI] [PubMed] [Google Scholar]
- 16.Mandal BK, Suzuki KT. Arsenic round the world: a review. Talanta. 2002;58:201–35. [PubMed] [Google Scholar]
- 17.Fox TR, Comerford NB. Low-molecular-weight organic-acids in selected forest soils of the southeastern USA. Soil Sci Soc Am J. 1990;54(4):1139–44. [Google Scholar]
- 18.Strobel BW. Influence of vegetation on low-molecular-weight carboxylic acids in soil solution - a review. Geoderma. 2001;99(3–4):169–98. [Google Scholar]
- 19.van Hees PAW, Lundstrom US, Giesler R. Low molecular weight organic acids and their Al-complexes in soil solution - composition, distribution and seasonal variation in three podzolized soils. Geoderma. 2000;94(2–4):173–200. [Google Scholar]
- 20.Jones DL. Organic acids in the rhizosphere - a cricial review. Plant Soil. 1998;205:25–44. [Google Scholar]
- 21.Shi R, Jia YF, Wang C, Shuhua Y. Mechanism of arsenate mobilization from goethite by aliphatic carboxylic acid. J Hazard Mater. 2009;163:1129–33. doi: 10.1016/j.jhazmat.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 22.Zhang SZ, Li W, Shan XQ, Lu AX, Zhou PJ. Effects of low molecular weight organic anions on the release of arsenite and arsenate from a contaminated soil. Water Air Soil Pollut. 2005;167(1–4):111–22. [Google Scholar]
- 23.Luo L, Zhang S, Shan XQ, Zhu YG. Effects of oxalate and humic acid on arsenate sorption by and desorption from a Chinese red soil. Water, Air, Soil Pollut. 2006;176:269–83. [Google Scholar]
- 24.Keon NE, Swartz CH, Brabander DJ, Harvey C, Hemond HF. Validation of an arsenic sequential extraction method for evaluating mobility in sediments. Environ Sci Technol. 2001;35:2778–84. doi: 10.1021/es001511o. [DOI] [PubMed] [Google Scholar]
- 25.Swartz CH, Blute NK, Badruzzman B, Ali A, Brabander D, Jay J, Besancon J, Islam S, Hemond HF, Harvey CF. Mobility of arsenic in a Bangladesh aquifer: Inferences from geochemical profiles, leaching data, and mineralogical characterization. Geochim Cosmochim Acta. 2004;68:4539–57. [Google Scholar]
- 26.Wenzel WW, Kirchbaumer N, Prohaska T, Stingeder G, Lombi E, Adriano DC. Arsenic fractionation in soils using an improved sequential extraction procedure. Anal Chim Acta. 2001;436:309–23. [Google Scholar]
- 27.Slowey AJ, Johnson SB, Newville M, Brown GE., Jr Speciation and colloid transport of arsenic from mine tailings. Appl Geochem. 2007;22:1884–98. [Google Scholar]
- 28.Klarup DG. The influence of oxalic acid on release rates of metals from contaminated river sediment. Sci Total Environ. 1997;204(3):223–31. [Google Scholar]
- 29.Mohapatra D, Singh P, Zhang W, Pullammanappallil P. The effect of citrate, oxalate, acetate, silicate, and phosphate on stability of synthetic arsenic-loaded ferrihydrite and Al- ferrihydrite. J Hazard Mater. 2005;B124:95–100. doi: 10.1016/j.jhazmat.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 30.US Environmental Protection Agency. Vineland Chemical Company site final draft, Feasibility study report. River areas, Vineland NJ: 1989. [Google Scholar]
- 31.US Environmental Protection Agency, Vineland Chemical Co., Inc. National Priority List site fact sheet. 2006 http://www.epa.gov/Region2/superfund/npl/0200209c.pdf.
- 32.EPA. Technical fact sheet: Final rule for arsenic in drinking water. 2001 EPA 815-F-00-016. [Google Scholar]
- 33.Keimowitz AR, Zheng Y, Chillrud SN, Mailloux B, Jung HB, Stute M, Simpson HJ. Arsenic redistribution between sediments and water near a highly contaminated source. Environ Sci Technol. 2005;39:8606–13. doi: 10.1021/es050727t. [DOI] [PubMed] [Google Scholar]
- 34.US Environmental Protection Agency. Groundwater pump and treat systems: summary of selected cost and performance information at Superfund-financed sites. 2001 (EPA 542-R-01- 021a) [Google Scholar]
- 35.US Army Core of Engineers. Vineland Chemical Company Superfund Site. Vineland, NJ: Prepared for USEPA, Region II; 2007. Classification Exception Area and Well Restriction Area Report. [Google Scholar]
- 36.Wovkulich K. PhD thesis. New York: Columbia University; 2011. Laboratory and Field Studies Directed toward Accelerating Remediaton at a Major US Superfund Site in New Jersey. [Google Scholar]
- 37.Wovkulich K, Stute M, Protus TJ, Sr, Mailloux BJ, Chillrud SN. Injection System for a Multiwell Injection Using a Single Pump. Ground Water Monit Rem. 2011;31(1):79–85. doi: 10.1111/j.1745-6592.2011.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fleisher MQ, Anderson R. Marine Particle Analysis and Characterization. American Geophysical Union; Washington, DC: 1991. Particulate matter digestion (from mg to 10’s of g) and radionuclide blanks; pp. 221–2. [Google Scholar]
- 39.NJ Department of Health and Senior Services. Right to Know, Hazardous Substance Fact Sheet. Oxalic Acid. 2010 [Google Scholar]
- 40.Baghel A, Singh B, Pandey P, Sekhar K. A rapid field detection method for arsenic in drinking water. Anal Sci. 2007;23(2):135–7. doi: 10.2116/analsci.23.135. [DOI] [PubMed] [Google Scholar]
- 41.Agency for Toxic Substances and Disease Registry. Toxicological profile for arsenic. US Department of Health and Human Services; 2007. [PubMed] [Google Scholar]
- 42.Sahin N. Oxalotrophic Bacteria. Res Microbiol. 2003;154:399–407. doi: 10.1016/S0923-2508(03)00112-8. [DOI] [PubMed] [Google Scholar]
- 43.Panias D, Taxiarchou M, Paspaliaris I, Kontopoulos A. Mechanisms of dissolution of iron oxides in aqueous oxalic acid solutions. Hydrometallurgy. 1996;42(2):257–65. [Google Scholar]
- 44.Lee SO, Tran T, Jung BH, Kim SJ, Kim MJ. Dissolution of iron oxide using oxalic acid. Hydrometallurgy. 2007;87:91–9. [Google Scholar]
- 45.Tao YQ, Zhang SZ, Jian W, Yuan CG, Shan XQ. Effects of oxalate and phosphate on the release of arsenic from contaminated soils and arsenic accumulation in wheat. Chemosphere. 2006;65(8):1281–7. doi: 10.1016/j.chemosphere.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 46.Hongshao Z, Stanforth R. Competitve adsorption of phosphate and arsenate on goethite. Environ Sci Technol. 2001;35:4753–7. doi: 10.1021/es010890y. [DOI] [PubMed] [Google Scholar]
- 47.Jain A, Loeppert RH. Effect of competing anions on the adsorption of arsenate and arsenite by ferrihydrite. J Environ Qual. 2000;29(5):1422–30. [Google Scholar]
- 48.Jia YF, Xu LY, Fang Z, Demopoulos GP. Observation of surface precipitation of arsenate on ferrihydrite. Environ Sci Technol. 2006;40(10):3248–53. doi: 10.1021/es051872+. [DOI] [PubMed] [Google Scholar]
- 49.Skelley, Loy . The Vineland Chemical Superfund site. Vineland, NJ: 2003. Draft report of hydrogeologic investigations/capture zone analysis. [Google Scholar]
- 50.Spayd SE, Johnson SW. Guidelines for delineation of well head protection areas in New Jersey. New Jersey Geological Survey, Open File Report OFR 03-1. 2003 [Google Scholar]
- 51.Creighton S. Personal communication. 2007.
- 52.Holzbecher EO. Modeling density-driven flow in porous media: principles, numerics, software. New York: Springer; 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.