Abstract
Surgery has traditionally been the preferred treatment for early stage esophageal cancer. Recent advances in endoscopic treatments have been shown to be effective and safe. Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) allow endoscopists to remove small, superficial lesions, providing tumor specimen that can be examined for accurate pathologic tumor staging and assessment of adequacy of resection. Endoscopic ablation procedures, including photodynamic therapy (PDT) and radio frequency ablation (RFA), have also been shown to safely and effectively treat esophageal dysplasia and early stage neoplasia, with excellent long-term disease control. Both approaches are becoming more widely available around the world, and provide an alternative, safe, low risk strategy for treating early stage disease, making combined endoscopic therapy the recommended treatment of choice for early stage esophageal cancers.
Keywords: Esophageal cancer, high grade dysplasia (HGD), endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), radio frequency ablation (RFA)
Introduction
In 2014-2015, the majority of esophageal cancers diagnosed in the United States will be esophageal adenocarcinoma (EAC); this represents a shift in incidence since the 1960s when 90% of all esophageal cancers were of the squamous cell type (1,2). Worldwide, however, esophageal squamous cell carcinoma (SCC) remains more common than EAC with the highest incidence seen in China (3). Increasing research into the pathogenesis of these two malignancies has revealed different risk factors, pathophysiology, and treatment options. EAC arises most commonly in the distal esophagus and is shown to be associated with gastroesophageal reflux disease (GERD) and obesity (4,5). The increasing incidence of EAC has also led to an increasing recognition of the precursor lesions of this disease including Barrett’s esophagus (BE), low-grade dysplasia (LGD), high-grade dysplasia (HGD), and intramucosal adenocarcinoma (ImCa)—defined as carcinoma limited to the mucosal layers of the esophagus. BE is histologically described as specialized intestinal metaplasia (SIM). SCC, however, is more common in the upper and middle esophagus and is associated with risk factors of smoking and alcohol use (3). SCC arises through a progression of squamous cell dysplasia from low-grade intra-epithelial neoplasia (LGIN) to high-grade intra-epithelial neoplasia (HGIN) to early squamous cell carcinoma (ESCC) or non-invasive SCC (historically referred to as carcinoma in situ) to invasive disease. These represent the World Health Organization (WHO) defined categories of disease progression.
With the increasing recognition of the association of GERD and BE, patients with GERD often undergo upper endoscopy (EGD) to screen for BE. National guidelines recommend surveillance EGD once every 3 years in patients with BE (6,7). This has resulted in an increase in diagnosis of early stage esophageal cancer of both EAC and SCC types. The remainder of patients, however, often don’t present until symptoms develop, generally representing more advanced disease. Approximately 50% of patients with esophageal cancer present with loco-regional disease and potentially curable disease; the remainder have distant metastatic disease or extra-regional nodal disease at the time of diagnosis (2). Patients with loco-regional disease are extensively evaluated for combination therapies to attempt to achieve the greatest success of cure with the least co-morbidity of treatment. Treatment options include a combination of endoscopic treatment, chemotherapy, radiation therapy, and surgical resection. The optimal course of therapy is largely defined by the histopathology of disease, the stage of disease at presentation, and patient co-morbidities. Cancer limited to the mucosal layer (AJCC classification T1aN0M0) may be treated with endoscopic methods yielding a greater than 80% cure rate of dysplasia in the adenocarcinoma sequence and a greater than 70% cure rate of all BE (8-10). While the use of endoscopic techniques is newer for treatment of early SCC, case series report similar cure rates for patients with mucosal disease (11-13). This article presents a focal discussion of the role of endoscopic evaluation in diagnosis and treatment of early stage esophageal cancer, of both, adenocarcinoma and squamous cell varieties. Understanding the tools available for diagnosis, patient selection criteria, and endoscopic treatment options for early stage esophageal cancer can improve patient outcomes and reduce patient morbidity and mortality.
Endoscopic techniques for diagnosis
Patients with esophageal cancer are identified on EGD and confirmed by histopathological review of biopsy specimens taken during this evaluation. EGD may be initiated as a result of screening in patients with a long history of GERD or as a diagnostic evaluation tool in patients with symptoms including dysphagia, dyspepsia, or atypical chest pain. Current guidelines recommend screening patients with BE every 3 years with targeted biopsies of any abnormal lesions within the Barrett’s mucosa and systemic 4-quadrant biopsies every 2 centimeters within the remaining mucosa to detect dysplastic tissue (6,7). While this technique increases the yield of diagnosis over random biopsies, there remains sampling error. Enhanced endoscopic technologies have significantly improved the ability of the endoscopist to identify subtle variations in the mucosal appearance and recognize lesions that are in the precancerous or early stages of cancer development. Further improvement in technologies to enhance the ability of the endoscopist to identify premalignant lesions and improve the diagnostic yield of endoscopy is ongoing.
High definition white light endoscopy (HDWLE) has increased the ability of the endoscopist to identify and differentiate normal squamous epithelium from abnormal SIM and dysplastic tissue (14). HDWLE has become the standard used in all modern EGDs. With HDWLE, BE appears as salmon colored mucosa, an alternation from the normal subdued pink mucosa. Within normal mucosa or BE, HDWLE will allow for the inspection and identification of any raised nodules or patches of nodular mucosa, which is one common presentation of dysplasia or ImCa in either the adeno or squamous cell pathways. Obvious masses can be inspected. Using HDWLE, the endoscopist can take targeted biopsies of any abnormal mucosa for further differentiation and identification. EGD should be used to describe the location of any abnormalities, best represented as distance from the incisors. The size and length of any abnormality should be described and the relationship of the abnormality relative to the gastroesophageal junction (GEJ) is described. The percentage of circumference involved or the location on a clock-face can be helpful in further characterizing the lesion (15). The extent of BE should also be characterized using the well described Prague classification system; this uses a “C” distinction to note the level of circumferential BE and an “M” distinction to note the maximal length of esophagus affected with BE (16). Additionally, the gastric cardia should be carefully examined and the degree of extension of esophageal or GEJ tumors into the gastric cardia should be documented using the Siewert classification system (Table 1) (17-19). Supplementing the use of HDWLE is the use of narrow band imaging (NBI) or virtual chromoendoscopy, a form of imaging using specific wavelengths of light in the blue and green part of the spectrum which enhances the mucosal and vascular pattern of BE and dysplastic tissue at the time of endoscopy. NBI is available on certain modern endoscopes with the use of a separate switching button, which turns on the blue-green filter. NBI has been shown to increase the real-time identification of HGD with sensitivity and specificity of greater than 90% by allowing for identification of irregular mucosal pit patterns and irregular microvasculature (20). This can increase the ability of the endoscopist to perform targeted biopsies to confirm the abnormal endoscopic findings and potentially the development of neoplasia (14).
Table 1. Siewert classification of EGJ tumors.
| Siewert | Description | Surgical approach |
|---|---|---|
| I | Tumor center located between 5 and 1 cm proximal to the anatomical cardia | Approached as esophageal or EGJ cancer |
| II | Tumor center located between 1 cm proximal and 2 cm distal to the anatomical cardia | Approached as esophageal or EGJ cancer |
| III | Tumor center located between 2 and 5 cm distal to the anatomical cardia | Approached as gastric cancer |
EGJ, esophago-gastric junction. Ref: NCCN guidelines Version 1. 2014.
Chromoendoscopy can additionally supplement the endoscopist’s ability to recognize subtle lesions. Chromoendoscopy refers to the use of dyes sprayed within the esophagus to detect mucosal variation secondary to dysplasia or early neoplasia. The most commonly used application of chromoendoscopy is the use of Lugol’s solution, a combination of potassium iodide and iodine, which has an affinity for glycogen in squamous cells. Lugol’s solution is sprayed to coat the squamous mucosa in the esophagus resulting in a brown staining of normal squamous cells; absence of uptake is associated with dysplasia and carcinoma. Lugol’s staining has been shown to have a sensitivity of 91-100% and a specificity of 40-95% for the detection of squamous cell neoplasia (21). The adjunct use of Lugol’s in patients with squamous cell dysplasia and carcinoma has been shown to increase the yield of diagnosis and to allow for better characterization in terms of size, location, and multi-focality of squamous dysplastic lesions. For detection of dysplasia and neoplasia in BE, dyes available include indigo carmine, methylene blue, crystal violet, and acetic acid (22), though these have been less well characterized than in the SCC pathway. A recent meta-analysis looking at the use of chromoendoscopy and advanced imaging techniques such as NBI for the detection of dysplasia or carcinoma in patients with BE demonstrated improvement in the yield of diagnosis with the use of these supplemental techniques (22).
Confocal laser endomicroscopy (CLE) is an enhanced optical technique designed to further enhance real-time assessment of dysplastic and neoplastic cells of the esophagus. After intravenous injection of fluorescein, a blue laser light probe is passed into the esophagus and used to assess cellular and subcellular structures within the esophagus. CLE allows for in vivo assessment of dysplasia and carcinoma. Studies have assessed its efficacy including a recent multicenter study, which suggested improved ability to detect dysplasia and neoplasia above HD-WLE and NBI (23,24); however, CLE requires specific training and expertise and while it is actively used in research studies and shows great promise, this technology is not yet widely available clinically.
Finally, newer techniques including optical coherence tomography and endocytoscopy have been developed and described as potential probe based enhanced imaging techniques to increase the visualization of the tissue microstructure in the esophagus. These techniques have only begun to be studied in vivo and remain experimental technologies that may have a role in real time diagnosis of esophageal cancer precursor lesions in the future.
Histopathology remains the gold standard for diagnosis of esophageal cancer and its precursor lesions. Histopathology should be reviewed by an expert GI pathologist and in cases of initial diagnosis of precursor lesions, should be confirmed by a second GI pathologist. Pathology should describe the presence of SCC or adenocarcinoma. The degree of differentiation, depth of invasion and any lymphovascular invasion should be described, as these factors affect prognosis and treatment plan. In the setting of precancerous lesions, the degree of dysplasia should be characterized and degree of focality should be described (unifocal or multifocal).
Staging
The 2010 American Joint Committee on Cancer (AJCC) has recommended that esophageal cancer be staged based on a T (tumor size), N (nodal involvement), and M (metastatic disease) based system. For patients with localized esophageal cancer, the T and N criteria are imperative to determining the optimal course of treatment. The 5-year survival for T1a adenocarcinoma (tumor invades lamina propria and/or muscularis mucosae) is between 88-90% versus 47-62% for T1b disease (tumor invades submucosa) (25-28). This significant decrease in survival is driven by the increase in lymphovascular invasion and development of lymph node involvement once the tumor has penetrated into the submucosa, but is also affected by the progression of histopathological grade (well/moderate differentiation to poor differentiation). Recent reviews of resected early surgical specimens of both, EAC and SCC, revealed that patients with T1a disease have 0% risk of lymph node involvement with increase to 4-46% of lymph node involvement when the tumor has reached T1b disease. Depth of submucosal (SM) involvement can be further delineated into SM1, SM2 and SM3 involvement with 0-21% nodal involvement seen in SM1 disease (upper 1/3 of SM) to 43-67% in SM3 disease (deepest 1/3 of SM) (29-31).
Accurate preoperative staging of patients with esophageal cancer is imperative to direct treatment and is dependent on a combination of techniques including EGD, endoscopic ultrasound (EUS), computed tomography (CT) scans, and positron emission tomography (PET) scans using fluorodeoxyglucose (FDG) activity. After initial diagnosis of esophageal cancer with EGD and biopsy, all patients should undergo CT scan of the chest and abdomen for evaluation of loco-regional disease and distant metastases. Metastatic disease should be described in terms of distant metastases (unresectable disease) or invasion into adjacent resectable structures (T4a disease) such as pleura, pericardium, and diaphragm, which is identified by loss of fat planes on CT scan. CT is otherwise not useful in distinguishing T stage. For nodal disease, CT has a sensitivity of 47-82% and a specificity of 25-92% (28,32,33). Supplementing CT information is the use of PET with FDG activity, which can identify occult metastatic disease in up to 10-20% of people not identified on standard imaging (34). CT combined with PET is the optimal study to combine these two roles (35).
In patients with no evidence of metastatic disease, further staging is completed with EUS evaluation. EUS allows for completion of staging with information on the T stage and N stage. The overall accuracy of EUS for T staging ranges between 72-76% (28,36-38). EUS is more accurate in staging T3 and T4 disease than in staging T1 disease. Additionally, in T1 disease, EUS using high frequency probes (12-20 MHz) can help distinguish between T1a (mucosal involvement) and T1b disease (SM involvement) and is able to successfully do this in 75-82% of cases (39). Thus, EUS staging for distinction between T1a and T1b disease is often augmented with the use of endoscopic resection of lesions to obtain more accurate staging and possibly curative treatment in the same session.
Endoscopic resection techniques
Endoscopic resection has been described with two approaches, including endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), both of which were initially developed in Japan for the treatment of early esophageal and gastric cancer. EMR procedures include strip biopsy using saline injection followed by cautery snare resection, EMR with a cap fitted onto the endoscope (EMRC), and EMR with the use of a band-ligating device (EMRL). ESD is a newer method of endoscopic resection developed to permit a wider, en bloc excision of the entire lesion with a surrounding margin, without cutting through the lesion.
Endoscopic mucosal resection (EMR)
One of the earliest descriptions of endoscopic resection of early gastric cancer was published in 1980 (40). Papazian et al. also described the technique using an insulated tip cautery knife to endoscopically resect a gastric leiomyoblastoma in the stomach of an elderly patient (41). In 1990, Inoue et al. reported successful mucosal resections of early esophageal cancers, with almost no complications, using a clear tube attached to the tip of the endoscope to perform EMR, leaving an intact muscularis propria (42,43). They also demonstrated that this technique can be safely performed in a patient with esophageal varices (44). Since then commercially available kits have been marketed around the world providing access to reliable devices, permitting endoscopists to safely perform the technique.
The two most used devices in the United States include the Olympus EMR kit (Olympus America; Center Valley, USA) and the Cook Medical Duette EMR kit (Cook Medical; Bloomington, Indiana). Both kits include a clear, short plastic tube, which fits onto the tip of a standard gastroscope, and is paired with a special snare to resect the desired lesion. In the case of the Olympus EMR kit, the snare opens inside the cap forming a noose, which will ensnare the desired lesion or mucosa. An injection needle is also provided to allow instillation of either saline or another liquid substance such as Hyaluronic acid into the submucosa, beneath the lesion, separating it from the muscularis propria, for safe resection. Although the original description of this technique included the injection of saline into the submucosa, EMR has also been described to be effective and safe for removing esophageal lesions without the injection (45).
During the procedure, the endoscopist identifies the lesion for resection with careful inspection, and may mark the area for resection by placing cautery marks a few millimeters around the lesion for easy localization during resection and also to ensure complete excision. The endoscopist then brings the lesion close to the edge of the clear cap, applies suction causing the lesion and surrounding mucosa to be pulled into the clear cap. The snare is then closed, cinching the mucosa surrounding the suctioned lesion, and high-frequency electrocautery is applied, cutting through the mucosa at the point it is cinched by the snare, simultaneously cauterizing any superficial blood vessels. The Duette kit provides multiple small rubber bands that are applied to the suctioned lesion, cinching the mucosa at the base of the lesion, forming a pseudo-polyp which can then be snared and resected in standard polypectomy fashion (Figure 1). Both devices work well for the resection of small lesions measuring up to 1.5 cm in diameter, but can be repeatedly applied to remove larger lesions in piecemeal fashion. In fact, this approach has also been described to completely remove large areas of BE by repeatedly applying the suction and snaring to neighboring areas of mucosa until the entire field of Barrett’s epithelium has been excised (46).
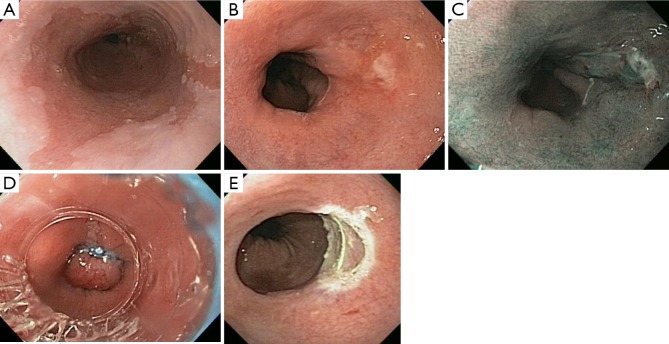
Figure 1.
Endoscopic mucosal resection. (A) HDWLE of a patient with long-segment BE; (B) image of a small nodular patch of mucosa at the distal end of the BE; (C) NBI image of the distal area of BE showing the small nodular patch on the right side; (D) EMRL being performed with band applied to the patch of nodular mucosa; (E) image of the site of EMR showing complete resection of the abnormal nodular mucosa, with healthy, non-bleeding submucosal visible. HDWLE, high definition white light endoscopy; BE, Barrett’s esophagus; NBI, narrow band imaging; EMR, endoscopic mucosal resection.
The earliest reports of EMR demonstrated success in small series of excising small superficial squamous cell esophageal cancers (42,43). In 2000, Ell et al. reported use of the strip biopsy technique or EMRC in the treatment of patients with HGD or superficial (T1) adenocarcinoma in the setting of BE (45). EMR was successful in achieving complete local resection based on histopathology in 97% of patients with well differentiated, non-ulcerated, mucosal lesions <2 cm in maximal diameter. The success rate was lower in those with more advanced disease, including size >2 cm, ulcerated lesions and higher differentiation grade. Other groups from other parts of the world have published similar results, indicating that EMR is an important tool in the evaluation and management of patients with superficial esophageal neoplasia (13,47-49). An important finding in several of these reports is the change in staging noted in 20-30% of patients, both up-staging and down-staging, following EMR (47). Also important are the reports of a metachronous rate of cancer as high as 21% (13).
Endoscopic submucosal dissection (ESD)
The technique of ESD was first developed in Japan to permit the en bloc resection of large superficial lesions of the GI tract. It is performed by initially marking the periphery of the lesion with cautery markings 5-10 mm around the edge of the lesion, making a circular mucosal incision around the lesion, then careful, meticulous dissection with a cautery device beneath the lesion through the submucosa, slowly separating it from the muscularis propria of the stomach wall, eventually removing the entire lesion with its surrounding margin of normal mucosa. The technique was originally described by Gotoda et al. in the treatment of a large flat rectal lesion, and then subsequently adapted to the treatment of early gastric cancers and now esophageal lesions (50-55).
In an initial series, Oyama et al. reported treating 102 patients with superficial esophageal squamous cell cancer ranging in size of lateral spread from 4-64 mm, using the hook knife to perform ESD. They achieved successful en bloc resection in 95% and had a local recurrence rate of 0% with mean follow-up period of 21 months (range, 3-54 months). They experienced no major bleeding events or perforations, but had six cases of mediastinal emphysema (6%) that they treated successfully with 2 days of IV antibiotics (54).
In another series, Fujishiro et al. (55) reported performing ESD for 58 esophageal squamous cell neoplasms in 43 patients (intraepithelial neoplasm or intramucosal invasive carcinoma). They achieved en bloc resection for 100% of the lesions, but had negative margins in only 78%. They experienced no significant bleeding, but had four perforations, which they were able to close endoscopically. Nine patients experienced subsequent esophageal strictures requiring balloon dilation. One patient developed a local recurrence 6 months after ESD, which was treated successfully by a second ESD.
Based on experiences such as these, some have suggested that ESD can be considered for curative treatment of patients with superficial esophageal neoplasia in Japan (56). This technique has been adopted in Korea for treatment of gastric cancer (57), but has very slowly been adopted in the United States and Europe. As the technology to perform the procedure becomes more widely available, greater experience should follow.
Endoscopic ablation techniques
Supplementing the use of endoscopic resection techniques is the use of ablation to eliminate all flat neoplastic or dysplastic disease and all precursor disease such as BE. In patients with early stage EAC treated with EMR or ESD, the remaining BE generally contains residual dysplasia; recurrence of carcinoma can occur in 19% to 30% of cases (58). Thus, the goal in endoscopic management is to eradicate all BE in the treatment process. Ablation techniques have evolved with the further development of technologies.
Laser
Laser therapy has been described and was previously used for ablation of BE. The 1,064-nm neodymium yttrium aluminum garnet (Nd:YAG) laser and 940-nm diode laser have been used for tissue destruction with results in the range of 65-67% complete eradication. Laser therapy has a limited area of treatment and requires numerous sessions to ablate large areas making this less favorable than other ablation techniques. Additionally variable levels of subsquamous BE have been described (59), possibly because of non-uniform application throughout the affected area. Laser therapy has therefore gone out of favor, and is infrequently used for early esophageal neoplasia.
Photodynamic therapy (PDT)
PDT requires multiple steps to achieve ablation ; the patient is first administered a light-sensitizing drug that accumulates in the BE or neoplastic tissue. A light-diffusing fiber is then placed in the esophagus and monochromatic laser light is applied resulting in free oxygen radical formation and ischemia of the treated tissue with controlled tissue destruction. Porfimer sodium or oral 5-aminolevulinic acid are the most commonly used photosensitizers (60). PDT has been studied for the treatment of LGD, HGD, and ImCa and has yielded successful ablation in 93%, 78%, and 44% respectively. Approximately 5% of patients developed subsquamous or “buried” adenocarcinoma. Complications including strictures have been described in up to 30% of patients with one session of PDT (61). PDT was previously widely used for treatment of BE but has become less common with the advent of safer methods of treatment such as radiofrequency ablation.
Radio frequency ablation (RFA)
RFA is the current standard of care of ablative techniques. RFA uses a bipolar electrode to apply 465 kHz of energy waveform to the affected tissue resulting in cauterization and destruction of the epithelial layer. RFA can be applied to the full circumference of the esophagus (Figure 2) using a balloon-based device or can be applied in smaller increments using a cap-based device for focal ablation (HALO360 or HALO90 system; BARRX Medical, Sunnyvale, California) (62). RFA has been evaluated for the treatment of dysplastic precursor stages in both adenocarcinoma and SCC pathways and has been shown to be safe and effective.
Figure 2.
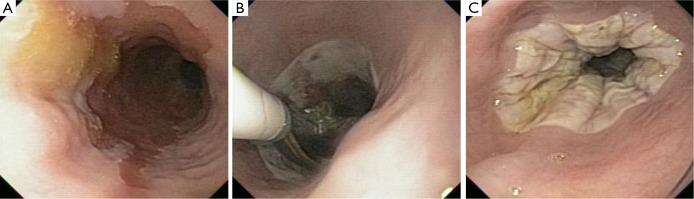
Radiofrequency ablation. (A) HDWLE of a patient with long-segment BE; (B) image of RFA being performed with the use of the Halo 360 catheter; (C) image of successful circumferential ablation of area of BE. HDWLE, high definition white light endoscopy; BE, Barrett’s esophagus; RFA, radio frequency ablation.
In patients with BE and dysplasia, a multicenter randomized control trial of 127 patients randomized to RFA versus sham procedure revealed significantly improved rates of complete eradication of BE, decreased rates of progression, and fewer cancers in patients who underwent RFA (63) with low rates of complication (chest pain, bleeding, esophageal stricture). A meta-analysis of 18 studies involving 3,802 patients who underwent RFA revealed complete eradication of BE in 78% of patients with recurrence of BE in 13% of patients and progression to cancer in 0.7% of patients after complete eradication of BE. Esophageal stricture, the most common complication reported, was noted in 5% of patients (64). Patients with longer segments BE (length >10 cm) and dysplasias have also successfully been treated using EMR and RFA (58).
Patients with squamous cell dysplasia and early flat SCC have been treated with RFA as well, though the literature remains unclear as to its role in this disease. The largest series to date presented a single center study of 29 patients treated with RFA resulting in 97% complete eradication of disease at 12 months (62). A second prospective cohort of patients treated with RFA from the United Kingdom revealed only 50% of patients with complete eradication of disease at 12 months (65). These small series with varying protocols and varying results indicate further research is needed to determine if RFA is effective in the treatment of early SCC.
Argon plasma coagulation (APC)
APC is a widely available, alternative way to ablate dysplastic tissue in the esophagus. APC uses a probe device that has a constant flow of ionized argon gas that transmits high-frequency current to tissue to cause superficial cautery effect and tissue destruction. Efficacy of APC varies in studies with 66% to 100% of complete eradication of BE and relapse rates of 3% to 11% per year (60,66,67). Complications have been reported with APC including strictures, pleural effusions, and perforations. Given this mixed profile, APC for BE is less routinely performed in favor of techniques such as RFA.
Cryoablation
Cryoablation is a form of ablative therapy that involves spraying liquid nitrogen onto the area of abnormal tissue, resulting in intracellular disruption and ultimate ischemia of the cells. Few studies have looked at outcomes after cryotherapy for ablation of dysplastic BE and further research is needed before this is used widely as a modality for treatment (60).
Endoscopic approach to the patient with early stage esophageal cancer
Patients with early stage esophageal cancer, staged as T1a lesions, are candidates for endoscopic approach for treatment and potential cure of their disease. Patients with T1a lesions have a less than 2% risk of lymph node metastases, making them appropriate candidates for this approach. Patients with T1b may be considered for endoscopic treatment on a case-by-case basis; in a recent study, 28% of patients with T1b disease had lymph node involvement and the rate of lymph node involvement increased with involvement of SM1 to SM3 with 54% of patients with SM3 disease having lymph node metastases. In patients who are surgical candidates, surgery is the recommended approach for definitive treatment; in select patients with multiple co-morbidities or higher surgical mortality risk and with SM1 involvement, endoscopic treatment may be considered for curative intent (68).
Patients who are selected to undergo endoscopic treatment are generally treated with combination treatment with the goal of eradication of all dysplastic tissue in addition to eradication of all precursor abnormalities such as BE. Patients are carefully inspected on initial exam to identify all raised or nodular lesions. These lesions are treated with either EMR or ESD depending on the depth of disease and size of the lesion. After endoscopic resection of all raised lesions is completed, patients are treated with high dose PPI therapy for 3 months for improved wound healing. Patients return at 3 months intervals for follow up evaluation; ablative therapy is applied to all residual flat disease at the next session with the goal of eradication of all precursor lesions. Patients generally undergo on average 2 to 3 sessions of ablative therapy for successful elimination of all flat dysplasia and BE (9). Patients are recommended to undergo surveillance endoscopy and retreatment every 3 months for the first year.
Several series have reported long-term outcomes after combination endoscopic treatment for HGD or early EAC. The largest published case series to date included long term follow up (mean 56.6±33.4 months) of 1,000 patients who underwent endoscopic treatment of intra-mucosal carcinomas (T1a) by combination therapy of EMR followed by ablative therapy including APC or RFA. In this series, complete remission of carcinoma was achieved in 96.3% of patients. Recurrence of HGD or intramucosal carcinoma occurred in 14.5% of patients and repeat endoscopic treatment was successful in 85% of these patients. Major complications (bleeding, perforation) from endoscopic treatment were seen in 1.5% of patients and minor complications (stricture) were seen in 1.3% of patients (8).
While combination therapy is the most described, some series have looked at patients treated with EMR alone or with ablation alone. A recent study described 107 patients treated with EMR only for complete eradication of all dysplasia and BE; 72% of patients achieved complete remission of HGD/ImCA and all BE with 40% of patients developing strictures that required dilation (69).
There is less reported literature on long-term outcomes after combination endoscopic treatment for squamous cell HGD or intramucosal carcinoma. A recent retrospective study, at a Japanese institution, presented 204 patients with early SCC, defined as histological confirmation of invasion limited to SM1, treated with endoscopic therapy followed by ablation if positive margins remained. In this group, 11% of patients experienced metachronous recurrence and 2% developed local recurrence during a median follow of 36 months. All patients were able to be treated with subsequent endoscopic therapy. Approximately 4% of patients developed complications including one perforation and eight strictures (70). While these results are promising, combined modality endoscopic treatment for early SCC continues to be investigated.
Surveillance after endoscopic treatment is essential to ensure complete eradication of dysplastic tissue and to observe for the possible recurrence of dysplasia in the treated area. Recurrence of “buried” BE or the development of subsquamous BE and cancer is a concern that requires close monitoring. “Buried” BE has been reported in case series in up to 5% of patients treated with endoscopic modalities. Surveillance endoscopy should include a high-resolution exam with diagnostic tools including HDWLE, NBI, or chromoendoscopy as clinically indicated. All surveillance endoscopies should include four quadrant biopsies of the entire length of previous BE or dysplastic tissue to evaluate for recurrence or subsquamous disease. Surveillance intervals are generally recommended to be every 3 months for the first year with lengthening of the interval following this.
Published guidelines
The National Comprehensive Cancer Network (NCCN) guidelines updated in January 2014 describe the addition of endoscopic resection or endoscopic resection followed by ablation as possible alternatives for surgery for patients with Tis or T1a SCC of the esophagus (71). Additionally, endoscopic resection followed by ablation can be considered in medically unfit patients with T1bN0 disease. In patients with Tis, T1a or superficial T1b EAC, endoscopic resection followed by ablation is considered the preferred treatment of choice. Patients are recommended to undergo endoscopic surveillance following endoscopic treatment every 3 months for the first year followed by annual surveillance thereafter.
Summary
Endoscopic treatment should be considered for patients with early esophageal cancer. A combination of modalities including endoscopic resection and ablation is safe and effective to achieve eradication of all dysplastic and neoplastic tissue. As technical skills improve and newer technology becomes available, more options will become available to affected patients. Comparative studies will become necessary to determine the best approach for the treatment of patients with early esophageal cancer. Although wide field resections such as with ESD requires greater technical skill and is associated with greater risk of bleeding and perforation, it is our impression that it provides a greater potential for accurate assessment of disease and stage, more definitive treatment of the disease, and a resulting greater accuracy in prognostication.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst 2008;100:1184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [DOI] [PubMed] [Google Scholar]
- 3.Lu CL, Lang HC, Luo JC, et al. Increasing trend of the incidence of esophageal squamous cell carcinoma, but not adenocarcinoma, in Taiwan. Cancer Causes Control 2010;21:269-74. [DOI] [PubMed] [Google Scholar]
- 4.Lagergren J, Bergström R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825-31. [DOI] [PubMed] [Google Scholar]
- 5.Lagergren J, Bergström R, Nyrén O.Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med 1999;130:883-90. [DOI] [PubMed] [Google Scholar]
- 6.American Gastroenterological Association , Spechler SJ, Sharma P, et al. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 2011;140:1084-91. [DOI] [PubMed] [Google Scholar]
- 7.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol 2008;103:788-97. [DOI] [PubMed] [Google Scholar]
- 8.Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014;146:652-660. [DOI] [PubMed] [Google Scholar]
- 9.Guarner-Argente C, Buoncristiano T, Furth EE, et al. Long-term outcomes of patients with Barrett’s esophagus and high-grade dysplasia or early cancer treated with endoluminal therapies with intention to complete eradication. Gastrointest Endosc 2013;77:190-9. [DOI] [PubMed] [Google Scholar]
- 10.Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut 2008;57:1200-6. [DOI] [PubMed] [Google Scholar]
- 11.Katada C, Muto M, Momma K, et al. Clinical outcome after endoscopic mucosal resection for esophageal squamous cell carcinoma invading the muscularis mucosae--a multicenter retrospective cohort study. Endoscopy 2007;39:779-83. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu Y, Takahashi M, Yoshida T, et al. Endoscopic resection (endoscopic mucosal resection/ endoscopic submucosal dissection) for superficial esophageal squamous cell carcinoma: current status of various techniques. Dig Endosc 2013;25:13-9. [DOI] [PubMed] [Google Scholar]
- 13.Pech O, May A, Gossner L, et al. Curative endoscopic therapy in patients with early esophageal squamous-cell carcinoma or high-grade intraepithelial neoplasia. Endoscopy 2007;39:30-5. [DOI] [PubMed] [Google Scholar]
- 14.Jayasekera C, Taylor AC, Desmond PV, et al. Added value of narrow band imaging and confocal laser endomicroscopy in detecting Barrett’s esophagus neoplasia. Endoscopy 2012;44:1089-95. [DOI] [PubMed] [Google Scholar]
- 15.Enestvedt BK, Lugo R, Guarner-Argente C, et al. Location, location, location: does early cancer in Barrett's esophagus have a preference? Gastrointest Endosc 2013;78:462-7. [DOI] [PubMed] [Google Scholar]
- 16.Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett's esophagus: the Prague C & M criteria. Gastroenterology 2006;131:1392-9. [DOI] [PubMed] [Google Scholar]
- 17.Siewert JR, Hölscher AH, Becker K, et al. Cardia cancer: attempt at a therapeutically relevant classification. Chirurg 1987;58:25-32. [PubMed] [Google Scholar]
- 18.Curtis NJ, Noble F, Bailey IS, et al. The relevance of the Siewert classification in the era of multimodal therapy for adenocarcinoma of the gastro-oesophageal junction. J Surg Oncol 2014;109:202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ajani JA, Barthel JS, Bekaii-Saab T, et al. Esophageal cancer. J Natl Compr Canc Netw 2008;6:818-49. [PubMed] [Google Scholar]
- 20.Mannath J, Subramanian V, Hawkey CJ, et al. Narrow band imaging for characterization of high grade dysplasia and specialized intestinal metaplasia in Barrett’s esophagus: a meta-analysis. Endoscopy 2010;42:351-9. [DOI] [PubMed] [Google Scholar]
- 21.Dawsey SM, Fleischer DE, Wang GQ, et al. Mucosal iodine staining improves endoscopic visualization of squamous dysplasia and squamous cell carcinoma of the esophagus in Linxian, China. Cancer 1998;83:220-31. [PubMed] [Google Scholar]
- 22.Qumseya BJ, Wang H, Badie N, et al. Advanced imaging technologies increase detection of dysplasia and neoplasia in patients with Barrett’s esophagus: a meta-analysis and systematic review. Clin Gastroenterol Hepatol 2013;11:1562-70.e1-2. [DOI] [PMC free article] [PubMed]
- 23.Sharma P, Meining AR, Coron E, et al. Real-time increased detection of neoplastic tissue in Barrett's esophagus with probe-based confocal laser endomicroscopy: final results of an international multicenter, prospective, randomized, controlled trial. Gastrointest Endosc 2011;74:465-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canto MI, Anandasabapathy S, Brugge W, et al. In vivo endomicroscopy improves detection of Barrett's esophagus-related neoplasia: a multicenter international randomized controlled trial (with video). Gastrointest Endosc 2014;79:211-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wijnhoven BP, Tran KT, Esterman A, et al. An evaluation of prognostic factors and tumor staging of resected carcinoma of the esophagus. Ann Surg 2007;245:717-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu L, Hofstetter WL, Rashid A, et al. Significance of the depth of tumor invasion and lymph node metastasis in superficially invasive (T1) esophageal adenocarcinoma. Am J Surg Pathol 2005;29:1079-85. [PubMed] [Google Scholar]
- 27.Cen P, Hofstetter WL, Correa AM, et al. Lymphovascular invasion as a tool to further subclassify T1b esophageal adenocarcinoma. Cancer 2008;112:1020-7. [DOI] [PubMed] [Google Scholar]
- 28.Glasgow RE, Ilson DH, Hayman JA, et al. Modern approaches to localized cancer of the esophagus. J Natl Compr Canc Netw 2011;9:902-11. [DOI] [PubMed] [Google Scholar]
- 29.Sepesi B, Watson TJ, Zhou D, et al. Are endoscopic therapies appropriate for superficial submucosal esophageal adenocarcinoma? An analysis of esophagectomy specimens. J Am Coll Surg 2010;210:418-27. [DOI] [PubMed] [Google Scholar]
- 30.Ancona E, Rampado S, Cassaro M, et al. Prediction of lymph node status in superficial esophageal carcinoma. Ann Surg Oncol 2008;15:3278-88. [DOI] [PubMed] [Google Scholar]
- 31.Sgourakis G, Gockel I, Lang H.Endoscopic and surgical resection of T1a/T1b esophageal neoplasms: a systematic review. World J Gastroenterol 2013;19:1424-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowe VJ, Booya F, Fletcher JG, et al. Comparison of positron emission tomography, computed tomography, and endoscopic ultrasound in the initial staging of patients with esophageal cancer. Mol Imaging Biol 2005;7:422-30. [DOI] [PubMed] [Google Scholar]
- 33.Räsänen JV, Sihvo EI, Knuuti MJ, et al. Prospective analysis of accuracy of positron emission tomography, computed tomography, and endoscopic ultrasonography in staging of adenocarcinoma of the esophagus and the esophagogastric junction. Ann Surg Oncol 2003;10:954-60. [DOI] [PubMed] [Google Scholar]
- 34.Downey RJ, Akhurst T, Ilson D, et al. Whole body 18FDG-PET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol 2003;21:428-32. [DOI] [PubMed] [Google Scholar]
- 35.Yuan S, Yu Y, Chao KS, et al. Additional value of PET/CT over PET in assessment of locoregional lymph nodes in thoracic esophageal squamous cell cancer. J Nucl Med 2006;47:1255-9. [PubMed] [Google Scholar]
- 36.Smith BR, Chang KJ, Lee JG, et al. Staging accuracy of endoscopic ultrasound based on pathologic analysis after minimally invasive esophagectomy. Am Surg 2010;76:1228-31. [PubMed] [Google Scholar]
- 37.Shimpi RA, George J, Jowell P, et al. Staging of esophageal cancer by EUS: staging accuracy revisited. Gastrointest Endosc 2007;66:475-82. [DOI] [PubMed] [Google Scholar]
- 38.Pech O, Günter E, Dusemund F, et al. Accuracy of endoscopic ultrasound in preoperative staging of esophageal cancer: results from a referral center for early esophageal cancer. Endoscopy 2010;42:456-61. [DOI] [PubMed] [Google Scholar]
- 39.Rampado S, Bocus P, Battaglia G, et al. Endoscopic ultrasound: accuracy in staging superficial carcinomas of the esophagus. Ann Thorac Surg 2008;85:251-6. [DOI] [PubMed] [Google Scholar]
- 40.Rösch W, Frühmorgen P.Endoscopic treatment of precanceroses and early gastric carcinoma. Endoscopy 1980;12:109-13. [DOI] [PubMed] [Google Scholar]
- 41.Papazian A, Gineston JL, Capron JP, et al. Leiomyoblastoma of the stomach: endoscopic treatment. Endoscopy 1984;16:157-9. [DOI] [PubMed] [Google Scholar]
- 42.Inoue H, Endo M.Endoscopic esophageal mucosal resection using a transparent tube. Surg Endosc 1990;4:198-201. [DOI] [PubMed] [Google Scholar]
- 43.Inoue H, Endo M, Takeshita K, et al. Endoscopic resection of early-stage esophageal cancer. Surg Endosc 1991;5:59-62. [DOI] [PubMed] [Google Scholar]
- 44.Inoue H, Endo M, Takeshita K, et al. Endoscopic resection of carcinoma in situ of the esophagus accompanied by esophageal varices. Surg Endosc 1991;5:182-4. [DOI] [PubMed] [Google Scholar]
- 45.Ell C, May A, Gossner L, et al. Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett’s esophagus. Gastroenterology 2000;118:670-7. [DOI] [PubMed] [Google Scholar]
- 46.Chennat J, Konda VJ, Ross AS, et al. Complete Barrett’s eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma--an American single-center experience. Am J Gastroenterol 2009;104:2684-92. [DOI] [PubMed] [Google Scholar]
- 47.Conio M, Repici A, Cestari R, et al. Endoscopic mucosal resection for high-grade dysplasia and intramucosal carcinoma in Barrett’s esophagus: an Italian experience. World J Gastroenterol 2005;11:6650-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujita H, Sueyoshi S, Yamana H, et al. Optimum treatment strategy for superficial esophageal cancer: endoscopic mucosal resection versus radical esophagectomy. World J Surg 2001;25:424-31. [DOI] [PubMed] [Google Scholar]
- 49.May A, Gossner L, Pech O, et al. Intraepithelial high-grade neoplasia and early adenocarcinoma in short-segment Barrett’s esophagus (SSBE): curative treatment using local endoscopic treatment techniques. Endoscopy 2002;34:604-10. [DOI] [PubMed] [Google Scholar]
- 50.Gotoda T, Kondo H, Ono H, et al. A new endoscopic mucosal resection procedure using an insulation-tipped electrosurgical knife for rectal flat lesions: report of two cases. Gastrointest Endosc 1999;50:560-3. [DOI] [PubMed] [Google Scholar]
- 51.Ono H, Kondo H, Gotoda T, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut 2001;48:225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamamoto H, Kawata H, Sunada K, et al. Success rate of curative endoscopic mucosal resection with circumferential mucosal incision assisted by submucosal injection of sodium hyaluronate. Gastrointest Endosc 2002;56:507-12. [DOI] [PubMed] [Google Scholar]
- 53.Yahagi N, Omata M.Trends in widening application of gastroscopic mucosal resection for early-stage stomach neoplasms. Nihon Naika Gakkai Zasshi 2003;92:29-35. [PubMed] [Google Scholar]
- 54.Oyama T, Tomori A, Hotta K, et al. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol 2005;3:S67-70. [DOI] [PubMed] [Google Scholar]
- 55.Fujishiro M, Yahagi N, Kakushima N, et al. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol 2006;4:688-94. [DOI] [PubMed] [Google Scholar]
- 56.Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009;70:860-6. [DOI] [PubMed] [Google Scholar]
- 57.Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 2009;69:1228-35. [DOI] [PubMed] [Google Scholar]
- 58.Alvarez Herrero L, van Vilsteren FG, Pouw RE, et al. Endoscopic radiofrequency ablation combined with endoscopic resection for early neoplasia in Barrett’s esophagus longer than 10 cm. Gastrointest Endosc 2011;73:682-90. [DOI] [PubMed] [Google Scholar]
- 59.Johnston MH. Technology insight: ablative techniques for Barrett’s esophagus--current and emerging trends. Nat Clin Pract Gastroenterol Hepatol 2005;2:323-30. [DOI] [PubMed] [Google Scholar]
- 60.Leggett CL, Gorospe EC, Wang KK. Endoscopic therapy for Barrett’s esophagus and early esophageal adenocarcinoma. Gastroenterol Clin North Am 2013;42:175-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Overholt BF, Lightdale CJ, Wang KK, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett’s esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc 2005;62:488-98. [DOI] [PubMed] [Google Scholar]
- 62.Bergman JJ, Zhang YM, He S, et al. Outcomes from a prospective trial of endoscopic radiofrequency ablation of early squamous cell neoplasia of the esophagus. Gastrointest Endosc 2011;74:1181-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med 2009;360:2277-88. [DOI] [PubMed] [Google Scholar]
- 64.Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for Barrett’s Esophagus: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013;11:1245-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haidry RJ, Butt MA, Dunn J, et al. Radiofrequency ablation for early oesophageal squamous neoplasia: outcomes form United Kingdom registry. World J Gastroenterol 2013;19:6011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ragunath K, Krasner N, Raman VS, et al. Endoscopic ablation of dysplastic Barrett’s oesophagus comparing argon plasma coagulation and photodynamic therapy: a randomized prospective trial assessing efficacy and cost-effectiveness. Scand J Gastroenterol 2005;40:750-8. [DOI] [PubMed] [Google Scholar]
- 67.Manner H, Rabenstein T, Pech O, et al. Ablation of residual Barrett’s epithelium after endoscopic resection: a randomized long-term follow-up study of argon plasma coagulation vs. surveillance (APE study). Endoscopy 2014;46:6-12. [DOI] [PubMed] [Google Scholar]
- 68.Manner H, Pech O, Heldmann Y, et al. Efficacy, safety, and long-term results of endoscopic treatment for early stage adenocarcinoma of the esophagus with low-risk sm1 invasion. Clin Gastroenterol Hepatol 2013;11:630-5; quiz e45. [DOI] [PubMed]
- 69.Konda VJ, Gonzalez Haba Ruiz M, Koons A, et al. Complete Endoscopic Mucosal Resection Is Effective and Durable Treatment for Barrett’s-Associated Neoplasia. Clin Gastroenterol Hepatol 2014;12:2002-2010.e2. [DOI] [PubMed]
- 70.Nakagawa K, Koike T, Iijima K, et al. Comparison of the long-term outcomes of endoscopic resection for superficial squamous cell carcinoma and adenocarcinoma of the esophagus in Japan. Am J Gastroenterol 2014;109:348-56. [DOI] [PubMed] [Google Scholar]
- 71.Ajani JA, Barthel JS, Bentrem DL, et al. Esophageal and Esophagogastric Junction Cancers. J Natl Compr Canc Netw 2011;9:830-87. [DOI] [PubMed] [Google Scholar]