Abstract
This study was conducted to expand the use of Lithospermum erythrorhizon, which is a good source of natural dye, in skin whitening and immune activation cosmetics. The goal was to provide cosmeceutical data about the extraction yield and shikonin contents of this plant by optimizing the ultrasonic extraction and high pressure extraction conditions. Under optimal extraction conditions, which consisted of 500 MPa for 60 min and 120 kHz for 90 min, 27.49 and 3.19 % (w/w) of the highest extraction yield and shikonin contents were obtained, compared to 16.32 and 1.81 % from a conventional ethanol extract (EE) control. Hyaluronidase inhibition activity was measured as 44.24 % after adding 1.0 mg/ml of ethanol extract, but it was as high as 64.19 % when using extract produced by ultrasonication with high pressure extraction (UE + HPE). The MMP-1 expression levels from skin fibroblast cells (CCD-986sk) treated with or without UV irradiation were also lowered by as much as 110.6 % after adding 1.0 mg/ml of the UE + HPE extract, relative to 126.9 % from the EE. After UVA exposure, prostaglandin E2 production from RAW 264.7 was also lower, at 110.6 %, which also indicates that the extract from the UE + HPE process enhanced skin immune activation activities. For the skin whitening activity, tyrosinase inhibitory activity was observed at 67.15 % in the HPE + UE extract, which was ca. 20 % higher than that of the EE extract (57.48 %). To reduce melanin production in Clone M-3 cells, 79.5 % of the melanin production was estimated after adding 1.0 mg/ml of the UE + HPE extract compared to that of the control (no treatment), which was similar to the 77.4 % result found in an ascorbic acid positive control. The highest shikonin secretion was conclusively obtained under the optimal conditions and resulted in a significant improvement of the cosmetic activities of L. erythrorhizon extracts.
Keywords: Lithospermum erythrorhizon, Cosmetic activity, Ultrasonication process, High-pressure process
Introduction
With rapid industrialization, environmental pollution is destroying the ozone layer and ultraviolet light exposure is increasing. In addition, as improved living standards and medical technology developments increase the average age of human beings, people are becoming increasingly interested in living long, healthy lives and growing old in good shape. Accordingly, the functional foods and cosmetics markets are expanding. Natural products studies are progressing quickly (Cho 2007), and people are becoming more interested in products that fight signs of aging and whiten the skin. With this trend, natural dyes, rather than synthetic dyes, are being studied as ingredients for the functional cosmetics industry. Although natural dyes were first used at the beginning of human history, they were not as widely used as synthetic dyes in recent years because of productivity, stability, and storage disadvantages. With improved living standards, people are becoming more interested in living environmentally friendly lives; therefore, natural materials are becoming popular for enhancing beauty. Interest in natural dyes is further increasing as information on the carcinogenicity and danger of synthetic dyes to the human body continues to emerge. Natural dyes have only slight effects on the environment, significantly low or almost no toxicity in the human body, and antibacterial, anticarcinogenic, and anti-inflammatory effects (Kim and Cho 2008). They also have natural and elegant colors that are stable and delicate even after they fade, and many different colors can be obtained from their diverse material characteristics (Park et al. 2010). Therefore, it is expected that healthy and eco-friendly products can be developed by applying natural dyes that have diverse biofunctions and natural colors for the production of make-up.
In this study, L. erythrorhizon was used to study the suitability of natural dyes as cosmetics ingredients. This perennial herb belongs to the Boraginaceae family and naturally grows throughout Korea, including in Gangwon-do and Jecheon. Its roots have long been used as a medicinal herb and edible dye in Korea, China, and Japan. L. erythrorhizon has been used for blood circulation stimulation, fever, detoxification, and for treating hematemesis, hematuria, constipation, burns, eczema, and urinary tract infections (Lee et al. 1988). It is known to have diverse effects, such as antibacterial, antivirus, antitumor, and coloration properties (Sasaki 2002; Bai 2004; Chen et al. 2003; Staniforth et al. 2004), which are explained by the presence of allantoin, cyanoglucoside, fumaric acid, succinic anhydride, shikonin, and acetyl shikonin (Yun et al. 1999). The representative dye ingredients in L. erythrorhizon include shikonin (Kim 2001; Kim et al. 2006a, b) and acetyl shikonin, which are naphthoquinone derivatives (Yoon et al. 1988). They have long been used as food, medicine, cosmetics, and clothing dyes. They also reportedly have excellent antibacterial and anti-oxidative effects (Sekine et al. 1998; Park and Lee 2011) as well as anti-inflammatory effects (Kang 2005).
Roots, such as those from L. erythrorhizon, have hard tissues; therefore, extracting useful natural products from them is difficult. To address this problem, the elution of useful components is increased by conducting a longer extraction at a high temperature to increase the penetrability and solubility of the solvent. In this case, chemical changes and a lack of heat resistance reduce the biological activity of the extract, and the resulting economic efficiency is low because of the high energy consumption needed for the long extraction (Zhang et al. 2007). Therefore, diverse extraction processes, such as ultrasonication and high-pressure processes, are being developed. There are no established optimal extraction conditions for each natural product and process; however, because natural products have different characteristics, solvent solubility in tissues varies. Therefore, a systematic empirical study on the extraction yield and bioactivity by process parameters is required. This study was conducted to establish optimal ultrasonication and high-pressure process conditions for L. erythrorhizon and to improve its cosmetic activity.
Ultrasonic energy functions by inducing cavitation through ultrasonic vibration, which increases the kinetic energy of adjacent molecules and leads to an impact effect and high pressure, and these effects present a mixed effect (Chung et al. 2000). An increased extraction yield and reduced extraction time were verified when using the ultrasonication process compared to a general extraction process. The high pressure from ultrasonic cavitation reportedly destroyed tissues, which shortened the travel distance and caused easier diffusion of the active components (Kim et al. 2001).
High-pressure treatment is a new alternative to the recently developed micro-organism control technology that is currently being used to sterilize foods (Koo et al. 2007). In addition, high-pressure extraction (HPE) can isolate the main components of medicinal plants, yields barely any impurities and easily provides a single component with a high degree of purity, seemingly because the cell membrane that is destroyed under high pressure allows the materials to easily move so that more components can be eluted out of the cell. Using HPE, weak bonds, such as hydrogen bonds, ionic bonds and Van der Waals interactions, can be broken to elute a new material (Zhang et al. 2004).
This study was conducted to provide relatively new cosmeceutical information about L. erythrorhizon, which has been widely used as a natural dye, and to increase its applicability by optimizing the ultrasonication and high pressure extraction conditions.
Materials and methods
Sample preparation
Lithospermum erythrorhizon was harvested in Yeongcheon, Gyeongsangbuk-do, Korea in November 2011 and collected from a market (Daegwang Pharmaceutical, Chuncheon, Korea). For the HPE, dried and ground L. erythrorhizon powder was mixed with 70 % ethyl alcohol at a ratio of 1:10 in a vinyl pack and was then vacuum-packed. A high-pressure extractor (FOOD CIP-70-350-80, Ilshin Autoclave, Daejeon, Korea) was used at pressures of 300, 400 and 500 MPa at 60 °C for 30, 60 and 90 min. For the UE, L. erythrorhizon samples were placed in a temperature-controlled ultrasonication extractor with 70 % ethyl alcohol (working volume of 1 L, AUT-S2-500, Asia industry, Incheon, Korea) and processed at frequencies of 40, 80, 120 and 160 kHz with 300 watts/vol input energy at 60 °C for 30, 60 and 90 min. Following the HPE and UE processes, the L. erythrorhizon extract was transferred to an extraction flask with a vertical reflux condenser, and an extraction was conducted for an additional 12 h at 60 °C to compare it with a conventional hot water control extraction. For the hot water extraction, the L. erythrorhizon was placed in an extraction flask with a vertical reflux condenser in a 1:10 water ratio, and the extraction was performed for 12 h at 60 °C. Subsequently, all of the extracts were filtered by vacuum filtration and evaporated using a rotary vacuum evaporator (N–N series, Evela, Tokyo, Japan). The concentrates were freeze-dried and stored at −20 °C before use.
Cell lines and culture media
A cell toxicity and MMP-1 expression inhibition test was conducted using the CCD-986sk human skin fibroblast (KCLB, Seoul, Korea) to measure the cosmetic activity of the L. erythrorhizon extract. Melanin production was also tested using a mouse melanocyte, which is known as Clone M-3 (KCLB, Seoul, Korea). Skin fibroblasts and melanocytes were cultured with 10 % heat-inactivated fetal bovine serum (FBS) and DMEM (GIBCO, Grand Island, NY, USA) or RPMI 1640 (GIBCO), respectively. All other nutrients, including HEPES buffer, gentamycin sulfate and trypsin–EDTA, were purchased from Sigma (St. Louis, MO, USA) as analyzed guaranteed or reagent grade.
Measurements of extraction yields and shikonin contents from several extraction processes
To compare the extraction yields and shikonin contents of L. erythrorhizon, the L. erythrorhizon extracts from each process were freeze-dried and redissolved in acetonitrile solvent with 0.01 M formic acid. Then, 20 μl of the sample was injected into a high-performance liquid chromatograph (BIO-TEK 535 UV detector, Bio-Tek Kontron Instruments, Basel, Switzerland) at 520 nm (BIO-TEK 535 UV detector, Bio-Tek Kontron Instruments) with a Prevail C18 column (5 μm, 4.6 × 150 nm, Alltech, KY, USA) at a 0.5 ml/min flow rate, in which solvent A was acetonitrile with 0.01 M formic acid and solvent B was a water gradient (0–10 min 50:50, 10–20 min 60:40, 20–40 min 75:25, 40–60 min 95:5 % (v/v)). To quantify the biologically active shikonin component in the extract (not its other derivatives), standard shikonin (Ksei Kogyo Co., Ltd., Tokyo, Japan) was first dissolved in CHCl3 and then filtered with 0.2 μm syringe filters before being injected into the HPLC (Kim et al. 2006a, b). After the calibration curve was determined by analyzing the standard shikonin material according to its concentration, the content was calculated by comparing the concentration in comparison with the peak area of the L. erythrorhizon extract and dividing it by the total L. erythrorhizon weight (Lee 1996).
Measuring cell cytotoxicity against skin fibroblasts
The cell toxicity of the extract was measured using CCD-986sk human fibroblasts according to an SRB (sulforhodamine B) assay (Doll and Peto 1983), which measures the proliferation and toxicity of cells by cell protein staining. The 100 μl cell aliquots were added to each well of a 96-well plate at a concentration of 4–5 × 104 cells/ml and incubated for 24 h (37 °C, 5 % CO2). Each new 100 μl of extract was added to achieve final concentrations of 0.2, 0.4, 0.6, 0.8 and 1.0 mg/ml and then incubated for 48 h. After cultivation, the supernatant was removed, and 100 μl of cold 10 % (w/v) TCA (trichloroacetic acid) was added and left at 4 °C for 1 h. Subsequently, the plate was washed with distilled water five times to remove the TCA. After the wells were dried at room temperature, 100 μl of a 0.4 % (w/v) SRB solution dissolved in 1 % (v/v) acetic acid was added to each well and then they were stained for 30 min at room temperature. The residual SRB staining solution was washed four or five times using 1 % acetic acid and then dried. A 100 μl quantity of 10 mM Tris buffer was added to melt the staining solution, and the resulting absorbance at 540 nm was measured using a microplate reader (Molecular Devices, Sunnyvale, CA, USA). Then, cytotoxicity was expressed as the ratio of dead cell numbers after addition of the different extract samples (for each concentration of the samples) to total viable cell numbers without addition as a control by the following equation:
where A is number of dead cells after addition of extract samples and B is the total viable cell numbers without adding the sample as a control, and 100 % cytotoxicity means that all of the cells were dead.
Measurement of hyaluronidase inhibition activity
Hyaluronic acid is a polysaccharide that prevents bacteria or toxic materials from penetrating the skin, and it is subject to hydrolysis upon the reaction of the catabolic enzyme hyaluronidase. Furthermore, it has been shown that the inhibition of hyaluronidase activity can improve skin immunity (Ghosh 1994). In this experiment, the quantity of N-acetyl glucosamine formed from rooster comb was measured to determine the hyaluronidase inhibition activity (Reissig et al. 1955). The method details are as follows: A 20 μl L. erythrorhizon extract was added to 50 μl hyaluronidase (Sigma-Aldrich, St. Louis, MO, USA) dissolved in 0.1 M acetate buffer (pH 3.5) to achieve final concentrations of 0.2, 0.4, 0.6, 0.8 and 1.0 mg/ml. To activate the enzyme, 200 μl of 12.5 mM CaCl2 was added, and the extract was incubated for 20 min at 37 °C in a water bath. DMSO solution was added to the control group and incubated for 20 min in a water bath. The 250 μl hyaluronic acid (12 mg/5 ml) that was dissolved in 0.1 M acetate buffer was added to the hyaluronidase solution that had been activated with Ca2+ and then incubated for 40 min in a water bath. After the culture, 100 μl of 0.4 N NaOH solution and 100 μl of 0.4 M potassium tetraborate were added to the reaction mixture, and it was incubated in boiling water for 3 min and cooled. A 3.3 ml dimethyl aminobenzaldehyde solution (4 g of p-dimethyl aminobenzaldehyde (Sigma-Aldrich, St. Louis, MO, USA), 350 ml of 100 % acetic acid, and 50 ml of 10 N HCl mixture) was added to the reaction mixture, which was then incubated for 20 min at 37 °C in a water bath, after which its absorbance was measured at 585 nm. The inhibition rate was calculated as follows:
where ODc is the optical density (OD) of the control group and ODs is the OD of the sample at 585 nm, and 100 % inhibition means that the enzyme activities were completely inhibited after addition of the extract samples.
Measurement of MMP-1 (matrix metalloproteinase-1) expression inhibition
CCD-986sk skin fibroblasts were cultured in a 96-well plate at a concentration of 1.5 × 105 viable cells/ml. UVA irradiation-induced MMP-1 expression was measured using a method from Dunsmore et al. (1996). Before the UV irradiation, the medium in the wells was removed by washing with PBS, then the UVA (6.3 J/cm2) was irradiated for 24 h by using a filter for the UV light (35 W, UV, Coralife, Franklin, WI, USA). After UVA irradiation, 1.0 mg/ml L. erythrorhizon extract was added to the wells with only DMEM medium and incubated for 24 h. The medium was then divided into the 96-well plate and left for a day at 4 °C. Subsequently, the wells were washed three times with PBS (phosphate buffered saline + 0.05 % Tween 20) and blocked with 3 % BSA in PBS at 37 °C for 1 h; then, primary antibody (Sigma-Aldrich, St. Louis, MO, USA) (the monoclonal anti-MMP-1 antibody) was added to the blocking buffer at a ratio of 1:3,000, after which they were subjected to 37 °C for 90 min. After a PBS wash, secondary antibody (Sigma-Aldrich, St. Louis, MO, USA) (the alkaline-phosphatase-conjugated Goat anti-mouse IgG) was added at a ratio of 1:3,000, subjected to 37 °C for 90 min, washed with PBS, and subjected to a reaction with alkaline phosphatase substrate solution p-nitrophenyl phosphate (Sigma-Aldrich, St. Louis, MO, USA) at room temperature for 30 min. The reaction was completely stopped using 3 N NaOH, and the absorbance was measured at 405 nm using a microplate reader. Then, the relative concentration of MMP-1 production from the cells was expressed as the ratio of the amounts of MMP-1 after addition of the extract samples to the amounts without the sample as a control by the following equation:
where A is the amounts of MMP-1 (pg/mL) after addition of the extract samples and B is the amount of MMP-1 produced without addition (pg/mL).
Prostaglandin E2 (PGE2) content measurement after UV exposure
UV irradiation is known to significantly change the quantity of the cyclooxygenase enzyme that is strongly related to phospholipase A2 (PLA2) in cell membranes (Bernstein and Chen 1994), and it also increases PGE2 expression levels (Liou et al. 2007). The PGE2 contents of the cells were measured as follows: 1 × 106 viable cells/ml of CCD-986sk human fibroblasts were suspended in a 96-well plate with 10 % FBS and DMEM media. Fifty micromolar aspirin was added to this suspension to irreversibly inhibit cellular Cox enzyme activity to maintain the PGE2 quantity constant in the wells before UV exposure. Then, several different concentrations of extract (0.2–1.0 mg/mL) were added to each well of the plate, and a UVA radiation intensity of 6.3 J/cm2 was applied inside a CO2 incubator at 37 °C for 2 h. Subsequently, the 96-well plate was washed twice with phosphate-buffered saline (PBS), and the attached cells in the wells were used for further experimentation. The PGE2 concentration in the culture medium was quantified using a competitive enzyme immunoassay kit according to the manufacturer’s instructions (R&D system, Minneapolis, MN, USA).
Measuring tyrosinase activity inhibition
The inhibition of tyrosinase activity has been shown to indicate skin whitening effects by the dopachrome method (Pomerantz 1963): One hundred fifty microliters of mushroom tyrosinase (150 units) was mixed with 225 μl of 2.5 mM l-tyrosine, 225 μl of 0.4 M HEPES buffer (pH 6.8) and 300 μl of ethanol solution or 1 mg/ml L. erythrorhizon extract and incubated for 15 min at room temperature. Then, the absorbance was measured at 475 nm, and the tyrosinase inhibition rates were calculated as follows:
where A is the absorbance of the sample solution after the reaction, B is the solution with the sample before the reaction, C is the solution without the sample before the reaction, and D is the solution without the sample after the reaction, and 100 % inhibition means that the enzyme activities were completely inhibited after addition of the extract samples.
Measurement of melanin production in Clone M-3 cells
The amounts of melanin produced in Clone M-3 cells were measured using the following procedures (Komiyama et al. 1993): 1 × 105 viable cells/well of Clone M-3 cells (KCLB 10053.1, Korean Cell Line Bank, Seoul, Korea) were inoculated into 96-well plates and then cultured in a CO2 incubator (5 %, 37 °C) until 80 % of the cells were attached to the wells. After 24 h, each well was treated with L. erythrorhizon extracts at 0.2, 0.4, 0.6, 0.8 and 1.0 mg/ml or with ascorbic acid as a positive control for 24 h. Then, the wells were washed with PBS and treated with trypsin–EDTA to detach and collect the cells by centrifugation at 5,000 rpm for 10 min. The supernatant was removed, and the pellets were dried at 60 °C. The melanin in the cells was obtained by placing the samples in a 60 °C thermostatic bath and adding 100 μl of 1 M NaOH with 10 % DMSO. The melanin quantity produced by the cells was calculated by measuring the absorbance at 490 nm in a microplate reader. Then, the relative concentration of melanin production from the cells was expressed as the ratio of the amounts of melanin after addition of the extract samples to the amounts without addition as a control by the following equation:
where A is the amounts of melanin (ug/mL) after addition of the extract sample and B is the amounts of melanin produced without addition as a control (ug/mL).
Statistical analysis
All the experiments were carried out in triplicates. The data points are the mean averages of the experiments and the bars are mean standard deviations calculated by the Statistical Analysis System (SAS, Cary, NC, USA), and the significance of the differences between the means was verified via Student t-test at P < 0.05.
Results
Extraction yields and shikonin contents from several extraction conditions
Table 1 and Fig. 1 shows the extraction yields and shikonin contents of the extracts under various conditions. With respect to HPE, the yield increased when the pressure and treatment time increased, and it was the highest at 500 MPa. According to the treatment time, the yield was 20.14 % at 30 min, 22.84 % at 60 min and 23.06 % at 90 min. Because the yields at 60 and 90 min were similar, the yield difference was very small according to the energy and time. Therefore, it appears that a 60 min process time at 500 MPa pressure would be considered the most efficient extraction condition. With respect to UE, the extraction yield also increased as the ultrasonic wave intensity and treatment time increased, and the highest yield was obtained with 26.03 % at 90 min and 160 kHz followed by 25.64 % at 90 min and 120 kHz. For the ultrasonic extraction, 120 kHz and a 90 min process time were the most efficient extraction conditions. The extract from the UE-HPE procedure had the highest yield at 27.49 %, and the conventional ethanol extraction (the control group) had the lowest yield at 16.32 %. The shikonin content trends of the extracts were similar to the extraction yields, with 3.19 % (w/v) for the highest content from UE after the HPE. In the HPE process alone, the shikonin content was higher when the pre-treatment pressure was higher, with 2.50 % for 500 MPa over 60 min and 2.53 % for 90 min. Therefore, the optimal extraction condition was the 60 min process time at 500 MPa, which provided the highest extraction yield and shikonin content. For the UE method, both the extraction yield and shikonin content were higher when the ultrasonic wave intensity was higher. For example, 2.90 % and 2.91 % were yielded at 120 kHz and 160 kHz, respectively, after 90 min. Therefore, the optimal condition was recognized as a 90 min. processing time at 120 kHz for the ultrasonic extraction. These results imply that extra physical energy, such as ultrasonication or ultra-high pressure, can clearly increase shikonin elution in comparison to conventional extraction conditions because shikonin and/or its derivatives could not be eluted out of the hard cell walls of L. erythrorhizon, but an ultra-high pressure of 500 MPa effectively destroyed the cell wall and increased solvent penetrability (Bennett et al. 1998). An ultrasonic wave energy of 120 kHz or higher could also increase the diffusion and solubility of the solvent, which resulted in an increase in the shikonin contents of the extracts. A similar result was also reported elsewhere (Kim et al. 2008). For ginseng (a natural product in roots), the extraction yield and ginsenoside content were greatly increased at 200 MPa over 5 min for HPE extraction or 40 kHz over 40 min for UE extraction, which also increased their anti-oxidation activities (Zhang et al. 2007).
Table 1.
Comparison of the extraction yields of the L. erythrorhizon in relation to the different extraction processes
| Extraction process | Pre-treatment time (min) | Extraction yield (%) | Shikonin (%) |
|---|---|---|---|
| EEa | 16.32 ± 0.84 | 1.81 ± 0.15 | |
| HPEb | |||
| 300 MPa | 30 | 16.38 ± 0.67 | 1.84 ± 0.20 |
| 60 | 16.84 ± 1.05 | 1.89 ± 0.16 | |
| 90 | 17.2 ± 0.44 | 1.92 ± 0.13 | |
| 400 MPa | 30 | 17.32 ± 0.88 | 1.91 ± 0.11 |
| 60 | 18.84 ± 0.74 | 2.04 ± 0.08 | |
| 90 | 19.23 ± 0.84 | 2.08 ± 0.12 | |
| 500 MPa | 30 | 20.14 ± 0.83 | 2.30 ± 0.04 |
| 60 | 22.84 ± 0.66 | 2.50 ± 0.06 | |
| 90 | 23.06 ± 0.51 | 2.53 ± 0.12 | |
| UEc | |||
| 40 kHz | 30 | 16.43 ± 0.15 | 1.90 ± 0.08 |
| 60 | 19.09 ± 0.32 | 2.21 ± 0.09 | |
| 90 | 20.16 ± 0.48 | 2.29 ± 0.05 | |
| 80 kHz | 30 | 18.04 ± 0.58 | 2.01 ± 0.03 |
| 60 | 20.11 ± 0.33 | 2.30 ± 0.06 | |
| 90 | 23.84 ± 0.81 | 2.71 ± 0.08 | |
| 120 kHz | 30 | 21.55 ± 0.64 | 2.46 ± 0.18 |
| 60 | 23.51 ± 0.35 | 2.64 ± 0.26 | |
| 90 | 25.64 ± 0.84 | 2.90 ± 0.15 | |
| 160 kHz | 30 | 21.94 ± 0.44 | 2.47 ± 0.09 |
| 60 | 24.16 ± 0.51 | 2.66 ± 0.06 | |
| 90 | 26.03 ± 0.39 | 2.91 ± 0.11 | |
| HPE + UEd | 27.49 ± 0.91 | 3.19 ± 0.08 | |
a EE 70 % ethyl alcohol extraction at 60 °C, control. b HPE high-pressure extraction at 60 °C with 70 % ethyl alcohol solvent. c UE ultrasonication extraction at 60 °C with 70 % ethyl alcohol solvent. d HPE + UE ultrasonication extraction for 90 min at 120 kHz after high-pressure extraction for 60 min at 500 MPa and at 60 °C with 70 % ethyl alcohol solvent
Fig. 1.
HPLC chromatograms of L. erythrorhizon extracts from various extraction conditions. The red arrow indicates the shikonin peak (standard). (EE 70 % ethyl alcohol at 60 °C as a control. UE ultrasonication extraction at 60 °C with 70 % ethyl alcohol. HPE high-pressure extraction at 60 °C with 70 % ethyl alcohol. HPE + UE ultrasonication extraction for 90 min at 120 kHz after high-pressure extraction for 60 min at 500 MPa). (Color figure online)
Extract cytotoxicity against skin fibroblast cells
As shown in Fig. 2, the cytotoxicity of the extracts against CCD-986sk human skin fibroblasts increased depending on the treatment concentrations. At the 1.0 mg/ml concentration, the HPE had the highest toxicity at 24.85 % and the UE extract with HPE had the lowest cytotoxicity of 20.14 %, which was ca. 16 % lower than the 23.36 % of the ethanol extract (EE) control. In the HPE-only case, the cell toxicity increased relative to that of the EE. This finding appears to have occurred because the HPE eluted the toxic substances and the existing active substances. However, the toxicity decreased in the extracts from the UE and the UE with HPE. This finding appears to have been caused by the deformation and destruction of toxic substances in the extracts because of the cavitation induced by the combined process (Kwon et al. 2008). In the combined process, the L. erythrorhizon internal tissues were loosened by high pressure, and the ultrasonic wave energy easily penetrated the internal tissues and deformed or destroyed the toxic substances. Therefore, considering that the bulbs and tubers of L. erythrorhizon have limitations in terms of the elution of active substances during the extraction process because of their hard tissues and toxicity, HPE and UE can help obtain bioactive substances and reduce the toxicity of natural products.
Fig. 2.
Cytotoxicity of L. erythrorhizon extracts against CCD-986sk human skin fibroblasts according to the different extraction processes (EE 70 % ethyl alcohol extraction at 60 °C, control. UE ultrasonication extraction at 60 °C with 70 % ethyl alcohol solvent. HPE high-pressure extraction at 60 °C with 70 % ethyl alcohol solvent. HPE + UE ultrasonication extraction for 90 min at 120 kHz after high-pressure extraction for 60 min at 500 MPa and at 60 °C with 70 % ethyl alcohol solvent). Cytotoxicity was calculated as described in “Materials and methods” section. The values are expressed as the mean ± SD of the data from three independent experiments. Means with different letters (a–b) within the same concentration are significantly different at p < 0.05
Measurement of hyaluronidase activity inhibition
As shown in Fig. 3, the hyaluronidase inhibitory activity was examined to verify the skin immunity effect of L. erythrorhizon extracts obtained from several extraction processes because hyaluronidase is a catabolic enzyme for hyaluronic acid that contributes to keeping the skin moist and elastic and is known to be a skin immunity inhibiting factor (Ghosh 1994). In all the extracts, the inhibition of hyaluronidase increased as the treatment concentrations increased. After adding 0.2–1.0 mg/ml of the extracts, the inhibition activities were measured as 15.15–48.24 % in the EE, 15.68–55.74 % in the UE, 16.12–59.18 % in the HPE and 17.98–64.19 % in the HPE + UE. At the highest concentration (1.0 mg/ml), the UW with HPE extract or the HPE inhibited ca. 18 % more than that of the EE. This finding also appears to have occurred because HPE and UE increased the elution of bioactive substances from L. erythrorhizon related to skin immunity.
Fig. 3.
The hyaluronidase inhibition ratio of L. erythrorhizon extracts from different extraction conditions (EE 70 % ethyl alcohol at 60 °C as a control. UE ultrasonication extraction at 60 °C with 70 % ethyl alcohol. HPE high-pressure extraction at 60 °C with 70 % ethyl alcohol. HPE + UE ultrasonic extraction for 90 min at 120 kHz after high-pressure extraction for 60 min at 500 MPa). The Hyaluronidase inhibition rate was calculated as described in “Materials and methods” section. The values are expressed as the mean ± SD of the data from three independent experiments. Means with different letters (a–c) within the same concentration are significantly different at p < 0.05
Inhibition of MMP-1 expression after UVA treatment
The expression of MMP-1, which plays an important role in wrinkle creation from photo-aging, is known to increase through the signal transmission path when UVA increases the activities of JNK/p38 and transcription factor AP-1, resulting in a lack of collagen in the skin (Chung et al. 2000). In Fig. 4, which shows the addition of 1.0 mg/ml, the HPE + UE extract showed the lowest MMP-1 expression at 110.6, and 120.4 and 116.8 % in the UE extract and the HPE extract, respectively, while 126.9 % was found for the EE control. The MMP-1 expression in the ascorbic acid positive control was observed at only 92.4 %. The MMP-1 expression level increased by 148.4 % after UVA irradiation without samples, and the addition of L. erythrorhizon extracts clearly inhibited MMP-1 expression by increasing in proportion to the concentration. This finding implies that the UE and HPE extraction processes can increase skin wrinkle inhibition by effectively controlling the MMP-1 expression level caused by the external UVA stress that contributes to collagen destruction (Pathak and Stratton 1968). In addition, these results can be used as reference cosmetic substance data to prevent the destruction of skin collagen and the creation of wrinkles.
Fig. 4.
Comparison of MMP-1 production from CCD-986sk human skin fibroblast after being exposed to UVA by adding the L. erythrorhizon extracts from different extraction conditions (EE 70 % ethyl alcohol extraction at 60 °C, control. UE ultrasonication extraction at 60 °C with 70 % ethyl alcohol solvent. HPE high-pressure extraction at 60 °C with 70 % ethyl alcohol solvent. HPE + UE ultrasonication extraction for 90 min at 120 kHz after high-pressure extraction for 60 min at 500 MPa and at 60 °C with 70 % ethyl alcohol solvent. Non-UVA-control (only medium without the samples) and Ascorbic acid as a positive control). MMP1 production was calculated as described in “Materials and methods” section. The values are expressed as the mean ± SD of the data that were obtained from three independent experiments. Means with different letters (a–f) within the same concentration (1.0 mg/ml) are significantly different at p < 0.05
Measuring Prostaglandin E2 (PGE2) expression
Prostaglandin E2 is one of the metabolic products of cyclooxygenase in arachidonic acid, which originates from the cell membrane lipid and is synthesized by prostaglandin synthetase. In addition, it is synthesized by fibroblasts and then secreted to influence the growth of surrounding cells and inflammatory cells, protein synthesis, and collagen synthesis (Kim and Jo 1990). Figure 5 shows that PGE2 expressions were observed at 2,250 and 820 pg/ml when they were and were not UV-irradiated, respectively. In both conditions, the PGE2 expression decreased following the addition of the L. erythrorhizon extract, depending on the treatment concentration; Fig. 5a shows 514 pg/ml for the lowest PGE2 expression without UV irradiation in adding 1.0 mg/ml of the UE + HPE extract while 525, 549 and 582 pg/ml of PGE2 production were observed in HPE, UE and EE, respectively. Figure 5b also illustrates that PGE2 production with UV irradiation was decreased as the treatment concentration increased by generating 933, 977, 1,002 and 1,092 pg/ml of PGE2 in HPE, UE and EE, respectively. These results were similar to results from a study on Radix sophorae tonkinensis extract (Kim et al. 2007). Especially for the case with UV irradiation, the L. erythrorhizon effectively reduced PGE2 production by yielding ca. 932 pg/mL after addition of 1.0 mg/mL of the extract, whose results seemed to be superior to those when using Perilla drutescens extracts (Kim et al. 2006a, b). These data indicate that the secretion of IL-1 or TNF caused by UV exposure might have been inhibited by a specific peptide, the PLA2 activity might have been directly inhibited later, or the final process wherein the inflammatory substance, namely arachidonic acid, was secreted from phospholipids and might have been inhibited. Thus, it is expected that the extracts from the UE + HPE process, which produced a relatively low PGE2, can be used as a valuable cosmetic substance that greatly improves skin immunity (Jeong et al. 2010).
Fig. 5.
Effect of extracts obtained by different extraction conditions on the PGE2 secretion by human CCD-986sk fibroblasts in the a) absence and b) presence of UV irradiation. (EE 70 % ethyl alcohol extraction at 60 °C, control. UE ultrasonication extraction at 60 °C with 70 % ethyl alcohol solvent. HPE high-pressure extraction at 60 °C with 70 % ethyl alcohol solvent. HPE + UE ultrasonication extraction for 90 min at 120 kHz after high-pressure extraction for 60 min at 500 MPa and at 60 °C with 70 % ethyl alcohol solvent). The values are expressed as the mean ± SD of the data that were obtained from three independent experiments
Whitening activities of extracts from various extraction treatments
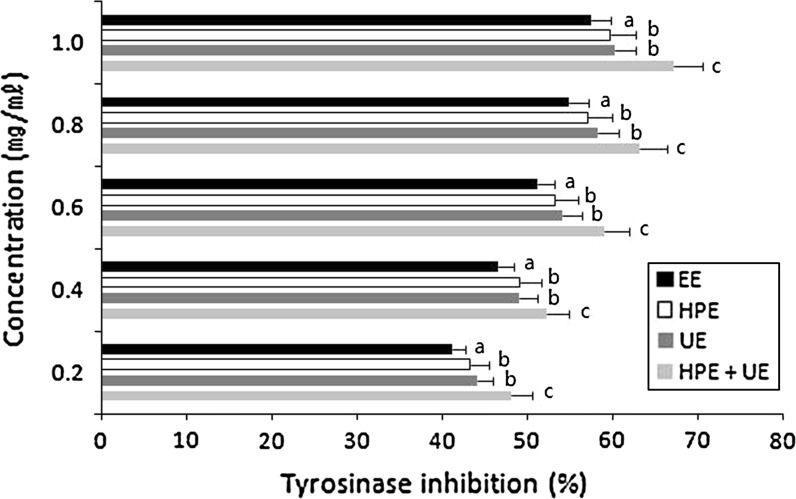
Melanin is produced from the biosynthesis of tyrosinase in skin cells, and it improves skin resistance to ultraviolet rays, dryness and extreme temperatures; however, too much melanin production leads to pigmentations, such as freckles and liver spots, and to skin damage. Therefore, the reduction of melanin biosynthesis by tyrosinase has been used as an indicator of the whitening activities of natural products (Ishihara et al. 1993) As shown in Fig. 6, all of the L. erythrorhizon extracts generally had inhibition effects of at least 40 % at 0.2 mg/ml, and the UE + HPE extract specifically inhibited ca. 67.15 % of the tyrosinase activity after adding 0.2 mg/ml, which was 20 % higher than the 57.48 % in the EE extract. Its inhibition activity increased with the increase in the concentration. This inhibition activity is higher than the case of Jung et al. (1995), in which the tyrosinase inhibition activity was approximately 30 % of that in the mume fruit, cinnamon, morus bark, cuscuta seed, etc. This result also indicates that the HPE + UE extract has a greater whitening effect by effectively reducing tyrosinase activity. In addition to the tyrosinase inhibition activity observation, the reduction in Clone M-3 cell melanin production after sample treatment has also been employed to measure the whitening activities of natural products (Komiyama et al. 1993). Therefore, to examine melanin synthesis in Clone M-3 cells, the extracts were treated for 3 days at concentrations of 0.2, 0.4, 0.6, 0.8 and 1.0 mg/ml, as shown in Fig. 7. However, the UE + HPE extract had 79.5 % of the highest inhibition ratio of melanin production compared to the production of melanin without any treatment as a control relative to the 1.0 mg/ml concentrations. This result indicates that the extract from the UE + HPE process has great potential whitening activity because ascorbic acid, a positive control, inhibited only 77.4 % of the inhibition ratio of melanin production in the same treatment concentration. In general, the EE process extract did not show inhibitory activity relative to those from the UE, HPE and UE + HPE processes. The HPE + UE extract resulted in an 80 % or lower melanin production compared with 87.5 % of the EE extract, especially at the highest (1.0 mg/ml) concentration, which indicates that the extract from UE + HPE effectively inhibits melanin production.
Fig. 6.
Tyrosinase inhibitory activity of L. erythrorhizon extracts according to the different extraction conditions (EE 70 % ethyl alcohol extraction at 60 °C, control. UE ultrasonication extraction at 60 °C with 70 % ethyl alcohol solvent. HPE high-pressure extraction at 60 °C with 70 % ethyl alcohol solvent. HPE + UE ultrasonication extraction for 90 min at 120 kHz after high-pressure extraction for 60 min at 500 MPa and at 60 °C with 70 % ethyl alcohol solvent). The tyrosinase inhibition rate was calculated as described in “Materials and methods” section. The values are expressed as the mean ± SD of the data that were obtained from three independent experiments. Means with a different letter (a–c) within the same concentration are significantly different at p < 0.05
Fig. 7.
Effects of the extracts from different extraction conditions on melanin production in Clone M-3 cells (EE 70 % ethyl alcohol extraction at 60 °C, control. UE ultrasonication extraction at 60 °C with 70 % ethyl alcohol solvent. HPE high-pressure extraction at 60 °C with 70 % ethyl alcohol solvent. HPE + UE ultrasonication extraction for 90 min at 120 kHz after high-pressure extraction for 60 min at 500 MPa and at 60 °C with 70 % ethyl alcohol solvent). The relative melanin production rate was calculated as described in “Materials and methods” section. The values are expressed as the mean ± SD of the data from three independent experiments. Means with different letters (a–c) within the same concentration are significantly different at p < 0.05
Comparison of cosmetic activities with extraction yields under several different extraction conditions
Table 2 and Fig. 8 compare the extract yields with the various cosmetic activities of extracts obtained from several extraction processes to determine the optimal extraction conditions for L. erythrorhizon. The extraction yield was estimated as 16.32 % in the EE process, 22.64 % for only HPE at 500 MPa for 60 min, 22.84 % in the UE at 120 kHz for 90 min, and the highest was 27.49 % in the HPE + UE condition. In comparison with the extraction yields, the shikonin content was also the highest at 3.19 % in the HPE + UE extract. The hyaluronidase inhibition activity that is related to skin immunity was 48.2 % in the EE extract, but it was the highest at 64.2 % in the HPE + UE process. For the skin whitening activities, the highest tyrosinase inhibition activity was observed as 67.2 % in the UE + HPE, which is ca. 10 % higher than that of the EE (57.5 %). To inhibit melanin production, 87.5 % melanin production was observed following the addition of EE relative to the control without a sample, and 80.1, 79.4 and 78.5 % were found in the UE, HPE and UE + HPE conditions, respectively. Thus, it can be concluded that the highest extraction yield from the UE + HPE process results in the highest cosmetic activities relative to those from other processes by eluting the highest amount of the shikonin bioactive compound from L. erythrorhizon. It appears that the treatment at 500 MPa, the optimal pressure for this HPE process, effectively destroyed the hard cell walls, which resulted in increased solvent penetrability and the elution of its useful components. The 120 kHz treatment, which was also determined to be the optimal ultrasonic wave intensity, increased the diffusion and solubility of the solvent and accelerated the elution of useful components from L. erythrorhizon. These results are very similar to other reported data that have shown that an increased shikonin extraction yield is related to the enhancement of biological activities, such as anticancer (Ruifa and Jie 2012), antioxidant (Rajasekar et al. 2012), atopic dermatitis (Ju et al. 2010) and wound healing (Min et al. 2005) properties.
Table 2.
Comparison of the extraction yields, shikonin contents, and cosmetic activities of the L. erythrorhizon specimens in relation to the different extraction processes
| Extraction process | ||||
|---|---|---|---|---|
| EEa | UEb | HPEc | HPE + UEd | |
| Extraction yield (%) | 16.32 ± 0.84 | 25.64 ± 0.84 | 22.84 ± 0.66 | 27.49 ± 0.91 |
| Shikonin (%) | 1.81 ± 0.15 | 2.90 ± 0.15 | 2.50 ± 0.06 | 3.19 ± 0.08 |
| Hyaluronidase inhibitory activity (%) | 48.24 ± 1.31 | 55.74 ± 1.58 | 59.18 ± 1.44 | 64.19 ± 1.85 |
| MMP-1 contents (%) | 559.94 ± 2.11 | 522.17 ± 1.38 | 514.23 ± 1.89 | 472.11 ± 2.68 |
| PGE2 secretion | ||||
| Non-UV PGE2 level (pg/ml) | 571.2 ± 1.87 | 539.9 ± 1.65 | 522.1 ± 2.08 | 514.2 ± 1.27 |
| UV PGE2 level (pg/ml) | 1,040.0 ± 1.57 | 972.1 ± 0.98 | 964.5 ± 1.56 | 912.3 ± 2.17 |
| Tyrosinase inhibitory activity (%) | 57.48 ± 1.05 | 59.74 ± 1.21 | 60.22 ± 0.74 | 67.15 ± 0.59 |
| Melanin production (%) | 87.5 ± 0.84 | 80.1 ± 0.54 | 79.4 ± 0.62 | 78.5 ± 0.71 |
a EE 70 % ethyl alcohol extraction at 60 °C, control. b UE ultrasonication extraction at 60 °C with 70 % ethyl alcohol solvent. c HPE high-pressure extraction at 60 °C with 70 % ethyl alcohol solvent. d HPE + UE ultrasonication extraction for 90 min at 120 kHz after high-pressure extraction for 60 min at 500 MPa and at 60 °C with 70 % ethyl alcohol solvent
Fig. 8.
Optimization of the cosmetic activities of the different extract samples generated by for different conditions with respect to extraction yield, shikonin contents, melanin production, and tyrosinase and hyalurodinase inhibition as follows: EE 70 % ethyl alcohol extraction at 60 °C, control; UE ultrasonication extraction at 60 °C with 70 % ethyl alcohol solvent; HPE high-pressure extraction at 60 °C with 70 % ethyl alcohol solvent; HPE + UE ultrasonication extraction for 90 min at 120 kHz after high-pressure extraction for 60 min at 500 MPa and at 60 °C with 70 % ethyl alcohol solvent
Discussion
This study was the first report to indicate that the various cosmetic activities of L. erythrorhizon relating to the extraction yield and shikonin content can be enhanced by optimizing the extraction conditions, such as the pressure, ultrasonic wave intensity, temperature and treatment time. A treatment employing HPE at 500 MPa for 60 min, after which UE was conducted at 120 kHz for 90 min, produced the highest yield and shikonin content as well as the highest whitening activity and skin immunity. This finding indicates that ultra-high pressure effectively destroyed the tissues and cell walls of the hard-surfaced plant L. erythrorhizon, which resulted in an improved elution of the active cell components. It appears that the higher pressure of the HPE process especially loosened the hard tissues that prevent the elution of active components and increased the area of contact with the solvent to accelerate active component diffusion and elution (Zhang et al. 2007). In addition, the high input energy produced by the cavitation from the ultrasonic device destroyed the inner tissues of the cells, which shortened the travel distance of the extracts, facilitated solvent diffusion, and increased solubility. The resulting breakage of bonds between the atoms in the high molecular polymer might have contributed to the elution of substances that were not easily eluted using the existing methods (Kim et al. 2001; Kim and Choi 2009). Therefore, the HPE + UE process can maintain the synergistic effect that improves both the extraction yield and the elution of shikonin, which resulted in an increase of its cosmetic activities, such as skin immunity and whitening, and also expands the use of this natural dye for multifunctional cosmetics.
Acknowledgments
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry of Health & Welfare, Republic of Korea (Grant NO. : A103017).
References
- Bai JH. Antimicrobial effect of Lithospermum erythorhizon extract on the food-borne pathogens. Korean J Food Sci Technol. 2004;36:823–827. [Google Scholar]
- Bennett PB, Marquis RE, Demchenko I. High pressure biology and medicine. New York: University of Rochester Press; 1998. [Google Scholar]
- Bernstein EF, Chen YQ. Enhanced elastin and fibrillin gene expression in chronically photo-damaged skin. J Invest Dermatol. 1994;103:182–186. doi: 10.1111/1523-1747.ep12392693. [DOI] [PubMed] [Google Scholar]
- Chen X, Yang L, Zhang N, Turpin JA, Buckheit RW, Osterling C, Oppenheim JJ, Howard OM. Shikonin, a component of Chinese herbal medicine, inhibits chemokine receptor function and suppresses human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2003;47:2810–2816. doi: 10.1128/AAC.47.9.2810-2816.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho WG. Comparison of drug delivery using hairless and pig skin. J Korean Oil Chem Soc. 2007;24:410–415. [Google Scholar]
- Chung KW, Kim WI, Hong IK, Park KA. Ultrasonic energy effects on squalene extraction from amaranth seed. Appl Chem. 2000;4:149–152. [Google Scholar]
- Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. Food Chem Toxicol. 1983;21:512–513. [PubMed] [Google Scholar]
- Dunsmore SE, Rubin JS, Kovacs SO, Chedid M, Parks WC, Welgus HG. Mechanisms of hepatocyte growth factor stimulation of keratinocyte metalloproteinase production. J Biol Chem. 1996;271:24576–24582. doi: 10.1074/jbc.271.40.24576. [DOI] [PubMed] [Google Scholar]
- Ghosh P. The role of hyaluronic acid in health and disease: interactions with cells, cartilage and components of synovial fluid. Clin Exp Rheumatol. 1994;12:82–85. [PubMed] [Google Scholar]
- Ishihara K, Takemura T, Hmada Y, Sakai C, Kondoh S, Nishiyama S, Urabe K, Hearing J. Pigment production in murine melanoma cell is regulated by tyrosinase, tyrosinase-related protein 1 (TRP1), DOPA chrome tautomerase (TRP2) and a melanogenic inhibitor. J Invest Dermatol. 1993;2:126–131. doi: 10.1111/1523-1747.ep12462778. [DOI] [PubMed] [Google Scholar]
- Jeong MH, Kim SS, Kim JS, Lee HJ, Choi GP, Lee HY. Skin whitening and skin immune activities of different parts of Acer mono and Acer okamotoanum. J Korean Soc. 2010;99:470–478. [Google Scholar]
- Ju JH, Cho HH, Lee YS. Progress on phytochemical and atopic dermatitis-related study of the root of Lithospermum erythrorhizon. Korean J Pharm. 2010;41:73–88. [Google Scholar]
- Jung SW, Lee NK, Kim SJ, Han D. Screening of tyrosinase inhibitor from plants. Korean J Food Sci Technol. 1995;27:891–896. [Google Scholar]
- Kang JH. The anti-inflammatory effect of Lithospermi Radix and suppression of iNOS and TNF-alpha production. Seoul: Kyunghee University; 2005. [Google Scholar]
- Kim MN. Studies on the constituents of Lithospermum erythrorhizon. J Sun Moon Univ Nat Sci. 2001;4:25–39. [Google Scholar]
- Kim SY, Cho JA. Research paper: a study based on natural dyes usage shown with shade makeup. J Soc Cosmet Sci Korea. 2008;14:106–113. [Google Scholar]
- Kim YS, Choi JM. Physiochemical properties and dyeability of safflower colorants extracted by ultrasonic treatment. J Korean Soc Cloth Ind. 2009;11:337–343. [Google Scholar]
- Kim IS, Jo JS. Modulation of human fibroblast proliferation and collegan production by prostaglandin E2: association of intracelluar cyclic AMP and changes in prostaglandin E2 responsiveness. Korean Biochem J. 1990;23:40–52. [Google Scholar]
- Kim WI, Chung KW, Lee SB, Hong IK, Park KA. Ultrasound energy effect on solvent extraction of amaranth seed oil. J Korean Ind Eng Chem. 2001;12:307–311. [Google Scholar]
- Kim JS, Han YS, Kang MH. Identification of shikonin and its derivatives from Lithospermum erthrorhizon. J Korean Soc Food Sci Nutr. 2006;35:177–181. doi: 10.3746/jkfn.2006.35.2.177. [DOI] [Google Scholar]
- Kim SN, Lee EJ, Lee HJ, Nam GS, Kim HS, Hwang SW, Hwang SY. Effect on inflammatory-cytokines production inhibition and analgesic activity of Perilla Frutescens extracts. Korean J Physiol Pathol. 2006;20:414–419. [Google Scholar]
- Kim KT, Eom HS, Chi GY. Antiproliferative effect of RST associated with the inhibition of cyclooxygenase-2 expression and prostaglandin E2 release in human lung carcinoma cells. Korean J Physiol Pathol. 2007;21:907–915. [Google Scholar]
- Kim CH, Kwon MC, Han JG, Na CS, Kwak HG, Choi GP, Park UY, Lee HY. Skin-whitening and UV protective effects of Angelica gigas Nakai extracts on ultra high pressure extraction process. Korean J Med Crop Sci. 2008;16:255–260. [Google Scholar]
- Komiyama K, Takamatsu S, Yakahashi Y, Shinse M, Hayashi M, Tanaka H, Iwai Y, Omura S. New inhibitors of melanogenesis, OH-3084 K1 and K2. J Antibiot. 1993;46:1520–1525. doi: 10.7164/antibiotics.46.1520. [DOI] [PubMed] [Google Scholar]
- Koo SY, Cha KH, Lee DU. Effects of high hydrostatic pressure on foods and biological system. Food Sci Ind. 2007;40:23–30. [Google Scholar]
- Kwon MC, Han JG, Ahn JH, Lee DH, Syed Abdul Q, Lee HY. Enhancement of immuno-potentiation of Cichorium endivia L. by ultrasonification extraction process. Korean J Med Crop Sci. 2008;16:9–15. [Google Scholar]
- Lee JH. The antioxidizing, nitrate-scavenging and antimutagenic activities of Ecklonia stolonifea. MS Theis: Bukyong National University, Busan, Korea; 1996. [Google Scholar]
- Lee KS, Ahn DK, Shin MK, Kim CM (1988) In: The encyclopedia of oriental herbal medicine. Jungdam Pubishing Co., Seoul, Korea 381-383
- Liou JY, Ellent DP, Lee S, Goldsby J, Ko BS, Matijevic N, Huang JC, Wu KK. Cyclooxygenase-2-derived prostaglandin E2 protects mouse embryonic stem cells from apoptosis. Stem Cells. 2007;25:1096–1103. doi: 10.1634/stemcells.2006-0505. [DOI] [PubMed] [Google Scholar]
- Min DH, Kim DK, Lim JP, Yang JH. Transdermal drug delivery and therapeutic effect of the preparations of Lithospermi Radix and Gardeniae fructus extracts on the burn and wound healing. J Korean Pharm Sci. 2005;35:255–263. [Google Scholar]
- Park YH, Lee CS. Efficacy of safflower on the acne skin and its application for facial cleansing biomedical material. J Korean Chem Soc. 2011;55:400–404. doi: 10.5012/jkcs.2011.55.3.400. [DOI] [Google Scholar]
- Park BR, Kim KS, Park HR, Shin KO, Ahn KM, Kim KY. A study on lip-balm usability from Lithospermum erthrohizon. J Korean Soc Cosmetol. 2010;6:239–247. [Google Scholar]
- Pathak MA, Stratton K. Free radical in human skin before and after exposure to light. Arch Biochem Biophys. 1968;123:468–476. doi: 10.1016/0003-9861(68)90168-9. [DOI] [PubMed] [Google Scholar]
- Pomerantz SH. Separation, purification and properties of two tyrosinases from hamster melanoma. J Biol Chem. 1963;238:2351–2357. [PubMed] [Google Scholar]
- Rajasekar S, Park DJ, Park C, Park SJ, Park YH, Kim ST, Choi YH, Choi YW. In vitro and in vivo anticancer effects of Lithospermum erythrorhizon extract on B16F10 murine melanoma. J Ethnopharmacol. 2012;144:335–345. doi: 10.1016/j.jep.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Reissig JL, Stronminger JL, Leloir LF (1955) A modified colorimetric method for the estimation of N-acetyl glucosamine sugars. J Biol Chem 217:959–966 [PubMed]
- Ruifa J, Jie L. Theoretical study of the radical scavenging activity of Shikonin and its derivatives. Chin J Chem. 2012;30:84–90. doi: 10.1002/cjoc.201100044. [DOI] [Google Scholar]
- Sasaki K, Abe H, Yoshizaki F. In vitro antifungal activity of naphthoquinone derivatives. Biol Pharm Bull Sci. 2002;25:669–670. doi: 10.1248/bpb.25.669. [DOI] [PubMed] [Google Scholar]
- Sekine T, Masumizu T, Maitani Y, Nagai T. Evaluation of superoxide anion radical scavenging activity of shikonin by electron spin resonance. Int J Pharm. 1998;174:133–139. doi: 10.1016/S0378-5173(98)00256-7. [DOI] [Google Scholar]
- Staniforth V, Wang SY, Shyur LF, Yang NS. Shikonins, phytocompounds from Lithospermum erythorhizon, inhibit the transcriptional activation of human tumor necrosis factor alpha promoter in vivo. J Biol Chem. 2004;279:5877–5885. doi: 10.1074/jbc.M309185200. [DOI] [PubMed] [Google Scholar]
- Yoon KJ, Lee HW, Yook CS. Studies on the constitutements and their antibacterial effect of the root of Lithospermum erythrorhizon Sieb. et Zucc. Bull Kyung Hee Pharm Sci. 1988;16:155–161. [Google Scholar]
- Yun KJ, Kim DH, Ryu JH, Yook CS. Studies on the constituents and their antibacterial effect of the root of Lithospermum erythorhizon. Bull Kyung Hee Pharm Sci. 1999;27:27–31. [Google Scholar]
- Zhang S, Zhu J, Wang C. Novel high pressure extraction technology. Int J Pharm. 2004;278:471–474. doi: 10.1016/j.ijpharm.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Zhang S, Chen R, Wang C. Experiment study on ultrahigh pressure extraction of Ginsenosides. J Food Eng. 2007;79:1–5. doi: 10.1016/j.jfoodeng.2005.12.048. [DOI] [Google Scholar]