Abstract
Bisphenol A (BPA) is an endocrine disrupting chemical used on a wide range in industry. Several studies reported that BPA may cause cardiovascular disorders in humans and animals. The present study aims to investigate the effect of BPA on the heart of adult male rats. The rats received a daily oral administration of BPA (25 mg/kg for 6 weeks and 10 mg/kg for 6 and 10 weeks). It was found that BPA at the two studied doses induced a significant increase in malondialdehyde, and a significant decrease in catalase after 6 weeks. Moreover, a significant decrease in reduced glutathione and acetylcholinesterase (AchE) activity was observed after treatment with the two doses of BPA throughout the studied time intervals. The two doses (25 and 10 mg/kg) resulted in a significant decrease in nitric oxide (NO) levels after 6 and 10 weeks, respectively. A significant increase in body weight gain occurred in all animals after BPA treatment. These results suggest that BPA has cardiotoxic effects which are mediated by the oxidative stress resulting from the overproduction of free radicals, the deficiency of NO and the inhibition of AchE leading to cholinergic activation. The obesity promoting effect of BPA may also participate in the observed cardiovascular disturbances.
Keywords: Bisphenol A, Heart, Oxidative stress, Acetylcholinesterase, Body weight-rat
Introduction
Bisphenol A (BPA) is one of the world’s highest production volume chemicals (Ritter 2011) used in polycarbonate plastics in many consumer products and epoxy resins lining food and beverage containers (EU 2008). The global population is subject to repeated exposure to BPA, primarily through packaged food but also through drinking water, dental sealants, dermal exposure, and inhalation of household dusts (Lakind and Naiman 2008) with detectable concentrations of metabolites in the urine of >90 % of the population worldwide (Calafat et al. 2005; Ye et al. 2008). Heat, repeated washing of polycarbonate products and contact with either acidic or basic compounds accelerate hydrolysis of the ester bond linking BPA molecules in polycarbonate plastics and resins resulting in an increase in the rate of leaching of BPA (Lim et al. 2009). In addition, another potential source of human exposure is water used for drinking or bathing. Studies conducted in Japan (Kawagoshi et al. 2003) and in the United States (Coors et al. 2003) have shown that BPA accounts for most estrogenic activity that leaches from landfills into the surrounding ecosystem.
BPA has been demonstrated in both in vivo and in vitro experiments to act as an endocrine disrupting chemical (vom Saal and Hughes 2005). The actions of chemicals such as BPA are mediated by endocrine-signaling pathways that evolved to act as powerful amplifiers, with the result that large changes in cell function can occur in response to extremely low concentrations (Welshons et al. 2003).
In humans, increased levels of BPA in adults have been correlated with various diseases, health outcomes and medical conditions. To date, reported health complications associated with increased levels of BPA exposure include diabetes (Lang et al. 2008), cardiovascular disease (Lang et al. 2008; Melzer et al. 2010), altered liver enzymes as increases in alanine aminotransferase and aspartate aminotransferase (Lang et al. 2008; Mourad and Khadrawy 2012) and obesity-promoting effects (Ropero et al. 2008, Trasande et al. 2012; Harley et al. 2013). Contributing to the potential for altered metabolic homeostasis, BPA has been shown to alter glucose homeostasis, increase pancreatic insulin content and induce insulin resistance in adult male mice (Alonso-Magdalena et al. 2006). Moreover, several studies have shown that BPA induces oxidative stress in vital organs as the liver, kidney and testis (Bindhumol et al. 2003; Chitra et al. 2003; Kabuto et al. 2004; Mourad and Khadrawy 2012).
There has been increasing interest in the concept that oxygen free radicals and nitric oxide (NO) play an important role in the pathogenesis of cardiovascular diseases (Das 2000). The cellular sources of reactive oxygen species (ROS) generation within the heart include cardiac myocytes, endothelial cells, and neutrophils (Tsutsui et al. 2009). The heart has the highest oxygen uptake rate within the human body, consuming about 0.1 ml O2/g per minute at basal rates (Antoni 1991). To meet the demand for synthesis of ATP by oxidative metabolism, cardiac myocytes have the highest volume density of mitochondria in the entire body. Under physiological conditions, small quantities of ROS are formed during mitochondrial respiration, which, however, can be detoxified by the endogenous scavenging mechanisms of myocytes. However, when the production of ROS exceeds the capacity of antioxidant defenses, oxidative stress might have a harmful effect on the functional and structural integrity of biological tissue. ROS cause contractile failure and structural damage in the myocardium (Tsutsui et al. 2009) possibly through the oxidation of membrane phospholipids, proteins, and DNA (McCord 1985).
Acetylcholinesterase (AchE) is an important component of the heart’s cholinergic system; it is known to regulate the cardiac parasympathetic responses by controlling acetylcholine levels (Hoover et al. 2004). Normally, AchE rapidly and efficiently degrades acetylcholine, thereby terminating its signaling action (Lefkowitz et al. 1996).
Nitric oxide as a gaseous free radical acts like a neurotransmitter and effective cardiovascular modulator. This gas plays a fundamental role in cardiovascular physiology and pathophysiology (Shah et al. 1999). Within the cardiovascular system, NO participates in the regulation of coronary blood flow and tension of vessel wall (Roy et al. 2005).
Several investigators found that higher BPA concentrations were associated with cardiovascular diagnoses (Lang et al. 2008; vom Saal and Myers 2008; Melzer et al. 2010) and incident coronary artery disease (Melzer et al. 2012). In addition, Asano et al. (2010) reported that BPA in the micromolar range activates Maxi-K (KCa1.1) ion channels in human coronary smooth muscle cells in culture to a degree sufficient to hyperpolarize the membrane potential. Echocardiography identified concentric remodeling in all BPA-treated males (Patel et al. 2013). The authors found that systolic and diastolic cardiac functions were essentially similar, but lifelong BPA enhanced male and reduced female sex-specific differences in velocity of circumferential shortening and ascending aorta velocity time integral while diastolic blood pressure (BP) was increased in all BPA females. They suggested that continual exposure to BPA impacts cardiac structure/function, protein expression, and epigenetic DNA methylation marks in males and females.
Bae et al. (2012) reported that exposure to BPA is associated with increased BP and decreased heart rate (HR) variability, which are risk factors of cardiovascular disorders. They found that the risk of hypertension increased with increasing concentrations of BPA in participants who had not reported previous history of hypertension. The authors suggested that these results have important implications in public health perspectives because of the almost ubiquitous usage and exposure of BPA.
The FDA recently indicated some concern about the safety of BPA and announced that more research is needed (Erickson 2010). Health Canada (2008) has also banned it from baby bottles. Similarly, the American Endocrine Society recommended further research on endocrine-disrupting chemicals including BPA, citing a strong basis for concern about possible links between these chemicals, obesity, and related disorders (Diamanti-Kandarakis et al. 2010). However, there is to-date no literature available on the effect of BPA on cardiac oxidative stress parameters and AchE activity.
The present study aims to investigate the effect of the daily oral administration of BPA at two dose levels (25 mg/kg for 6 weeks and 10 mg/kg for 6 and 10 weeks) on some oxidative stress parameters as malondialdehyde (an indicator of lipid peroxidation), reduced glutathione and NO levels and catalase and glutathione-S-transferase (GST) activities in the heart of adult male albino rats. In addition, the effects of the two doses of BPA on cardiac AchE activity and body weight of rats were also determined.
Materials and methods
Materials
Animals
Adult male Wistar albino rats weighing 120–180 g were used as experimental animals. The animals were obtained from the animal house of the National Research Center (Cairo, Egypt). They were maintained on stock diet and kept under fixed appropriate conditions of housing and handling. All experiments were carried out in accordance with the research protocols established by the Animal Care Committee of the National Research Center (Cairo, Egypt), which followed the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985).
Chemicals
Pure BPA powder was purchased from Sigma (St. Louis, MO, USA) and suspended in distilled water. Reagents used for the measurement of the different parameters were also obtained from Sigma (USA).
Experimental design
A total of 40 animals were divided randomly into 4 groups. Group (1) served as control and received an oral administration of distilled water, five times a week, throughout the experimental protocol. They were divided to group 1–6 W which received distilled water for 6 weeks and group 1–10 W which were administered distilled water for 10 weeks. In Group (2), rats were administered orally with 25 mg/kg of BPA daily for 6 weeks. Animals of groups (3) and (4) received an oral administration of 10 mg/kg of BPA for 6 and 10 weeks, respectively. The doses of BPA were administered five times a week. The higher dose of BPA (25 mg/kg) in this study was chosen on the base of previous studies (Bian et al. 2006; Richter et al. 2007). A group of the control animals was sacrificed simultaneously with each group of the treated animals.
Handling of tissue samples
Both treated and control animals were sacrificed after being fasted. The heart of each animal was quickly removed, washed and rapidly weighed and frozen until analyzed. The heart tissue was homogenized in 10 ml of ice cold phosphate buffer (50 mM pH 7.4, 0.1 % triton X and 0.5 mM EDTA). The homogenates were centrifuged at 1,753g for 15 min at 4 °C using a high speed cooling centrifuge (Type 3 K-30, Sigma, Osterode-am-Harz, Germany). The clear supernatants were separated and used for analysis.
Methods
Determination of lipid peroxidation
Lipid peroxidation was assayed by measuring the thiobarbituric-acid-reactive substances (TBARS) in heart homogenates, using the method of Ruiz-Larrea et al. (1994) in which the TBARS react with thiobarbituric acid to produce a red colored complex having peak absorbance at 532 nm and analyzed in a Helios Alpha Thermospectronic (UVA 111615, Cambridge, England) spectrophotometer.
Determination of reduced glutathione
Reduced glutathione (GSH) was determined in heart tissue by Ellman’s method (1959). The procedure is based on the reduction of Ellman’s reagent by –SH groups of GSH to form 2-nitro-S-mercaptobenzoic acid, the nitromercaptobenzoic acid anion has an intense yellow color which can be determined spectrophotometrically at 412 nm. GSH concentration was calculated by comparison with a standard curve.
Determination of nitric oxide level
Nitric oxide levels, measured as nitrite, were determined using Griess reagent according to the method of Moshage et al. (1995), where nitrite, a stable end product of the NO radical, is primarily used as an indicator for the production of NO. Nitrite is converted to a deep purple azo compound after the addition of Griess reagents. The purple/magenta color developed is read at 540 nm. The quantity was measured via a standard curve.
Determination of enzyme activities
Catalase activity was measured using the Biodiagnostic Kit No. CA 25 17 (Giza, Egypt) which is based on the spectrophotometric method described by Aebi (1984). Catalase reacts with a known quantity of hydrogen peroxide and the reaction is stopped after 1 min with catalase inhibitor. In the presence of peroxidase, the remaining hydrogen peroxide reacts with 3,5-dichloro-2-hydroxybenzene sulfonic acid and 4-aminophenazone to form a chromophore with a color intensity inversely proportional to the amount of catalase in the sample. The absorbance was measured at 510 nm.
Glutathione-S-transferase was assayed by the method of Habig et al. (1974) which measures the conjugation of 1-chloro-2,4-dinitrobenzene with reduced glutathione. This conjugation is accompanied by an increase in absorbance at 340 nm, the rate of increase being directly proportional to GST activity.
The procedure used for the determination of AchE activity in the heart was a modification of the method of Ellman et al. (1961) as described by Gorun et al. (1978). The principle of the method is the measurement of the thiocholine produced as acetylthiocholine is hydrolyzed. The color was read immediately at 412 nm.
Determination of body weight
The body weight was measured daily for each rat then the body weight gain was estimated by subtracting the initial body weight from the final body weight for each rat.
Statistical analysis
The data were expressed as mean ± standard error of mean (SEM). All variables were tested for normal distribution and compared using analysis of variance (ANOVA) followed by the Duncan multiple range test when the F test was significant (p < 0.05). All analyses were performed using the statistical package for social sciences (SPSS) software in a PC-compatible computer and the significance was set at p < 0.05.
Results
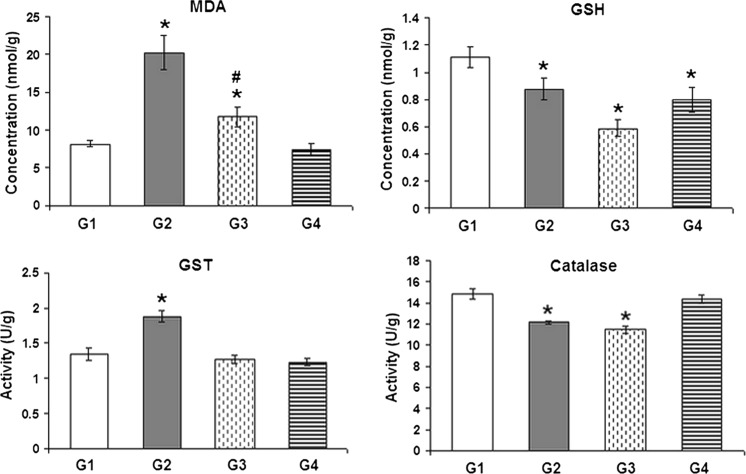
ANOVA revealed significant differences between the four groups in all tested parameters. As shown in Fig. 1, the oral administration of BPA at the two dose levels (25 and 10 mg/kg, five times a week) resulted in a significant increase in malondialdehyde (MDA) levels after 6 weeks when compared to the control values. Moreover, MDA levels in animals receiving 25 mg/kg of BPA also showed a significant increase when compared to animals administered 10 mg/kg of BPA. However, a significant decrease in GSH levels below the control values occurred after the two doses of BPA, recording −20.72 % after the administration of 25 mg/kg of BPA for 6 weeks and −46.85 % and −27.93 % after treatment with 10 mg/kg of BPA for 6 and 10 weeks, respectively. Moreover, the oral administration of 25 and 10 mg/kg of BPA (five times a week) decreased catalase activity in the heart by 17.95 and 22.47 % (p < 0.05) after 6 weeks, respectively. However, the only significant increase in GST activity was obtained in rats treated with the highest dose level of BPA (25 mg/kg) for 6 weeks, recording 39.26 % above the control.
Fig. 1.
The effect of BPA on malondialdehyde (MDA) and reduced glutathione (GSH) levels and the activities of glutathione-S-transferase (GST) and catalase in the heart of male albino rats. G1 control, G2 BPA-treated (25 mg/kg for 6 weeks), G3 BPA-treated (10 mg/kg for 6 weeks), G4 BPA-treated animals (10 mg/kg for 10 weeks). Asterisk Significant difference with respect to the control, Number sign significant difference with respect to the highest dose (25 mg/kg)
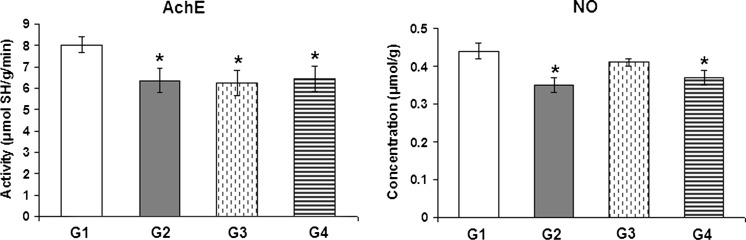
As clear from Fig. 2, cardiac NO levels recorded a significant decrease after the administration of 25 mg/kg of BPA for 6 weeks (−20.46 %) and 10 mg/kg for 10 weeks (−15.91 %) as compared to the control levels. Meanwhile, the oral administration of BPA for 6 weeks (five times a week) decreased AchE activity by 21.14 % after administration of the highest dose (25 mg/kg) and by 22.26 % after administration of the lowest dose (10 mg/kg) when compared to the control values. In addition, a decrease in AchE activity by 19.78 % was recorded after the daily oral administration of 10 mg/kg of BPA for 10 weeks.
Fig. 2.
The effect of BPA on acetylcholinesterase activity (AchE) and nitric oxide (NO) levels in the heart of male albino rats. G1 control, G2: BPA-treated (25 mg/kg for 6 weeks), G3: BPA-treated (10 mg/kg for 6 weeks), G4: BPA-treated animals (10 mg/kg for 10 weeks). Asterisk Significant difference with respect to the control
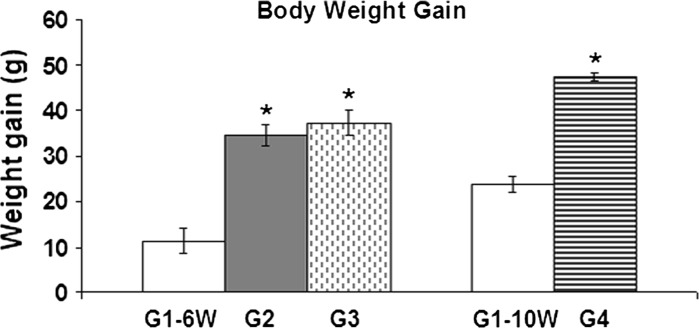
As indicated by ANOVA, the oral treatment with the two tested dose levels (25 and 10 mg/kg, five times a week) resulted in a significant increase in body weight gain after all investigated time intervals (Fig. 3). The body weight gain showed an increase from 11.43 ± 0.6 g in the control rats to 34.63 ± 2.31 g after the administration of 25 mg/kg of BPA and was increased to 37.33 ± 2.64 g after treatment with 10 mg/kg for 6 weeks. After 10 weeks, the body weight gain increased from 23.67 ± 1.67 g in control rats to 47.47 ± 1.04 g after 10 mg/kg of BPA.
Fig. 3.
The effect of BPA on the body weight gain of male albino rats. G1–6W control after 6 weeks, G1–10W control after 10 weeks, G2 BPA-treated (25 mg/kg for 6 weeks), G3 BPA-treated (10 mg/kg for 6 weeks), G4 BPA-treated animals (10 mg/kg for 10 weeks). Asterisk Significant difference with respect to the control
Discussion
The present study revealed that BPA administration induced a state of oxidative stress in the heart of rats as evident from the increase in MDA levels and decrease in catalase activity at the two tested doses (10 and 25 mg/kg) after 6 weeks and the decrease in GSH levels after the administration of the two doses of BPA at all tested time segments. Increased lipid peroxidation may indicate an increased oxygen free radical generation. BPA induces ROS production and significantly compromises mitochondrial function.
Catalase is an enzyme that converts hydrogen peroxide into hydrogen oxide. Therefore, the present results suggest that the exposure to BPA induces overproduction of hydrogen peroxide in the heart. The reduction in the activity of catalase may be due to the exhaustion of the enzyme in attempting to eliminate the hydrogen peroxide generated after the exposure to BPA. This may also be due to enzyme inactivation caused by excess ROS production in mitochondria and microsomes (Pigeolet et al. 1990).
Glutathione provides a first line of defence against ROS, as it can scavenge free radicals and reduce H2O2 (Pastorea et al. 2003). The present study revealed a significant decrease in GSH levels after BPA administration at the two dose levels (25 and 10 mg/kg) for different time intervals. It is clear from the percentage differences that BPA (10 mg/kg) for 6 weeks produced a larger effect than BPA (25 mg/kg) for 6 weeks and BPA (10 mg/kg) for 10 weeks. This pattern is typical of the non-monotonic dose–response curves that have been reported for many actions of BPA (Vandenberg et al. 2006; Alonso-Magdalena et al. 2008; Hugo et al. 2008; Vandenberg et al. 2009). There are currently 18 published reports of inverted-U dose response curves (vom Saal 2006).
It may be concluded that GSH, in the present study, is consumed during the conversion of hydrogen peroxide into hydrogen oxide. The peroxide is readily converted to the hydroxyl radical which may be involved in the observed decrease in GSH levels as GSH itself is also a general hydroxy-radical scavenger. This finding is supported by various studies demonstrating that glutathione is reduced in the tissues by oxidative stress (Sian et al. 1994; Melchiorri et al. 1996).
Glutathione-S-transferase catalyses the conjugation of GSH with several compounds produced in vivo during oxidative stress. In the present study, a significant increase in GST activity occurred at the high dose level of BPA (25 mg/kg for 6 weeks). This may eventually lead to the consumption of GSH during the generation of glutathione-S-conjugates by GSTs thus lowering the level of total intracellular glutathione after prolonged treatment. The present non significant changes observed in GST after 10 mg/kg of BPA suggests that GSH is utilized in the degradation of H2O2 resulting from the generation of ROS after the two time intervals.
In the present study, a significant decrease was obtained in NO levels in the heart of rats having received an oral administration of 25 mg/kg of BPA for 6 weeks and 10 mg/kg for 10 weeks (five times a week).
Nitric oxide is a free radical synthesized by the nitric oxide synthase (NOS) (Cannon 1998). All three NOS isoforms such as constitutive (nNOS and eNOS) and inducible (iNOS) are expressed in the cardiovascular system (Kelly et al. 1996). Although normal endothelial release of NO through endothelium-derived NO (eNOS) reaction mediates physiologic vasodilation, excessive release through iNOS induction may play a role in regulating BP (blood pressure), HR (heart rate) and endogenous antioxidants in septic shock (Petros et al. 1991). Acute or chronic administration of NOS inhibitors has been shown to cause BP elevation and changes in HR in normal rats (Tribulová et al. 2000).
It is known that NO is deeply involved in vascular pathologies, such as hypertension (Moncada et al. 1991; Ignarro et al. 1999). It is hypothesized that chronic NO-deficient hypertension and alteration in HR is associated with depletion of antioxidants and oxidative injury to the heart (Husain and Hazelrigg 2002). In addition, deficiency in eNOS has been shown to promote the development of heart failure postmyocardial infarction (Scherrer-Crosbie et al. 2001). Melzer et al. (2010) postulated several mechanisms by which BPA might increase the risk of cardiovascular disease, including reduced NO bioavailability, altered vascular reactivity to endothelin-1, oxidative stress and inflammation.
It may be postulated that the decreased level of NO under the effect of BPA in the heart may result in vasoconstriction which in turn may lead to decreased blood supply to the cardiac tissue. This may lead to a state of myocardial ischemia and consequently oxidative stress. Moreover, the decreased NO availability may represent one of the most important mechanisms underlying the reported CVDs related to BPA administration.
In the present study, a significant decrease in the activity of AchE enzyme was observed in the rat heart after the oral administration of 25 mg/kg of BPA for 6 weeks and also after the administration of 10 mg/kg of BPA for 6 and 10 weeks.
Acetylcholinesterase (AchE; acetylcholine acetylhydrolase EC 3.1.1.7) hydrolyzes acetylcholine and thereby terminates the action of this neurotransmitter at the cholinergic neuroeffector junctions of the heart. Very few evidences suggest that inhibition of AchE is mediated by oxidative stress (Wyse et al. 2004). This was supported by the notion that hydroxyl radicals are involved in the AchE inhibition (Tsakiris et al. 2000). Thus, the present inhibition of AchE activity may be related to the state of oxidative stress induced by BPA in rat heart.
Pant et al. (2011) found that BPA decreased the rate and force of atrial contractions simultaneously and depressed the functioning of the pacemaker cells and the contractile machinery of the heart. Decreased rate and force of contractions can be due to the activation of cholinergic system or NO (Deshpande et al. 2008; Kanoo et al. 2009). From the present data, it may be postulated that the deficiency in NO levels together with the increased cholinergic activation resulting from AchE enzyme inhibition induced by BPA administration may lead to the previously reported reduction in the rate and force of cardiac contractions.
There are considerable data linking oxidative stress and ROS to the physiology and pathophysiology of CVD (cardiovascular disease) (Sugamura and Keaney 2011).
In addition, elevated levels of oxidative stress markers are detected in several pathologic conditions of cardiovascular disorders, including hypertension, ventricular hypertrophy, atherosclerosis, and congestive heart failure (Carlos et al. 1998; Keith et al. 1998; Miller et al. 1998; Harjai 1999).
Both experimental and clinical studies suggested that the generation of ROS increases in heart failure (Hill and Singal 1996, 1997; Mallat et al. 1998). Levels of lipid peroxides and 8-iso-prostaglandin F2a, the major biochemical markers of ROS generation, have been shown to be elevated in the plasma and pericardial fluid of patients with heart failure and are also positively correlated to its severity (Mallat et al. 1998).
On the other hand, depletion of GSH and GSH/GSSG ratio in blood has been reported to be a good marker in hypertension (Vaziri et al. 2000; Husain 2002). It is clear that ROS may contribute to myocyte injury resulting from ischemic–reperfusion (Zweier et al. 1987), reduction of endogenous antioxidants in the myocardium (Hill and Singal 1997), and the remodeling response (Dhalla et al. 1996).
Several reports found a link between urinary BPA concentrations and prevalence of heart diseases using 2003–2006 NHANES data, suggesting an association between BPA exposure and CVD (Lang et al. 2008; Melzer et al. 2010). Recently, Pant et al. (2011) reported that acute exposure to BPA depressed cardiac activity even up to the stage of asystole. They suggested that the decreased contractility may lead to coronary insufficiency. There is also a recent study suggesting that exposure to BPA, measured in serum, is associated with atherosclerosis (Lind and Lind 2011) and atherosclerosis development (Olsén et al. 2012).
It may be concluded that the increase in lipid peroxidation and the reduction in the antioxidant mechanisms, NO level and AchE activity in the heart after the daily oral administration of BPA at the present dose levels may lead to the generation of ROS and the development of a state of oxidative stress which may underlie the CVDs linked to BPA exposure.
The present data revealed an increase in the body weight gain of animals treated with the two doses of BPA (25 and 10 mg/kg) for different time intervals. These results are consistent with those of other investigators who showed that perinatal BPA exposure increased body weight relative to controls (Rubin et al. 2001; Miyawaki et al. 2007). In addition, several investigators confirmed that BPA has a role in weight gain and the development of obesity (Newbold et al. 2009; Rubin and Soto 2009; Shankar et al. 2012). Trasande et al. (2012) found that among 6–19-year-olds participating in NHANES, increasing urinary BPA concentrations were cross-sectionally associated with increased body mass index (BMI) z-score and increased odds of obesity. Moreover, in a low-income Mexican–American population, Harley et al. (2013) found that higher BPA concentrations in children’s urine at 9 years of age were associated with increased odds of obesity and increased BMI z-score, waist circumference, and percent body fat at the age 9.
Recently, Li et al. (2013) reported that exposure to high BPA level may contribute to childhood obesity. The authors suggested that BPA could be a potential new environmental obesogen and that widespread exposure to BPA in the human population may contribute to the worldwide obesity epidemic.
Thus, the present increase in body weight gain emphasizes the ability of BPA to promote obesity which in turn could exacerbate many of the metabolic and cardiovascular disorders reported after BPA exposure.
This effect of BPA could be explained by the report of Hugo et al. (2008) who showed the ability of low levels of BPA to decrease adiponectin release from human adipose tissue explants. Adiponectin is known to play a positive role in cardiovascular health. Another possible explanation of enhanced weight gain in BPA-exposed animals is an increase in food intake as the estrogenic action of BPA can affect neuronal circuits that control appetite by acting on the hypothalamus (Wade and Schneider 1992). The above data confirm that BPA exposure could be a major public health concern in relation to the epidemic of childhood and adult obesity (Laron 2004; Reilly 2005).
In conclusion, it is evident that BPA has an adverse effect on the heart of rats which is mediated principally by the generation of ROS and reduction of antioxidant defenses of the heart aggravating a state of oxidative stress. The concomitant reduction in NO levels and AchE activity and the increase in body weight may contribute to the cardiovascular disturbances resulting from BPA. It is clear that these pathological conditions may occur after prolonged exposure to BPA even at extremely low levels which raises the demands for prohibiting the use of BPA in plastic industries.
References
- Aebi H. Catalase in vitro. Meth Enzym. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A. The estrogenic effect of bisphenol A disrupts pancreatic β-cell function in vivo and induces insulin resistance. Environ Health Perspect. 2006;114:106–112. doi: 10.1289/ehp.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Ropero AB, Carrera MP, Cederroth CR, Baquié M, Gauthier BR, Nef S, Stefani E, Nadal A (2008) Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS One 30:e2069 [DOI] [PMC free article] [PubMed]
- Antoni H. Function of the heart. In: Schmidt RF, Thews G, editors. Human Physiology. Berlin: Springer; 1991. pp. 358–396. [Google Scholar]
- Asano S, Tune JD, Dick GM. Bisphenol A activates Maxi-K (KCa1.1) channels in coronary smooth muscle. Br J Pharmacol. 2010;160:160–170. doi: 10.1111/j.1476-5381.2010.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae S, Kim JH, Lim Y-H, Park HY, Hong Y-C. Associations of bisphenol A exposure with heart rate variability and blood pressure. Hypertension. 2012;60:786–793. doi: 10.1161/HYPERTENSIONAHA.112.197715. [DOI] [PubMed] [Google Scholar]
- Bian Q, Qian J, Xu L, Chen J, Song L, Wang X. The toxic effect of 4-tert-octylphenol on the reproductive system of male rats. Food Chem Toxicol. 2006;44:1355–1361. doi: 10.1016/j.fct.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Bindhumol V, Chitra KC, Mathur PP. Bisphenol A induces reactive oxygen species generation in the liver of male rats. Toxicology. 2003;188:117–124. doi: 10.1016/S0300-483X(03)00056-8. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113:391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon RO. Role of nitric oxide in cardiovascular disease: focus on the endothelium. Clin Chem. 1998;44:1809–1819. [PubMed] [Google Scholar]
- Carlos DM, Goto S, Urata Y, Iida T, Cho S, Niwa M, Tsuji Y, Kondo T. Nicardipine normalizes elevated levels of antioxidant activity in response to xanthine oxidase-induced oxidative stress in hypertensive rat heart. Free Radic Res. 1998;29:143–150. doi: 10.1080/10715769800300161. [DOI] [PubMed] [Google Scholar]
- Chitra KC, Latchoumycandane C, Mathur PP. Induction of oxidative stress by bisphenol A in the epididymal sperm of rats. Toxicology. 2003;185:119–127. doi: 10.1016/S0300-483X(02)00597-8. [DOI] [PubMed] [Google Scholar]
- Coors A, Jones PD, Giesy JP, Ratte HT. Removal of estrogenic activity from municipal waste landfill leachate assessed with a bioassay based on reporter gene expression. Environ Sci Technol. 2003;37:3430–3434. doi: 10.1021/es0300158. [DOI] [PubMed] [Google Scholar]
- Das UN. Free radicals, cytokines and nitric oxide in cardiac failure and myocardial infarction. Mol Cell Biochem. 2000;215:145–152. doi: 10.1023/A:1026579422132. [DOI] [PubMed] [Google Scholar]
- Deshpande SB, Kanoo S, Alex AB. Bradycardia induced by Mesobuthus tamulus scorpion venom involves muscarinic receptor-G-protein-coupled cell signaling pathways. Indian J Exp Biol. 2008;46:229–233. [PubMed] [Google Scholar]
- Dhalla AK, Hill MF, Singal PK. Role of oxidative stress in transition of hypertrophy to heart failure. J Am Coll Cardiol. 1996;28:506–514. doi: 10.1016/0735-1097(96)00140-4. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Palioura E, Kandarakis SA, Koutsilieris M. The impact of endocrine disruptors on endocrine targets. Horm Metab Res. 2010;42:543–552. doi: 10.1055/s-0030-1252034. [DOI] [PubMed] [Google Scholar]
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Erickson B. FDA raises flag on bisphenol A. Chem Eng News. 2010;88:8. [Google Scholar]
- European Union (EU) (2008) Updated European Risk Assessment Report: 4,4-isopropylidenediphenol (bisphenol-A) (CAS Number: 80-05-7, EINECS Number: 201-245-8). Available from: http://ecb.jrc.it/documents/Existingchemicals/ RISK ASSESSMENT/ADDENDUM/bisphenolaadd 325.pdf. Accessed 01. 02. 11
- Gorun V, Proinov I, Baltescu V, Balaban G, Barzu O. Modified Ellman procedure for assay of cholinesterase in crude-enzymatic preparations. Anal Biochem. 1978;86:324–326. doi: 10.1016/0003-2697(78)90350-0. [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Harjai KJ. Potential new cardiovascular risk factors: left ventricular hypertrophy, homocysteine, lipoprotein(a), triglycerides, oxidative stress, and fibrinogen. Ann Intern Med. 1999;131:376–386. doi: 10.7326/0003-4819-131-5-199909070-00009. [DOI] [PubMed] [Google Scholar]
- Harley KG, Schall RA, Chevrier J, Tyler K, Aguirre H, Bradman A, Holland NT, Lustig RH, Calafat AM, Eskenazi B. Prenatal and postnatal bisphenol A exposure and body mass index in childhood in the CHAMACOS cohort. Environ Health Perspect. 2013;121:514–520. doi: 10.1289/ehp.1306866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Canada (2008) Government of Canada takes action on another chemical of concern: bisphenol A [Press release]. Available: http://www.hc-sc.gc.ca/ahc-asc/media/nr-cp/_2008/2008_59-eng.php. Accessed 25 Sept 2009
- Hill MF, Singal PK. Antioxidant and oxidative stress changes during heart failure subsequent to MI in rats. Am J Pathol. 1996;148:291–300. [PMC free article] [PubMed] [Google Scholar]
- Hill MF, Singal PK. Right and left myocardial antioxidant responses during heart failure subsequent to myocardial infarction. Circulation. 1997;96:2414–2420. doi: 10.1161/01.CIR.96.7.2414. [DOI] [PubMed] [Google Scholar]
- Hoover DB, Ganote CE, Ferguson SM, Blakely RD, Parsons RL. Localization of cholinergic innervation in guinea pig heart by immunohistochemistry for high-affinity choline transporters. Cardiovasc Res. 2004;62:112–121. doi: 10.1016/j.cardiores.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect. 2008;116:1642–1647. doi: 10.1289/ehp.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain K. Exercise conditioning attenuates the hypertensive effects of nitric oxide synthase inhibitor in rat. Mol Cell Biochem. 2002;231:129–137. doi: 10.1023/A:1014416915643. [DOI] [PubMed] [Google Scholar]
- Husain K, Hazelrigg SR. Oxidative injury due to chronic nitric oxide synthase inhibition in rat: effect of regular exercise on the heart. Biochim Biophys Acta. 2002;1587:75–82. doi: 10.1016/S0925-4439(02)00070-4. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ, Cirino G, Casini A, Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. J Cardiovasc Pharmacol. 1999;34:879–886. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- Kabuto H, Amakawa M, Shishibori T. Exposure to bisphenol A during embryonic/fetal life and infancy increases oxidative injury and causes underdevelopment of the brain and testis in mice. Life Sci. 2004;74:2931–2940. doi: 10.1016/j.lfs.2003.07.060. [DOI] [PubMed] [Google Scholar]
- Kanoo S, Mandal MB, Alex AB, Deshpande SB. Cardiac dysrhythmia produced by Mesobuthus tamulus venom involves NO-dependent G-Cyclase signaling pathway. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:525–532. doi: 10.1007/s00210-008-0375-7. [DOI] [PubMed] [Google Scholar]
- Kawagoshi Y, Fujita Y, Kishi I, Fukunaga I. Estrogenic chemicals and estrogenic activity in leachate from municipal waste landfill determined by yeast two-hybrid assay. J Environ Monit. 2003;5:269–274. doi: 10.1039/b210962j. [DOI] [PubMed] [Google Scholar]
- Keith M, Geranmayegan A, Sole MJ, Kurian R, Robinson A, Omran AS, Jeejeebhoy KN. Increased oxidative stress in patients with congestive heart failure. J Am Coll Cardiol. 1998;31:1352–1356. doi: 10.1016/S0735-1097(98)00101-6. [DOI] [PubMed] [Google Scholar]
- Kelly RA, Balligand JL, Smith TW. Nitric oxide and cardiac function. Circ Res. 1996;79:363–380. doi: 10.1161/01.RES.79.3.363. [DOI] [PubMed] [Google Scholar]
- Lakind JS, Naiman DQ. Daily intake of bisphenol A and potential sources of exposure: 2005–2006 National Health and Nutrition Examination Survey. J Expo Sci Environ Epidemiol. 2008;21:272–279. doi: 10.1038/jes.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300:1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- Laron Z. Increasing incidence of childhood obesity. Pediatr Endocrinol Rev. 2004;1:443–447. [PubMed] [Google Scholar]
- Lefkowitz RJ, Hoffman BB, Taylor P. Neurotransmission: the autonomic and somatic motor nervous systems. In: Hardman JG, Limbird LL, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman and Gilman’s the pharmacological basis of therapeutics. 9. New York: McGraw-Hill; 1996. pp. 105–139. [Google Scholar]
- Li D-K, Miao M, Zhou Z, Wu C, Shi H, Liu X, Wang S, Yuan W. Urine bisphenol-A level in relation to obesity and overweight in school-age children. PLoS ONE. 2013;8:e65399. doi: 10.1371/journal.pone.0065399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DS, Kwack SJ, Kim KB, Kim HS, Lee BM. Potential risk of bisphenol A migration from polycarbonate containers after heating, boiling, and microwaving. J Toxicol Environ Health A. 2009;72:1285–1291. doi: 10.1080/15287390903212329. [DOI] [PubMed] [Google Scholar]
- Lind PM, Lind L. Circulating levels of bisphenol A and phthalates are related to carotid atherosclerosis in the elderly. Atherosclerosis. 2011;218:207–213. doi: 10.1016/j.atherosclerosis.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Mallat Z, Philip I, Lebret M, Chatel D, Maclouf J, Tedgui A. Elevated levels of 8-iso-prostaglandin F2α in pericardial fluid of patients with heart failure: a potential role for in vivo oxidant stress in ventricular dilatation and progression to heart failure. Circulation. 1998;97:1536–1539. doi: 10.1161/01.CIR.97.16.1536. [DOI] [PubMed] [Google Scholar]
- McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Melchiorri D, Reiter RJ, Sewerynek E, Hara M, Chen L, Nistico G. Paraquat toxicity and oxidative damage. Reduction by melatonin. Biochem Pharmacol. 1996;51:1095–1099. doi: 10.1016/0006-2952(96)00055-X. [DOI] [PubMed] [Google Scholar]
- Melzer D, Rice NE, Lewis C, Henley WE, Galloway TS. Association of urinary bisphenol A concentration with heart disease: evidence from NHANES 2003/06. PLoS ONE. 2010;5:e8673. doi: 10.1371/journal.pone.0008673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer D, Osborne NJ, Henley WE, Cipelli R, Young A, Money C, McCormack P, Luben R, Khaw KT, Wareham NJ, Galloway TS. Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation. 2012;125:1482–1490. doi: 10.1161/CIRCULATIONAHA.111.069153. [DOI] [PubMed] [Google Scholar]
- Miller FJJ, Gutterman DD, Rios CD, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res. 1998;82:1298–1305. doi: 10.1161/01.RES.82.12.1298. [DOI] [PubMed] [Google Scholar]
- Miyawaki J, Sakayama K, Kato H, Yamamoto H, Masuno H. Perinatal and postnatal exposure to bisphenol a increases adipose tissue mass and serum cholesterol level in mice. J Atheroscler Thromb. 2007;14:245–252. doi: 10.5551/jat.E486. [DOI] [PubMed] [Google Scholar]
- Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Moshage H, Kok B, Huizenga JR. Nitrite and nitrate determination in plasma: a critical evaluation. Clin Chem. 1995;41:892–896. [PubMed] [Google Scholar]
- Mourad IM, Khadrawy YA. The sensitivity of liver, kidney and testis of rats to oxidative stress induced by different doses of bisphenol A. Int J Life Sci Pharma Res. 2012;2:L19–L28. doi: 10.5963/LSMR0202002. [DOI] [Google Scholar]
- Newbold RR, Padilla-Banks E, Jefferson WN. Environmental estrogens and obesity. Mol Cell Endocrinol. 2009;304:84–89. doi: 10.1016/j.mce.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsén L, Lind L, Lind PM. Associations between circulating levels of bisphenol A and phthalate metabolites and coronary risk in the elderly. Ecotoxicol Environ Saf. 2012;80:179–183. doi: 10.1016/j.ecoenv.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Pant J, Ranjan P, Deshpande SB. Bisphenol A decreases atrial contractility involving NO-dependent G-cyclase signaling pathway. J Appl Toxicol. 2011;31:698–702. doi: 10.1002/jat.1647. [DOI] [PubMed] [Google Scholar]
- Pastorea A, Federicia G, Bertini E, Piemonte F. Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta. 2003;333:19–39. doi: 10.1016/S0009-8981(03)00200-6. [DOI] [PubMed] [Google Scholar]
- Patel BB, Raad M, Sebag IA, Chalifour LE. Lifelong exposure to bisphenol a alters cardiac structure/function, protein expression, and DNA methylation in adult mice. Toxicol Sci. 2013;133:174–185. doi: 10.1093/toxsci/kft026. [DOI] [PubMed] [Google Scholar]
- Petros A, Bennett D, Vallance P. Effect of nitric oxide synthase inhibitors on hypotension in patients with septic shock. Lancet. 1991;338:1557–1558. doi: 10.1016/0140-6736(91)92376-D. [DOI] [PubMed] [Google Scholar]
- Pigeolet E, Corbisier P, Houbion A, Lambert D, Michiels DC, Raes M, Zachary D, Ramacle J. Glutathione peroxidase, superoxide dismutase and catalase inactivation by peroxides and oxygen derived free radicals. Mech Ageing Dev. 1990;51:283–290. doi: 10.1016/0047-6374(90)90078-T. [DOI] [PubMed] [Google Scholar]
- Reilly JJ. Descriptive epidemiology and health consequences of childhood obesity. Best Pract Res Clin Endocrinol Metab. 2005;19:327–341. doi: 10.1016/j.beem.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Richter CA, Birnabaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vaderbergh JC, Walser-Kuntz DR, vom Saal FS (2007) In vivo effect of bisphenol A in laboratory rodent studies. Reprod Toxicol 24:199–224 [DOI] [PMC free article] [PubMed]
- Ritter S. Debating BPA’s toxicity. Chem Eng News. 2011;89:5–13. [Google Scholar]
- Ropero AB, Alonso-Magdalena P, Garcia–Garcia E, Ripoll C, Fuentes E, Nadal A. Bisphenol-A disruption of the endocrine pancreas and blood glucose homeostasis. Int J Androl. 2008;31:194–200. doi: 10.1111/j.1365-2605.2007.00832.x. [DOI] [PubMed] [Google Scholar]
- Roy P, Venkat RG, Naidu MUR, Usha RP. Recent trends in the nitrergic nervous system. Educational Forum. 2005;37:69–76. [Google Scholar]
- Rubin BS, Soto AM. Bisphenol A: perinatal exposure and body weight. Mol Cell Endocrinol. 2009;304:55–62. doi: 10.1016/j.mce.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol-A affects body weight, patterns of estrous cyclicity and plasma LH levels. Environ Health Perspect. 2001;109:675–680. doi: 10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Larrea MB, Leal AM, Liza M. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids. 1994;59:383–388. doi: 10.1016/0039-128X(94)90006-X. [DOI] [PubMed] [Google Scholar]
- Scherrer-Crosbie M, Ullrich R, Bloch KD, Nakajima H, Nasseri B, Aretz HT, Lindsey ML, Vançon AC, Huang PL, Lee RT, Zapol WM, Picard MH. Endothelial nitric oxide synthase limits left ventricular remodeling after myocardial infarction in mice. Circulation. 2001;104:1286–1291. doi: 10.1161/hc3601.094298. [DOI] [PubMed] [Google Scholar]
- Shah AM, Vallance P, Harrison D. NO in the cardiovascular system. Cardiovasc Res. 1999;43:507–508. doi: 10.1016/S0008-6363(99)00181-9. [DOI] [PubMed] [Google Scholar]
- Shankar A, Teppala S, Sabanayagam C (2012) Urinary bisphenol-A levels and measures of obesity: results from the National Health and Nutrition Examination Survey 2003–2008. ISRN Endocrinol 2012:965243. doi:10.5402/2012/965243 [DOI] [PMC free article] [PubMed]
- Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting the basal ganglia. Annals Neurol. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- Sugamura K, Keaney JF. Reactive oxygen species in cardiovascular disease. Free Radic Biol Med. 2011;51:978–992. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Attina TM, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA. 2012;308:1113–1121. doi: 10.1001/2012.jama.11461. [DOI] [PubMed] [Google Scholar]
- Tribulová N, Okruhlicová L, Bernátová I, Pechánová O. Chronic disturbances in NO production results in histochemical and subcellular alterations of the rat heart. Physiol Res. 2000;49:77–88. [PubMed] [Google Scholar]
- Tsakiris S, Angelogianni P, Schulpis KH, Stavridis JC. Protective effect of l-phenylalanine on rat brain acetylcholinesterase inhibition induced by free radicals. Clin Biochem. 2000;33:103–106. doi: 10.1016/S0009-9120(99)00090-9. [DOI] [PubMed] [Google Scholar]
- Tsutsui H, Kinugawa S, Matsushima S. Mitochondrial oxidative stress and dysfunction in myocardial remodeling. Cardiovas Res. 2009;81:449–456. doi: 10.1093/cvr/cvn280. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM (2009) Bisphenol-a and the great divide: a review of controversies in the field of endocrine disruption. Endocrine Rev 30 :75–95 [DOI] [PMC free article] [PubMed]
- Vandenberg LN, Wadia PR, Schaeberle CM, Rubin BS, Sonnenschein C, Soto AM (2006) The mammary gland response to estradiol: monotonic at the cellular level, non-monotonic at the tissue-level of organization? J Steroid Biochem Molec Biol 101:263–274 [DOI] [PubMed]
- Vaziri ND, Wang XQ, Oveisi F, Rad B. Induction of oxidative stress by glutathione depletion causes severe hypertension in normal rats. Hypertension. 2000;36:142–146. doi: 10.1161/01.HYP.36.1.142. [DOI] [PubMed] [Google Scholar]
- vom Saal FS (2006) Bisphenol A references: list of published articles. http://endocrinedisruptors.missouri.edu/vomsaal/vomsaal.html
- vom Saal FS, Hughes C. An extensive new literature concerning low dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Myers JP. Bisphenol A and risk of metabolic disorders. JAMA. 2008;300:1353–1355. doi: 10.1001/jama.300.11.1353. [DOI] [PubMed] [Google Scholar]
- Wade GN, Schneider JE. Metabolic fuels and reproduction in female mammals. Neurosci Biobehav Rev. 1992;16:235–272. doi: 10.1016/S0149-7634(05)80183-6. [DOI] [PubMed] [Google Scholar]
- Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect. 2003;111:994–1006. doi: 10.1289/ehp.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse AT, Stefanello FM, Chiarani F, Delwing D, Wannmacher CM, Wajner M. Arginine administration decreases cerebral cortex acetylcholinesterase and serum butyrylcholinesterase probably by oxidative stress induction. Neurochem Res. 2004;29:385–389. doi: 10.1023/B:NERE.0000013741.81436.e8. [DOI] [PubMed] [Google Scholar]
- Ye XB, Pierik FH, Hauser R, Duty S, Angerer J, Park MM, Burdorf A, Hofman A, Jaddoe VWV, Mackenbach JP, Steegers EAP, Tiemeier H, Longnecker MP. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: the Generation R study. Environ Res. 2008;108:260–267. doi: 10.1016/j.envres.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier JL, Rayburn BK, Flaherty JT, Weisfeldt ML. Recombinant superoxide dismutase reduces oxygen free radical concentrations in reperfused myocardium. J Clin Invest. 1987;80:1728–1734. doi: 10.1172/JCI113264. [DOI] [PMC free article] [PubMed] [Google Scholar]