Abstract
The purpose of this study was to test whether mesenchymal stem cells (MSCs) transplantation with application of granulocyte colony-stimulating factor (G-CSF) would have beneficial effects on damaged heart in a rabbit model of myocardial infarction (MI). MI was created by ligation of the left anterior descending coronary artery. After induction of MI, 40 New Zealand white rabbits were randomly divided into 8 groups: (1) MSCs injection at 3 days after MI; (2) G-CSF injection at 3 days after MI; (3) MSCs + G-CSF (20 u/kg/day) injection at 3 days after MI; (4) PBS injection at 3 days after MI; (5) MSCs injection at 7 days after MI; (6) G-CSF injection at 7 days after MI; (7) MSCs + G-CSF (20 u/kg/day) injection 7 days after MI; and (8) PBS injection 7 days after MI. TUNEL analysis showed that the apoptotic cells were distributed in the marginal area of MI. In both 3 and 7 days after MI groups, there were less apoptotic cells in the MSCs and MSCs + G-CSF groups as compared with the PBS group (P < 0.05). However, no decrease in apoptosis was observed in the G-CSF only group (P > 0.05). Immunohistochemistry analysis demonstrated that the expression level of vascular endothelial growth factor was higher in the MSCs, MSCs + G-CSF and G-CSF groups as compared with the PBS group. The present study demonstrated a beneficial effect of MSCs transplantation with application of G-CSF in the treatment of rabbit MI.
Keywords: Mesenchymal stem cells, Granulocyte colony-stimulating factor, Superparamagnetic iron oxide, Magnetic resonance imaging, Myocardium infarction
Introduction
With improved economic development and changing lifestyles, the incidence of ischemic heart disease has increased nowadays (Labarthe et al. 2005). Traditional medication treatment, interventional treatment, and surgical coronary artery bypass grafting, are based on improving cardiac function through repairing damaged cells without promoting the regeneration of myocardial cells. Recently, cell transplantation has become the favorable treatment for such diseases (Rosenstrauch et al. 2005). Cell transplantation is conducted through transplanting exogenous stem cells to promote the regeneration of myocardial cells, reshape ventricular wall structure, and finally improve heart function. Cell transplantation has thus been viewed as an ischemic new strategy for the treatment of heart disease.
Bone marrow mesenchymal stem cells (MSCs) have good application prospects for cell transplantation because they possess multiple differentiation potential. With addition of either environmental or chemical substances, MSCs can differentiate into a variety of cell types (Tülpar et al. 2012; Wang et al. 2012). This high plasticity of cultured MSCs has great beneficial effects in transplantation treatment of myocardial infarction (MI). A study from Tomita et al. was the first to confirm that MSCs under in vitro conditions can differentiate into cardiac-like muscle cells (Tomita et al. 1999). These differentiated cells express troponin I and myosin heavy chain genes, and promote angiogenesis at 8 weeks after injection to cryoinjury-derived myocardial scar. Numerous animal experiments have shown that MSCs transplantation can promote the formation of new blood vessels and myocardial infarct size, reduce the formation of scar tissue and ventricular remodeling, and improve cardiac functions (Kamihata et al. 2001; Kocher et al. 2001). In addition, MSCs transplantation has great advantages due to the non-rejection of the transplant from the body itself, due to convenient withdrawal of materials (bone marrow), due to no ethical controversy, and due to high clinical feasibility.
Granulocyte colony-stimulating factor (G-CSF) is a glycoprotein that stimulates the bone marrow to produce granulocytes and stem cells and release them into the bloodstream. A study by Orlic et al. showed that the concomitant administration of G-CSF and stem cell factor (SCF) mobilized bone MSCs and repaired infarcted myocardium in the acute MI animal experiments. They decreased mortality by 68 %, infarct size by 40 %, and cavitary dilation by 26 %, showing that cytokines such as G-CSF could promote mobilization of bone MSCs to the injured heart after MI (Orlic et al. 2001). These conclusions were further proven by subsequent studies (Cheng et al. 2008; Fan et al. 2008). However, the effect of cell-based therapies in improving cardiac function remains promising and further experimental and clinical studies are warranted to determine the role of G-CSF (Ripa and Kastrup 2008).
The survival and proof of homing and engraftment of implanted stem cells in the targeted tissues is important for the success of cell therapy (Reddy et al. 2010). Traditional labeling methods, such as fluorescent labeling, nucleic acid labeling, or genetically modified labeling, require in vitro histological analysis and identification, making it difficult to get a comprehensive and objective evaluation of the efficacy of stem cell transplantation. Therefore, to meet the current need for stem cell transplantation for clinical applications, we applied magnetic resonance imaging (MRI), which is a non-invasive, dynamic, and highly reproducible in vivo method to monitor transplanted cells (Cerqueira et al. 2002). However, MRI alone cannot distinguish between the transplanted cells and the target tissue cells. To actively visualize transplanted stem cells, we used superparamagnetic iron oxide nanoparticles (SPIOs) to label the transplanted cells in MRI.
In this study, we established a rabbit MI model by ligation of the proximal left anterior descending (LAD) coronary artery. Injection of SPIO-labeled MSCs was conducted by direct vision intra-myocardial transplantation at different periods after MI. Moreover, MSCs transplantation combined with G-CSF were applied to evaluate their effect in the treatment of MI, as well as to explore its clinical value.
Materials and methods
Animals
Forty New Zealand white rabbits (offered by the animal experiment center at the Tongji hospital, Shanghai, China), male or female, 12–14 weeks old, weighing 500–1,000 g, were used in this study. All animals were bred under climate-controlled conditions with 12-h light-dark cycle and provided with standardized food and water. All animal studies were performed in accordance with the guidelines for the care and use of laboratory animals at our university.
Preparation of labeled MSCs
MSCs were isolated from the femora and tibiae of a 1-month-old rabbit and subcultured for three generations as previously described (Redzić et al. 2010). When cells were grown to 75 % confluence, the original Dulbecco’s modified Eagle medium (DMEM; Invitrogen, Carlsbad, CA, USA) was replaced by DMEM containing SPIO resovist (25 μg/ml, Schering AG, Berlin, Germany) and poly-l-lysine (PLL; 0.75 μg/ml, Sigma, St. Louis, MO, USA), and incubated at 37 °C under 5 % CO2. MSCs were collected after 48 h and digested with 0.25 % trysin and 0.02 % EDTA (Invitrogen). Then, MSCs suspension was prepared after centrifuging at 1,000 rpm for 5 min, and adjusted to 5 × 107/ml by PBS.
MI model and MSCs transplantation
Myocardial infarction was induced by the ligation of LAD coronary artery as previously described (Kawamoto et al. 2001). In brief, rabbits were anesthetized by ear marginal vein injection of 3 % phenobarbitol sodium (1 mg/kg, Zhongxin Chemical CO. Ltd, Hainan, China) on a volume-cycled ventilator. The LAD coronary artery was ligated using a 7-0 silk suture. The procedure of establishment of the MI model is shown in Fig. 1. After induction of MI, 40 rabbits were randomly divided into 8 groups: (1) MSCs injection at 3 days after MI; (2) G-CSF injection (20 u/kg/day) at 3 days after MI for 7 consecutive days; (3) MSCs + G-CSF (20 u/kg/day for 7 consecutive days) injection at 3 days after MI; (4) PBS injection at 3 days after MI for 7 days; (5) MSCs injection at 7 days after MI; (6) G-CSF (20 u/kg/day) injection at 7 days after MI for 7 consecutive days; (7) MSCs + G-CSF (20 u/kg/day for 7 consecutive days) injection at 7 days after MI; and (8) PBS injection at 7 days after MI for 7 days.
Fig. 1.
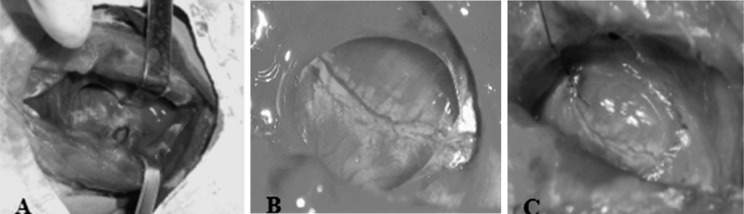
The procedure of establishment of rabbit MI model: a Median sternotomy incision thoracotomy was used to expose the heart. b LAD was fully exposed. c A silk ligature was looped under the LAD coronary artery ≈5 mm from its origin. Distal blood vessels were thinning, angioplerosis was not obvious and left ventricular wall displayed pale characteristics of MI
Intra-myocardial injection of MSCs was conducted after animals were anesthetized. A midline skin incision was made and the sternohyoid muscle was divided in the middle to expose the heart. Then, the labeled MSCs suspension was slowly injected at 4 points at the border of MI area (50 μl/point). Totally 1 × 107 cells were injected for one rabbit.
Determination of ejection fraction (EF) and the thickness of left ventricular anterior wall (LVAW) by cine-MRI
Cine-MRI was performed in conscious rabbit by using Marconi 1.5 T Edge Eclipse (Cleveland, OH, USA) at 4 weeks after MSCs transplantation. The end-diastolic volume (EDV) and end-systolic volume (ESV) were measured to determine the increase rate of the thickness of LVAW and EF in all above 8 groups. In addition, the preoperative rabbits served as the normal control. EF was calculated by the formula: EF = (EDV − ESV)/EDV. The increase rate of the thickness of LVAW was calculated by the formula: increase rate = (ESV − EDV)/EDV.
Hematoxylin and eosin (HE) staining
The rabbits in each group were euthanized and their hearts were removed after cardiac MRI. The excised heart was fixed in 4 % paraformaldehyde, dehydrated in graded ethanol series, cleared in dimethylbenzene and embedded in paraffin. Sections (5 μm) were deparaffinized through immersing in dimethylbenzene and rehydrated. The sections were stained with HE using standard procedures and visualized and captured with an optical microscope.
TUNEL assay
After deparaffinization and dehydration, apoptotic cardiomyocytes were evaluated by TUNEL assay with a TUNEL Kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions. In brief, the sections were incubated in proteinase K (Thermo Fisher, Beijing, China) working solution (20 μg/ml in 10 mmol/l Tris/HCl, pH 7.4–8) at room temperature in a humidified atmosphere for 15 min, and then added with 50 μl TUNEL reaction mixture each and incubated for 60 min at 37 °C. After being rinsed 3 times with PBS, the sections were added with 100 μl horse radish peroxidase (HRP) and incubated for 30 min at 37 °C, and then rinsed 3 times with PBS again and 100 μl of diaminobenzidine (DAB) as substrate was added. Finally, the sections were counterstained with hematoxylin and analyzed by light microscope. Apoptotic index (calculated as the total number of apoptotic cells divided by the total number of myocardial cells) was assessed in 5 randomly selected fields in the border zone of the ischemic region.
Immunohistochemical assay
Formalin-fixed samples were dehydrated with an ethanol gradient and embedded in paraffin. The paraffin sections (4 μm) were mounted onto PLL-coated glass slides and dried for 1 h at 60 °C followed by deparaffinating and rehydrating according to a standard protocol. For antigen retrieval, slides were immersed in sodium citrate buffer (pH = 6.0, 0.01 M) and boiled twice for 10 min in a microwave oven. The slides were then washed with PBS (3 min) for three times and treated with 3 % H2O2 for 20 min to inhibit endogenous peroxidase. This was followed by incubation with primary rabbit polyclonal antibody against anti-human vascular endothelial growth factor (VEGF) (Santa Cruz Biotechnology, Santa Cruz, CA, USA; dilution 1:100) at 37 °C for 2 h. After PBS washes, the slides were incubated with secondary antibody (HRP-conjugated goat anti-rabbit IgG, Envision Reagent, Dako Cytomation, Glostrup, Denmark) for 30 min at room temperature. The slides were developed in DAB, counterstained with hematoxylin, and mounted.
Statistical analysis
All data were presented as mean ± SD. SPSS 17.0 program (SPSS Inc, Chicago, IL, USA) was used for data analysis. Differences between two groups were evaluated by t test; mean values were compared by one-way ANOVA and multiple comparisons were evaluated by Fisher’s least significant difference t (LSD-t) test. Data were considered statistically significant at P < 0.05.
Results
The EF and increase rate of thickness of LVAW in each group
To test if myocardial function was restored in response to the different treatment groups, EDV and ESV were measured to determine the increase rate of the thickness of LVAW (Table 1) and EF (Table 2). As shown in Tables 1 and 2, the increase rate of thickness of LVAW and EF were significantly decreased in the four treatment groups (P < 0.05), compared with those in the preoperative control group, in either 3 or 7 days groups. For the treatment groups at 3 days after MI, there were no significant differences for both increase rate of thickness of LVAW and EF values in MSCs, G-CSF, and MSCs + G-SCF groups, compared with the PBS injection group, respectively (P > 0.05). However, for the treatment groups at 7 days after MI, these two indicators were significantly higher in the MSCs and MSCs + G-SCF groups than those in the PBS group (P < 0.05). Besides, both of these two indicators were highest in the MSCs + G-SCF group, suggesting that both MSCs and G-CSF are needed for improving myocardial function. By comparing the four groups at 3 days and those at 7 days, we found that the increase rate of thickness of LVAW in the MSCs, G-CSF and MSCs + G-CSF groups at 7 days was higher than that in their corresponding groups at 3 days (P < 0.05), while there was no significant difference between the PBS groups at 3 and 7 days (P > 0.05). In addition, the EF in the MSCs, G-CSF and MSCs + G-CSF groups at 7 days was higher than that in their corresponding groups at 3 days, but without statistical significance (P > 0.05).
Table 1.
The increase rate of thickness of left ventricular anterior wall in each group (Increase rate = (ESV − EDV)/EDV)
| Groups | EDV (mm) | ESV (mm) | Increase rate of thickness of LVAW |
|---|---|---|---|
| Normal control | 3.338 ± 0.22 | 4.954 ± 0.62 | 0.484 ± 0.01 |
| MSC 3 days | 3.293 ± 0.15 | 4.248 ± 0.17 | 0.290 ± 0.02* |
| G-CSF 3 days | 3.278 ± 0.36 | 4.195 ± 0.23 | 0.280 ± 0.01* |
| MSC + G-CSF 3 days | 3.320 ± 0.28 | 4.343 ± 0.43 | 0.308 ± 0.06* |
| PBS 3 days | 3.252 ± 0.44 | 4.122 ± 0.14 | 0.268 ± 0.03* |
| MSC 7 days | 3.322 ± 0.15 | 4.517 ± 0.25 | 0.362 ± 0.01*,#,&, % |
| G-CSF 7 days | 3.305 ± 0.37 | 4.480 ± 0.38 | 0.351 ± 0.03*,#,&, % |
| MSC + G-CSF 7 days | 3.330 ± 0.13 | 4.665 ± 0.21 | 0.401 ± 0.02*,#, % |
| PBS 7 days | 3.282 ± 0.41 | 4.178 ± 0.36 | 0.273 ± 0.03* |
EDV end-diastolic volume, ESV end-systolic volume, LVAW left ventricular anterior wall; Data were expressed as mean ± SD
* P < 0.05, compared with normal control
# P < 0.05, compared with PBS group
& P < 0.05, compared with MSC + G-CSF group
% P < 0.05, compared with corresponding treatment groups at 3 days
Table 2.
The ejection fraction in each group [EF = (EDV − ESV)/EDV]
| Groups | EDV (ml) | ESV (ml) | EF |
|---|---|---|---|
| Normal control | 8.326 ± 0.63 | 3.988 ± 0.31 | 0.521 ± 0.03 |
| MSC 3 days | 8.368 ± 0.15 | 5.054 ± 0.22 | 0.396 ± 0.01* |
| G-CSF 3 days | 8.307 ± 0.29 | 5.109 ± 0.15 | 0.385 ± 0.02* |
| MSC + G-CSF 3 days | 8.352 ± 0.22 | 4.953 ± 0.08 | 0.407 ± 0.06* |
| PBS 3 days | 8.349 ± 0.39 | 5.143 ± 0.22 | 0.384 ± 0.02* |
| MSC 7 days | 8.431 ± 0.26 | 4.915 ± 0.27 | 0.417 ± 0.03*# |
| G-CSF 7 days | 8.443 ± 0.37 | 5.074 ± 0.13 | 0.399 ± 0.01* |
| MSC + G-CSF 7 days | 8.481 ± 0.36 | 4.902 ± 0.15 | 0.422 ± 0.05*# |
| PBS 7 days | 8.299 ± 0.06 | 5.137 ± 0.42 | 0.381 ± 0.01* |
EDV end-diastolic volume, ESV end-systolic volume; Data were expressed as mean ± SD
* P < 0.05, compared with normal control
# P < 0.05, compared with PBS group
Morphology of cardiac muscular tissue before and after MSCs transplantation
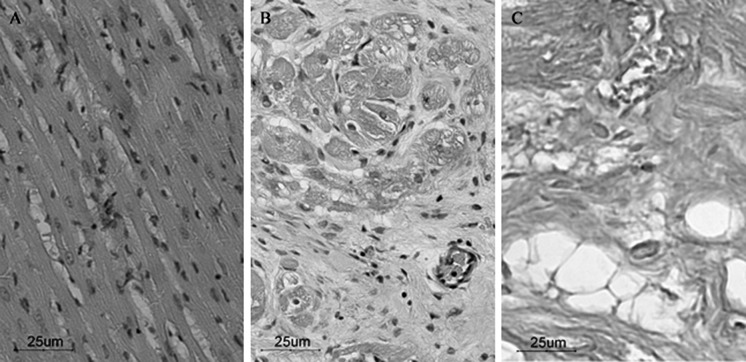
HE staining showed the morphology of normal cardiac muscular tissue, including the size of the myocardial cells and the hematoxylin stained nuclei, in which chromatin was equally distributed in the nucleus (Fig. 2a). Four weeks after transplantation, HE staining showed extensive scar formation at the infarcted area. Besides, a large cohort of fibroblasts and dense collagen fiber bundles was observed. Nest-like necrotic nidus accompanied by neovascularization could be observed in the fibrous tissue, while remnant myocardial tissue could be observed at the edges of the infarcted zone (Fig. 2b, c).
Fig. 2.
HE staining. a Normal myocardial cells are arranged in neat rows and have normal cell size and morphology; b infarcted area of the junction with normal myocardium, and remnants of infarct border myocardial tissue can be seen; c infarct zone has mainly scar formation, with a large number of collagen fibers and focal steatosis (inverted phase contrast microscope, ×200)
TUNEL assay of cardiomyocyte apoptosis
As shown in Fig. 3, brown nuclei were defined as positive apoptotic cells, while normal cell nuclei were stained blue. Comparison of AI between treatment groups at 3 days and 7 days show a statistically significant difference (P > 0.05). However, apoptotic cells were less in the MSCs group and MSCs + G-CSF group as compared with the PBS group (P < 0.05, Table 3). In the G-CSF only group, no reduction in apoptosis was observed (P > 0.05).
Fig. 3.
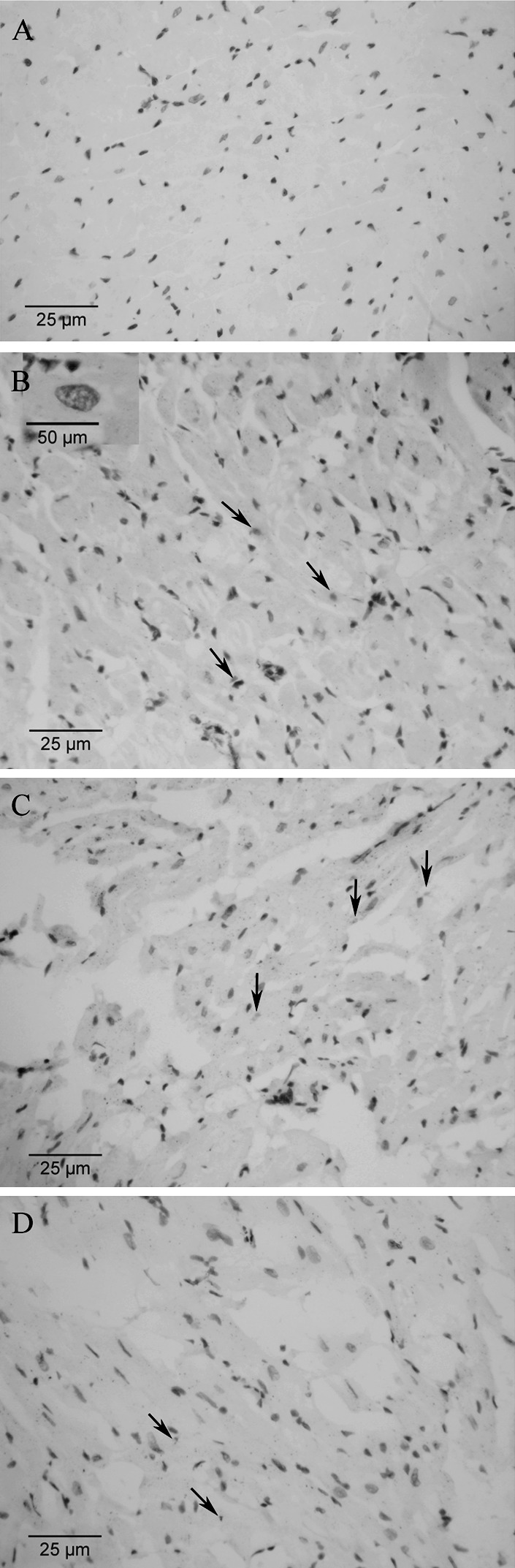
TUNEL assay: apoptotic cells were stained brown (indicated by an arrow), while non-apoptotic cells were stained blue. a Normal myocardial tissue; b myocardial tissue in G-CSF group at 3 days after MI; c myocardial tissue in MSCs group at 3 days after MI; d myocardial tissue in PBS group at 3 days after MI (inverted phase contrast microscope, ×200; enlarged view for apoptotic cells in b, ×400)
Table 3.
Apoptotic index (% apoptotic cells/ total cells) evaluated by TUNEL assay after 4 weeks of transplantation
| Group | PBS | MSCs | G-CSF | MSCs + G-CSF |
|---|---|---|---|---|
| Injection at 3 days after MI | 0.121 ± 0.05 | 0.060 ± 0.02* | 0.115 ± 0.02 | 0.059 ± 0.02* |
| Injection at 7 days after MI | 0.129 ± 0.02 | 0.060 ± 0.01* | 0.134 ± 0.06 | 0.068 ± 0.03* |
* P < 0.05, compared with PBS group; Data were expressed as mean ± SD
Expression of VEGF in the MI tissues
The expression of VEGF in each group was detected by immunohistochemical staining. Figure 4 displays the staining results of the infarcted area in the PBS injection at 7 days after MI group and the MSCs + G-CSF injection at 7 days after MI group. From Fig. 4, we could easily find that expression of VEGF was significantly higher in the MSCs + G-CSF group, compared with that in the PBS group. By using the KS400 image analysis system to quantify the staining results, we obtained the average grey value of VEGF at 4 weeks after cell transplantation in each group (Table 4). As shown in Table 4, the expression of VEGF was significantly higher in the MSCs, G-CSF and MSCs + G-CSF groups in comparison to the PBS group. The increased VEGF expression was seen in both the 3 and 7 days groups, however, there was no statistically significant difference between each group at 3 and that at 7 days (P > 0.05).
Fig. 4.
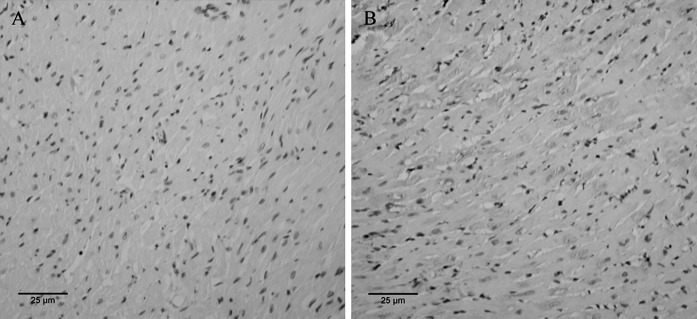
Immunohistochemical staining results of VEGF expression at 4 weeks after cell transplantation. The brown areas denote VEGF positive expression. a PBS injection at 7 days after MI, b MSCs + G-CSF injection at 7 days after MI (inverted phase contrast microscope, ×200)
Table 4.
Expression of VEGF in the myocardial tissues 4 weeks after transplantation (average grey value)
| Group | PBS | MSCs | G-CSF | MSCs + G-CSF |
|---|---|---|---|---|
| Injection at 3 days after MI | 72.4 ± 3.8 | 126.1 ± 2.3* | 118.4 ± 3.8* | 129.5 ± 3.5* |
| Injection at 7 days after MI | 78.6 ± 2.6 | 132.6 ± 4.5* | 122.6 ± 2.4* | 128.1 ± 3.2* |
* P < 0.05, compared with PBS group; Data were expressed as mean ± SD
Discussion
MSCs have been extensively characterized because they are relatively easy to isolate and can be extensively expanded in culture (Peister et al. 2004). Using either environmental or chemical induction, MSCs can differentiate into multiple cell phenotypes, including myocardial cells (Vogel 2000; Derubeis and Cancedda 2004). Further evidence from animal studies has confirmed that bone MSCs improve myocardial function and perfusion in the setting of ischemic heart disease (Kawamoto et al. 2001; Fuchs et al. 2001). However, due to its low number in the bone marrow cells (approximately 1 × 104–105), it is therefore necessary to isolate and culture MSCs in vitro.
Currently, there are four methods for isolation of MSCs (Hung et al. 2002), including whole bone marrow adherence method, density gradient centrifugation, immunomagnetic separation, and flow cytometry sorting method. In this study, we used whole bone marrow direct adherence screening to isolate and culture MSCs. With this method, injury and contamination of MSCs can be avoided, which will be a highly favorable factor for practical applications.
The changes in cardiac anatomy in combination with elevated diastolic pressure and decreased systolic pressure, induce large increases in diastolic stress and modest increases in systolic stress (Olivetti et al. 1991; Pfeffer and Braunwald 1990). Formation of new myocardium within the infarct attenuated the anatomical alterations, led to chronic increases in EF and thickness of LVAW, and reduced the abnormalities in cavitary pressure (Orlic et al. 2001). In this study, EF and the thickness of LVAW were significantly higher in the MSCs and MSCs + G-CSF groups than those in the PBS group at 7 days, suggesting the myocardial repair effect of MSCs and the concomitant administration of MSCs and G-CSF.
In addition, by comparing the results at 3 days with those at 7 days, we found only that the increase rate of thickness of LVAW in the MSCs, G-CSF and MSCs + G-CSF groups at 7 days was higher than that in their corresponding groups at 3 days, and the other parameters, including EF, AI, and VEGF expression were not significantly different between these groups. Park et al. compared the results of neural stem cells injection at 4 h, 1, 3 days, 1, 2, and 5 weeks after hypoxic-ischemic injury, and suggested that stem cells transiently re-enter the cell cycle, migrate preferentially to the ischemic site with a 3–7 days “window” following hypoxic-ischemic injury (Park et al. 2006). Our results suggest that though the increase rate of thickness of LVAW was higher at 7 days, the cardiac function was not recovered. Therefore, the “window” of MSC transplant of MI needs further investigation.
Nowadays, in-depth research on the mechanism of stem cell therapy for MI and stem cell transplantation to improve heart function have gone through several different stages: (1) MSCs differentiate into cardiomyocytes and endothelial cells in vivo when transplanted to the heart in MI models; (2) Cell-to cell communication between MSCs and cardiomyocytes was enhanced because of cellular or nuclear fusion (Sánchez et al. 2006; Balsam et al. 2004; Koyanagi et al. 2005); 3) Promoting revascularization and cytokine paracrine mechanisms: the transplanted stem cells are able to secrete a variety of cytokines to promote blood vessel growth, reconstruction of the collateral circulation (Kinnaird et al. 2004; Tang et al. 2005), providing more blood supply to the transplanted cells, thereby improving myocardial ischemia, saving hibernating myocardium, and reducing ventricular remodeling. A series of experiments have shown that MSCs have protective and anti-apoptotic effects on myocardial cells in the early implanted phase in MI (Murry et al. 2004). Gnecchi et al. suggested that MSCs achieve the effect of heart function protection by paracrine pathway on the undamaged and damaged myocardial tissues (Gnecchi et al. 2005).
G-CSF is used to increase bone MSCs mobilization for transplant and has been proposed as a means of mobilizing stem cells to the uterus (Du et al. 2012). G-CSF has good application prospects in the treatment of MI because it can be directly subcutaneous injected, and circumvent thoracotomy or surgical intervention. The underlying mechanisms of G-CSF include the following aspects: (1) G-CSF stimulates stem cells to differentiate into myocardial cells and vascular tissue. (2) G-CSF stimulates bone MSCs through paracrine pathways, leading to vascular regeneration and cell apoptosis. (3) G-CSF acts directly on receptors in the target cells to play a cytoprotective function. In 2001, Orlic et al. reported that the concomitant administration of SCF (200 μg/kg) and G-CSF (50 μg/kg) to bone MSCs can promote myocardial revascularization after MI in the mouse, and reduce the myocardial infarct size by 40 % and the mortality by 68 % (Orlic et al. 2001). Numerous animal studies and clinical trials have reported that G-CSF can improve functional capacity after MI. However, the effect of G-CSF has become controversial in the recent years. In two rigorously designed randomized controlled trials, although the application of G-CSF was confirmed to be highly safe, the 6-month follow-up results showed that G-CSF neither reduced infarct size nor improved left ventricular function (Ripa et al. 2006; Zohlnhöfer et al. 2006). The effect of G-CSF for treatment of acute MI is greatly challenged by these results.
In this study, we found that the EF value in the G-CSF injection at both 3 and 7 days after MI groups did not show significant difference as compared to the PBS group 30 days after the injection. This result could be explained by: (1) Limited mobilization of MSCs. Hematopoietic stem cells are the main cell groups activated by G-CSF, but the hematopoietic stem cells cannot differentiate into myocardial cells. Thus the limited amount of MSCs could not improve the infarct heart function by homing effect. (2) Inappropriate mobilization timing. The microenvironment in MI area may receive stem cell homing only at a certain time frame and the inappropriate timing causes the stem cells not arrive to the infarction area.
Apoptosis is a gene-regulated programmed cell death process. Myocardial ischemia is a major factor to induce cardiomyocyte apoptosis and thereby changes the post-infarction pathophysiological process of both infarcted and non-infarcted myocardium (Condorelli et al. 1999). Although TUNEL analysis is not specific to apoptotic cells, it is not difficult to distinguish apoptotic and necrotic cells using histological analysis. MSCs secrete a variety of cytokines and growth factors, such as VEGF (Besse et al. 2000). VEGF is a major angiogenic factor and it plays an important role in the formation of new blood vessels for the blood supply of local tissues (Su et al. 2000). It also promotes endothelial cell proliferation and survival by mediating the phosphorylation of protein kinase B and endothelial NO synthesis (Dai et al. 2005; Amano et al. 2003). In addition, VEGF acts through ras to inhibit cell apoptosis by activating PI3K/Akt pathway, and upregulating Bcl-2 expression (Zachary 2001). MSCs transplantation increases Bcl-2 expression to reduce post-infarction myocardial apoptosis in rat. In his study, Kaminhata found that the transplanted MSCs can not only improve heart function and reduce infarction size, but also improve collateral circulation due to increased VEGF levels around the infarction zone (Kamihata et al. 2001). Tang et al. also confirmed the increased VEGF level and the formation of new blood vessels around the infarction zone (Tang et al. 2004). In our study, myocardial apoptosis and VEGF expression were analyzed by TUNEL assay and immunohistochemistry. We showed that in the 3 and 7 days post-infarction groups, treatment with MSCs or in combination with G-CSF increased VEGF expression, and decreased apoptosis. These results were highly consistent with the previous studies (Tang et al. 2004; Kamihata et al. 2001). It is believed that the reduction of myocardial apoptosis might be associated with increased capillary density and thereby improved blood supply around the infarction zone (Schuster et al. 2004). Like the MSCs alone or the MSCs + G-CSF groups, the G-CSF only group showed increased VEGF expression in the 3 and 7 days post-infarction group. However, AI in this G-CSF group was not significantly reduced than that in the PBS group, suggesting that improved blood supply is not the reason that MSCs can reduce myocardial apoptosis. It is more likely that MSCs secrete anti-apoptotic growth factors to reduce myocardial apoptosis (Gnecchi et al. 2005).
Data from our study cannot definitively state that G-CSF can improve heart function after MI and reduce cellular apoptosis. However, we observed an increase in VEGF expression in the infarction area in the G-CSF injection group in comparison to control, suggesting that G-CSF might improve the vascular regeneration process through paracrine mechanisms. EF values are increased in both MSCs group and MSCs + G-CSF group. In addition, the thicker LVAW and increased EF values in the MSCs + G-CSF group as compared with the MSCs group suggest that MSCs in combination with G-CSF treatment of myocardial infarction show a better therapeutic effect than the MSCs group.
Footnotes
Jia Yang and Jindong Xia are co-first authors.
Contributor Information
Jiangmin Zhao, Phone: +86-021-56692066, Email: zhaojiangminzjm@hotmail.com.
Guixiang Zhang, Email: guixiangzhanggxz@163.com.
References
- Amano K, Matsubara H, Iba O, Okigaki M, Fujiyama S, Imada T, Kojima H, Nozawa Y, Kawashima S, Yokoyama M, Iwasaka T. Enhancement of ischemia-induced angiogenesis by eNOS overexpression. Hypertension. 2003;41:156–162. doi: 10.1161/01.HYP.0000053552.86367.12. [DOI] [PubMed] [Google Scholar]
- Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- Besse A, Trimoreau F, Praloran V, Denizot Y. Effect of cytokines and growth factors on the macrophage colony-stimulating factor secretion by human bone marrow stromal cells. Cytokine. 2000;12:522–525. doi: 10.1006/cyto.1999.0580. [DOI] [PubMed] [Google Scholar]
- Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart a statement for healthcare professionals from the cardiac imaging committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Liu X, Ou L, Zhou X, Liu Y, Jia X, Zhang J, Li Y, Kong D. Mobilization of mesenchymal stem cells by granulocyte colony-stimulating factor in rats with acute myocardial infarction. Cardiovasc Drugs Ther. 2008;22:363–371. doi: 10.1007/s10557-008-6110-2. [DOI] [PubMed] [Google Scholar]
- Condorelli G, Morisco C, Stassi G, Notte A, Farina F, Sgaramella G, de Rienzo A, Roncarati R, Trimarco B, Lembo G. Increased cardiomyocyte apoptosis and changes in proapoptotic and antiapoptotic genes bax and bcl-2 during left ventricular adaptations to chronic pressure overload in the rat. Circulation. 1999;99:3071–3078. doi: 10.1161/01.CIR.99.23.3071. [DOI] [PubMed] [Google Scholar]
- Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, Kloner RA. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112:214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- Derubeis AR, Cancedda R. Bone marrow stromal cells (BMSCs) in bone engineering: limitations and recent advances. Ann Biomed Eng. 2004;32:160–165. doi: 10.1023/B:ABME.0000007800.89194.95. [DOI] [PubMed] [Google Scholar]
- Du H, Naqvi H, Taylor HS (2012) Ischemia/reperfusion injury promotes and granulocyte-colony stimulating factor inhibits migration of bone marrow-derived stem cells to endometrium. Stem Cells Dev 21:3324–3331 [DOI] [PMC free article] [PubMed]
- Fan L, Chen L, Chen X, Fu F. A meta-analysis of stem cell mobilization by granulocyte colony-stimulating factor in the treatment of acute myocardial infarction. Cardiovasc Drugs Ther. 2008;22:45–54. doi: 10.1007/s10557-007-6072-9. [DOI] [PubMed] [Google Scholar]
- Fuchs S, Baffour R, Zhou YF, Shou M, Pierre A, Tio FO, Weissman NJ, Leon MB, Epstein SE, Kornowski R. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol. 2001;37:1726–1732. doi: 10.1016/S0735-1097(01)01200-1. [DOI] [PubMed] [Google Scholar]
- Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- Hung SC, Chen NJ, Hsieh SL, Li H, Ma HL, Lo WH. Isolation and characterization of size-sieved stem cells from human bone marrow. Stem cells. 2002;20:249–258. doi: 10.1634/stemcells.20-3-249. [DOI] [PubMed] [Google Scholar]
- Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, Asahara T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–637. doi: 10.1161/01.CIR.103.5.634. [DOI] [PubMed] [Google Scholar]
- Kinnaird T, Stabile E, Burnett M, Lee C, Barr S, Fuchs S, Epstein S. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- Kocher A, Schuster M, Szabolcs M, Takuma S, Burkhoff D, Wang J, Homma S, Edwards N, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- Koyanagi M, Brandes RP, Haendeler J, Zeiher AM, Dimmeler S. Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes a novel mechanism for cell fate changes? Circ Res. 2005;96:1039–1041. doi: 10.1161/01.RES.0000168650.23479.0c. [DOI] [PubMed] [Google Scholar]
- Labarthe DR, Biggers A, Goff DC, Jr, Houston M. Translating a plan into action: a public health action plan to prevent heart disease and stroke. Am J Prev Med. 2005;29:146–151. doi: 10.1016/j.amepre.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KBS, Virag JI, Bartelmez SH, Poppa V. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- Olivetti G, Capasso JM, Meggs LG, Sonnenblick EH, Anversa P. Cellular basis of chronic ventricular remodeling after myocardial infarction in rats. Circ Res. 1991;68:856–869. doi: 10.1161/01.RES.68.3.856. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KI, Hack MA, Ourednik J, Yandava B, Flax JD, Stieg PE, Gullans S, Jensen FE, Sidman RL, Ourednik V, Snyder EY. Acute injury directs the migration, proliferation, and differentiation of solid organ stem cells: evidence from the effect of hypoxia-ischemia in the CNS on clonal “reporter” neural stem cells. Exp Neurol. 2006;199:156–178. doi: 10.1016/j.expneurol.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.CIR.81.4.1161. [DOI] [PubMed] [Google Scholar]
- Reddy AM, Kwak BK, Shim HJ, Ahn C, Lee HS, Suh YJ, Park ES (2010) In vivo tracking of mesenchymal stem cells labeled with a novel chitosan-coated superparamagnetic iron oxide nanoparticles using 3.0T MRI. J Korean Med Sci 25:211–219 [DOI] [PMC free article] [PubMed]
- Redzić A, Smajilagić A, Aljicević M, Berberović L (2010) In vivo osteoinductive effect and in vitro isolation and cultivation bone marrow mesenchymal stem cells. Coll Antropol 34:1405–1409 [PubMed]
- Ripa RS, Kastrup J. G-CSF therapy with mobilization of bone marrow stem cells for myocardial recovery after acute myocardial infarction—A relevant treatment? Exp Hematol. 2008;36:681–686. doi: 10.1016/j.exphem.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Ripa RS, Jørgensen E, Wang Y, Thune JJ, Nilsson JC, Søndergaard L, Johnsen HE, Køber L, Grande P, Kastrup J. Stem cell mobilization induced by subcutaneous granulocyte-colony stimulating factor to improve cardiac regeneration after acute ST-elevation myocardial infarction result of the double-blind, randomized, placebo-controlled stem cells in myocardial infarction (STEMMI) trial. Circulation. 2006;113:1983–1992. doi: 10.1161/CIRCULATIONAHA.105.610469. [DOI] [PubMed] [Google Scholar]
- Rosenstrauch D, Poglajen G, Zidar N, Gregoric ID. Stem cell therapy for ischemic heart failure. Tex Heart Inst J. 2005;32:339–347. [PMC free article] [PubMed] [Google Scholar]
- Sánchez PL, San Román JA, Villa A, Fernández ME, Fernández-Avilés F. Contemplating the bright future of stem cell therapy for cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2006;3:S138–S151. doi: 10.1038/ncpcardio0456. [DOI] [PubMed] [Google Scholar]
- Schuster MD, Kocher AA, Seki T, Martens TP, Xiang G, Homma S, Itescu S. Myocardial neovascularization by bone marrow angioblasts results in cardiomyocyte regeneration. Am J Physiol Heart Circ Physiol. 2004;287:H525–H532. doi: 10.1152/ajpheart.00058.2004. [DOI] [PubMed] [Google Scholar]
- Su H, Lu R, Kan YW. Adeno-associated viral vector-mediated vascular endothelial growth factor gene transfer induces neovascular formation in ischemic heart. Proc Natl Acad Sci USA. 2000;97:13801–13806. doi: 10.1073/pnas.250488097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YL, Zhao Q, Zhang YC, Cheng L, Liu M, Shi J, Yang YZ, Pan C, Ge J, Phillips MI. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept. 2004;117:3–10. doi: 10.1016/j.regpep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Tang YL, Zhao Q, Qin X, Shen L, Cheng L, Ge J, Phillips MI (2005) Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg 80:229–236 [DOI] [PubMed]
- Tomita S, Li RK, Weisel RD, Mickle DAG, Kim EJ, Sakai T, Jia ZQ. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100:II-247–II-256. doi: 10.1161/01.CIR.100.suppl_2.II-247. [DOI] [PubMed] [Google Scholar]
- Tülpar S, Poyrazoglu MH, Özbilge H, Bastug F, Gündüz Z, Torun YA, Kaya EG, Akgün H, Dursun I, Düsünsel R (2012) Modulation of inflammation by mesenchymal stem cell transplantation in peritoneal dialysis in rats. Ren Fail 34:1317–1323 [DOI] [PubMed]
- Vogel G. Stem cells: new excitement, persistent questions. Science. 2000;290:1672–1674. doi: 10.1126/science.290.5497.1672. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li J, Lei L, Jiang C, An S, Zhan Y, Cheng Q, Zhao Z, Wang J (2012) Effects of hypoxia on osteogenic differentiation of rat bone marrow mesenchymal stem cells. Mol Cell Biochem 362:25–33 [DOI] [PubMed]
- Zachary I. Signaling mechanisms mediating vascular protective actions of vascular endothelial growth factor. Am J Physiol Cell Physiol. 2001;280:C1375–C1386. doi: 10.1152/ajpcell.2001.280.6.C1375. [DOI] [PubMed] [Google Scholar]
- Zohlnhöfer D, Ott I, Mehilli J, Schömig K, Michalk F, Ibrahim T, Meisetschläger G, von Wedel J, Bollwein H, Seyfarth M. Stem cell mobilization by granulocyte colony-stimulating factor in patients with acute myocardial infarction. JAMA. 2006;295:1003–1010. doi: 10.1001/jama.295.9.1003. [DOI] [PubMed] [Google Scholar]