Abstract
Knee osteoarthritis is a degenerative disease of diarthrodial joints. Biomechanical factors are considered as risk factors for the disease, the knee joint being normally subject to pressure. Some studies have examined the biomechanical environment of the knee joint in vitro. The aim of this study was to establish a culture model to mimic the knee joint environment. As a first step, synoviocytes induced contraction of three-dimensional collagen gels. Next, contracted collagen gels containing synoviocytes underwent cyclical compression ranging from 0 to 40 kPa at a frequency of 1.0 Hz for 1.5, 3, 6 and 12 h using the FX-4000C™ Flexercell® Compression Plus™ System. RNA in collagen gels was extracted immediately after compression and mRNA expression levels of HAS genes were analyzed by quantitative RT-PCR. Culture medium was collected 48 h after compression and analyzed by agarose gel electrophoresis and cellulose acetate electrophoresis. Synoviocytes in contracted collagen gels were stimulated by cyclic compressive load. Long-term compressive stimulation led to the production of higher molecular weight hyaluronic acid, whereas, short-term, compressive stimulation increased the total amount of hyaluronic acid. Furthermore, mRNA expression levels of both HAS-1 and HAS-2 were significantly higher than without compression. Taken together, using this gel culture system, synoviocytes synthesized higher molecular weight hyaluronic acid and produced large quantities of hyaluronic acid through up-regulation of HAS gene expression. Therefore, the contracted collagen gel model will be a useful in vitro three-dimensional model of the knee joint.
Keywords: Knee osteoarthritis, Hyaluronic acid, Synoviocytes, Three-dimensional collagen gel culture, Gel contraction, Cyclic compressive load
Introduction
Knee osteoarthritis (Knee OA) is a degenerative disease of diarthrodial joints in which degrading and reparative processes in articular cartilage, subchondral bone, and synovium occur concurrently. The pathological features of cartilage in knee OA, including changes in matrix composition, chondrocyte clustering, cartilage fissuring and flaking, are well recognized. Aging, mechanical stress, traumatic injury, genetic susceptibility, and metabolic predispositions are considered risk factors for the disease (Martel-Pelletier et al. 1999). The knee joint is normally subject to pressure which affects the synoviocytes that produce hyaluronic acid (HA). Momberger et al. (2005, 2006) showed that synovial HA secretion was activated under tensile stress and regulated by a mechanosensitive pathway in statically stretched rabbit intimal synoviocytes. They suggested that mechanical deformation is likely to be a major regulator of intra-articular HA content to lubricate the surfaces between the synovial membrane and the articular cartilage. HA is an anionic polysaccharide, a glycosaminoglycan polymer of repeating N-acetyl-glucosamine β (1–4) and glucuronic acid β (1–3) disaccharide units, which is widely distributed in connective, epithelial, and neural tissues (DeAngelis 1999). In cartilage, HA is present in synovial fluid, on cartilage surfaces and in the synovial interstitial matrix, and forms a complex with proteoglycans. Synovial fluid HA is mainly synthesized by fibroblast-related synoviocytes, which form 67 % of the cell population lining the joint cavity (Levick and McDonald 1989). One of the roles of HA is to increase the viscosity and elasticity of the synovial fluid (Peyron and Balazs 1974). HA is synthesized by hyaluronic acid synthase (HAS), which occurs in three mammalian isoforms (HAS-1, HAS-2 and HAS-3) (Rittig et al. 1993). Generally, the viscosity of synovial fluid depends on the concentration and molecular weight of HA. HA is essential for absorbing mechanical stress in cartilage during periods of increased pressure, such as during sustained joint flexion (Coleman et al. 2000; Lu et al. 2004). In the synovial fluid of OA patients, both this viscosity and the high molecular weight of HA decrease (Saari et al. 1993). This decrease in the molecular weight of HA leads to joint degradation, as HA loses its ability to interact with proteoglycans (Palmoski and Brandt 1976; Rizkalla et al. 1992; Takahashi et al. 2004).
Some decades ago, Bell et al. (1979) developed an in vitro culture system in which fibroblasts were embedded in a three-dimensional collagen lattice. Such a fibroblast-populated collagen gel contracts to produce a dermal-like tissue, and this cell-collagen gel system is useful as an in vitro model for the study of various aspects of wound healing and connective tissue regeneration. Collagen gel contraction has been used to investigate the effects of several growth factors on fibroblasts and also epithelial cells and the extracellular matrix (Montesano and Orci 1988; Nishiyama et al., 1991). Tissue-engineering studies of the collagen gel matrix have been done to evaluate cell responses to a glycosaminoglycan-collagen lattice cross-linked with a water soluble cross-linker (Hanthamrongwit et al. 1996).
There have been few studies on the influence of pressure on synoviocytes. Therefore, there are no suitable in vitro three-dimensional models of the knee joint. The aim of this study was to evaluate the contracted collagen gel model. Furthermore, we studied the effect of HA synthesis by synoviocytes under cyclic compressive load.
Materials and methods
Materials
The synoviocyte line HIG-82 was purchased from Dainippon Sumitomo Pharma Co., Ltd. (Osaka, Japan). Native type I collagen (AteloCell® IAC-30) was purchased from Koken Co., Ltd. (Tokyo, Japan). Foetal bovine serum (FBS), Ham’s F-12 and TRIzol® Reagent were purchased from Life Technologies (Carlsbad, CA, USA). Stains-All was purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). Cellulose acetate membrane (SELECA®-V) was purchased from Toyo Roshi Kaisha, Ltd. (Tokyo, Japan). Sodium bicarbonate, ascorbic acid 2-glucoside, Alcian Blue 8GX, and other chemical reagents were all of special grade from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Preparation of three-dimensional collagen gels
The preparation of three-dimensional collagen gels was done following the method of Vernon and Gooden (2002), with slight modifications. First, the 0.2 % collagen solution and synoviocytes in double-strength culture medium were each cooled on an ice bath. The collagen solution and the cells were then mixed slowly using a pipette, and then placed in a 6-well plate. The plate was then transferred to a CO2 incubator, where the collagen solution containing the cells became transformed into a gel. The collagen gel was then separated from the edge of the plate.
The composition of the culture medium was Ham’s F-12, 10 % FBS, 4.1 % sodium bicarbonate and 8.6 × 10−2 % ascorbic acid 2-glucoside. The final concentration of native type I collagen was 0.1 %. Cells were seeded at 2.5 × 105, 5.0 × 105, and 10 × 105 cells/gel in a humidified atmosphere of 5 % CO2/95 % air.
Cyclic loading compression
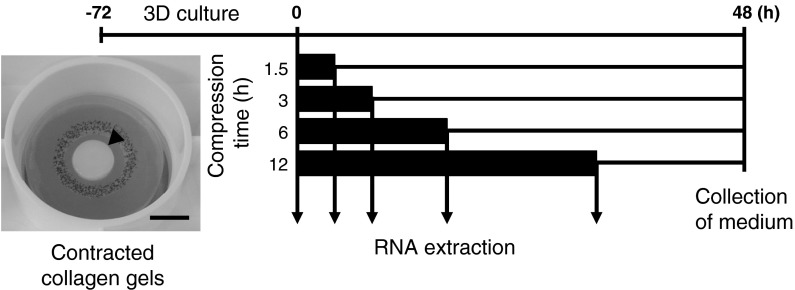
The experimental schedules are shown in Fig. 1. Each contracted collagen gel was placed on an FX-4000C™ bio plate (Flexcell International Corp., Hillsborough, NC, USA). Synoviocytes in contracted collagen gels (10 × 105 cells/gel) were cultured with the FX-4000C™ Flexercell® Compression Plus™ System (Flexcell International Corp.). The same air pressures were applied to the device in a dynamic setup controlled by the Flexercell. In this apparatus, positive pressure constantly compressed a three-dimensional cell culture between a piston and a stationary plate. Contracted collagen gels underwent cyclic compression ranging from 0 to 40 kPa at a frequency of 1.0 Hz for 0, 1.5, 3, 6 and 12 h.
Fig. 1.
Experimental schedules. Contracted collagen gels containing synoviocytes were pre-cultured for 72 h then placed in the FX-4000C™ Flexercell® Compression Plus™ System. Gels then underwent cyclical compression ranging from 0 to 40 kPa at a frequency of 1.0 Hz for 1.5, 3, 6 and 12 h. The black columns represent period of compressive load. Arrows indicate when RNA in the collagen gels was extracted, immediately after compression, for analysis of mRNA expression levels of HAS genes by quantitative real-time RT-PCR. The culture medium was collected 48 h after the start of compression and analyzed by agarose gel electrophoresis and cellulose acetate electrophoresis. The arrowhead indicates a contracted collagen gel in the image. Bar 10 mm
Cells in contracted collagen gels were collected just after compression for RNA extraction. To prepare HA, the medium was collected after 48 h of culture.
HA analysis
Hyaluronic acid was prepared from the culture medium following the method of Ohara et al. (2010), with slight modifications. The medium was subjected to protease (Actinase E; Kaken Pharmaceutical Co., Ltd., Tokyo, Japan) overnight digestion using protease in 0.1 M Tris–HCl (pH 7.8) containing 5 mM CaCl2 at 50 °C. The digest was TCA precipitated on ice overnight, centrifuged, and the acid soluble HA was dialyzed extensively against water.
The molecular weight of HA in the culture medium was determined by agarose gel electrophoresis. The separation buffer used was 0.1 M pyridine and 0.47 M formic acid (pH 3.0). Agarose gels (0.1 %) were run at a constant current (200 mA, 50 V). HA markers (1 mg/ml) were 1,500, 1,000, 700, 500 and 50 k (Kewpie Corp., Tokyo, Japan). After 5 h, agarose gels were further stained with Stains-All (10 mg in 100 ml water–dioxane 90:10 plus 0.01 M ascorbic acid 5 ml and 0.1 M acetic acid 100 μl, overnight in the dark and destained with water) to reveal HA.
Hyaluronic acid content was determined by cellulose acetate electrophoresis, following the method of Hata and Nagai (1973). The membranes were stained with Alcian Blue (1 g in 200 ml water–ethanol–acetic acid 65:25:10, and destained with water–acetic acid 90:10) to reveal HA.
Bands detected on the agarose gels and cellulose acetate membranes were analysed after densitometry using image analysis software (ImageJ; Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, MD, USA, http://imagej.nih.gov/ij/, 1997–2012.).
Quantitative real-time RT-PCR analysis of HAS genes
Constructs after 0, 1.5, 3, 6 and 12 h compression were processed for extraction of total RNA using Trizol® Reagent. Fifteen constructs were used for this experiment with three constructs at each time point. After washing with 75 % ethanol, RNA was treated with RNase-free DNase (DNA-free™; Life Technologies) to remove genomic DNA, and purified with phenol–chloroform. Single strand cDNA was synthesized by reverse transcriptase (PrimeScript™ RT reagent Kit; Takara Bio Inc., Otsu, Shiga, Japan). Complementary DNA was synthesized by 15 min incubation at 37 °C with reverse transcriptase and a random hexamer primer, followed by enzyme inactivation at 85 °C for 5 s. Messenger RNA expression in the specimens was quantitatively determined by real-time reverse transcriptase polymerase chain reaction (real-time RT-PCR) using a Thermal Cycler Dice Real Time System TP800 (Takara Bio Inc.) with associated enzyme and reagents (SYBR® Premix Ex Taq™; Takara Bio Inc.). Primer sequences were as follows: HAS-1, AGTGTGGCGCTGACCATCTC (forward) and TTGCCATCCACCACCATGA (reverse); HAS-2, AGTCATGTACACAGCCTTCAGAGCA (forward), CACCTCCAACCATGGGATCTTC (reverse); HAS-3, AAGTGCCTCACAGAGACCCC (forward) and AAGATCATCTCTGCATTGCC (reverse); GAPDH, GCACCGTCAAGGCTGAGAAC (forward), and TGGTGAAGACGCCAGTGGA (reverse). PCR amplification of the cDNA samples was carried out by initial denaturation for 10 s at 95 °C, followed by 40 cycles of denaturation for 5 s at 95 °C and annealing for 30 s at 60 °C, then denaturation for 15 s at 95 °C and annealing for 30 s at 60 °C, and final denaturation for 15 s at 95 °C (n = 3).
Statistical analysis
Data were expressed as means and standard errors of the mean (SEM) for the number of measurements shown in the Figures. Statistical analysis was carried out by analysis of variance (ANOVA), using Excel 2007 (Microsoft, USA) with the add-in software Statcel 3 (OMS Publishing Inc., Saitama, Japan). The significance of differences was determined using Dunnett’s multiple comparison test. The probability level used to determine statistical significance was P < 0.05.
Result
Average diameters of three-dimensional collagen gels
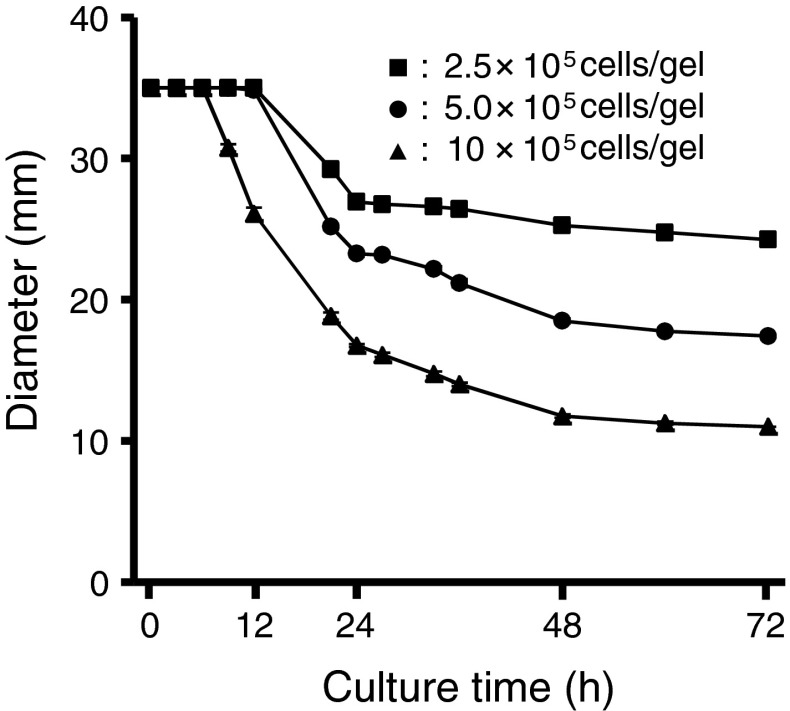
The FX-4000C™ bio plate has 6 wells each 13 mm in diameter (in Fig. 2 initial diameter is 35 mm). Accordingly, the number of cells used was 10 × 105 cells/gel, and a 72 h culture period was applied.
Fig. 2.
Effect of synoviocyte numbers on the contraction of collagen gels. Squares, 2.5 × 105 cells/gel; Circles, 5.0 × 105 cells/gel; Triangles, 1.0 × 106 cells/gel. Data are shown as mean ± SEM., n = 6
Figure 2 shows the contraction curves for collagen gels containing synoviocytes. Synoviocytes were able to contract the collagen gels. The contraction rate of the collagen gels depended on the number of cells. After 72 h, the average diameters of the collagen gels had contracted from 35 to 24.3 mm (2.5 × 105 cells/gel), 17.4 mm (5.0 × 105 cells/gel), and 11.0 mm (10 × 105 cells/gel).
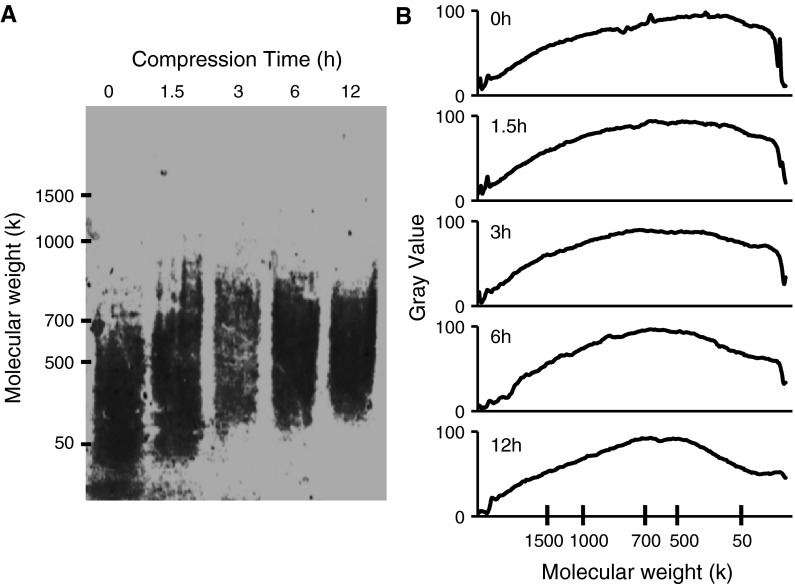
Molecular weight of HA
Figure 3 shows the agarose gel electrophoresis pattern of HA from the culture medium. Without compression (0 h), synoviocytes secreted HA with molecular weights of 50–500 k. At 6 and 12 h of compression, the molecular weight of the HA increased to approximately 700 k.
Fig. 3.
Effect of compression stimulus on the molecular weight of HA produced by synoviocytes. Electrophoresis was performed on agarose gels in 0.1 M pyridine/0.2 M formic acid, pH 3.0, at 200 mA, 50 V for 5 h. Gels were stained using Stains-All. a Agarose gel electrophoretic pattern of HA. b Densitometric pattern of HA by agarose gel electrophoresis. Graphs show the molecular weight distribution of the bands shown in a, obtained using ImageJ software from NIH (http://rsb.info.nih.gov/ij/download/)
This suggests that the molecular weight of the HA secreted into the extracellular matrix had increased due to compressive stimulation.
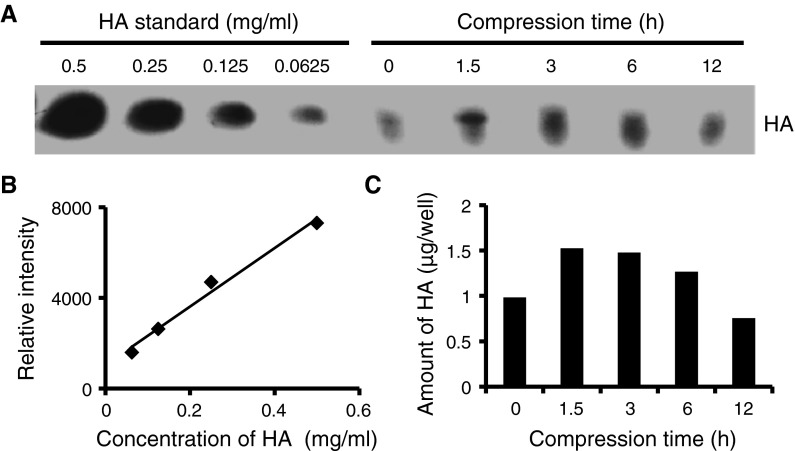
Amount of HA
The amount of HA in the culture medium increased with compressive stimulation (Fig. 4). HA content without compression was 0.98 μg/well. In the compression group, this increased to 1.53, 1.48 and 1.27 μg/well at 1.5, 3 and 6 h of compression, respectively.
Fig. 4.
Effect of compression stimulus on the amount of HA produced by synoviocytes. Electrophoresis was performed on cellulose acetate membranes in 0.1 M pyridine/0.47 M formic acid, pH 3.0, at 1 mA/cm for 60 min. Membranes were stained using Alcian Blue. a Cellulose acetate electrophoretic pattern of HA. b Calibration curve based on standard concentrations of HA. (R2 = 0.985). c The amount of HA produced by synoviocytes, obtained by densitometric quantification of the bands shown in a, obtained using ImageJ software from NIH (http://rsb.info.nih.gov/ij/download/)
Therefore synoviocytes in contracted collagen gels increased the amount of HA by compressive stimulation.
Quantification of mRNA expression levels by real-time RT-PCR
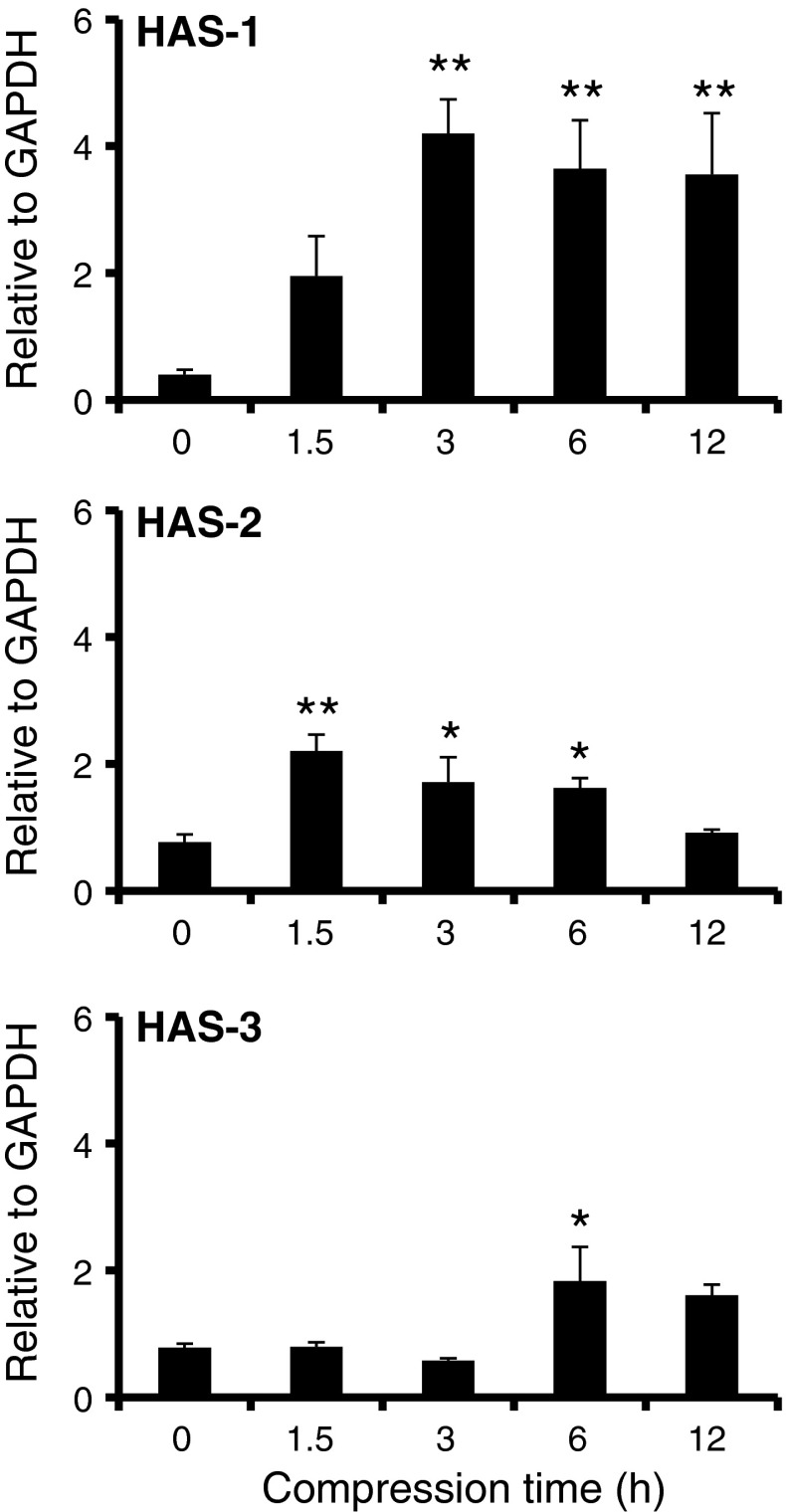
mRNA expressions levels are shown in Fig. 5. The mRNA expression levels of HAS-1 after 3, 6 and 12 h compression were approximately 10 times higher than without compression. In addition, the mRNA expression levels of HAS-2 at 1.5, 3 and 6 h of compression were significantly higher than without compression. Finally, the mRNA expression levels of HAS-3 at 6 h of compression were significantly higher than without compression.
Fig. 5.
Effect of compression stimulus on HAS gene expression by synoviocytes. Messenger RNA expression levels of HAS-1, HAS-2 and HAS-3 were analysed with real-time RT-PCR. All mRNA of HAS genes were normalized by GAPDH, a housekeeping gene. Data are shown as mean ± SEM., n = 3. *, **, Significant difference from without compression (0 h) at P < 0.05 and 0.01, respectively
These results suggest that the compressive stimulation of synoviocytes activated HAS gene expression.
Discussion
It has been reported that biomechanical factors affect cellular function in knee joint tissues (Bougault et al. 2009; Buschmann et al. 1995; Gabay et al. 2008; Knight et al. 1998; Momberger et al. 2005, 2006). HA secretion by synoviocytes is activated under tensile stress and regulated by a mechanosensitive pathway (Momberger et al. 2005). Some researchers have demonstrated the compressive loading of three-dimensional culture models using the FX-4000C™ Flexercell® Compression Plus™ System, where they used a maximum of 40 kPa so as not to deform their model. For example, in the agarose gel model, 20 kPa was chosen as the strain regimen because this preserves agarose gel integrity, which is required to achieve the correct transmission of mechanical stress to the isolated cells embedded within it (Bougault et al. 2009; Buschmann et al. 1995). However, the material containing the cells must have sufficient flexibility for repetitive compressive stimulation. Collagen is a major component of extracellular matrix and is used as an in vitro organization model (Chevallay and Herbage 2000; Fox et al. 2006; Stone et al. 1992, 1997; Vernon and Gooden 2002). Contracted collagen gels (spheroids), in particular, are flexible under mechanical stress. Synoviocytes caused collagen gel contraction, as shown in Fig. 2, where the HIG-82 cells used are fibroblast-related synoviocytes derived from rabbit synovial tissue. As also shown here, in collagen gels, the degree of gel contraction depends on the number of fibroblasts per gel (Vernon and Gooden 2002). Synoviocytes at 10 × 105 cells/gel contracted the collagen gel to resist the repetitive compressive stimulation.
In this article, we focused on the effect of compressive stimulation on both the molecular weight and amount of HA. Over the long-term, compressive stimulation resulted in HA with a higher molecular weight (Fig. 3). The mRNA expression levels of both HAS-1 at 3, 6 and 12 h compression, and HAS-2 at 1.5, 3 and 6 h compression were significantly higher than without compression (Fig. 5). It has been reported that the synthesis of high molecular weight HA in synoviocytes is accelerated through the up-regulation of HAS-1 and HAS-2 (David-Raoudi et al. 2009). This mechanism might be reflected in the results obtained here; synoviocytes synthesized with HA a high molecular weight through the up-regulation of HAS gene expression. In addition, the duration of short-term compressive stimulation modified the amount of HA (Fig. 4). In agreement with the mRNA expression, levels of HAS-2 after 1.5, 3 and 6 h compression were significantly higher than without compression (Fig. 5). It has been reported that when cultured rabbit intima synoviocytes were subjected to 10 % maintained stretch for 10 min, the rate of HA secretion increased over 3 h by 30–75 % (Momberger et al. 2005). These results suggest that when stimulated with cyclic compressive loads, synoviocytes produced more HA through up-regulation of HAS-2 expression. In contrast, long-term compressive stimulation decreased HA levels. The amount of HA after 12 h compressive stimulation was smaller than that without compressive stimulation. These results suggest that compressive stimulation may affect the synthesis of low molecular weight HA. Here we demonstrate that HA secretion by synoviocytes alters under cyclic compressive load in contracted collagen gels.
In conclusion, under cyclic compressive load in contracted collagen gels, synoviocytes synthesized higher molecular weight HA and produced large quantities of HA through up-regulation of HAS gene expression. Therefore, the contracted collagen gel model will be a useful in vitro three-dimensional model of the knee joint.
References
- Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci USA. 1979;76:1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougault C, Paumier A, Aubert-Foucher E, Mallein-Gerin F. Investigating conversion of mechanical force into biochemical signaling in three-dimensional chondrocyte cultures. Nat Protoc. 2009;4:928–938. doi: 10.1038/nprot.2009.63. [DOI] [PubMed] [Google Scholar]
- Buschmann MD, Gluzband YA, Grodzinsky AJ, Hunziker EB. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108:1497–1508. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- Chevallay B, Herbage D. Collagen-based biomaterials as 3D scaffold for cell cultures: applications for tissue engineering and gene therapy. Med Biol Eng Comput. 2000;38:211–218. doi: 10.1007/BF02344779. [DOI] [PubMed] [Google Scholar]
- Coleman PJ, Scott D, Mason RM, Levick JR. Role of hyaluronan chain length in buffering interstitial flow across synovium in rabbits. J Physiol. 2000;526:425–434. doi: 10.1111/j.1469-7793.2000.00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-Raoudi M, Deschrevel B, Leclercq S, Galera P, Boumediene K, Pujol JP. Chondroitin sulfate increases hyaluronan production by human synoviocytes through differential regulation of hyaluronan synthases: role of p38 and Akt. Arthritis Rheum. 2009;60:760–770. doi: 10.1002/art.24302. [DOI] [PubMed] [Google Scholar]
- DeAngelis PL. Molecular directionality of polysaccharide polymerization by the Pasteurella multocida hyaluronan synthase. J Biol Chem. 1999;274:26557–26562. doi: 10.1074/jbc.274.37.26557. [DOI] [PubMed] [Google Scholar]
- Fox DB, Cook JL, Kuroki K, Cockrell M. Effects of dynamic compressive load on collagen-based scaffolds seeded with fibroblast-like synoviocytes. Tissue Eng. 2006;12:1527–1537. doi: 10.1089/ten.2006.12.1527. [DOI] [PubMed] [Google Scholar]
- Gabay O, Hall DJ, Berenbaum F, Henrotin Y, Sanchez C. Osteoarthritis and obesity: experimental models. Joint Bone Spine. 2008;75:675–679. doi: 10.1016/j.jbspin.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanthamrongwit M, Reid WH, Grant MH. Chondroitin-6-sulphate incorporated into collagen gels for the growth of human keratinocytes: the effect of cross-linking agents and diamines. Biomaterials. 1996;17:775–780. doi: 10.1016/0142-9612(96)81414-1. [DOI] [PubMed] [Google Scholar]
- Hata R, Nagai Y. A micro colorimetric determination of acidic glycosaminoglycans by two dimensional electrophoresis on a cellulose acetate strip. Anal Biochem. 1973;52:652–656. doi: 10.1016/0003-2697(73)90075-4. [DOI] [PubMed] [Google Scholar]
- Knight MM, Lee DA, Bader DL. The influence of elaborated pericellular matrix on the deformation of isolated articular chondrocytes cultured in agarose. Biochim Biophys Acta. 1998;1405:67–77. doi: 10.1016/S0167-4889(98)00102-5. [DOI] [PubMed] [Google Scholar]
- Levick JR, McDonald JN. Ultrastructure of transport pathways in stressed synovium of the knee in anaesthetized rabbits. J Physiol. 1989;419:493–508. doi: 10.1113/jphysiol.1989.sp017882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Levick JR, Wang W. Concentration polarization of hyaluronan on the surface of the synovial lining of infused joints. J Physiol. 2004;561:559–573. doi: 10.1113/jphysiol.2004.073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel-Pelletier J, Di Battista JA, Lajeunesse D. Biochemical factors in joint articular tissue degradation in osteoarthritis. In: Reginster JY, Pelletier JP, Martel-Pelletier J, Henrotin Y, editors. Osteoarthritis: clinical and experimental aspects. Berlin: Springer-Verlag; 1999. pp. 156–187. [Google Scholar]
- Momberger TS, Levick JR, Mason RM. Hyaluronan secretion by synoviocytes is mechanosensitive. Matrix Biol. 2005;24:510–519. doi: 10.1016/j.matbio.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momberger TS, Levick JR, Mason RM. Mechanosensitive synoviocytes: a Ca2+-PKCα-MAP kinase pathway contributes to stretch-induced hyaluronan synthesis in vitro. Matrix Biol. 2006;25:306–316. doi: 10.1016/j.matbio.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Montesano R, Orci L. Transforming growth factor beta stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proc Natl Acad Sci USA. 1988;85:4894–4897. doi: 10.1073/pnas.85.13.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama T, Akutsu N, Horii I, Nakayama Y, Ozawa T, Hayashi T. Response to growth factors of human dermal fibroblasts in a quiescent state owing to cell-matrix contact inhibition. Matrix. 1991;11:71–75. doi: 10.1016/S0934-8832(11)80210-6. [DOI] [PubMed] [Google Scholar]
- Ohara H, Iida H, Ito K, Takeuchi Y, Nomura Y. Effects of Pro-Hyp, a collagen hydrolysate-derived peptide, on hyaluronic acid synthesis using in vitro cultured synovium cells and oral ingestion of collagen hydrolysates in a guinea pig model of osteoarthritis. Biosci Biotechnol Biochem. 2010;74:2096–2099. doi: 10.1271/bbb.100193. [DOI] [PubMed] [Google Scholar]
- Palmoski M, Brandt K. Hyaluronate-binding by proteoglycans. Comparison of mildly and severely osteoarthritic regions of human femoral cartilage. Clin Chim Acta. 1976;70:87–95. doi: 10.1016/0009-8981(76)90008-5. [DOI] [PubMed] [Google Scholar]
- Peyron JG, Balazs EA. Preliminary clinical assessment of Na-hyaluronate injection into human arthritic joints. Pathol Biol (Paris) 1974;22:731–736. [PubMed] [Google Scholar]
- Rittig M, Flugel C, Prehm P, Lutjen-Drecoll E. Hyaluronan synthase immunoreactivity in the anterior segment of the primate eye. Graefes Arch Clin Exp Ophthalmol. 1993;231:313–317. doi: 10.1007/BF00919026. [DOI] [PubMed] [Google Scholar]
- Rizkalla G, Reiner A, Bogoch E, Poole AR. Studies of the articular cartilage proteoglycan aggrecan in health and osteoarthritis. Evidence for molecular heterogeneity and extensive molecular changes in disease. J Clin Invest. 1992;90:2268–2277. doi: 10.1172/JCI116113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saari H, Konttinen YT, Friman C, Sorsa T. Differential effects of reactive oxygen species on native synovial fluid and purified human umbilical cord hyaluronate. Inflammation. 1993;17:403–415. doi: 10.1007/BF00916581. [DOI] [PubMed] [Google Scholar]
- Stone KR, Rodkey WG, Webber R, McKinney L, Steadman JR. Meniscal regeneration with copolymeric collagen scaffolds. In vitro and in vivo studies evaluated clinically, histologically, and biochemically. Am J Sports Med. 1992;20:104–111. doi: 10.1177/036354659202000202. [DOI] [PubMed] [Google Scholar]
- Stone KR, Steadman JR, Rodkey WG, Li ST. Regeneration of meniscal cartilage with use of a collagen scaffold. Analysis of preliminary data. J Bone Joint Surg Am. 1997;79:1770–1777. doi: 10.2106/00004623-199712000-00002. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Tominaga K, Takano H, Ariyoshi W, Habu M, Fukuda J, Maeda H. A decrease in the molecular weight of hyaluronic acid in synovial fluid from patients with temporomandibular disorders. J Oral Pathol Med. 2004;33:224–229. doi: 10.1111/j.0904-2512.2004.00024.x. [DOI] [PubMed] [Google Scholar]
- Vernon RB, Gooden MD. An improved method for the collagen gel contraction assay. In Vitro Cell Dev Biol Anim. 2002;38:97–101. doi: 10.1290/1071-2690(2002)038<0097:AIMFTC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]