Abstract
We compared the osteoblastic differentiation abilities of dedifferentiated fat cells (DFATs) and human bone marrow mesenchymal stem cells (hMSCs) as a cell source for bone regeneration therapies. In addition, the utility of DFATs in bone tissue engineering in vitro was assessed by an alpha-tricalcium phosphate (α-TCP)/collagen sponge (CS). Human DFATs were isolated from the submandibular of a patient by ceiling culture. DFATs and hMSCs at passage 3 were cultured in control medium or osteogenic medium (OM) for 14 days. Runx2 gene expression, alkaline phosphatase (ALP) activity, as well as osteocalcin (OCN) and calcium contents were analyzed to evaluate the osteoblastic differentiation ability of both cell types. DFATs seeded in a α-TCP/CS and cultured in OM for 14 days were analyzed by scanning electron microscopy (SEM) and histologically. Compared with hMSCs, DFATs cultured in OM generally underwent superior osteoblastogenesis by higher Runx2 gene expression at all days tested, as well as higher ALP activity at day 3 and 7, OCN expression at day 14, and calcium content at day 7. In SEM analyses, DFATs seeded in a α-TCP/CS were well spread and covered the α-TCP/CS by day 7. In addition, numerous spherical deposits were found to almost completely cover the α-TCP/CS on day 14. Von Kossa staining showed that DFATs differentiated into osteoblasts in the α-TCP/CS and formed cultured bone by deposition of a mineralized extracellular matrix. The combined use of DFATs and an α-TCP/CS may be an attractive option for bone tissue engineering.
Keywords: Dedifferentiated fat cells, Ceiling culture, Alpha-tricalcium phosphate/collagen sponge, Bone tissue engineering
Introduction
Repair of osseous defects in alveolar clefts allows for closure of oronasal fistulas, fusion of the maxilla, tooth eruption, and support of the nasal alar base. Autogenous bone grafts are the gold standard for such reconstruction because of their osteoconductive, osteoinductive, and non-immunogenic properties (Gimbel et al. 2007). Although autologous bone grafting is widely performed for bone reconstruction in the maxillofacial field, it is very invasive and there is a limited amount of collectable bone. Therefore, bone formation using bone marrow mesenchymal stem cells (MSCs) has attracted attention as an alternative procedure.
MSCs are fibroblast-like cells established from bone marrow, which attach to tissue culture surfaces (Colter et al. 2000). Human MSCs (hMSCs) are multipotent and have been differentiated into several kinds of mesodermal tissues, including bone, fat, cartilage, and bone in vitro (Pittenger et al. 1999). Induced pluripotent stem cells and embryonic stem cells have great potential in tissue engineering because they proliferate indefinitely while retaining pluripotency, and can differentiate into all cell types found in the body (Shimada et al. 2012). However, there are many problems to be resolved regarding these pluripotent cell types, including ethical issues and tumorigenic transformation. In contrast, somatic stem cells such as MSCs and adipose stem cells have shown considerable safety in basic research. Previously, Yamada et al. (2004) used a mixture of hMSCs from bone marrow and platelet-rich plasma for craniofacial reconstruction and dental implants that are bone graft materials with predictable grafting success.
However, painful bone marrow aspiration is required to obtain these cells from patients and the procedure is technically demanding, particularly in elderly patients. In contrast to these disadvantages, somatic stem cells derived from subcutaneous fat can be obtained from patients of all ages by low-invasive procedures (Planat-Benard et al. 2004). Mature adipocytes are the most abundant cell type in adipose tissue. Mature adipocytes isolated from fat tissue can be dedifferentiated into fibroblast-like cells, which have been named dedifferentiated fat cells (DFATs), by an in vitro dedifferentiation strategy known as ceiling culture (Matsumoto et al. 2008; Yagi et al. 2004).
We have previously investigated bone tissue engineering using a combination of DFATs and the self-assembling peptide RADA16 or a titanium fiber mesh as scaffolds, and found that DFATs proliferate and differentiate into osteoblasts (Kishimoto et al. 2013; Kishimoto et al. 2011; Kishimoto et al. 2008). However, a study has not been performed to compare the osteoblastic differentiation abilities of DFATs and MSCs. Therefore, the purpose of this study was to perform such an evaluation of DFATs and MSCs. In addition, the utility of DFATs in bone tissue engineering in vitro was assessed by an alpha-tricalcium phosphate (α-TCP)/collagen sponge (CS) scaffold.
Materials and methods
Isolation and culture of DFATs
Human adipose tissue was obtained from a male patient (63 years old) who underwent submandibular surgery at the Amagasaki Chuo Hospital. The patient was healthy and had no systemic disease. This study conformed to the tenets of the declaration of Helsinki, and the protocol was approved by the ethics committee of the Osaka Dental University and the Amagasaki Chuo Hospital (approval number: 110714). We isolated DFATs using the ceiling culture method as described in our previous report (Kishimoto et al. 2013) (Fig. 1). Cell culture medium consisting of Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 20 % fetal bovine serum (FBS; Invitrogen, Life Technologies, Carlsbad, CA, USA) and an antibiotic/antimycotic mixed stock solution (Nacalai Tesque, Kyoto, Japan) was replaced twice a week. At confluency, the cells were passaged and used for experiments.
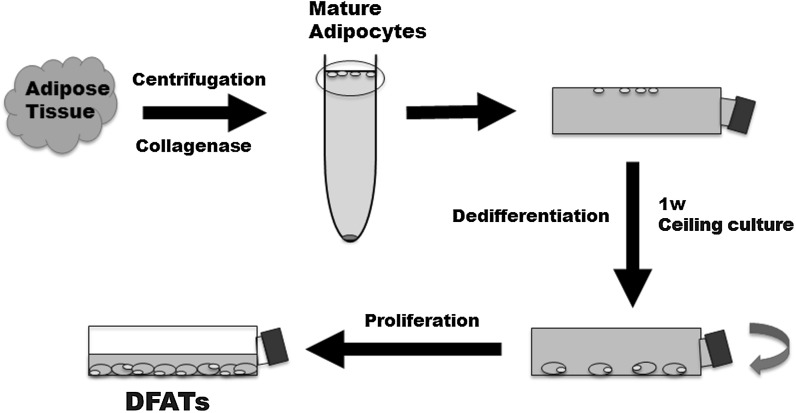
Fig. 1.
Isolation of human dedifferentiated fat cells (DFATs) by “ceiling culture”
Osteogenic differentiation of DFATs and hMSCs
hMSCs were provided by RIKEN BRC through the National Bio-Resource Project of the Ministry of Education, Science, Sports Culture and Technology, Japan (Tsutsumi et al. 2001). To evaluate the osteogenic potential of DFATs and hMSCs at passage 3, they were replated in standard medium onto a 12-well plate at a density of 3 × 104 cells/well and incubated at 37 °C with 5 % CO2. At confluency, both cell types were cultured in control medium (CM; DMEM supplemented with 10 % FBS and the antibiotic/antimycotic mixed stock solution) or osteogenic medium (OM; CM supplemented with 10 % FBS, 100 nM dexamethasone, 50 μM L-ascorbic acid 2-phosphate, and 10 mM β-glycerophosphate) (Sigma-Aldrich, St. Louis, MO, USA) for 14 days. Both media were replaced twice a week.
DNA content analysis
DNA content was measured on days 3, 7, and 14. The medium was removed and the cells were washed twice with PBS. Then, 300 μL of 0.2 % Triton X-100 was added to each well, and the cells were removed by a cell scraper (Becton–Dickinson, Franklin Lakes, NJ, USA) for DNA extraction. DNA content was measured by a Quant-iT™ PicoGreen® dsDNA Reagent and Kit (Invitrogen). Fifty microliters of a sample was mixed with a DNA-binding fluorescent dye solution (0.5 μL Picogreen reagent in 100 μL 1 × TE buffer), and the fluorescent intensity was measured by a microplate reader (Ex 450 nm/Em 510 nm, SpectraMax® M5; Molecular Devices, Sunnyvale, CA, USA).
Quantitative real-time RT-PCR
Total RNA was isolated using a Mag Extractor (Toyobo, Osaka, Japan) according to the manufacturer’s protocol, and single-stranded cDNA was synthesized using a High-Capacity RNA-to-cDNA Master Mix (Applied Biosystems, Foster City, CA, USA). Runx2 mRNA levels were analyzed by quantitative real-time PCR using a TaqMan® Gene Expression Assay (Hs00231692_m1; Applied Biosystems) on a Step One Plus PCR system (Applied Biosystems). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was co-amplified as an internal standard (Human GAPDH endogenous control; Applied Biosystems). Gene expression was measured using the ΔΔCt method (Livak and Schmittgen 2001).
Measurement of alkaline phosphatase activity
Alkaline phosphatase (ALP) activity was measured by LabAssay™ ALP (Wako Pure Chemical Industries, Osaka, Japan). One-hundred microliters of working assay solution (6.7 mmol/L p-nitrophenylphosphate disodium) was added to 20 μL of the same sample used for DNA measurement, and then mixed thoroughly and incubated at 37 °C with 5 % CO2 for 15 min. After 80 μL of stop solution (0.2 mol/L sodium hydroxide) was added to the mixture, the absorbance was measured at 405 nm with the SpectraMax® M5. The levels of ALP activity were normalized to the amount of total DNA in the cell lysates.
Osteocalcin measurement
DFATs and hMSCs were cultured under the same condition described in DNA content analysis, and then the expression of osteocalcin (OCN) in both cell types was determined by a Gla-Type Osteocalcin EIA Kit (Takara Bio Inc., Shiga, Japan). On days 3, 7, and 14 of culture, the medium was removed, the cells were washed with PBS, and 300 μL of 10 % formic acid was added to each well, followed by removal of the cells using a cell scraper. Samples (100 μL) were added to each well of an anti-OCN antibody-coated microtiter plate and then incubated at room temperature for 2 h. After three washes with PBS, 100 μL of a peroxidase-conjugated anti-OCN antibody solution was added to each well, followed by incubation at room temperature for 1 h. After four washes with PBS, 100 μL of substrate solution (tetramethylbenzidine) was added to each well, followed by incubation at room temperature for 15 min. After 100 μL of stop solution was added to each well, the absorbance was measured at 450 nm with the SpectraMax® M5.
Calcium measurement
Calcium content was measured with a Calcium E-test Wako (Wako Pure Chemical Industries). Two milliliters of monoethanolamine buffer was added to 50 μL of the same sample used for OCN measurement, followed by thorough mixing. After 1 mL methylxylenol blue coloring agent was added to the mixture, the absorbance was measured at 610 nm with the SpectraMax® M5.
Preparation of the α-TCP/CS
Porous α-TCP granules were kindly supplied by Taihei Chemical (Osaka, Japan). The collagen used was extracted from porcine skin by enzymatic treatment with pepsin (Nippon Meat Packers, Osaka, Japan). α-TCP particles were mixed with the homogenized collagen solution at 150 mg α-TCP particles/mL of collagen solution. The mixture was poured into plastic molds, and then immediately frozen at −30 °C and freeze dried for 24 h. The freeze-dried α-TCP/CS resembled a sponge-like structure, and was subsequently cross-linked in vacuo at 140 °C for 10 h. The prepared α-TCP/CS was shaped with a 5 mm diameter and 2 mm thickness. The microstructures of the obtained scaffolds were then observed by scanning electron microscopy (SEM) (S-450; Hitachi, Tokyo, Japan).
Cell seeding technique
Briefly, DFATs were detached with 0.25 % trypsin in 1 mM EDTA (Nacalai Tesque, Kyoto, Japan) and centrifuged at 135 g for 5 min. Seeding was then performed by droplet seeding. α-TCP/CS scaffolds were placed in 96-well plates. Cells were resuspended in OM, and 50 μl of 1 × 105 cells/ml was pipetted into the α-TCP/CS scaffolds. DFATs seeded into α-TCP/CS scaffolds were cultured in OM for 14 days.
SEM
DFATs loaded in the αTCP/CS were fixed with 2 % glutaraldehyde in 0.1 M phosphate buffer for 1 h followed by 1 % OsO4 in 0.1 M phosphate buffer for 1 h (Wako Pure Chemical Industries). After dehydration through a graded series of ethanol and ethanol isoamyl acetate solutions, samples were dried by a critical pointdryer (VFD-21; VACUUM DEVICE, Ibaraki, Japan). Samples were subsequently shadowed with gold using an iron sputter (MSP-1S, VFD-21; VACUUM DEVICE) and then observed under a scanning electron microscope (4700-S; Hitachi).
Histological analysis
DFATs seeded in the αTCP/CS were fixed in 4 % formaldehyde on day 14 of culture. The fixed samples were dehydrated, embedded in paraffin, cut into 4 μm-thick sections, and then stained with hematoxylin and eosin (H&E). Von Kossa staining was performed to detect calcium in DFAT-seeded αTCP/CS scaffolds. Samples were incubated in a 5 % silver nitrate solution (Wako Pure Chemical Industries) for 1 h, washed with distilled water, and then fixed in 5 % sodium thiosulphate (Wako Pure Chemical Industries) for 3 min. An unseeded αTCP/CS was also subjected to Von Kossa staining, because αTCP contains calcium. The samples were then analyzed by automated fluorescence microscopy (BZ-9000; Keyence, Osaka, Japan).
Statistical analysis
All experiments were conducted in quintuplicate and repeated at least twice. All data were expressed as the mean and standard deviation. Differences were evaluated by analysis of variance with Tukey’s test. Differences were considered significant at p < 0.05.
Results
DNA content
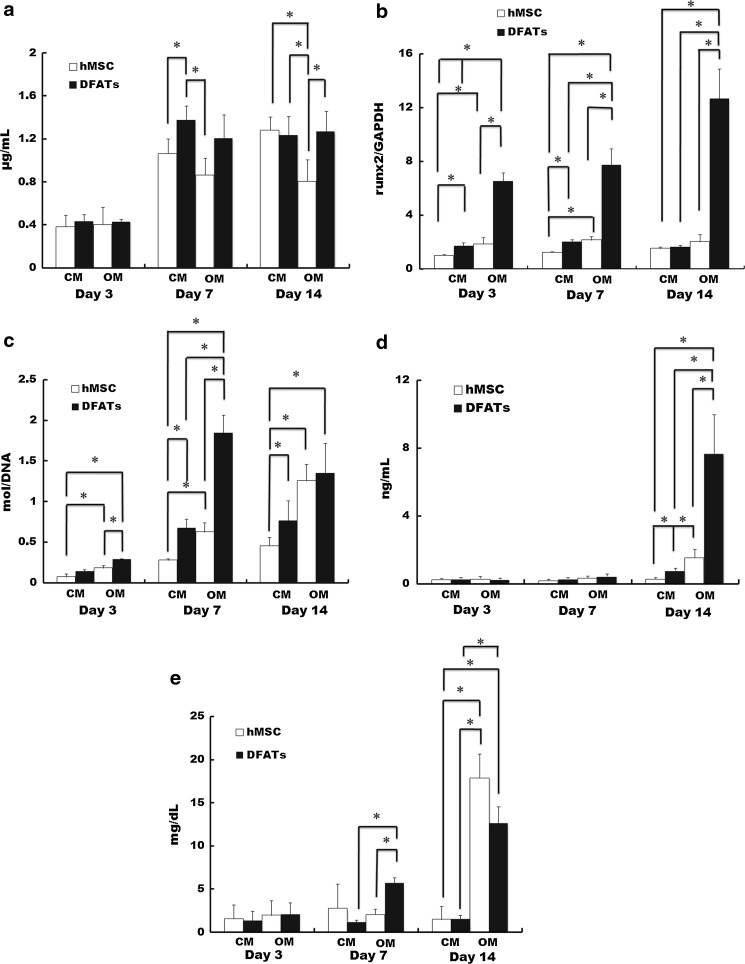
As an indicator of cell proliferation, no significant differences were observed in the DNA contents of DFATs and hMSCs on day 3. However, the DNA content of DFATs cultured in CM was significantly higher than that of hMSCs cultured in CM and OM on day 7 (p < 0.05). The DNA content of hMSCs cultured in OM was the lowest of all groups on day 14 (p < 0.05) (Fig. 2a).
Fig. 2.
Proliferation and osteoblastic differentiation of hMSCs and DFATs. Quantification of DNA (a), Runx2 expression (b), alkaline phosphatase activity (c), osteocalcin expression (d), and calcium content (e) of hMSCs and DFATs cultured in CM or OM. Data are the mean and standard deviation. *p < 0.05
Runx2 expression analysis
Runx2 expression of DFATs cultured in OM was the highest and that of hMSCs cultured in CM was the lowest of all groups on days 3 and 7 (p < 0.05). Moreover, Runx2 expression of DFATs cultured in OM was the highest of all groups on 14 (p < 0.05) (Fig. 2b).
ALP activity
As a marker of early stage osteoblastic differentiation (Wang et al. 2011), the ALP activity of DFATs cultured in OM was the highest and that of hMSCs cultured in CM was the lowest of all groups on days 3 and 7 (p < 0.05). ALP activity of hMSCs cultured in CM was the lowest of all groups on day 14 (p < 0.05) (Fig. 2c).
Expression of OCN
OCN is a marker of late stage osteoblastic differentiation (Zhao et al. 2009). No significant differences were observed in the OCN contents of all groups on days 3 and 7. The OCN contents of DFATs cultured in OM was the highest of all groups on day 14 (p < 0.05) (Fig. 2d).
Calcium content
Calcium content is a marker of terminal stage osteoblastic differentiation (Lian and Stein 1992). No significant differences were observed in the calcium contents of DFATs and hMSCs on day 3. However, the calcium content of DFATs cultured in OM was significantly higher than those of hMSCs cultured in OM and DFATs cultured in CM on day 7 (p < 0.05). The calcium contents of DFATs and hMSCs cultured in OM were higher than those of DFATs and hMSCs cultured in CM on day 14 (p < 0.05) (Fig. 2e).
SEM analysis
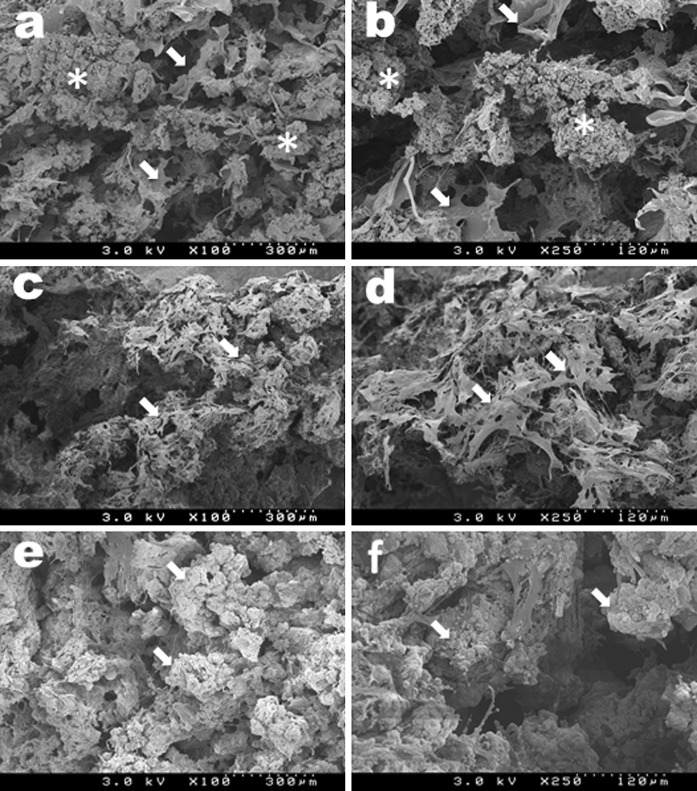
In SEM analysis, Fig. 3a and b shows the microstructure of the α-TCP/CS. The composites were composed of α-TCP granules (0.2 mm diameter) and a three-dimensional porous structure with an anatomizing network. Well-spread cells covered the α-TCP/CS on day 7 (indicated by white arrows Fig. 3c and d). Numerous large and small spherical deposits were found to almost completely cover the α-TCP/CS on day 14 (indicated by the white arrows in Fig. 3e and f).
Fig. 3.
SEM images of an intact α-TCP/CS (a, b). Arrows and asterisks indicate collagen fibrils and porous a-TCP granules, respectively. SEM images of a DFAT-seeded α-TCP/CS on day 7 (c, d). Arrows indicate DFATs. SEM images of a DFAT-seeded α-TCP/CS on day 14 (e, f). Arrows indicate spherical deposits
Histological analysis
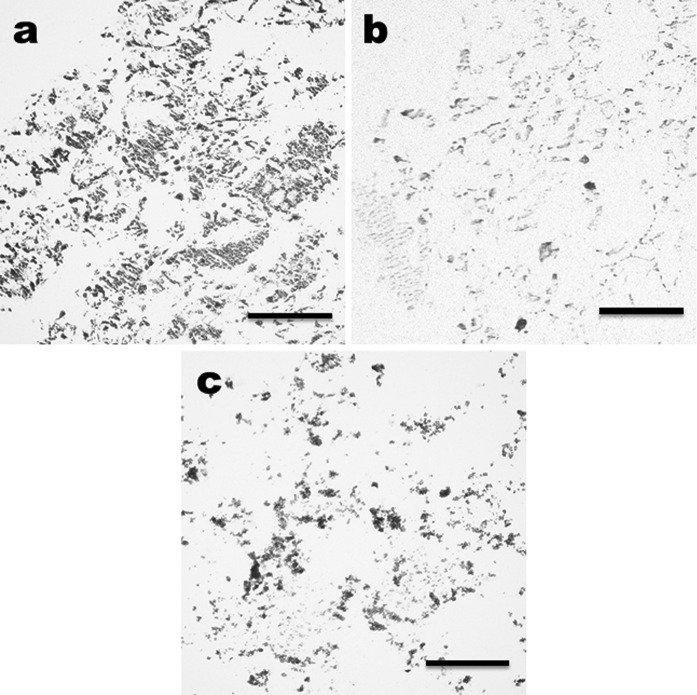
Figure 4 shows the H&E and von Kossa staining of the α-TCP/CS with or without DFATs cultured in OM for 14 days. DFATs in the α-TCP/CS were partially stained strongly as blackish-brown by von Kossa staining and eosin (Fig. 4a and b). Areas stained blackish-brown by von Kossa staining in the α-TCP/CS without cultured DFATs indicated calcium in the α-TCP (Fig. 4c).
Fig. 4.
Histological evaluation of DFATs seeded in a α-TCP/CS on day 14. Von Kossa (a) and H&E (b) staining of a DFAT-seeded α-TCP/CS cultured in OM. Von Kossa staining of an intact α-TCP/CS (c). Scale bars indicate 100 μm
Discussion
MSCs have been isolated from almost all tissues of the body, including bone marrow, umbilical cord, umbilical cord blood, adipose tissue, dental pulp, periosteum, tendons, skin, synovial membrane, amniotic fluid, limbal tissue, and menstrual blood (Nekanti et al. 2010; Vishnubalaji et al. 2012; Zhu et al. 2008). Furthermore, MSCs possess the ability to differentiate into osteoblasts, adipocytes, and chondroblasts in vitro (Dominici et al. 2006). Sakaguchi et al. (2005) demonstrated that the osteogenic ability of bone marrow-, synovium-, and periosteum-derived cells is greater than that of adipose tissue- and muscle-derived cells by the rate of alizarin red-positive colony formation. A previous study (Matsumoto et al. 2008) has indicated that lipid-filled adipocytes can dedifferentiate into fibroblast-like DFATs that have the potential to transdifferentiate into lineages of mesenchymal tissue, which is similar to the differentiation potential of MSCs. However, quantitative investigations are not sufficient to compare osteoblastic differentiation of MSCs and DFATs, and additional studies are needed to evaluate the utility of DFATs in bone tissue engineering in vitro.
In this study, cell proliferation was measured by the DNA content of cultured hMSCs and DFATs. Dexamethasone as a component of OM can either promote or inhibit cell proliferation depending on the species, cell maturation stage, and culture conditions (Canalis and Giustina 2001; Patschan et al. 2001). OM inhibited the cell proliferation of hMSCs on day 14, but not that of DFATs. Real-time RT-PCR analysis showed that DFATs cultured in OM expressed the osteogenic marker Runx2, suggesting that DFATs retain the properties of osteoblast lineage-committed progenitor cells. Matsumoto et al. (2008) revealed increased expression of not only Runx2, but also the osteogenic marker osteopontin, and osterix in DFATs during induction culture. Here, the Runx2 expression of hMSCs cultured in OM was higher than that of hMSCs cultured in CM on days 3 and 7, but it was remarkably lower than that of DFATs. ALP activity of DFATs and hMSCs was significantly higher with cultivation in OM than that of DFATs and hMSCs cultured in CM. Moreover, ALP activity of DFATs was higher than that of hMSCs on days 3 and 7 in OM. ALP is a marker of early osteogenic differentiation and commitment of stem cells toward the osteoblastic phenotype. OCN has been shown to be a late stage marker of osteoblastic differentiation (Zhao et al. 2009), whereas calcium deposition is a terminal stage marker (Lian and Stein 1992). In osteoblastic cells, transcription of the bone-specific OCN gene is principally regulated by the Runx2 transcription factor (Sierra et al. 2003). Runx2 expression of DFATs was higher than that of hMSCs in the early stage. As a result, OCN protein expression of DFATs was higher than that of hMSCs. Our previous study (Kishimoto et al. 2011) has demonstrated that the calcium content of DFATs increases on day 7 in three-dimensional culture using the self-assembling peptide RADA16. This previous result supports our data in two-dimensional culture. Collectively, compared with hMSCs, our results demonstrate that DFATs retain a high potential for osteogenic differentiation.
MSCs and DFATs appear to be an attractive cell source for regenerative medicine because of their high proliferation rates and multilineage differentiation capacity. In this study, the osteoblastic differentiation capacity of DFATs was superior to that of hMSCs, although the reasons are not clarified in this study. MSCs are obtained by expansion of a very small number of stem cells from highly heterogeneous cell populations by exploiting the phenotype of plastic adherence (Matsumoto et al. 2008). On the other hand, DFATs have been advocated as quite homogeneous cells isolated from mature adipocytes in adipose tissue using the ceiling culture technique (Fig. 1). After collagenase digestion and filtration of adipose tissue, mature adipocytes containing lipid droplets are suspended and can be separated from the stromal-vascular fraction by centrifugation. Almost all floating cells (over 98 %) have been reported to be mature adipocytes that comprise a highly homogeneous fraction (Gao et al. 2012). Based on these reports, we considered that almost all of the cells isolated in the present study might also consist of highly homogenous mesenchymal lineage cells and therefore induce superior osteoblastogenesis.
Our previous studies (Kishimoto et al. 2013; Kishimoto et al. 2011; Kishimoto et al. 2008) showed that the self-assembling peptide RADA16 or a titanium fiber mesh as scaffolds induce three-dimensional expansion and osteoblastic differentiation of DFATs. However, these scaffolds are biocompatible but not osteoconductive. Among the various calcium phosphate materials, a previous study has highlighted the potential of α-TCP particles as a bone-rebuilding material, because they gradually biodegrade while the bone regenerates around them (Wiltfang et al. 2002). Our previous study (Arima et al. 2013) demonstrated that an α-TCP/CS supports bone regeneration in a rat calvarial defect model. The composites were composed of α-TCP particles and a three-dimensional porous structure with an anatomizing network and were expected to have a high seeding efficiency.
Tissue engineering involves control of the initial seeding density and uniform cell numbers within the scaffold. In this study, the droplet seeding method was used to seed cells into the three-dimensional scaffolds (Van Den Dolder et al. 2003; Zhu et al. 2010). Fifty microliters of medium containing 1 × 105 cells/ml was pipetted into the α-TCP/CS with a three-dimensional porous structure that did not leak, thereby retaining the cells in the scaffold. In fact, SEM observation showed numerous well-spread cells in the α-TCP/CS on day 7. On the other hand, the identification of individual cells was difficult on day 14, and we found numerous spherical deposits. OCN is a vitamin K-dependent protein produced by osteoblasts, which binds strongly to calcium and hydroxyapatite (Park et al. 2009). Thus, these spherical deposits might be calcified matrix resulting from OCN secreted by osteoblasts binding calcium contained in the FBS and α-TCP. In addition, von Kossa staining provided histological evidence that DFATs differentiated into osteoblasts in the α-TCP/CS and formed cultured bone by deposition of a mineralized extracellular matrix.
Conclusion
Compared with MSCs, DFATs can be obtained less invasively. Furthermore, the present study revealed another advantage of using DFATs: the osteoblastic differentiation ability of DFATs is higher than that of hMSCs. This study also histologically revealed that, under the three-dimensional culture conditions provided by the α-TCP/CS, DFATs differentiate into osteoblasts as early as day 14 of culture to form cultured bone in vitro. Therefore, the combined use of DFATs and an α-TCP/CS may be an attractive option for bone tissue engineering. The clinical application of DFATs and an α-TCP/CS for alveolar cleft defects should be successful. Further in vivo study of bone tissue engineering will be required.
Acknowledgments
We thank Dr. Noboru Sasaki (Department of Oral and Maxillofacial Surgery, Amagasaki Chuo Hospital) for his cooperation with collecting the adipose tissue. This study was supported by a Grant-in-Aid for Scientific Research (B) No. 24792263 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- Arima Y, Uemura N, Hashimoto Y, Baba S, Matsumoto N. Evaluation of bone regeneration by porous alpha-tricalcium phosphate/atelocollagen sponge composite in rat calvarial defects. Orthod Waves. 2013;72:23–29. doi: 10.1016/j.odw.2012.11.001. [DOI] [Google Scholar]
- Canalis E, Giustina A. Glucocorticoid-induced osteoporosis: summary of a workshop. J Clin Endocrinol Metab. 2001;86:5681–5685. doi: 10.1210/jcem.86.12.8066. [DOI] [PubMed] [Google Scholar]
- Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci USA. 2000;97:3213–3218. doi: 10.1073/pnas.97.7.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Gao Q, Zhao L, Song Z, Yang G. Expression pattern of embryonic stem cell markers in DFAT cells and ADSCs. Mol Biol Rep. 2012;39:5791–5804. doi: 10.1007/s11033-011-1371-4. [DOI] [PubMed] [Google Scholar]
- Gimbel M, Ashley RK, Sisodia M, Gabbay JS, Wasson KL, Heller J, Wilson L, Kawamoto HK, Bradley JP. Repair of alveolar cleft defects: reduced morbidity with bone marrow stem cells in a resorbable matrix. J Craniofac Surg. 2007;18:895–901. doi: 10.1097/scs.0b013e3180a771af. [DOI] [PubMed] [Google Scholar]
- Kishimoto N, Momota Y, Mori R, Hashimoto Y, Imai K, Omasa T, Kotani J. Bone regeneration using dedifferentiated fat cells with PuraMatrixTM. J Oral Tissue Eng. 2008;6:127–134. [Google Scholar]
- Kishimoto N, Momota Y, Hashimoto Y, Omasa T, Kotani J. Self-assembling peptide RADA16 as a scaffold in bone tissue engineering using dedifferentiated fat cells. J Oral Tissue Engin. 2011;8:151–161. [Google Scholar]
- Kishimoto N, Momota Y, Hashimoto Y, Ando K, Omasa T, Kotani J. Dedifferentiated fat cells differentiate into osteoblasts in titanium fiber mesh. Cytotechnology. 2013;65:15–22. doi: 10.1007/s10616-012-9456-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian JB, Stein GS. Concepts of osteoblast growth and differentiation: basis for modulation of bone cell development and tissue formation. Crit Rev Oral Biol Med. 1992;3:269–305. doi: 10.1177/10454411920030030501. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408 [DOI] [PubMed]
- Matsumoto T, Kano K, Kondo D, Fukuda N, Iribe Y, Tanaka N, Matsubara Y, Sakuma T, Satomi A, Otaki M, Ryu J, Mugishima H. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol. 2008;215:210–222. doi: 10.1002/jcp.21304. [DOI] [PubMed] [Google Scholar]
- Nekanti U, Rao VB, Bahirvani AG, Jan M, Totey S, Ta M. Long-term expansion and pluripotent marker array analysis of Wharton’s jelly-derived mesenchymal stem cells. Stem Cells Dev. 2010;19:117–130. doi: 10.1089/scd.2009.0177. [DOI] [PubMed] [Google Scholar]
- Park KH, Kim H, Moon S, Na K. Bone morphogenic protein-2 (BMP-2) loaded nanoparticles mixed with human mesenchymal stem cell in fibrin hydrogel for bone tissue engineering. J Biosci Bioeng. 2009;108:530–537. doi: 10.1016/j.jbiosc.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Patschan D, Loddenkemper K, Buttgereit F. Molecular mechanisms of glucocorticoid-induced osteoporosis. Bone. 2001;29:498–505. doi: 10.1016/S8756-3282(01)00610-X. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Penicaud L, Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthr Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- Shimada H, Hashimoto Y, Nakada A, Shigeno K, Nakamura T. Accelerated generation of human induced pluripotent stem cells with retroviral transduction and chemical inhibitors under physiological hypoxia. Biochem Biophys Res Commun. 2012;417:659–664. doi: 10.1016/j.bbrc.2011.11.111. [DOI] [PubMed] [Google Scholar]
- Sierra J, Villagra A, Paredes R, Cruzat F, Gutierrez S, Javed A, Arriagada G, Olate J, Imschenetzky M, Van Wijnen AJ. Regulation of the bone-specific osteocalcin gene by p300 requires Runx2/Cbfa1 and the vitamin D3 receptor but not p300 intrinsic histone acetyltransferase activity. Mol Cell Biol. 2003;23:3339–3351. doi: 10.1128/MCB.23.9.3339-3351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi S, Shimazu A, Miyazaki K, Pan H, Koike C, Yoshida E, Takagishi K, Kato Y. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun. 2001;288:413–419. doi: 10.1006/bbrc.2001.5777. [DOI] [PubMed] [Google Scholar]
- Van Den Dolder J, Spauwen PHM, Jansen JA. Evaluation of various seeding techniques for culturing osteogenic cells on titanium fiber mesh. Tissue Eng. 2003;9:315–325. doi: 10.1089/107632703764664783. [DOI] [PubMed] [Google Scholar]
- Vishnubalaji R, Al-Nbaheen M, Kadalmani B, Aldahmash A, Ramesh T. Comparative investigation of the differentiation capability of bone-marrow- and adipose-derived mesenchymal stem cells by qualitative and quantitative analysis. Cell Tissue Res. 2012;347:419–427. doi: 10.1007/s00441-011-1306-3. [DOI] [PubMed] [Google Scholar]
- Wang J, Ye Y, Tian H, Yang S, Jin X, Tong W, Zhang Y (2011) In vitro osteogenesis of human adipose-derived stem cells by coculture with human umbilical vein endothelial cells. Biochem Biophys Res Commun 412:143–149. doi:10.1016/j.bbrc.2011.07.062 [DOI] [PubMed]
- Wiltfang J, Merten HA, Schlegel KA, Schultze-Mosgau S, Kloss FR, Rupprecht S, Kessler P. Degradation characteristics of alpha and beta tri-calcium-phosphate (TCP) in minipigs. J Biomed Mater Res. 2002;63:115–121. doi: 10.1002/jbm.10084. [DOI] [PubMed] [Google Scholar]
- Yagi K, Kondo D, Okazaki Y, Kano K. A novel preadipocyte cell line established from mouse adult mature adipocytes. Biochem Biophys Res Commun. 2004;321:967–974. doi: 10.1016/j.bbrc.2004.07.055. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Ueda M, Naiki T, Takahashi M, Hata KI, Nagasaka T. Autogenous injectable bone for regeneration with mesenchymal stem cells and platelet-rich plasma: tissue-engineered bone regeneration. Tissue Eng. 2004;10:955–964. doi: 10.1089/1076327041348284. [DOI] [PubMed] [Google Scholar]
- Zhao J, Shinkai M, Takezawa T, Ohba S, Chung UI, Nagamune T. Bone regeneration using collagen type I vitrigel with bone morphogenetic protein-2. J Biosci Bioeng. 2009;107:318–323. doi: 10.1016/j.jbiosc.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z. Adipose-derived stem cell: a better stem cell than BMSC. Cell Biochem Funct. 2008;26:664–675. doi: 10.1002/cbf.1488. [DOI] [PubMed] [Google Scholar]
- Zhu H, Schulz J, Schliephake H. Human bone marrow stroma stem cell distribution in calcium carbonate scaffolds using two different seeding methods. Clin Oral Implants Res. 2010;21:182–188. doi: 10.1111/j.1600-0501.2009.01816.x. [DOI] [PubMed] [Google Scholar]