Abstract
Mesenchymal stem cells (MSCs) are adult multipotent cells currently employed in several clinical trials due to their immunomodulating, angiogenic and repairing features. The adipose tissue is certainly considered an eligible source of MSCs. Recently, putative adipose tissue derived MSCs (ADMSCs) have been isolated from the mediastinal depots. However, very little is known about the properties, the function and the potential of human mediastinal ADMSCs (hmADMSCs). However, the lack of standardized methodologies to culture ADMSCs prevents comparison across. Herein for the first time, we report a detailed step by step description to optimize the isolation and the expansion methodology of hmADMSCs using a virally inactivated good manufacturing practice (GMP)-grade platelet lysate, highlighting the critical aspects of the procedure and providing useful troubleshooting suggestions. Our approach offers a reproducible system which could provide standardization across laboratories. Moreover, our system is time and cost effective, and it can provide a reproducible source of adipose stem cells to enable future studies to unravel new insights regard this promising stem cell population.
Keywords: Adipose tissue, Mesenchymal stem cells, Platelet lysate, Good manufacturing practices
Introduction
Mesenchymal stem cells (MSCs) are adult multipotent stem cells, which can be isolated from most organs such as bone marrow, placenta, fat and lung (Hass et al. 2011). MSCs are an attractive stem cell source for cell therapy applications, because of their intriguing characteristics such as easy isolation and expansion. In addition, due to their immunological tolerance properties, MSCs can be also engineered ex vivo and re-introduced without immunomodulation in animal models (Krampera et al. 2005). In the light of this, MSCs have been the focus of intensive efforts worldwide directed not only at elucidating their nature and unique properties but also at developing cell-based therapies for a diverse range of diseases including diabetes, myocardial infarction or multiple sclerosis (Cai et al. 2009; Yamout et al. 2010; Si et al. 2012; Wang et al. 2013).
Among all tissue sources of MSCs, the fat depot has represented one of the most important reservoir (Zuk et al. 2001). ADMSCs exhibit phenotype, morphology, differentiation ability and colony formation activity similarly to bone marrow derived MSCs (BMMSCs) (Gronthos et al. 2001; Sengenès et al. 2005). However, over the years ADMSCs have been demonstrated to display different properties (Toma et al. 2001; Lee et al. 2004; Beltrami et al. 2007). Specifically, there are evidences indicating that ADMSCs and BMMSCs show comparable but not identical epigenetic states (Collas 2010). Moreover, ADMSCs are better candidates than other sources, as they hold the advantages of easy accessibility, greater availability of adipose tissue, combined with the higher percentage of MSCs that fat depots hold compared to bone marrow (Fraser et al. 2008; De Ugarte et al. 2003; Pittenger et al. 1999).
New tissue sources of MSCs are continuously discovered as for instance the human mediastinal ADMSCs (hmADMSCs) recently isolated (Patel et al. 2013). To date, hmADMSCs are very poorly described in literature and it is unclear what function they might exert in the mediastinal fat. Similarly, it would be significant to unravel potential properties of hmADMSCs and to investigate whether they could play a key role both in metabolic disorders or in other relevant diseases as suggested (Patel et al. 2013). In addition, the isolation of novel ADMSCs from a different adipose source, points out the possibility that MSCs derived from different adipose depots within the body could also display different properties. For instance, regional heterogeneity, metabolic and apoptotic differences between the omental and the mesenteric adipose tissue have been already described (Hassan et al. 2012; Tchkonia et al. 2005).
The standardization of laboratory procedures represents a mandatory step when stem cells are required to be employed in the regenerative medicine field (De Falco et al. 2013). Specifically, major limitations occur during the isolation of ADMSCs through optimized methodologies: the huge variability observed between donors (due to sex, race, clinical history, body mass index), the surgical technique employed to obtain the adipose biopsy or even the choice of the appropriate culture medium and the use of the fetal bovine serum (FBS) or platelet lysate (PL). In this regard, the PL, a hemocomponent enriched with growth factors and cytokines, has been shown to effectively substitute FBS during ex vivo expansion of ADMSCs (Blande et al. 2009; Shih et al. 2011). However, the composition and the methodology itself to obtain PL may also vary between laboratories. All the above mentioned issues may play a critical role on the viability and the expansion of ADMSCs, thus complicating interpretation of results.
In this study we describe for the first time a reproducible and detailed method to isolate, expand and characterize in vitro hmADMSCs with a virally inactivated good manufacturing practice (GMP)-grade PL (patent pending, PCT/IB2012/055062), identifying the critical steps through the procedure and providing troubleshooting advices.
Materials and methods
Specimen collection and transport
Human anterior mediastinal fat specimens were obtained from patients undergoing thoracic surgery at S. Andrea Hospital, Rome. Written informed consent and negative serological tests for HIV, Hepatitis B and C were obtained from patients, before starting all the surgical and laboratory procedures. The methodology described has been conducted in compliance with the tenets of the Declaration of Helsinki for experiments involving human tissues. After surgery, biopsies were kept in physiological solution (NaCl 0.9 %, B. Braun, Milan S.p.A., Italy) in a sterile Falcon tube, transported to the laboratory at room temperature and processed within 12 h. All data were de-identified and analysed anonymously.
Preparation of PL
Platelets concentrates were collected from 12 volunteer donors in a sterile multiple component system according to manufacturer’s instructions (Fresenius Kabi AG, Bad Homburg, Germany). The procedures were performed at San Camillo-Forlanini Hospital, Rome. The donors provided the informed consent and they were previously screened for their suitability to this procedure. The buffy coats (BC) were centrifuged at 6477 (rcf) xg for 12 min and constantly shaken during incubation at 22 °C within 24 h of collection. Afterwards, the BC were processed using the InterSol solution (318 mg Na-citrate 2H2O, 305 mg Na 2-phosphate anhydro, 105 mg Na dihydrogen phosphate 2H2O, 442 mg Na-Acetate 3H2O, 452 mg NaCl, 100 ml H2O, Fenwal Inc. Lake Zurich, IL, USA). The procedure to inactivate the pathogens was performed using a photochemistry treatment with psoralens and UV exposition (Intercept™ Blood System for Platelets, Cerus Corporation, Concord, CA, USA). At the end of the procedure, the BC were resuspended in InterSol and 20–30 % of human plasma. Platelets counts were performed using the hematology analyzer ABX Pentra DX 120 (Horiba ABX, Montpellier, France). Sterility tests were checked with the BacT/ALERT System, using defined media for aerobic/anaerobic bacteria and fungi (bioMérieux SA, Marcy l’Etoile, France). The Platelet pools were collected through sterile connecting devices (TSCD II, Terumo, Tokyo, Japan) in a single final bag and stored at −80 °C for 24 h before thawing at 37 °C for 60 min. This procedure was repeated three times to enrich the pool of growth factors. The PL was stored at −80 °C until use.
Isolation of hmADMSCs
The fat biopsy was transported to the validated cell culture facility. The specimen was cleaned of connective tissue, rinsed three times with PBS and then weighed. Subsequently, in order to remove red blood cells, the biopsy was washed twice with an appropriate volume of erythrocytes lysis buffer (ELB, Qiagen, Germantown, USA; Cat. N. 79217), taking care to cover the biopsy and then manually shaken for 1 min. Next, in order to remove the ELB, the biopsy was transferred to a fresh tube and rinsed with PBS. After that, the specimen was chopped using a sterile n.21 scalpel in a 100 mm petri dish into small, pinhead size pieces, then minced and transferred into a clean tube for enzymatic digestion. 0.05 %Trypsin/0.02 %EDTA (Biowest, Nuaillè, France, Cat. N. X0930-100)/1 mg/ml collagenase type-I solution (Gibco, Monza, Italy; Cat. N. 17100) (approximately 10 ml) was added to cover all tissue fragments and incubated at 37 °C at shaking water bath for 45 min. Every 5–10 min the sample was manually shaken, vigorously. Thereafter, the enzymatic digestion was blocked with PBS/20 % FBS (Gibco). The digested tissue was filtered with a 100 μM cell strainer, seeping first the liquid suspension then the fragments. Afterwards, the strainer was washed with PBS, applying a gentle pressure on the strainer to allow the filtration of the fragments. At this step, the cell suspension was centrifuged at 377 g for 5 min, the supernatant was discarded and the pellet was transferred into a new sterile tube filled with 1 ml of PBS using a pipette Gilson, avoiding contact with the tube wall. The cell suspension was spun again at 377 g for 5 min and the supernatant was discarded. This last step was repeated twice using EBL (three min of incubation, with regular shaking) instead of PBS. After centrifugation, the pellet was resuspended in 4–5 ml of complete growth medium (Low glucose-DMEM/1 % penicillin–streptomycin/1 % glutamine/1 % non-essential aminoacids (All: Biowest, Nuaillé, France)/20 % PL and 5U/ml heparin (Hospira Italia s.r.l., Neapoli, Italy) to avoid fibrin gel formation) and seeded in a T25 ventilated flask. Cells were incubated at 37 °C in 5 % CO2. To allow the initial adhesion to the plastic surface of the flask, cells were left undisturbed in the incubator for 72 h.
Maintenance of hmADMScs cultures
After 72 h the medium was removed and 5 ml of complete growth medium were added into the T25 flask. When cells reached the 70–80 % confluence, they were detached by 0.05 %Trypsin/0.02 %EDTA (Biowest, Nuaillè, France, Cat. N. X0930-100), centrifuged at 377 g for 5 min. HmADMSCs were counted by Trypan Blue (Sigma, St. Louis, MO, USA) and resuspended in complete growth medium at a density of 4,000 cells/cm2. The medium was changed every 3 days. Although hmADMSCs growth varied between donors, cells usually reached the confluence at day 7. In addition, the supernatant was collected at each passage and stored at −80 °C for further analysis if necessary. As we previously reported (De Falco et al. 2013), the Number of doubling cells through passages can be calculated according to the following formula: Number of doubling cells = 3.322 log10 N/N0 (N is the number of cells obtained and N0 is the number of cell plated). HmADMSCs were cultured until passage 5.
Quality control tests: FACS analysis, colony forming unit fibroblasts assay (CFU-F) and trans-differentiation assay
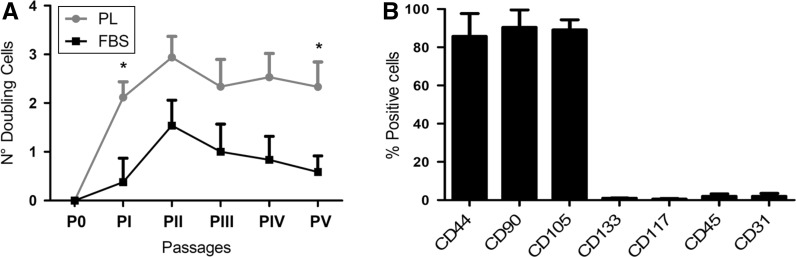
Quality control tests were performed at passage 3. For FACS analysis (Fig. 3b), 2 × 106 cells were stained for 30 min with the following panel of primary antibodies: 0.2 mg/ml anti-CD105, 0.1 mg/ml anti-CD44, 0.1 mg/ml anti-CD90, 0.3 mg/ml anti-CD45 (Abcam, Cambridge, UK, human Mesenchymal stromal cell marker panel Cat. N. Ab93758), 1:100 anti-CD31 (Sigma, St. Louis, MO, USA, Cat. N. P8590), 0.25 mg/ml anti-CD117 (Abcam, Cambridge, UK, Cat. N. Ab5506), 1 mg/ml anti-CD133 (Abcam, Cambridge, UK, Cat. N. Ab19898). Cells were then washed and incubated with the following secondary antibodies: anti-rabbit Alexafluor-488 and mouse FITC-conjugated anti-mouse IgG (H + L) (Jackson ImmunoResearch, Suffolk, UK). Flow cytometry was performed using FACSAria II (B&D, San Jose, CA, USA) and data were acquired and analysed by DiVa Software (v6.1.1, B&D, San Jose, CA, USA).
Fig. 3.
a Proliferation trends of hmADMSCs cultured with PL or FBS. Number of cell doublings = 3.322 log10 N/N0. N is the number of cells obtained, N0 the number of cells plated, *p < 0.05; b Representative FACS Analysis of hmADMSCs at passage 3 cultured with PL showing the mesenchymal positive and hematopoietic/endothelial negative immunophenotype
For CFU-F test (Fig. 4a), hmADMSCs were seeded at low density (20–40 cells/cm2) in a 100 mm petri dish. After 2 weeks of incubation, the petri dish was washed with PBS, fixed with 4 % paraformaldehyde and incubated for 2 min with absolute Giemsa (Sigma, St. Louis, MO, USA), then washed again in distilled water and incubated for 13 min with diluted Giemsa (1:20 distilled water, Sigma, St. Louis, MO, USA). The Petri dish was finally rinsed with distilled water. Clusters of cells with a diameter >5 mm were considered colonies and observed by optical microscope.
Fig. 4.
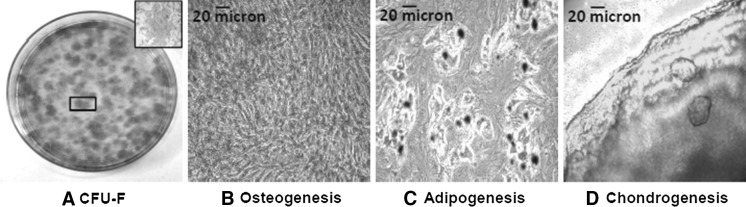
a Appearance of CFU-F assay positive for Giemsa staining. Inset contains higher magnification (×5) panel of the selected area showing a single hmADMSC clone; b-d Osteogenic (Alizarin Red staining), adipogenic (Oil Red Oil staining) and chondrogenic (Alcian Blue staining) transdifferentiation of hmADMSCs after 2–3 weeks in culture. All images ×10 magnification
For trans-differentiation test (Fig. 4b–d), hmADMSCs were detached and incubated with defined media for the three mesodermal lineage differentiation. Briefly, hmADMSCs were plated in 6-well plates at density of 5 × 103, 1 × 104 cells/cm2 and 2.5 × 105 cells and cultured with osteogenic (Stem-Pro® Osteogenic Differentiation kit Gibco, Monza, Italy, Cat. N.A10072-01), adipogenic (StemPro® Adipogenesis Differentiation kit, Gibco, Monza, Italy, Cat. N.A10070-01) and chondrogenic medium (StemPro® Chondrogenic Differentiation kit, Gibco, Monza, Italy Cat. N.A10071-01), respectively. The medium was changed every 3 days. After 14 days of incubation, cells were fixed with 4 % paraformaldehyde. Calcium deposition was analysed by incubating the cells for 1 h at room temperature with a 2 % Alizarin red solution at pH 4.1–4.3 (Sigma, St. Louis, MO, USA, Cat. N. A5533). Accumulation of lipid droplets was analysed by staining hmADMSCs with Oil Red Oil solution (3:2 distillate water, Sigma, St. Louis, MO, USA, Cat. N.01391). For chondrogenic differentiation, 2,5 × 105 cells were centrifuged in a 15 ml-tube at 377 g for 5 min, resuspended in StemPro® Chondrogenic differentiation basal medium and chondrogenesis supplement (StemPro® Chondrogenic differentiation kit) and centrifuged at 377 g for 5 min. The pellet in the 15 ml-tube was incubated in an upright position in the incubator and the medium was gradually added every 3 days avoiding pellet resuspension. After 3 weeks of culture the pellet was transferred in a 6-well plate and fixed with 4 % paraformaldehyde. The chondrospheres were stained with 1 % Alcian blue 8GX solution at pH 2.5 (Sigma, St. Louis, MO, USA, Cat. N.05500) to detect the presence of glycosaminoglycans.
Results and discussion
So far, the biology and the properties of hmADMSCs have not been described. The possibility to retrieve hmADMSCs from the mediastinal fat confirms several reports, indicating that ADMSCs can be isolated from multiple anatomical districts (Hass et al. 2011). Nevertheless, a structural and functional heterogeneity within the adipose tissue depots in the body has been reported (Hassan et al. 2012). Consequently, it is possible that the ADMSC pool might also show distinct capacities and metabolic differences. In fact, several evidences have shown that both differentiation and proliferation abilities of adipose precursor cells vary between fat depots (Schipper et al. 2008). Specifically, the fat localized in the proximity of the heart, such as the epicardial and the mediastinal adipose tissue, seems to play a role in cardiovascular diseases, although the mechanisms still remain unknown. On the other hand, it is clear that the adipose tissue displays a higher stem-cell density compared to bone marrow (Fraser et al. 2006) and more importantly that ADMSCs are more versatile multipotent progenitors, due to their ability to differentiate into more lineages than bone marrow itself (Zuk et al. 2002; Rangappa et al. 2003; Fischer et al. 2009; Brzoska et al. 2005; Corre et al. 2006; Seo et al. 2005). Additionally, ADMSCs and BMMSCs display dissimilar epigenetic states (Collas 2010).
ADMSCs have been under clinical evaluation for regenerative applications (Barry et al. 2001; Krebsbach et al. 1997), however the precise mechanism underlying their potential ability to regenerate is still unclear. Several studies have reported that ADMSCs can both give rise to cells of different organs through either the engraftment into the tissue, or recruiting tissue specific stem cells, or even through indirect immunomodulating mechanisms like increasing anti-inflammatory cytokines (Granero-Molto et al. 2008).
Despite both hmADMSCs and ADMSCs are promising stem cells sources, however, one major drawback is the lack of standardization for their isolation (Woodbury et al. 2000). In fact, the main issue regarding the reproducibility can be explained by the inconsistent methodologies, which exist between laboratories, resulting in isolation of non-homogenous ADMSCs populations (Bernardo et al. 2011). The reason underlying the heterogeneity of ADMSCs cultures includes different surgical-subspecialties and techniques employed to obtain the adipose tissue, as well as the time elapsed between the biopsy collection and the isolation of ADMSCs in the laboratory. In addition, clinical characteristics of patients such as age, body mass index and sex, can all influence both isolation and cell yield (Baer and Geiger 2012).
Besides, the true “ADMSC precursor” has not been determined, thus researchers have to rely on a panel of multiple markers and clonogenic/differentiation tests after the isolation process, in order to confirm the identity of ADMSCs (Woodbury et al. 2000; Campioni et al. 2003; Majumdar et al. 2003; Vogel et al. 2003).
Accordingly, this study has been based on the setting up of a standardized methodology to isolate hmADMSCs from 30 patients (mean age 63.3 ± 14.3) with no history of inflammatory diseases. To overcome the variability due to the operator and to the anatomical heterogeneity of the adipose tissue, the procedures were always performed by a designated surgeon and fat biopsies always obtained from the anterior mediastinum during thoracic surgery. We focused on the steps that occur during the isolation and expansion of hmADMSCs, pointing out key issues and providing suggestions through a useful troubleshooting table (Table 1). In particular, we have identified a set of steps, which we have considered critical during this procedure: weight of the starting biopsy, substitution of FBS with PL, cell density, use of Low-glucose medium, correct handling of the oily pellet during centrifugation. We have observed a strong correlation between the weight of the biopsy and successful isolation of hmADMSCs. Specifically, we have experienced that mediastinal fat biopsies weighting less than 10 g, negatively affect the outcome of the cell cultures. In this regard, we could suggest that the percentage of ADMSCs obtained after isolation could be in some way inversely proportional to the amount of the starting biopsy. However, we have quantified the variable “weight of the fat biopsy”, considering 10 g as our in-house minimum threshold, to ensure the success of the methodology.
Table 1.
Troubleshooting table
| Step | Issue | Possible reason | Solution |
|---|---|---|---|
| Biopsy digestion | Red blood cells contamination | Excessive vascularized tissue | Wash twice with ELB |
| Cells isolation (T25 passage 0) | No cells adhesion 1 | Biopsy weight <10 g | Obtain a biopsy ≥10 g. Alternatively, for a biopsy <10 g seed the cell in a 35 mm petri dish instead of a T25 after the enzymatic digestion of the sample |
| Cells isolation (T25 passage 0) | No cells adhesion 2 | Pellet loss and adipocytes contamination (tube oily walls) | Change tube at every wash, avoiding touch falcon greasy walls |
| Cells isolation (T25 passage 0) | Fiber particulate observed in culture | Filtration was not performed carefully and gently | Filter again cellular suspension with a 40 μm filter and do not touch the membrane filter |
| Passage 0-6 | Bacterial/Fungal contamination | Bad shipment conditions, poor aseptic culture techniques | Careful manipulation of the specimen when shipped and double the antibiotic concentration |
| hmADMSCs maintenance | Few cells in the flask or cell growth delay | Cells plated at low density | Seed the cells at higher density (8-10 x 103/cm2) |
Ideal hmADMSCs cultures should be “free” of adipose cell contaminants. This aspect is particularly significant to avoid the growth of adipocytes which interfere with that of hmADMSCs. For this reason, we recommend to carefully handle the pellet when the supernatant is discarded (see Isolation of hmADMSCs). In fact, it is very likely at this step to contaminate the pellet of hmADMSCs with adipocytes present in the fatty supernatant. Contamination can be avoided by both transferring the pellet into a clean tube and by avoiding contact with the tube walls.
One of the most important criteria to define ADMSCs is their adherence to a plastic surface (Engler et al. 2006). The adhesion certainly influences the outcome of the isolation procedure, as well as the proliferation of ADMSCs through passages. Therefore, to allow the adherence, cultures should be left undisturbed within the first 72 h (Fig. 1a). Afterwards, adherence can be preserved if hmADMSCs are seeded at the appropriate cell densities, 8–10 × 103 at passage ≤1 and 4 × 103 cells/cm2 at passage >1 (Fig. 1b, c).
Fig. 1.
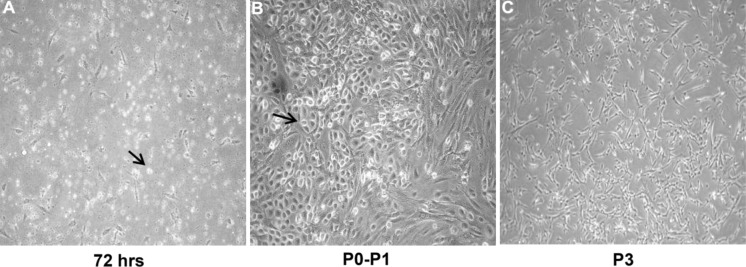
Characterization of hmADMSCs. a Optical images at 72 h of hmADMSCs cultured with DMEM-low glucose and 10 % PL, showing round proliferating cells detectable both at the initial adhesion phase and b between P0-P1 (black arrows); c whereas at P3 they display a flat morphology becoming monolayers
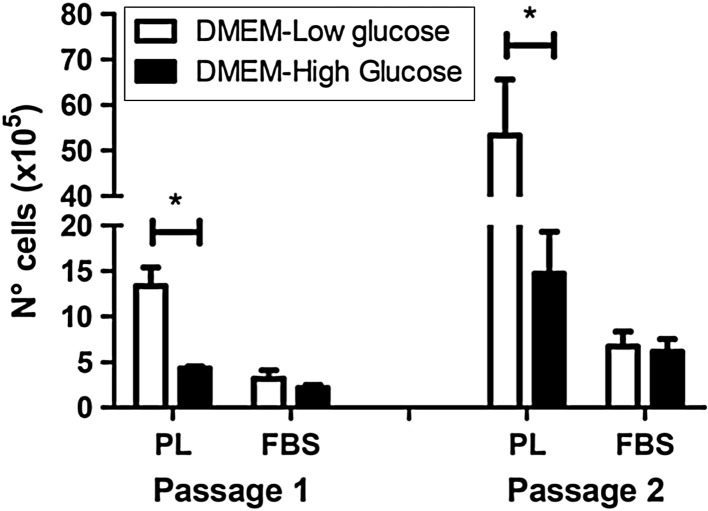
Moreover, it is well known that medium composition, FBS or growth factors highly influence stem cell growth (Baer and Geiger 2012; Sotiropoulou et al. 2006). In this study we used DMEM with low glucose content, which represents a more physiological-like concentration (Baer and Geiger 2012). Our results (Fig. 2) indicate that the number of hmADSMSCs significantly increases in presence of DMEM-Low glucose and PL compared to DMEM-High glucose and PL at both passage 1 (p = 0.01) and 2 (p = 0.04). On the contrary, neither the low nor the high concentration of glucose affect cell growth in presence of FBS (p = 0.35 at passage 1 and p = 0.8 at passage 2), confirming PL as better proliferative agent of hmADMSCs than FBS. Besides, it has been recently shown that subcutaneous and visceral fat depots take up glucose differently (Christen et al. 2010), thus suggesting that glucose might influence also ADMSC metabolism. In our hands, PL and DMEM-Low glucose have resulted as the best effect combination to maximize hmADMSC proliferation.
Fig. 2.
The number of hmADMSCs significantly increases in presence of PL and DMEM-Low glucose compared to PL and DMEM-High glucose at both passage 1 and 2, differently from FBS, *p < 0.05
PL has been recently used as substitute of FBS, showing greater efficient properties and preserving the stem cell pool during the isolation and expansion of ADMSCs (Beltrami et al. 2007). However, a major issue is that preparations of clinical grade PL still differ between laboratories, causing inconsistencies in the concentration of growth factors and cytokines in the PL at the end of the manufacturing process, thus limiting the possibility to achieve reproducible results between laboratories. Previous studies have also attempted to investigate alternative supplements to expand ADMSCs, such as AB-serum and thrombin activated platelet-rich plasma (tPRP) (Bieback et al. 2009). However, the effects of human serum on MSCs have been contradictory and tPRP requires difficult manufacturing process (Kocaoemer et al. 2007). Therefore, to date PL seems to represent a superior adjuvant both to culture MSCs regardless the source (Crespo-Diaz et al. 2011) and to comply with the current clinical good manufacturing practices (cGMPs) for cell preparation. In addition, given that PL is a hemoderivate, standard procedures are strictly now required for clinical safety in order to minimize potential risks due to infectious diseases. To date and to the best of our knowledge, only three articles (Shih et al. 2011; Burnouf et al. 2010; Lee et al. 2013) report the use of virally inactivated PL. However, the methodology described is not standardized neither patented and most importantly, the procedures to inactivate pathogens are not licensed.
Accordingly, in this protocol we used a GMP-grade PL (Patent pending) having been virally inactivated using the Intercept Technology™. The Intercept™ is the first system available to treat both platelets and plasma, allowing to efficiently inactivate pathogens for a broad range of enveloped and non-enveloped viruses, bacteria and intracellular and extracellular protozoa. In addition, several phase III clinical trials have been already performed in Europe, to evaluate the product’s performance and safety (www.clinicaltrials.gov). Besides, this is the first time that hmADMSCs are isolated and expanded using a virally inactivated PL. Specifically, PL has been derived from a pool of multiple donors (12 donors) to balance the individual variability and to achieve similar concentrations of growth factors and cytokines (PDGF-AB, VEGF, b-FGF, TGF-β1) between clinical batches (Table 2). More importantly, our viral inactivation procedure did not affect the concentration of mitogenic soluble factors in the final preparations of PL compared to non-virally inactivated samples (Table 2), indicating that both the manufacturing process for the PL and the Intercept™ system for the inactivation of pathogens, ensured the optimal release of the growth factors and cytokines in the clinical batches.
Table 2.
Soluble factors detected in non-virally and virally inactivated GMP-grade PL preparations
| PDGF-AB (pg/ml) | VEGF (pg/ml) | bFGF (pg/ml) | TGF-β1 (pg/ml) | |
|---|---|---|---|---|
| Non-virally inactivated PL preparation No. | ||||
| 0001 | 20,700 | 920 | 110 | 15,000 |
| 0002 | 28,830 | 751 | 99 | 11,000 |
| 0003 | 21,710 | 530 | 100 | 14,000 |
| Mean ± SE | 23,750 ± 2,560 | 733,67 ± 112,91 | 103 ± 3,51 | 13,330 ± 1,200 |
| Virally inactivated PL preparation No. | ||||
| 0004 | 22,760 | 560 | 118 | 10,200 |
| 0005 | 20,530 | 490 | 88 | 16,990 |
| 0006 | 27,500 | 880 | 97 | 13,000 |
| 0004 | 24,360 | 990 | 100 | 12,000 |
| Mean ± SE | 23,790 ± 1,460 | 730 ± 121,31 | 100,75 ± 6,29 | 13,740 ± 1,440 |
In addition, our data (Fig. 3a, b) have shown that the number of cell doublings through passages of hmADMSCs resulted between 2.12–2.94 versus 0.38–1.54 in presence of PL and FBS, respectively (p = 0.04 Passage 1 and 4). We also suggest the use of chemically-defined media to induce the differentiation of hmADMSCs into osteogenic, adipogenic and chondrogenic cells (Fig. 4a–d). In our hands the commercially available media were much more reliable and efficient to control the cell differentiation environment than the use of “in-house” differentiation media, often expensive and not consistent.
The reproducibility of our method can be particularly useful for all researchers who intend to better explore the potentialities of hmADMSCs or to establish this procedure for the first time in the laboratory. Moreover, our system can help researchers to contain the economic costs and can also allow to save time to the operators.
Acknowledgments
We dedicate this manuscript to Maria Pia Docimo. We also acknowledge Fondazione Roma. We thank, Colin Murdoch, Rosa Puca and Isotta Chimenti for their help and support. This study was supported by the grant University of Rome “Sapienza”, Ateneo 2011 prot. C26A11J528.
Footnotes
Giacomo Frati and Elena De Falco have contributed equally to this work.
References
- Baer P, Geiger H (2012) Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cells Int 2012:812693 [DOI] [PMC free article] [PubMed]
- Barry FP, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- Beltrami AP, Cesselli D, Bergamin N, et al. Multipotent cells can be generated in vitro from several adult human organs (heart, liver, and bone marrow) Blood. 2007;110:3438–3446. doi: 10.1182/blood-2006-11-055566. [DOI] [PubMed] [Google Scholar]
- Bernardo ME, Cometa AM, Pagliara D, Vinti L, Rossi F, Cristantielli R, Palumbo G, Locatelli F (2011) Ex vivo expansion of mesenchymal stromal cells. Best Pract Res Clin Haematol 24:73–81 [DOI] [PubMed]
- Bieback K, Hecker A, Kocaömer A, Lannert H, Schallmoser K, Strunk D, Klüter H (2009) Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem cells 27:2331–2341 [DOI] [PubMed]
- Blande IS, Bassaneze V, Lavini-Ramos C, Fae KC, Kalil J, Miyakawa AA, Schettert IT, Krieger JE (2009) Adipose tissue mesenchymal stem cell expansion in animal serum-free medium supplemented with autologous human platelet lysate. Transfusion 49:2680–2685 [DOI] [PubMed]
- Brzoska M, Geiger H, Gauer S, Baer P. Epithelial differentiation of human adipose tissue-derived adult stem cells. Biochem Biophys Res Commun. 2005;330:142–150. doi: 10.1016/j.bbrc.2005.02.141. [DOI] [PubMed] [Google Scholar]
- Burnouf PA, Juan PK, Su CY, Kuo YP, Chou ML, Su CH, Tseng YH, Lin CT, Burnouf T (2010) A novel virally inactivated human platelet lysate preparation rich in TGF-β, EGF and IGF, and depleted of PDGF and VEGF. Biotechnol Appl Biochem 56:151–160 [DOI] [PubMed]
- Cai L, Johnstone BH, Cook TG, Tan J, Fishbein MC, Chen PS, March KL (2009) IFATS collection: human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells 27:230–237 [DOI] [PMC free article] [PubMed]
- Campioni D, Lanza F, Moretti S, Dominici M, Punturieri M, Pauli S, Hofmann T, Horwitz E, Castoldi GL (2003) Functional and immunophenotypic characteristics of isolated CD105(+) and fibroblast(+) stromal cells from AML: implications for their plasticity along endothelial lineage. Cytotherapy 5:66–79 [DOI] [PubMed]
- Christen T, Sheikine Y, Rocha VZ. Increased glucose uptake in visceral versus subcutaneous adipose tissue revealed by PET imaging. JACC Cardiovasc Imaging. 2010;3:843–851. doi: 10.1016/j.jcmg.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collas P. Programming differentiation potential in mesenchymal stem cells. Epigenetics. 2010;5:476–482. doi: 10.4161/epi.5.6.12517. [DOI] [PubMed] [Google Scholar]
- Corre J, Barreau C, Cousin B, Chavoin JP, Caton D, Fournial G, Penicaud L, Casteilla L, Laharrague P (2006) Human subcutaneous adipose cells support complete differentiation but not self-renewal of hematopoietic progenitors. J Cell Physiol 208:282–288 [DOI] [PubMed]
- Crespo-Diaz R, Behfar A, Butler GW, Padley DJ, Sarr MG, Bartunek J, Dietz AB, Terzic A (2011) Platelet lysate consisting of a natural repair proteome supports human mesenchymal stem cell proliferation and chromosomal stability. Cell Transplant 20:797–811 [DOI] [PubMed]
- De Falco E, Scafetta G, Napoletano C, Puca R, Vingolo EM, Ragona G, Iorio O, Frati G (2013) A standardized laboratory and surgical method for in vitro culture isolation and expansion of primary human Tenon’s fibroblasts. Cell Tissue Banking 14:277–287 [DOI] [PubMed]
- De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, Fraser J, Hedrick MH (2003) Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 174:101–109 [DOI] [PubMed]
- Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126:677–689 [DOI] [PubMed]
- Fischer LJ, McIlhenny S, Tulenko T, Golesorkhi N, Zhang P, Larson R, Lombardi J, Shapiro I, DiMuzio PJ (2009) Endothelial differentiation of adipose-derived stem cells: effects of endothelial cell growth supplement and shear force. J Surg Res 152:157–166 [DOI] [PMC free article] [PubMed]
- Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–154. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Fraser JK, Zhu M, Wulur I, Alfonso Z (2008) Adipose derived stem cells. Methods Mol Biol 449:59–67 [DOI] [PubMed]
- Granero-Molto F, Weis JA, Longobardi L (2008) Role of mesenchymal stem cells in regenerative medicine: application to bone and cartilage repair. Expert Opin Biol Ther 8:255–268 [DOI] [PubMed]
- Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM (2001) Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol 189:54–63 [DOI] [PubMed]
- Hass R, Kasper C, Böhm S, Jacobs R (2011) Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun and Signal 14:12 [DOI] [PMC free article] [PubMed]
- Hassan M, Latif N, Yacoub M. Adipose tissue: friend or foe? Nat Rev Cardiol. 2012;9:689–702. doi: 10.1038/nrcardio.2012.148. [DOI] [PubMed] [Google Scholar]
- Kocaoemer A, Kern S, Klüter H, Bieback K (2007) Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells 25:1270–1278 [DOI] [PubMed]
- Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, Romagnani P, Maggi E, Romagnani S, Annunziato F (2005) Role of the IFN-γ in the immunomodulatory activity of human mesenchymal stem cell. Stem Cells 24:386–398 [DOI] [PubMed]
- Krebsbach PH, Kuznetsov SA, Satomura K, Emmons RV, Rowe DW, Robey PG (1997) Bone formation in vivo: comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation 63:1059–1069 [DOI] [PubMed]
- Lee RH, Kim B, Choi I, Kim H, Choi HS, Suh K, Bae YC, Jung JS (2004) Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem 14:311–324 [DOI] [PubMed]
- Lee YL, Lee LW, Su CY, Hsiao G, Yang YY, Leu SJ, Shieh YH, Burnouf T (2013) Virally inactivated human platelet concentrate lysate induces regulatory T cells and immunosuppressive effect in a murine asthma model. Transfusion 53:1918–1928 [DOI] [PubMed]
- Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, Mosca JD (2003) Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci 10:228–241 [DOI] [PubMed]
- Patel AN, Yockman J, Vargas V, Bull DA (2013) Putative population of adipose derived stem cells isolated from mediastinal tissue during cardiac surgery. Cell Transplant 22:507–511. doi:10.3727/096368912X636849 [DOI] [PubMed]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147 [DOI] [PubMed]
- Rangappa S, Fen C, Lee EH, Bongso A, Sim EK (2003) Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann Thorac Surg 75:775–779 [DOI] [PubMed]
- Schipper BM, Marra KG, Zhang W, Donnenberg AD, Rubin JP (2008) Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plast Surg 60:538–544 [DOI] [PMC free article] [PubMed]
- Sengenès C, Lolmède K, Zakaroff-Girard A, Busse R, Bouloumié A (2005) Preadipocytes in the human subcutaneous adipose tissue display distinct features from the adult Mesenchymal and hematopoietic stem cells. J Cell Physiol 205:114–122 [DOI] [PubMed]
- Seo MJ, Suh SY, Bae YC, Jung JS (2005) Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun 328:258–264 [DOI] [PubMed]
- Shih DT, Chen JC, Chen WY, et al. Expansion of adipose tissue mesenchymal stromal progenitors in serum-free medium supplemented with virally inactivated allogeneic human platelet lysate. Transfusion. 2011;51:770–778. doi: 10.1111/j.1537-2995.2010.02915.x. [DOI] [PubMed] [Google Scholar]
- Si Y, Zhao Y, Hao H, Liu J, Guo Y, Mu Y, Shen J, Cheng Y, Fu X, Han W (2012) Infusion of mesenchymal stem cells ameliorates hyperglycemia in type 2 diabetic rats: identification of a novel role in improving insulin sensitivity. Diabetes 61:1616–1625 [DOI] [PMC free article] [PubMed]
- Sotiropoulou PA, Perez SA, Salagianni M, Baxevanis CN, Papamichail M (2006) Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells 24:462–471 [DOI] [PubMed]
- Tchkonia T, Tchoukalova YD, Giorgadze N, Pirtskhalava T, Karagiannides I, Forse RA, Koo A, Stevenson M, Chinnappan D, Cartwright A, Jensen MD, Kirkland JL (2005) Abundance of two human preadipocyte subtypes with distinct capacities for replication, adipogenesis, and apoptosis varies among fat depots. Am J Physiol Endocrinol Metab 288:E267–E277 [DOI] [PubMed]
- Toma JG, Akhavan M, Fernandes KJ, Barnabé-Heider F, Sadikot A, Kaplan DR, Miller FD (2001) Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol 3:778–784 [DOI] [PubMed]
- Vogel W, Grünebach F, Messam CA, Kanz L, Brugger W, Bühring HJ (2003) Heterogeneity among human bone marrow derived mesenchimal stem cells and neural progenitors. Haematologica 88:126–132 [PubMed]
- Wang S, Li Y, Zhao J, Zhang J, Huang Y (2013) Mesenchymal stem cells ameliorate podocyte injury and proteinuria in a type 1 diabetic nephropathy rat model. Biol Blood Marrow Transplant 19:538–546. doi:10.1016/j.bbmt.2013.01.001 [DOI] [PubMed]
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB (2000) Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res 61:364–370 [DOI] [PubMed]
- Yamout B, Hourani R, Salti H, Barada W, El-Hajj T, Al-Kutoubi A, Herlopian A, Baz EK, Mahfouz R, Khalil-Hamdan R, Kreidieh NM, El-Sabban M, Bazarbachi A (2010) Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J Neuroimmunol 227:185–189 [DOI] [PubMed]
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7:211–228 [DOI] [PubMed]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295 [DOI] [PMC free article] [PubMed]