Abstract
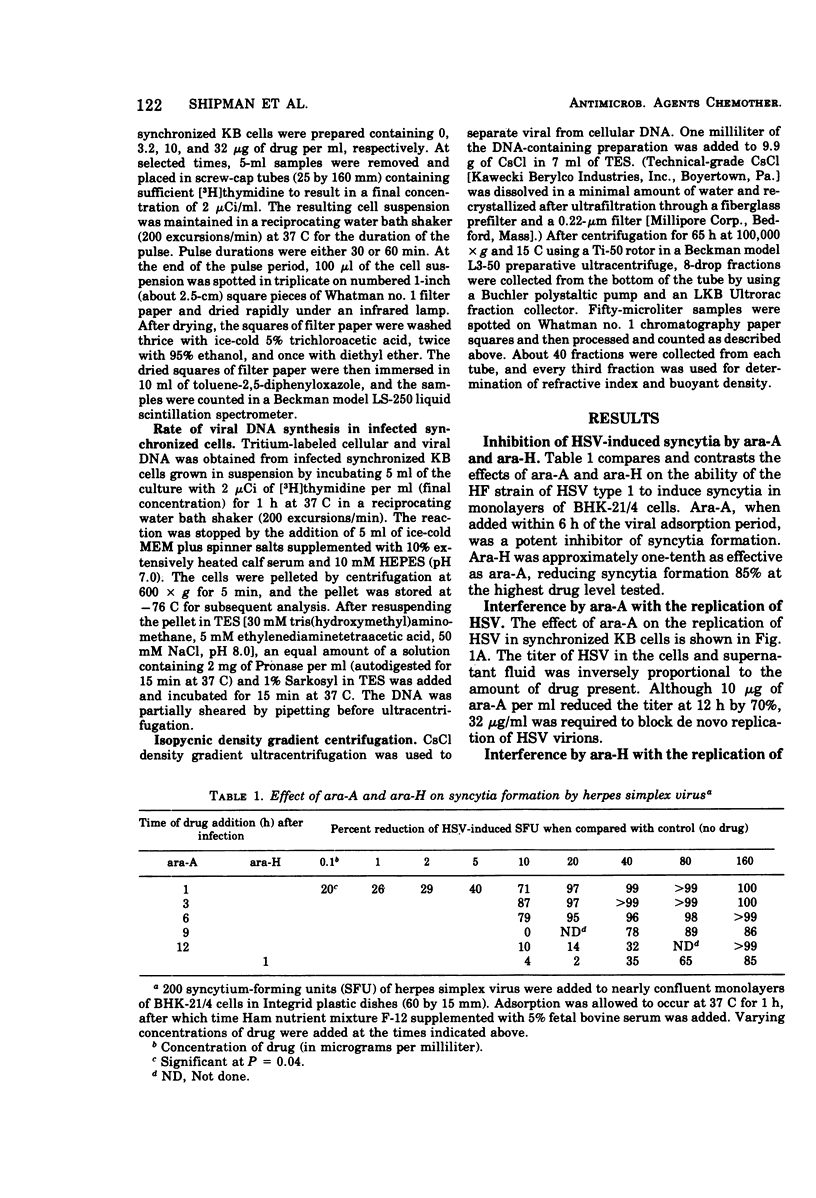
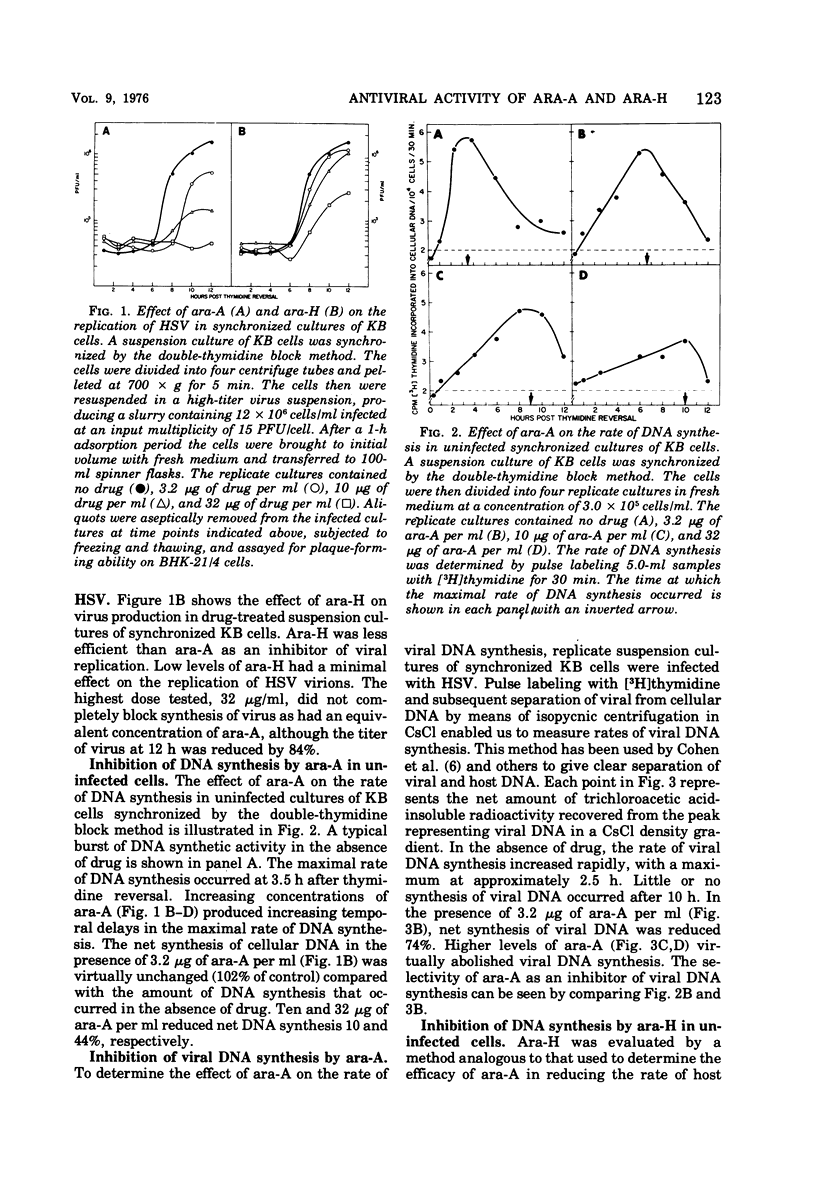
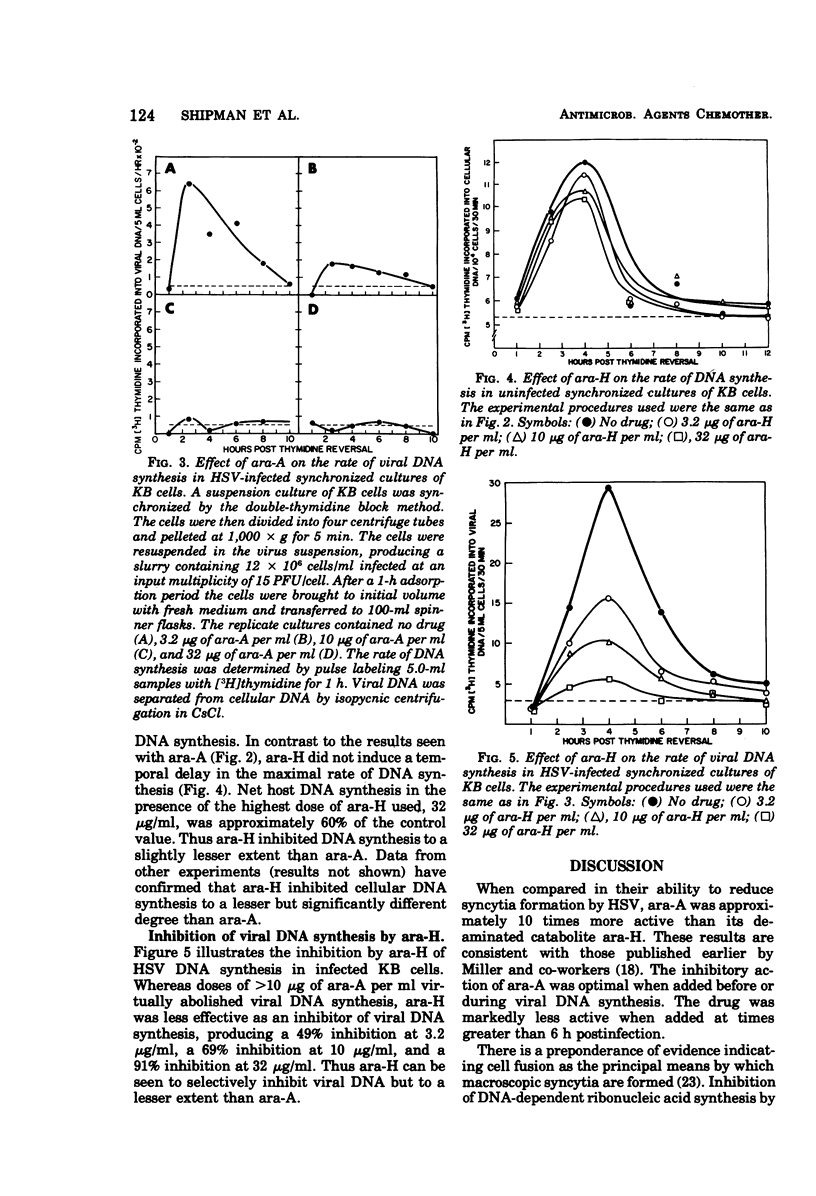
The drug 9-β-d-arabinofuranosyladenine (ara-A) significantly suppressed the formation of herpes simplex virus type 1-induced syncytia in BHK-21/4 cells at concentrations as low as 0.1 μg/ml. Optimal activity was noted when the drug was added before initiation of viral deoxyribonucleic acid (DNA) synthesis (3.5 h postinfection). The deaminated derivative of ara-A, 9-β-d-arabinofuranosylhypoxanthine (ara-H), was at least 10 times less effective in suppressing the development of herpes simplex virus-induced syncytia. The replication of herpes simplex virus was measured by assaying fluids and cells from infected drug-treated cultures by using a plaque production technique. Ara-A at drug levels of >10 < 32 μg/ml completely blocked the replication of infectious virus particles. Ara-H was less effective than ara-A in reducing the replication of virions. Rates of host and viral DNA synthesis were monitored by pulse labeling herpes simplex virus-infected synchronized KB cells with [3H]thymidine and subsequently separating viral from cellular DNA in CsCl density gradients. During synthetic (S) phase, ara-A or ara-H at concentrations ranging from 3.2 to 32 μg/ml selectively inhibited viral DNA synthesis. At 3.2 μg of ara-A per ml, viral DNA synthesis was reduced 74% although total cellular DNA synthesis was unaffected. Increasing concentrations of ara-A produced increasing temporal delays in the maximal rate of host DNA synthesis. This time shift was not observed in cells treated with ara-H.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOOTSMA D., BUDKE L., VOS O. STUDIES ON SYNCHRONOUS DIVISION OF TISSUE CULTURE CELLS INITIATED BY EXCESS THYMIDINE. Exp Cell Res. 1964 Jan;33:301–309. doi: 10.1016/s0014-4827(64)81035-1. [DOI] [PubMed] [Google Scholar]
- Becker Y., Olshevsky U. Inhibition of Herpes simplex virus replication by cordycepin. Isr J Med Sci. 1973 Nov-Dec;9(11):1581–1585. [PubMed] [Google Scholar]
- Becker Y., Weinber A. Distamycin A inhibition of Epstein-Barr virus replication in arginine-deprived Burkitt lymphoblasts. Isr J Med Sci. 1972 Jan;8(1):75–78. [PubMed] [Google Scholar]
- Bello L. J. Synthesis of DNA-like RNA in synchronized cultures of mammalian cells. Biochim Biophys Acta. 1968 Mar 18;157(1):8–15. doi: 10.1016/0005-2787(68)90258-x. [DOI] [PubMed] [Google Scholar]
- Bennett L. L., Jr, Shannon W. M., Allan P. W., Arnett G. Studies on the biochemical basis for the antiviral activities of some nucleoside analogs. Ann N Y Acad Sci. 1975 Aug 8;255:342–358. doi: 10.1111/j.1749-6632.1975.tb29242.x. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Vaughan R. K., Lawrence W. C. Deoxyribonucleic acid synthesis in synchronized mammalian KB cells infected with herpes simplex virus. J Virol. 1971 Jun;7(6):783–791. doi: 10.1128/jvi.7.6.783-791.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALKE D. UNTERSUCHUNGEN -UEBER DIE BEZIEHUNGEN ZWISCHEN RIESENZELLBILDUNG UND INFEKTIOSITAET VON HERPES-SIMPLEX-VIRUS. Arch Gesamte Virusforsch. 1965;15:387–401. [PubMed] [Google Scholar]
- Falke D., Peterknecht W. DNS-, RNS- und Proteinsynthese und ihre Relation zur Riesenzellbildung in vitro nach infektion mit Herpesvirus hominis. Arch Gesamte Virusforsch. 1968;24(3):267–287. [PubMed] [Google Scholar]
- Furth J. J., Cohen S. S. Inhibition of mammalian DNA polymerase by the 5'-triphosphate of 1-beta-d-arabinofuranosylcytosine and the 5'-triphosphate of 9-beta-d-arabinofuranoxyladenine. Cancer Res. 1968 Oct;28(10):2061–2067. [PubMed] [Google Scholar]
- Furth J. J., Cohen S. S. Inhibition of mammalian DNA polymerase by the 5'-triphosphate of 9-beta-D-arabinofuranosyladenine. Cancer Res. 1967 Sep;27(9):1528–1533. [PubMed] [Google Scholar]
- HUBERT-HABART M., COHEN S. S. The toxicity of 9-beta-D-arabinofuranosyladenine to purine-requiring Escherichia coli. Biochim Biophys Acta. 1962 May 21;59:468–471. doi: 10.1016/0006-3002(62)90198-1. [DOI] [PubMed] [Google Scholar]
- Hampar B., Derge J. G., Martos L. M., Tagamets M. A., Burroughs M. A. Sequence of spontaneous Epstein-Barr virus activation and selective DNA synthesis in activated cells in the presence of hydroxyurea. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2589–2593. doi: 10.1073/pnas.69.9.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay J., Perera P. A., Morrison J. M., Gentry G. A., Subak-Sharpe J. H. Herpes virus-specified proteins. In: strategy of the viral genome. Ciba Found Symp. 1971:355–376. [PubMed] [Google Scholar]
- Lowry S. P., Melnick J. L., Rawls W. E. Investigation of plaque formation in chick embryo cells as a biological marker for distinguishing herpes virus type 2 from type 1. J Gen Virol. 1971 Jan;10(1):1–9. doi: 10.1099/0022-1317-10-1-1. [DOI] [PubMed] [Google Scholar]
- Moore E. C., Cohen S. S. Effects of arabinonucleotides on ribonucleotide reduction by an enzyme system from rat tumor. J Biol Chem. 1967 May 10;242(9):2116–2118. [PubMed] [Google Scholar]
- Overby L. R., Robishaw E. E., Schleicher J. B., Rueter A., Shipkowitz N. L., Mao J. C. Inhibition of herpes simplex virus replication by phosphonoacetic acid. Antimicrob Agents Chemother. 1974 Sep;6(3):360–365. doi: 10.1128/aac.6.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRIVATDEGARILHE M., DE RUDDER J. EFFET DE DEUX NUCL'EOSIDES DE L'ARABINOSE SUR LA MULTIPLICATION DES VIRUS DE L'HERP'ES ET DE LA VACCINE EN CULTURE CELLULAIRE. C R Hebd Seances Acad Sci. 1964 Oct 19;259:2725–2728. [PubMed] [Google Scholar]
- Person D. A., Sheridan P. J., Herrmann E. C. Sensitivity of Types 1 and 2 Herpes Simplex Virus to 5-Iodo-2'-Deoxyuridine and 9-beta-d-Arabinofuranosyladenine. Infect Immun. 1970 Dec;2(6):815–820. doi: 10.1128/iai.2.6.815-820.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROIZMAN B. Polykaryocytosis. Cold Spring Harb Symp Quant Biol. 1962;27:327–342. doi: 10.1101/sqb.1962.027.001.031. [DOI] [PubMed] [Google Scholar]
- Schabel F. M., Jr The antiviral activity of 9-beta-D-arabinofuranosyladenine (ARA-A). Chemotherapy. 1968;13(6):321–338. doi: 10.1159/000220567. [DOI] [PubMed] [Google Scholar]
- Schildkraut I., Cooper G. M., Greer S. Selective inhibition of the replication of herpes simplex virus by 5-halogenated analogues of deoxycytidine. Mol Pharmacol. 1975 Mar;11(2):153–158. [PubMed] [Google Scholar]
- Schwartz P. M., Shipman C., Jr, Carlson R. H., Drach J. C. Thermal inactivation as a means of inhibiting the serum-associated deamination of 9-beta-D Arabinofuranosyladenine in tissue culture media. Antimicrob Agents Chemother. 1974 Mar;5(3):337–343. doi: 10.1128/aac.5.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipman C., Jr Evaluation of 4-(2-hydroxyethyl)-1-piperazineëthanesulfonic acid (HEPES) as a tissue culture buffer. Proc Soc Exp Biol Med. 1969 Jan;130(1):305–310. doi: 10.3181/00379727-130-33543. [DOI] [PubMed] [Google Scholar]
- Shipman C., Jr, Smith S. H., Drach J. C. Selective inhibition of nuclear DNA synthesis by 9- -D-arabinofuranosyl adenine in rat cells transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1753–1757. doi: 10.1073/pnas.69.7.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumaru T. The nature of toxins of herpes simplex virus. I. Syncytial giant cell producing components in tissue culture. Arch Gesamte Virusforsch. 1968;24(1):104–122. doi: 10.1007/BF01242905. [DOI] [PubMed] [Google Scholar]
- Waqar M. A., Burgoyne L. A., Atkinson M. R. Deoxyribonucleic acid synthesis in mammalian nuclei. Incorporation of deoxyribonucleotides and chain-terminating nucleotide analogues. Biochem J. 1971 Mar;121(5):803–809. doi: 10.1042/bj1210803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York J. L., LePage G. A. A proposed mechanism for the action of 9-beta-D-arabinofuranosyladenine as an inhibitor of the growth of some ascites cells. Can J Biochem. 1966 Jan;44(1):19–26. doi: 10.1139/o66-003. [DOI] [PubMed] [Google Scholar]
- Yoshirkura H. Effect of arabinosyl-cytosine on the G1-arrested cells which were subsequently induced to divide by medium change. Exp Cell Res. 1974 Mar 30;85(1):123–126. doi: 10.1016/0014-4827(74)90221-3. [DOI] [PubMed] [Google Scholar]



