Abstract
Scrotal pain is a common complaint in a urological practice. Its diagnosis can prove challenging in both acute and chronic forms and requires a thorough and complete history and physical examination. This article discusses the evaluation and management of several entities of scrotal pain, including testicular torsion, epididymitis, postvasectomy pain, varicocele, and chronic orchialgia.
Keywords: Pain; Pain management; Physical examination, Scrotum
INTRODUCTION
Scrotal pain, in both its acute and chronic forms, is a diagnostic challenge that must be carefully evaluated with a full patient history and physical examination. Pain, the dependent nature of the scrotum, and the edema and skin changes that accompany many scrotal pathologies may prevent a complete examination. The patient's age, sexual history, and duration, severity, and onset (gradual vs. sudden) of pain are necessary to focus the clinician's attention on the correct diagnostic path. In its acute form, scrotal pain is a medical emergency requiring prompt attention to rule out testicular torsion. The physical exam must include a careful evaluation of the abdomen and inguinal region and a genital exam to assess possible herniation [1,2,3].
This article will focus on the more common urological causes of scrotal pain (Table 1). However, nonurological causes of scrotal pain must also be entertained. These include peritonitis, incarcerated hernia, ruptured abdominal aortic aneurysm, and referred scrotal pain. Sensory fibers from both the upper ureter and the testis travel through spinal cord segments T11 and T12. Therefore, upper ureteral distension (e.g., due to a ureteral stone) may cause referred pain to the testis and lower ureteral distension may result in ipsilateral scrotal pain [4]. Other differential diagnoses to be aware of include Henoch-Schonlein purpura, testicular tumors, and lower back strain. Henoch-Schonlein purpura is a systemic vasculitis that typically affects patients aged 20 years or less and has a peak incidence at 4 to 5 years of age. Henoch-Schonlein purpura is reported to involve the scrotum in 2% to 38% of cases and can be misdiagnosed as one of pathologies that require prompt surgical intervention (e.g., testicular torsion or incarcerated hernia) [5]. Characteristic features include marked edema of the scrotal skin and contents with intact vascular flow in the testicle, epididymal enlargement, and a hydrocele, which help to distinguish this from testicular torsion and prevent unnecessary surgical exploration [5,6]. Testicular tumors most often present as a painless scrotal mass, a finding that must be assumed to be testicular cancer until proven otherwise. However, the astute clinician must be aware that a rapidly growing testicular mass, especially one that is accompanied by internal bleeding or areas of infarction, may present with scrotal pain. Lower back strain is a diagnosis of exclusion and must be considered after ruling out all other causes of scrotal pain described below. Lower back strain can be secondary to radiculitis at T10 to L1, causing nerve root irritation and subsequent scrotal referred pain [6].
Table 1.
Summary of entities of scrotal pain discussed in the article
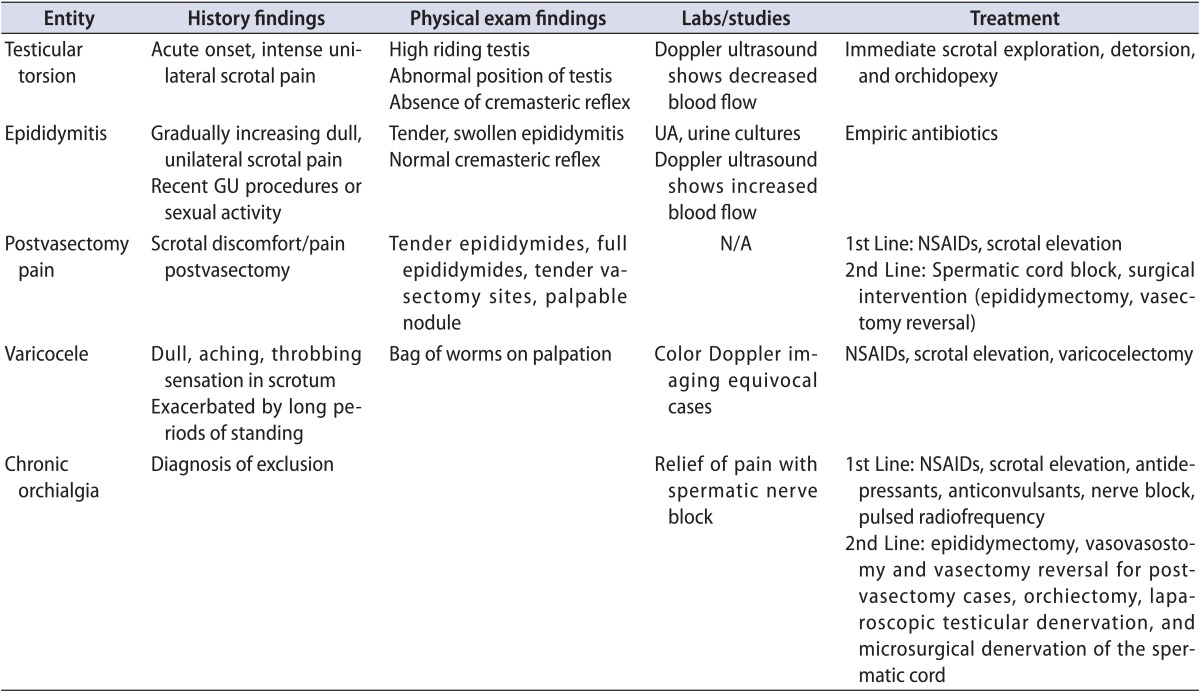
GU, genitourinary; UA, urine analysis; N/A, not applicable; NSAID, nonsteroidal anti-inflammatory drug.
Lastly, chronic scrotal pain can be idiopathic in nature. Holland et al. [7] proposed that idiopathic orchialgia or 'phantom orchialgia' may be caused by noxious stimuli that activate an abnormal neuronal pathway causing referred pain to the scrotum. In these cases, the authors recommend correction of bad posture, avoidance of heavy straining or lifting, use of a scrotal support for 6 weeks, and psychiatric referral for psychosocial evaluation as a possible source of stress. More invasive recommendations include spermatic cord anesthetic infiltration at the pubic tubercle with lidocaine 1% and bupivacaine 0.5% and a possible trial of transcutaneous electrical nerve stimulation. Patients who are refractory to the aforementioned treatments can elect to have selective ilioinguinal/genitofemoral blocks or paravertebral nerve root blocks at T10 to L1 [7].
TESTICULAR TORSION
Testicular torsion is a medical emergency, requiring prompt treatment or risking the loss of the testicle. The incidence is 1 in 4,000 males under the age of 25 years [8]. It may be intravaginal or extravaginal and is typically seen in neonatal patients and in adolescents prior to complete testicular descent and scrotal wall fusion. Intravaginal torsion occurs when the testicle can freely rotate within the tunica vaginalis; this can be due to a congenital anomaly called the bell clapper deformity. This deformity is due to failure of posterior anchorage of the gubernaculum, epididymis, and testis, thus allowing the testis to freely rotate within the tunica vaginalis. Extravaginal torsion occurs when the testis rotates within the scrotum owing to inadequate fusion of the testicle to the scrotal wall or increased mobility [8,9]. The torsion follows rotation of the spermatic cord and results in ischemia. The degree of testicular torsion is directly correlated with the possibility of salvage after torsion and time to necrosis. Torsion of the testicular appendages, often presenting in 7- to 13-year-old children, presents similarly to testicular torsion and accounts for 24% to 46% of acute scrotal presentations [1,10].
1. Diagnosis
The classic presentation of testicular torsion is acute-onset, intense, unilateral scrotal pain. Patients may also complain of nausea and vomiting, likely secondary to pain [1]. Intensity increases owing to edema and resultant capsular stretching [11]. Patients may also have a history of scrotal pain that may be related to prior ischemic episodes that resolved spontaneously. On examination, the hallmark of testicular torsion is a 'high riding' testis due to shortening of the cord. Additionally, the testis may have an abnormal (e.g., transverse) position in the scrotum [1]. Absence of the cremasteric reflex is a characteristic of torsion in the pediatric population [12]. A normal cremasteric reflex would result in elevation of the ipsilateral testis after an extra gentle stroke of the inner thigh. The cremasteric reflex is rarely seen in patients with testicular torsion. Labs and studies should include urinalysis and scrotal ultrasound to confirm the diagnosis. Urinalysis showing hematuria or leukocytosis is more typical of epididymo-orchitis than torsion. Doppler ultrasonography can be used to assess testicular blood flow, which is reduced or absent in testicular torsion. This technique, however, is highly operator dependent and may have significantly high false interpretations in young children or neonates with small vessels [13]. Although torsion usually occurs around puberty and epididymitis typically affects sexually active men after the age of 20 years, age distribution may be clinically misleading and should not be relied upon for a diagnosis.
In contrast to testicular torsion, patients with torsion of a testicular appendage (appendix testis or appendix epididymis) rarely have systemic complaints and typically present with a gradual-onset, less intense, unilateral scrotal pain that is localized to the superior pole of the testis [14]. Careful examination of the scrotal skin early in the course may reveal the 'blue dot sign' owing to the nonviable appendage [15].
2. Treatment
Testicular torsion requires immediate surgical intervention with scrotal exploration, detorsion, and orchidopexy. In a review of 543 surgical explorations for acute scrotal pain in boys, Van Glabeke et al. [2] found a 16.6% incidence of testicular torsion and a 46% incidence of appendage torsion. The authors recommended surgical intervention in all male children complaining of acute scrotal pain. If immediate operative intervention is not possible, manual detorsion may be attempted.
It is helpful to recall the anatomy of testicular torsion. Specifically, torsion typically occurs in a medial direction, and detorsion should therefore be initially tried in a clockwise direction on the left and counterclockwise on the patient's right side [1]. In contrast to testicular torsion, appendicular torsion does not necessarily require surgical intervention. However, the distinction between appendicular torsion and testicular torsion remains difficult. As a general rule, surgical intervention is required in all cases where Doppler sonography demonstrates decreased or absent testicular blood flow. Failure to treat promptly will result in loss of the affected testicle.
EPIDIDYMITIS
Epididymitis is another common cause of acute scrotal pain that must be differentiated from the more severe testicular torsion. According to the Centers for Disease Control and Prevention's ambulatory health care data, epididymitis accounted for 1 in 144 outpatient visits in 2002 in the United States in men aged 18 to 50 years [16]. The pathophysiology involves the spread of microorganisms from the urethra, prostate, or seminal vesicles, as well as hematogenous spread as in tuberculosis, causing a painful, parenchymal inflammatory process resulting in epididymal swelling. The swelling may affect the testicles, which is known as epididymo-orchitis. The common causative agents are dependent on age and sexual activity.
Sexually active men younger than 35 years are usually infected with Chlamydia trachomatis and Neisseria gonorrhea, whereas older patients, patients who have undergone recent genitourinary surgery, and patients with anatomical abnormalities often have infection with gram-negative enterococci associated with urinary tract infections [1,17,18]. Fungal agents such as Candida species, very rarely, can also cause epididymitis [19].
Epididymitis in the pediatric population has a peak incidence during puberty. The pathophysiology of infantile epididymitis is, in contrast to adult cases, rarely due to bacterial pathogens and remains largely unknown. Bacterial causes have been implicated in 6.2% to 9.9% of pediatric cases. Other etiologies may include anatomical abnormalities causing reflux of sterile or infected urine into the ejaculatory ducts or sequelae of a viral illness [18,20,21]. It has been suggested that the association of infantile epididymitis with other urogenital abnormalities mandates further diagnostic evaluation [22]. However, this recommendation remains controversial. One study assessed 49 boys for acute epididymis and found only 1 to have a relevant structural urinary tract malformation [23].
Finally, there has been an association of sterile epididymitis with the antiarrhythmic agent amiodarone in up to 11% of adult patients and rarely in children. The mechanism behind this is unknown but may be related to the accumulation of amiodarone in high concentrations in the testicular tissue [24,25].
1. Diagnosis
Epididymitis, unlike testicular torsion, presents with gradually increasing dull, unilateral scrotal pain. Involvement of the vasa may result in exquisite pain that affects the entire hemiscrotum as well as the spermatic cord. Discerning epididymitis from torsion may be difficult; however, a history of prior genitourinary tract procedures and sexual activity are more suggestive of epididymitis [15]. On physical examination, a tender and swollen epididymis and normal cremasteric reflex is observed. Urinalysis, urine cultures, and urethral cultures must be performed to identify possible causative agents. A positive urinalysis and urine cultures, along with elevated white blood cell count, favor a diagnosis of epididymitis but do not exclude torsion. Color Doppler ultrasound or nuclear scintigraphy to assess blood flow to the scrotum and its contents will help to differentiate between the two entities. Doppler ultrasound would show increased blood flow, because this is an inflammatory condition [15].
Although the incidence of tuberculous epididymitis (TE) has gradually declined in the Western hemisphere, it is important to be familiar with the typical presentation. Genital-urinary tuberculosis is the most common form of extrapulmonary tuberculosis, with TE commonly being the first manifestation [26]. The frequency of genital-urinary tuberculosis among those diagnosed with tuberculosis is reported to range from 2.3% to 40.9%, with TE having an incidence of 7.4% to 34.1% within this population. TE is most common in the 35- to 55-year-old age group and can present as acute epididymitis refractory to conventional antibiotic treatment. On exam, a painful, hard, bulky mass can be appreciated. Rapid diagnosis is essential and can be made with direct examination by using auramine staining as well as genomic amplif ication polymerase chain reaction. Cultures can be concurrently taken and can be used to confirm the diagnosis and determine sensitivities for treatment [26].
2. Treatment
Empiric antibiotic treatment should be started if the clinical suspicion is high. Once cultures and sensitivities are back from the lab, antibiotics should be adjusted accordingly. In the pediatric population, epididymitis should be treated with antibiotics if the urine analysis or urine culture suggests bacterial etiology; if there is no evidence of bacteria, supportive measures are suggested [18,21]. Surgery is an option for patients with genitourinary abnormalities [20,21]. In sexually active males younger than 35 years, empiric treatment includes ceftriaxone and doxycycline or ofloxacin. Sexual partners should also be evaluated and treated. Older patients (age greater than 35 years old) should be treated with oral levofloxacin or ofloxacin [1,27]. Treatment also includes symptomatic relief with bed rest, scrotal elevation, analgesics, and nonsteroidal anti-inflammatory drugs (NSAIDs). In severe cases, a cord block may be used to alleviate pain. It is also important to discontinue or reduce the dosage of offending agents such as amiodarone for rapid resolution and avoidance of unnecessary surgical interventions in high-risk groups [25,27]. The patient should be advised that the pain and edema usually subside in 7 to 10 days, but the epididymal induration may persist for a few weeks. If symptoms do not improve within 3 days, early follow-up is advised. Cases that persist for 6 to 8 weeks after completion of antimicrobial therapy should be reevaluated with a higher index of suspicion for unusual organisms (i.e., tuberculous or fungal etiology) [1].
POSTVASECTOMY PAIN
1. Diagnosis and pathophysiology
The most common adverse event af fecting the patient's quality of life after a vasectomy is pain [28]. Chronic scrotal discomfort is seen in up 15% of men after vasectomy and half of these men consider the pain bothersome enough to seek further therapy for relief [29,30,31]. According to one study of 13 patients with postvasectomy pain, the mean time to onset was 2 years. The presenting symptoms included testicular pain (9 cases), pain during intercourse (8 cases), pain with ejaculation (4 cases), and epididymal pain (2 cases). Physical examination of this cohort also revealed tender epididymides (6 cases), full epididymides (6 cases), tender vasectomy site (4 cases), and a palpable nodule (4 cases) [32]. Ultrasonography with Doppler study has been used a diagnostic modality to evaluate epididymal thickening, epididymal tubular ectasia, and blood flow. A recent study by Cho et al. [33], however, found no statistical differences in ultrasonographic findings between those with scrotal pain and those without.
The pathophysiology of this condition is not entirely known, but possible mechanisms include tender sperm granuloma, nerve entrapment, nerve proliferation at the site of vasectomy, perineural fibrosis, and mechanical duct obstruction with epididymal congestion [27,32,34]. Histological findings in these patients are contrasting and include epididymal engorgement, complex cystic disease, chronic epididymitis, and no histological difference [31,32].
2. Treatment
First-line conservative treatments include NSAIDs, scrotal elevation, heat or ice, and various analgesics. These measures should be continued for a period of 3 months. If ineffective, a cord block using bupivacaine or similar longer acting local anesthetic agents may be attempted, but this approach requires multiple (possibly lifelong) treatments and may not be a practical option for all patients. Incidence of postvasectomy pain was reported to be significantly reduced by the injection of 1 mL of 0.5% bupivacaine into the vasal lumen at the time of vasectomy [35]. Bupivacaine is thought to inhibit excitatory nerves at the dorsal horn of the spinal cord, thereby preventing transmission of nociceptive impulses from injured tissue [36].
Surgical intervention (i.e., epididymectomy) can be considered if there is specif ic point tenderness on examination of the epididymis [27]. Some reports showed an initial improvement in 14 of 16 patients and lasting symptomatic relief in 9 of 10 patients who were interviewed 3 to 8 years after epididymectomy [31,34]. The same authors cited atypical symptoms including testicular or groin pain, erectile dysfunction, and normal sonographic appearance of epididymis as poor prognostic indictors of the procedure [31]. Although this option precludes future ipsilateral vasectomy reversals, advances in reproductive techniques in combination with sperm retrieval provide the potential for future fatherhood.
The other option is microsurgical vasectomy reversal, which has been used for the treatment of postvasectomy pain syndrome [32,37,38,39]. One study by Bruning 3rd [37] reported relief of postvasectomy pain in 24 of 32 men who underwent a single microsurgical vasectomy reversal and in 3 of 6 with recurrent pain who had undergone a second procedure. A recent study by Lee et al. [40] found no significant difference in pain reduction or patient satisfaction between epididymectomy and vasectomy reversals; selection of the procedure should be optimized for the individual patient's needs.
VARICOCELE
1. Pathophysiology and diagnosis
A varicocele is an abnormal dilation of the spermatic veins commonly due to an anatomical abnormality with an incidence of 10% to 20% in the general male population and 2% to 15% in adolescent males [41,42,43]. The mechanism is thought to involve absent or malfunctioning vein valves, thus resulting in retrograde flow into the pampiniform plexus located in the spermatic cord and scrotum from the internal spermatic and cremasteric veins. Some investigators have also demonstrated lack of valves as proximal as the renal veins and absent or incomplete valves along the internal spermatic vein [44,45,46,47]. Furthermore, it has been postulated that the longer length of the left gonadal vein, as well as insertion into the left renal vein, results in greater hydrostatic pressures and thus more common expression of varicocele on the left side [48]. Varicoceles have been associated with male infertility, although the exact cause-and-effect relationships have not been established. Leading theories suggest that elevated scrotal temperature, hypoxia secondary to stasis, and reflux of renal and adrenal metabolites result in impaired spermatogenesis and infertility [49].
Varicocele pain typically presents as a dull, aching, and throbbing sensation in the scrotum without sharp or radiating components [50]. It can be exacerbated by long periods of standing owing to the resultant increased hydrostatic pressure in the valveless veins of the pampiniform plexus. Careful physical examination of the scrotum and spermatic cord is required and the classic finding is a 'bag of worms' on palpation [48]. Cremasteric relaxation should be facilitated by allowing the patient to stand in a warm room for a few minutes. Varicoceles are classified in grades: grade 0, no palpable varicocele; grade 1, palpable during a Valsalva maneuver; grade 2, visible in the standing position during a Valsalva maneuver; and grade 3, visible in the standing position through scrotal skin without Valsalva maneuver. Although varicocele is considered a clinical diagnosis, color Doppler imaging can be used as an adjunctive diagnostic tool in equivocal cases. Evidence of venous dilation and reflux during a Valsalva maneuver are sonographic criteria for the diagnosis of varicocele [48]. Pilatz et al. [51] demonstrated that grades 1 to 3 varicoceles can be predicted with a greater than 80% sensitivity and specificity by using cutoff vein diameters of 2.45 mm at rest and 2.95 mm during Valsalva maneuvers. Reflux is determined by calculating the retrograde flow of contrast material via an internal spermatic vein. A study conducted by Chiou et al. [52] used a scoring system with the following parameters: maximal venous diameter, presence of a venous plexus, sum of the diameters of veins in the plexus, and change of flow during a Valsalva maneuver to diagnose varicoceles with a sensitivity of 93% and a specificity of 85%. Each parameter is given a score from 0 to 3 and a total score of greater than 4 is considered positive for varicocele [48,52].
2. Treatment
Varicoceles can affect various seminal parameters including sperm production. Most authors agree that surgical therapy of varicoceles is an accepted intervention in the treatment of male factor infertility; microsurgical varicocelectomy is regarded as the gold standard [53]. When compared to androgen replacement therapy, a study by Cho and Seo [53] found varicocele repair to be more cost-effective in treating infertility. Pain secondary to varicoceles is estimated to affect 2% to 10% of men, but surgical intervention for relief of pain is controversial [50].
Several recent studies, including a large study from Turkey evaluating 119 men, have evaluated the results of varicocelectomy performed for relief of pain. In the study from Turkey, microsurgical varicocele ligation was performed in patients diagnosed on the basis of findings from the physical examination and Doppler ultrasound [54]. At the end of the study, 82 patients were evaluated. A total of 72 patients (88%) reported complete resolution of pain, 4 (5%) reported partial resolution, 5 (6%) reported no change, and 1 (1%) reported epididymal discomfort that resolved with conservative measures. Another study reviewed 58 patients who underwent varicocele ligation for pain relief and obtained follow-up on 35 of the 58 patients in whom initial conservative measures had failed. The study showed resolution of pain postoperatively in 86% and partial resolution in 1 patient, whereas 4 patients (11%) had persistent or worsened symptoms [50]. A more recent study conducted by Kim et al. [55] reported that 91.2% had complete or marked resolution of pain at a 1-year follow-up. The authors used microsurgical inguinal varicocelectomy, with 8.8% of patients having recurrent pain and 0.8% having recurrence. None were found to have hydroceles or evidence of testicular loss or atrophy.
CHRONIC ORCHIALGIA
1. Pathophysiology
Chronic orchialgia is a common and f rustrating problem that most urologists will encounter frequently during their careers. Its prevalence, frequently idiopathic etiology, psychosocial impact, and treatment dilemma often lead to patient distress, physician frustration, and incomplete care. It is defined as intermittent or constant testicular pain for a period of 3 or more months that interferes with daily activities [56]. The pain may involve any of the scrotal contents including the testicle, epididymis, paratesticular structures, and spermatic cord [57]. The etiology of chronic orchialgia remains largely unknown with up to 50% of patients presenting with an idiopathic etiology, but has been found to be associated with nerve damage to the spermatic cord after vasectomy, trauma, inguinal herniorrhaphy, and epididymitis [58]. Similarly, the pathophysiology is not well understood, but involves nerve sensitization following repeated stimulation leading to modulation of these pathways, ultimately resulting in spontaneous firing. These chronically up-regulated pathways are thought to be responsible for chronic orchialgia [59].
2. Diagnosis
This entity is a diagnosis of exclusion, thus requiring a great emphasis on the history and physical examination [27]. The history should be focused on identifying other causes of pain including tumor, torsion, infection, postvasectomy pain, and varicocele. The character of the pain, onset, duration, severity (using a standardized pain scale), location, referral of pain, and psychosocial impact should be investigated. Activities that exacerbate or alleviate the pain, including urinary habits, bowel movements, as well as sexual and physical activities, should be carefully documented. Previous surgeries, history of trauma, or infections localized to the back, inguinal, scrotal, and retroperitoneal areas should also be elicited. As with all sexual pain, it is important to ask questions about sexual health and abuse [57]. A focused and detailed examination of the genitalia followed by a rectal exam should be performed to identify other causes of the chronic pain. Laboratory tests, including urinalysis and urine and semen cultures, should be obtained when indicated (i.e., suspicion of malignancy or infection). Initial imaging should be limited to duplex scrotal ultrasound to exclude structural abnormalities; more advanced imaging may be obtained if indicated. If history and physical examination do not identify a cause, a spermatic cord block using 20 mL of 0.25% bupivacaine without epinephrine should be performed. Temporary pain relief is highly suggestive of chronic orchialgia [56,57].
3. Treatment
The first-line treatment is nonsurgical therapy including NSAIDs, antibiotics (if indicated), antidepressants, anticonvulsants, nerve block, and pulsed radiofrequency. Antibiotics of choice include doxycycline and quinolones, as they have the highest penetration into the affected structures [57]. Antidepressants used are amitriptyline (10-25 mg per oral at bed time) or nortriptyline (10-150 mg per oral daily), which inhibits norepinephrine release, thereby attenuating the neuronal pain pathways. The anticonvulsant gabapentin (300 mg per oral titrated up to 3,600 mg daily) is used for neuropathic pain and works by modulating calcium channels in the central nervous system [60]. Therapeutic nerve blocks with or without steroids can be done to provide relief from pain. Psychological counseling may be offered to the patient to better deal with the pain [57]. Lastly, a novel technique using pulsed radiofrequency to denervate the spermatic cord has been attempted. The efficacy of the procedure has yet to be determined [61]
Patients who fail nonsurgical therapy may consider surgical therapy. Unfortunately, there is no standard of care f or the surgical intervention of chronic orchialgia. Current treatment includes epididymectomy, vasovasostomy and vasectomy reversal for postvasectomy cases, orchiectomy, laparoscopic testicular denervation, and microsurgical denervation of the spermatic cord (MDSC). Epididymectomy has success rates ranging from 10% to 80% and is reserved for patients whose pain is localized to the epididymis [62]. A report on vasovasostomy for chronic pain found that 75% had complete relief, 10% had partial relief (improved by 30%), and 10% had no improvement [63]. Other studies have shown that vasectomy reversals have success rates ranging from 69% to 84% [39]. The major drawback for vasovasostomy and vasectomy reversal is loss of desired sterility. Orchiectomy success rates range from 20% to 80%. However, it is important to take into consideration the physiological and psychological damage that is frequently associated with this procedure [56,64].
Laparoscopic testicular denervation has been shown to result in 78% achieving partial relief and 28% with no relief of pain. However, the efficacy of this treatment has not been fully studied [64,65]. MDSC treats chronic orchialgia by denervating the chronically up-regulated pathways theorized to cause the pain. With the use of this technique, 71% had complete resolution of pain, 17% had greater than 50% reduction, and 12% had no resolution of pain. The advantage to this technique is preservation of organs and scrotal contents [64]. Temporary relief from a spermatic cord block may be a positive predictor for success [66]. The aforementioned surgical procedures all come with similar risks, including infection, bleeding (hematoma), hydrocele formation, testicular atrophy, possibility of no relief or worsened pain, and hypogonadism [57].
MDSC, which was popularized by Levine [57], is performed under general anesthesia with the use of an operating microscope powered at 8× to 14×. A low inguinal incision is made and carried down to the level of the external inguinal ring. The spermatic cord is isolated and the ilioinguinal nerve and branches are identified and divided. To reduce neuroma formation, the proximal end of the ilioinguinal nerve should be buried under external oblique fascia. Next, all fascia and cremasteric fibers in the cord should be divided with electrocautery while taking care to isolate and spare arterial structures (testicular, cremasteric, and deferential). Internal spermatic veins should be ligated and divided. In addition to arterial structures, one or two lymphatic vessels should be identified and spared to maintain proper drainage postoperatively and to reduce the chance of a hydrocele formation. Next, the vas deferens is identified and the nerve-rich perivasal fascia is stripped away because it contains afferent nerve pathways that can carry noxious stimuli and propagate scrotal pain. If the patient underwent a vasectomy, the vas and fascia should be stripped again. At the end of the operation, the remaining structures include the spermatic arteries, one to two lymphatic vessels, and the vas deferens (unless there is a prior history of vasectomy) [57]. At the risk of oversimplification, the procedure may be described as a more extensive or 'radical' varicocelectomy. Since 2012, we have performed this operation at our center on several patients refractory to other therapies and have found success rates similar to the rates reported by Levine [57]. A more recent study found that success rates with MDSC were not statistically significantly different between men who had undergone surgical treatment in the past and surgery-naive men [66].
Footnotes
The authors have nothing to disclose.
References
- 1.Marcozzi D, Suner S. The nontraumatic, acute scrotum. Emerg Med Clin North Am. 2001;19:547–568. doi: 10.1016/s0733-8627(05)70203-0. [DOI] [PubMed] [Google Scholar]
- 2.Van Glabeke E, Khairouni A, Larroquet M, Audry G, Gruner M. Acute scrotal pain in children: results of 543 surgical explorations. Pediatr Surg Int. 1999;15:353–357. doi: 10.1007/s003830050598. [DOI] [PubMed] [Google Scholar]
- 3.McAndrew HF, Pemberton R, Kikiros CS, Gollow I. The incidence and investigation of acute scrotal problems in children. Pediatr Surg Int. 2002;18:435–437. doi: 10.1007/s00383-002-0806-3. [DOI] [PubMed] [Google Scholar]
- 4.Hayden LJ. Chronic testicular pain. Aust Fam Physician. 1993;22:1357–1359. 1362, 1365. [PubMed] [Google Scholar]
- 5.Ben-Sira L, Laor T. Severe scrotal pain in boys with Henoch-Schönlein purpura: incidence and sonography. Pediatr Radiol. 2000;30:125–128. doi: 10.1007/s002470050029. [DOI] [PubMed] [Google Scholar]
- 6.Laor T, Atala A, Teele RL. Scrotal ultrasonography in Henoch-Schönlein purpura. Pediatr Radiol. 1992;22:505–506. doi: 10.1007/BF02012993. [DOI] [PubMed] [Google Scholar]
- 7.Holland JM, Feldman JL, Gilbert HC. Phantom orchalgia. J Urol. 1994;152(6 Pt 2):2291–2293. doi: 10.1016/s0022-5347(17)31660-9. [DOI] [PubMed] [Google Scholar]
- 8.Ringdahl E, Teague L. Testicular torsion. Am Fam Physician. 2006;74:1739–1743. [PubMed] [Google Scholar]
- 9.Dogra V, Bhatt S. Acute painful scrotum. Radiol Clin North Am. 2004;42:349–363. doi: 10.1016/j.rcl.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Knight PJ, Vassy LE. The diagnosis and treatment of the acute scrotum in children and adolescents. Ann Surg. 1984;200:664–673. doi: 10.1097/00000658-198411000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerber GS, Brendler CB. Evaluation of the urologic patient: history, physical examination, and urinalysis. In: Walsh PC, Retik AB, Vaughan ED Jr, Wein AJ, Kavoussi LR, Novick AC, et al., editors. Campbell's urology. 8th ed. Philadelphia: Saunders; 2002. pp. 83–110. [Google Scholar]
- 12.Rabinowitz R. The importance of the cremasteric reflex in acute scrotal swelling in children. J Urol. 1984;132:89–90. doi: 10.1016/s0022-5347(17)49476-6. [DOI] [PubMed] [Google Scholar]
- 13.Herbener TE. Ultrasound in the assessment of the acute scrotum. J Clin Ultrasound. 1996;24:405–421. doi: 10.1002/(SICI)1097-0096(199610)24:8<405::AID-JCU2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 14.Burgher SW. Acute scrotal pain. Emerg Med Clin North Am. 1998;16:781–809. doi: 10.1016/s0733-8627(05)70033-x. [DOI] [PubMed] [Google Scholar]
- 15.Boettcher M, Bergholz R, Krebs TF, Wenke K, Treszl A, Aronson DC, et al. Differentiation of epididymitis and appendix testis torsion by clinical and ultrasound signs in children. Urology. 2013;82:899–904. doi: 10.1016/j.urology.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Trojian TH, Lishnak TS, Heiman D. Epididymitis and orchitis: an overview. Am Fam Physician. 2009;79:583–587. [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. 1998 Guidelines for treatment of sexually transmitted diseases. MMWR Morb Mortal Wkly Rep. 1998;47(RR-01):1–118. [PubMed] [Google Scholar]
- 18.Joo JM, Yang SH, Kang TW, Jung JH, Kim SJ, Kim KJ. Acute epididymitis in children: the role of the urine test. Korean J Urol. 2013;54:135–138. doi: 10.4111/kju.2013.54.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hori S, Tsutsumi Y. Histological differentiation between chlamydial and bacterial epididymitis: nondestructive and proliferative versus destructive and abscess forming: immunohistochemical and clinicopathological findings. Hum Pathol. 1995;26:402–407. doi: 10.1016/0046-8177(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 20.Redshaw JD, Tran TL, Wallis MC, deVries CR. Epididymitis: a 21-year retrospective review of presentations to an outpatient urology clinic. J Urol. 2014;192:1203–1207. doi: 10.1016/j.juro.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Santillanes G, Gausche-Hill M, Lewis RJ. Are antibiotics necessary for pediatric epididymitis? Pediatr Emerg Care. 2011;27:174–178. doi: 10.1097/PEC.0b013e31820d647a. [DOI] [PubMed] [Google Scholar]
- 22.Hamdan M, Cerabona V. Epididymitis in infants. Apropos of a case and review of the literature. J Urol (Paris) 1991;97:228–229. [PubMed] [Google Scholar]
- 23.Haecker FM, Hauri-Hohl A, von Schweinitz D. Acute epididymitis in children: a 4-year retrospective study. Eur J Pediatr Surg. 2005;15:180–186. doi: 10.1055/s-2004-830355. [DOI] [PubMed] [Google Scholar]
- 24.Cicek T, Cicek Demir C, Coban G, Coner A. Amiodarone induced epididymitis: a case report. Iran Red Crescent Med J. 2014;16:e13929. doi: 10.5812/ircmj.13929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutcheson J, Peters CA, Diamond DA. Amiodarone induced epididymitis in children. J Urol. 1998;160:515–517. [PubMed] [Google Scholar]
- 26.Gomez Garcia I, Gomez Mampaso E, Burgos Revilla J, Molina MR, Sampietro Crespo A, Buitrago LA, et al. Tuberculous orchiepididymitis during 1978-2003 period: review of 34 cases and role of 16S rRNA amplification. Urology. 2010;76:776–781. doi: 10.1016/j.urology.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 27.Kavoussi PK, Costabile RA. Orchialgia and the chronic pelvic pain syndrome. World J Urol. 2013;31:773–778. doi: 10.1007/s00345-013-1092-5. [DOI] [PubMed] [Google Scholar]
- 28.Choe JM, Kirkemo AK. Questionnaire-based outcomes study of nononcological post-vasectomy complications. J Urol. 1996;155:1284–1286. [PubMed] [Google Scholar]
- 29.Awsare NS, Krishnan J, Boustead GB, Hanbury DC, McNicholas TA. Complications of vasectomy. Ann R Coll Surg Engl. 2005;87:406–410. doi: 10.1308/003588405X71054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMahon AJ, Buckley J, Taylor A, Lloyd SN, Deane RF, Kirk D. Chronic testicular pain following vasectomy. Br J Urol. 1992;69:188–191. doi: 10.1111/j.1464-410x.1992.tb15494.x. [DOI] [PubMed] [Google Scholar]
- 31.West AF, Leung HY, Powell PH. Epididymectomy is an effective treatment for scrotal pain after vasectomy. BJU Int. 2000;85:1097–1099. doi: 10.1046/j.1464-410x.2000.00656.x. [DOI] [PubMed] [Google Scholar]
- 32.Nangia AK, Myles JL, Thomas AJ., JR Vasectomy reversal for the post-vasectomy pain syndrome: a clinical and histological evaluation. J Urol. 2000;164:1939–1942. [PubMed] [Google Scholar]
- 33.Cho SH, Min SK, Lee ST. Associations of ultrasonographic features with scrotal pain after vasectomy. Korean J Urol. 2011;52:782–786. doi: 10.4111/kju.2011.52.11.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sweeney P, Tan J, Butler MR, McDermott TE, Grainger R, Thornhill JA. Epididymectomy in the management of intrascrotal disease: a critical reappraisal. Br J Urol. 1998;81:753–755. doi: 10.1046/j.1464-410x.1998.00636.x. [DOI] [PubMed] [Google Scholar]
- 35.Paxton LD, Huss BK, Loughlin V, Mirakhur RK. Intra-vas deferens bupivacaine for prevention of acute pain and chronic discomfort after vasectomy. Br J Anaesth. 1995;74:612–613. doi: 10.1093/bja/74.5.612. [DOI] [PubMed] [Google Scholar]
- 36.Woolf CJ, Chong MS. Preemptive analgesia: treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362–379. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- 37.Bruning CO., 3rd Re: Vasectomy reversal for treatment of the post-vasectomy pain syndrome. J Urol. 1997;158:1528. doi: 10.1016/s0022-5347(01)64267-8. [DOI] [PubMed] [Google Scholar]
- 38.Edwards IS. Vasectomy reversal for treatment of the postvasectomy pain syndrome. J Urol. 1997;158:2252. doi: 10.1016/s0022-5347(01)68223-5. [DOI] [PubMed] [Google Scholar]
- 39.Myers SA, Mershon CE, Fuchs EF. Vasectomy reversal for treatment of the post-vasectomy pain syndrome. J Urol. 1997;157:518–520. [PubMed] [Google Scholar]
- 40.Lee JY, Cho KS, Lee SH, Cho HJ, Cho JM, Oh CY, et al. A comparison of epididymectomy with vasectomy reversal for the surgical treatment of postvasectomy pain syndrome. Int Urol Nephrol. 2014;46:531–537. doi: 10.1007/s11255-013-0517-9. [DOI] [PubMed] [Google Scholar]
- 41.Meacham RB, Townsend RR, Rademacher D, Drose JA. The incidence of varicoceles in the general population when evaluated by physical examination, gray scale sonography and color Doppler sonography. J Urol. 1994;151:1535–1538. doi: 10.1016/s0022-5347(17)35295-3. [DOI] [PubMed] [Google Scholar]
- 42.Saypol DC. The varicocele. J Androl. 1981;2:61–71. [Google Scholar]
- 43.Lee HJ, Cheon SH, Ji YH, Moon KH, Kim KS, Park S, et al. Clinical characteristics and surgical outcomes in adolescents and adults with varicocele. Korean J Urol. 2011;52:489–493. doi: 10.4111/kju.2011.52.7.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahlberg NE, Bartley O, Chidekel N, Fritjofsson A. Phlebography in varicocele scroti. Acta Radiol Diagn (Stockh) 1966;4:517–528. doi: 10.1177/028418516600400506. [DOI] [PubMed] [Google Scholar]
- 45.Comhaire F, Kunnen M, Nahoum C. Radiological anatomy of the internal spermatic vein(s) in 200 retrograde venograms. Int J Androl. 1981;4:379–387. doi: 10.1111/j.1365-2605.1981.tb00722.x. [DOI] [PubMed] [Google Scholar]
- 46.Coolsaet BL. The varicocele syndrome: venography determining the optimal level for surgical management. J Urol. 1980;124:833–839. doi: 10.1016/s0022-5347(17)55688-8. [DOI] [PubMed] [Google Scholar]
- 47.Kohler FP. On the etiology of varicocele. J Urol. 1967;97:741–742. doi: 10.1016/S0022-5347(17)63109-4. [DOI] [PubMed] [Google Scholar]
- 48.Kwak N, Siegel D. Imaging and interventional therapy for varicoceles. Curr Urol Rep. 2014;15:399. doi: 10.1007/s11934-014-0399-0. [DOI] [PubMed] [Google Scholar]
- 49.Agarwal A, Sharma RK, Desai NR, Prabakaran S, Tavares A, Sabanegh E. Role of oxidative stress in pathogenesis of varicocele and infertility. Urology. 2009;73:461–469. doi: 10.1016/j.urology.2008.07.053. [DOI] [PubMed] [Google Scholar]
- 50.Peterson AC, Lance RS, Ruiz HE. Outcomes of varicocele ligation done for pain. J Urol. 1998;159:1565–1567. doi: 10.1097/00005392-199805000-00043. [DOI] [PubMed] [Google Scholar]
- 51.Pilatz A, Altinkilic B, Kohler E, Marconi M, Weidner W. Color Doppler ultrasound imaging in varicoceles: is the venous diameter sufficient for predicting clinical and subclinical varicocele? World J Urol. 2011;29:645–650. doi: 10.1007/s00345-011-0701-4. [DOI] [PubMed] [Google Scholar]
- 52.Chiou RK, Anderson JC, Wobig RK, Rosinsky DE, Matamoros A, Jr, Chen WS, et al. Color Doppler ultrasound criteria to diagnose varicoceles: correlation of a new scoring system with physical examination. Urology. 1997;50:953–956. doi: 10.1016/S0090-4295(97)00452-4. [DOI] [PubMed] [Google Scholar]
- 53.Cho KS, Seo JT. Effect of varicocelectomy on male infertility. Korean J Urol. 2014;55:703–709. doi: 10.4111/kju.2014.55.11.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yaman O, Ozdiler E, Anafarta K, Gogus O. Effect of microsurgical subinguinal varicocele ligation to treat pain. Urology. 2000;55:107–108. doi: 10.1016/s0090-4295(99)00374-x. [DOI] [PubMed] [Google Scholar]
- 55.Kim HT, Song PH, Moon KH. Microsurgical ligation for painful varicocele: effectiveness and predictors of pain resolution. Yonsei Med J. 2012;53:145–150. doi: 10.3349/ymj.2012.53.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davis BE, Noble MJ, Weigel JW, Foret JD, Mebust WK. Analysis and management of chronic testicular pain. J Urol. 1990;143:936–939. doi: 10.1016/s0022-5347(17)40143-1. [DOI] [PubMed] [Google Scholar]
- 57.Levine L. Chronic orchialgia: evaluation and discussion of treatment options. Ther Adv Urol. 2010;2:209–214. doi: 10.1177/1756287210390409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levine LA, Matkov TG. Microsurgical denervation of the spermatic cord as primary surgical treatment of chronic orchialgia. J Urol. 2001;165(6 Pt 1):1927–1929. doi: 10.1097/00005392-200106000-00020. [DOI] [PubMed] [Google Scholar]
- 59.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 60.Sinclair AM, Miller B, Lee LK. Chronic orchialgia: consider gabapentin or nortriptyline before considering surgery. Int J Urol. 2007;14:622–625. doi: 10.1111/j.1442-2042.2007.01745.x. [DOI] [PubMed] [Google Scholar]
- 61.Basal S, Ergin A, Yildirim I, Goktas S, Atim A, Sizlan A, et al. A novel treatment of chronic orchialgia. J Androl. 2012;33:22–26. doi: 10.2164/jandrol.110.010991. [DOI] [PubMed] [Google Scholar]
- 62.Siu W, Ohl DA, Schuster TG. Long-term follow-up after epididymectomy for chronic epididymal pain. Urology. 2007;70:333–335. doi: 10.1016/j.urology.2007.03.080. [DOI] [PubMed] [Google Scholar]
- 63.Werthman P. Vasectomy reversal for post-vasectomy pain syndrome: a ten-year experience. J Urol. 2010;183(Suppl):e752. [Google Scholar]
- 64.Strom KH, Levine LA. Microsurgical denervation of the spermatic cord for chronic orchialgia: long-term results from a single center. J Urol. 2008;180:949–953. doi: 10.1016/j.juro.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 65.Cadeddu JA, Bishoff JT, Chan DY, Moore RG, Kavoussi LR, Jarrett TW. Laparoscopic testicular denervation for chronic orchalgia. J Urol. 1999;162(3 Pt 1):733–735. doi: 10.1097/00005392-199909010-00028. [DOI] [PubMed] [Google Scholar]
- 66.Larsen SM, Benson JS, Levine LA. Microdenervation of the spermatic cord for chronic scrotal content pain: single institution review analyzing success rate after prior attempts at surgical correction. J Urol. 2013;189:554–558. doi: 10.1016/j.juro.2012.09.026. [DOI] [PubMed] [Google Scholar]


