Abstract
Purpose
To evaluate the impact of adjuvant chemotherapy (AC) in patients with upper tract urothelial carcinoma and lymphovascular invasion (LVI) after radical nephroureterectomy (RNU).
Materials and Methods
We retrospectively analyzed the clinical records and clinicopatholgic outcomes of patients (n=552) treated with RNU between 1986 and 2013. Patients treated with neoadjuvant chemotherapy and those for whom LVI status was not recorded were excluded. Patients were divided into two groups according to LVI (n=86) or no LVI (n=256).
Results
The study included 344 patients (240 men and 104 women) with a median of 53.9 months of follow-up (range, 1-297 months) after RNU. Tumors were organ confined (T2/N0) in 211 (61.3%) and tumor grade high in 291 (84.6%). AC was administered in 64 patients (18.6%). A total of 280 patients (81.4%) were treated with surgery alone. Patients with LVI tended to be older (p=0.049), have a higher pT stage (pT3/T4, p<0.001), be pN+ (p<0.001), have a high tumor grade (p<0.001), and experience recurrence (p<0.001). In the multivariate analysis, LVI was an independent prognostic factor for cancer-specific survival and overall survival (p=0.002 and p<0.001, respectively). The multivariate analysis demonstrated that in the subgroup of patients with LVI, AC was a significant prognostic factor for cancer-specific survival and overall survival (hazard ratio, 0.51; p=0.027 and hazard ratio, 0.50; p=0.025, respectively).
Conclusions
AC does not seem to reduce mortality in patients with advanced upper tract urothelial carcinoma after RNU. In the subgroup of patients with LVI, AC had a positive impact on cancer-specific survival and overall survival. LVI would be helpful for selecting patients who are appropriate for AC.
Keywords: Adjuvant chemotherapy, Kidney pelvis, Transitional cell carcinoma, Ureter, Urinary tract
INTRODUCTION
Upper tract urothelial carcinoma (UTUC) is a rare malignancy, with a reported annual incidence in the Western literature of 2 per 100,000 persons [1]. Despite advances in endoscopic and minimally invasive treatments during the past two decades, UTUC continues to portend a poor prognosis, with approximately 28% of patients experiencing a recurrence of disease outside the bladder and 23% of patients dying of the disease within 5 years [2].
Although advanced UTUC is associated with poor oncologic outcomes, there is no standardized therapy conferring a survival benef it af ter radical nephroureterectomy (RNU). Currently, no randomized trials that have investigated the role of adjuvant chemotherapy (AC) for UTUC exist, and there is insufficient evidence to integrate neoadjuvant chemotherapy or AC into a treatment strategy for advanced UTUC. The majority of the available data are from retrospective analyses and the results have been inconclusive [3,4,5,6]. A recent meta-analysis showed that cisplatin-based AC was beneficial in terms of overall survival (OS) and disease-specific survival; however, the results were also limited by the relatively small sample size and the retrospective nature of the studies included in the analysis [7].
Numerous studies have investigated prognostic factors in patients with UTUC, and tumor stage and tumor grade have been reported to be the most significant prognostic factors [3,4,5]. In addition, lymphovacular invasion (LVI) has been demonstrated as a predictor of poor prognosis in several studies [8,9,10,11,12,13,14]. Moreover, the latest European guidelines on UTUCs suggested LVI as an independent predictor of survival [15]. In our previous study, we also reported that LVI was a significant prognostic factor for recurrence-free and cancer-specific survival (CSS) in patients with UTUC [9]. In this study, we extended our previous UTUC cohort and investigated whether AC improved survival in patients with UTUC and LVI.
MATERIALS AND METHODS
Patient information was retrospectively collected from a database approved by the Yonsei University Institutional Review Board. A review of medical records of our institute identified 552 patients with primary UTUC who underwent RNU between 1986 and 2013. We excluded 181 patients who had incomplete data on LVI and 27 patients treated with neoadjuvant chemotherapy. Thus, a total of 344 patients were included in our study.
Each patient underwent preoperative evaluation, including blood test, urine cytology, cystoscopy, chest x-ray, abdominopelvic computed tomography, and bone scan. Clinicopathological variables including age, gender, tumor stage, tumor grade, tumor location, and LVI status were investigated.
All surgical specimens were analyzed by uropathologists of our institution. Tumor grades were assessed according to the 1998 World Health Organization/International Society of Urologic Pathology consensus classification [16]. Staging was performed according to the American Joint Committee on Cancer system [17]. LVI was defined as the unequivocal presence of cancer cells in endothelium-lined lymphatic and vascular channels without underlying muscular walls [18]. Routine light microscopic examination with hematoxylin and eosin was performed; no immunohistochemical staining was used to identify LVI.
Postoperative follow-up was performed every 3 months for 2 years, and then every 6 months thereafter. Follow-up included history, physical examination, serum chemistry studies, urinary cytology, chest radiography, cystoscopic evaluation of the bladder, and radiographic evaluation of the contralateral upper urinary tract.
The cause of death was corroborated by reviewing the charts. Death certificates were used to assess cause of death.
Patients treated with at least one cycle of chemotherapy within 3 months after RNU were regarded to have received AC based on pathological stage pT3 or greater or node positivity. Two types of platinum-based chemotherapies were used: methotrexate, vinblastine, doxorubicin (Adriamycin), and cisplatin (MVAC) or gemcitabine and cisplatin (GC). Cisplatin was substituted with carboplatin if the glomerular filtration rate was between 30 and 49 mL/min. The treatment protocols and follow-up schedules were surgeon-dependent. There was no routine restaging postoperatively before AC.
Patients were stratified into two groups in accordance with the presence of LVI, and clinicopathological features were compared between the two groups. Qualitative variables were compared by using a chi-square test and quantitative variables were compared by using Student t-test. The prognostic significance of LVI was examined in the entire cohort and we also evaluated whether AC was independently associated with OS and CSS in accordance with the presence of LVI. Univariate and multivariate Cox's proportional hazard regression models were used to identify the independent parameters associated with OS and CSS.
All reported p-values were two-sided, and a p-value of ≤0.05 was considered to indicate statistical significance. All statistical tests were carried out with IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA).
RESULTS
Patients were divided into two groups according to the presence of LVI: group 1 (n=86, LVI (+)) and group 2 (n=258, LVI (-)). Patients with LVI were significantly older and had higher pathologic stage and tumor grade (Table 1). The 5-year OS and CSS rates were 31.0% and 45.0%, respectively. OS and CSS were lower in patients with LVI than in patients without LVI (p<0.001, Fig. 1A; p<0.001, Fig. 1B, respectively).
Table 1.
Clinical and pathological characteristics of 344 patients according to LVI
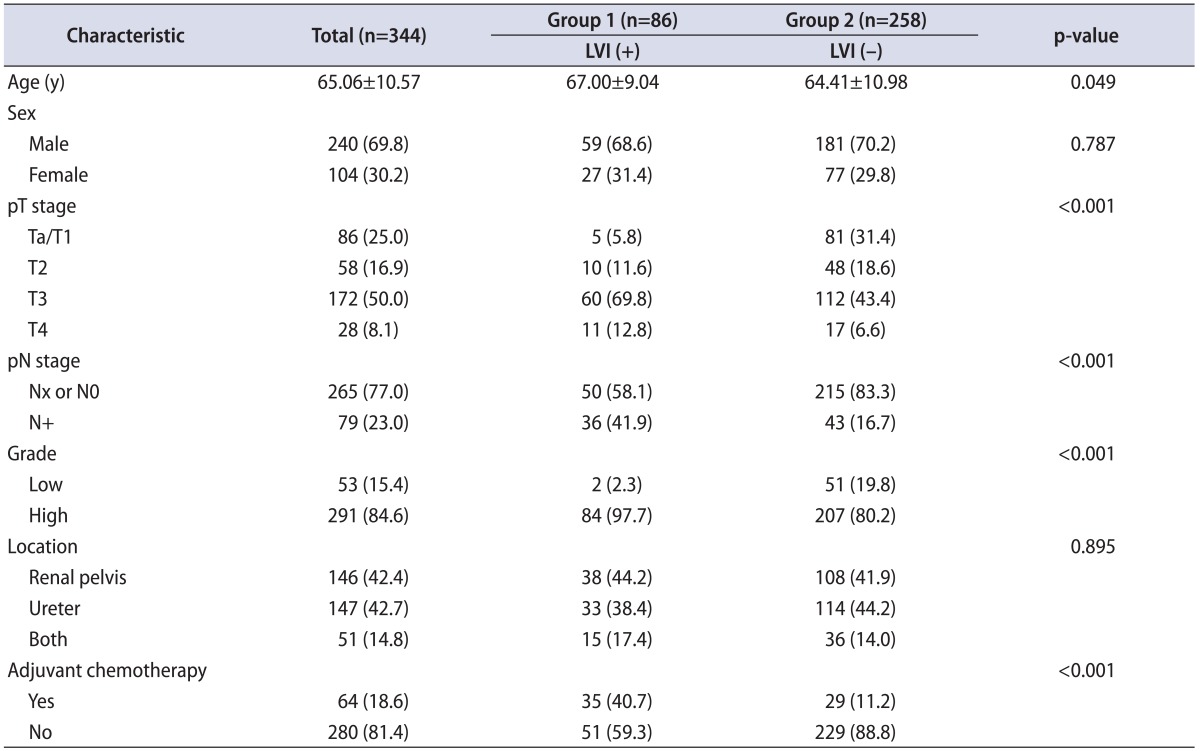
Values are presented as mean±standard deviation or number (%).
LVI, lymphovascular invasion.
Fig. 1.
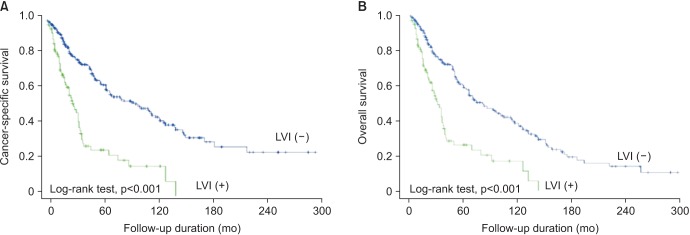
Impact of lymphovascular invasion (LVI) in cancer-specific (A) and overall survivals (B) in patients with upper urinary tract transitional cell carcinoma.
Of the 344 patients, 64 (18.6%) received AC and 280 (81.4%) were treated with surgery alone. Also, LVI was independently associated with worse CSS and OS after adjustment for age, gender, pathologic stage, and tumor grade. In the entire cohort, AC was not independently associated with survival (CSS, p=0.161; OS, p=0.144) (Table 2). In patients with LVI (group 1), AC was independently associated with improved CSS (hazard ratio [HR], 0.51; p=0.027) and OS (HR, 0.50; p=0.025) after adjustment for other variables (Table 3). In patients without LVI (group 2), AC was not significantly associated with survival and had no impact on CSS or OS (Table 3).
Table 2.
Univariatate and multivariate analyses predicting CSS and OS in patients with UTUC after RNU
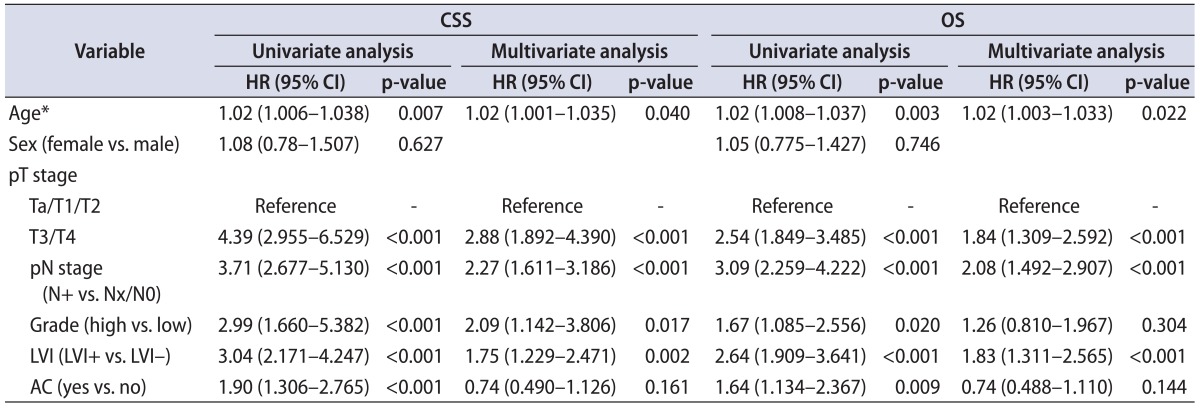
CSS, cancer-specific survival; OS, overall survival; UTUC, upper tract urothelial carcinoma; RNU, radical nephroureterectomy; HR, hazard ratio; CI, confidence interval; LVI, lymphovascular invasion; AC, adjuvant chemotherapy.
*At the time of surgery.
Table 3.
Univariatate and multivariate analyses predicting CSS and OS in patients with or without lymphovascular invasion after RNU
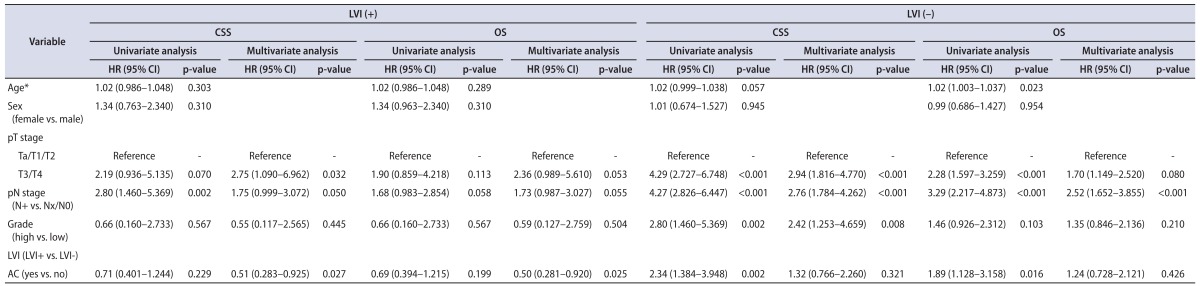
CSS, cancer-specific survival; OS, overall survival; RNU, radical nephroureterectomy; LVI, lymphovascular invasion; HR, hazard ratio; CI, confidence interval; AC, adjuvant chemotherapy.
*At the time of surgery.
Patients with LVI were further stratified by pathological stage (pT1-2 vs. pT3-4) and we further evaluated the impact of AC. A favorable impact of AC on CSS and OS was found in patients with pT3-4 and LVI but not in patients with pT1-2 and LVI in the univariate Cox's proportional hazard regression analyses (Table 4). In patients with pT3-4, AC was not significantly associated with CSS or OS in the univariate or multivariate analysis (data not shown).
Table 4.
Univariatate analyses predicting CSS and OS in patients stratified by pathological stage
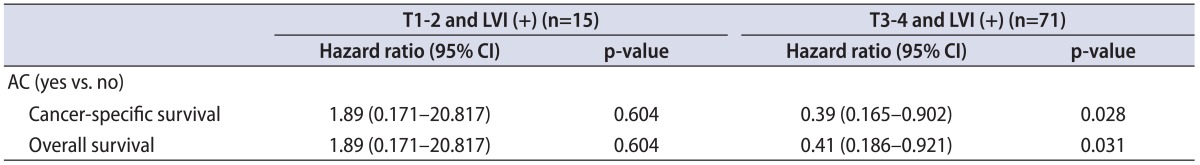
LVI, lymphovascular invasion; HR, hazard ratio; CI, confidence interval; AC, adjuvant chemotherapy.
DISCUSSION
We analyzed clinicopathologic features and survival outcomes of patients treated with RNU for UTUC over two decades. AC was not significantly associated with CSS or OS in all patients. However, AC was independently associated with CSS and OS in patients with LVI. Specifically, there was a significant difference in survival after AC in patients with T3-4 and LVI but not in those with T1-2 and LVI.
There is no standardized chemotherapy conferring a survival benefit after RNU. Although UTUC is similar morphologically to lower tract urothelial carcinoma, there are occasional phenotypic and genotypic differences [7]. Therefore, these findings suggest a difference in chemotherapy response between UTUC and lower tract urothelial carcinoma. Nevertheless, many clinicians treat patients with high-risk UTUC with AC. Several platinum-based regimens have been proposed, although AC protocols are not standardized. The efficacy of platinum-based AC was expected to be similar to that seen in bladder cancer because of similarities in the pathological features. Clinical decision-making regarding AC is dependent on multiple factors such as performance and impaired renal function after RNU. Urologists should consider that most patients with AC underwent cisplatin-based chemotherapy with nephrotoxicity.
The prospective randomized controlled perioperative chemotherapy versus surveillance in upper tract urothelial cancer (POUT) trial has enrolled patients with UTUC staged pT2-pT4 N0-3 M0 or pTany N1-3 M0 after RNU starting in 2012 [17]. Until the results of the POUT trial are revealed, the efficacy of AC after RNU will remain controversial. However, although there is no well-powered level 1 evidence to support AC for advanced UTUC, several studies have examined the role of AC. Kwak et al. [3] suggested that in a multivariable Cox proportional hazard analysis of 43 patients with UTUC, AC was strongly associated with OS. Another recent study reported that cisplatin-based AC for UTUC had no influence on CSS; however, AC can reduce bladder recurrence [4]. Lucca et al. [6] showed that only patients at the highest risk for disease recurrence with pT3/T4 N+ disease may benefit from AC. A recent retrospective analysis suggested that AC had no survival benefit for CSS and OS in patients with advanced UTUC (pT3N0, pT4N0, and/or lymph node positive) [5].
Our study showed that there was no significant difference in CSS or OS between an AC group and a no-adjuvant-chemotherapy group. However, this study presents a favorable response to AC in patients with LVI+ and pT3-pT4N0/x. A recent meta-analysis investigating AC for UTUC found an OS and disease-free survival benefit among patients treated with cisplatin-based AC compared with those who had surgery alone [7]. Established studies have shown that AC is not significantly associated with outcomes of patients with lymph-node-positive disease. We found that AC was effective in patients with high-risk UTUC and LVI. AC reduced the relative risk of UTUC-related CSS by 50% (HR, 0.50; p=0.025). However, these studies suffer from selection biases in which high-risk patients are selected to receive AC compared with their counterparts who undergo observation.
Several studies have reported that LVI is a significant prognostic factor in patients with UTUC as well as other urologic malignancies. Recent studies reported the significance of LVI for bladder cancer. LVI was strongly associated with lymph node invasion because of the presence of circulating cancer cells and the formation of micrometastasis, which suggested LVI as a predictor of poor prognosis [8,19]. Our previous study reported that LVI was significantly associated with recurrence-free survival or CSS in patients who had only localized UTUC (pTa-3N0M0) [9]. This study showed that LVI was independently associated with worse CSS and OS after adjustment for age, gender, pathologic stage, and tumor grade. A meta-analysis confirmed that patients with LVI were associated with LN metastases and suggested an opportunity for identifying patients for AC.
Hurel et al. [11] showed that the incidence of LVI (+) was slightly higher (29.6%) than previously described, because they did not classify unknown LVI status as LVI (-). In pathologic reports, LVI status was not routinely described. In previous studies, LVI was detected in -15% to 20% of cases [10]. In our study, we excluded patients without LVI status in pathologic reports. The incidence of LVI (+) was 25.0%.
This study had limitations. Several characteristics could account for the heterogeneity in the results, including the small size cohort, the multiple physicians, and the variability of intraoperative management. Heterogeneity of patients treated with AC existed. Multiple factors influenced the clinical decision making regarding the use of AC such as age, performance status, and renal function. Although AC regimens in the study cohort were not standardized, cisplatin-based chemotherapy was used for the standard AC regimen. We analyzed the impact of AC by including all 3 regimens and comparison between regimens was not performed.
Another limitation was the extent of lymph node dissection. pN stage and lymph node dissection may influence the decision-making regarding AC. However, in our study, regional lymph node dissection was not routinely performed in patients with clinically apparent lymphadenopathy on preoperative radiologic imaging or lymphadenopathies found intraoperatively.
Finally, the practice to demonstrate LVI status was performed only for select cases suspicious for invasion across the study period. Although the patients without LVI status were excluded and our incidence of LVI is similar to previous results, the potential for selection bias exists.
Despite the limitations of our study, we demonstrated that AC improved oncologic outcomes in patients with LVI who underwent RNU for UTUC. We expect a large, prospective randomized investigation to verify our conclusions.
CONCLUSIONS
In the current study, LVI and pT stage were significant prognostic factors for CSS and OS in patients with UTUC. The subgroup of patients with T3-4 and LVI is the target population for AC.
ACKNOWLEDGMENTS
This work was financially supported by the National Research Foundation Grant funded by the Korean Government (NRF-2012R1A1A1042968).
Footnotes
The authors have nothing to disclose.
References
- 1.Raman JD, Messer J, Sielatycki JA, Hollenbeak CS. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973-2005. BJU Int. 2011;107:1059–1064. doi: 10.1111/j.1464-410X.2010.09675.x. [DOI] [PubMed] [Google Scholar]
- 2.Margulis V, Shariat SF, Matin SF, Kamat AM, Zigeuner R, Kikuchi E, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115:1224–1233. doi: 10.1002/cncr.24135. [DOI] [PubMed] [Google Scholar]
- 3.Kwak C, Lee SE, Jeong IG, Ku JH. Adjuvant systemic chemotherapy in the treatment of patients with invasive transitional cell carcinoma of the upper urinary tract. Urology. 2006;68:53–57. doi: 10.1016/j.urology.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 4.Kim TS, Oh JH, Rhew HY. The efficacy of adjuvant chemotherapy for locally advanced upper tract urothelial cell carcinoma. J Cancer. 2013;4:686–690. doi: 10.7150/jca.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hellenthal NJ, Shariat SF, Margulis V, Karakiewicz PI, Roscigno M, Bolenz C, et al. Adjuvant chemotherapy for high risk upper tract urothelial carcinoma: results from the Upper Tract Urothelial Carcinoma Collaboration. J Urol. 2009;182:900–906. doi: 10.1016/j.juro.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Lucca I, Kassouf W, Kapoor A, Fairey A, Rendon RA, Izawa JI, et al. The role of adjuvant chemotherapy for lymph nodepositive upper tract urothelial carcinoma following radical nephroureterectomy: a retrospective study. BJU Int. 2014 May 13; doi: 10.1111/bju.12801. [Epub] http://dx.doi.org/10.1111/bju.12801. [DOI] [PubMed] [Google Scholar]
- 7.Leow JJ, Martin-Doyle W, Fay AP, Choueiri TK, Chang SL, Bellmunt J. A systematic review and meta-analysis of adjuvant and neoadjuvant chemotherapy for upper tract urothelial carcinoma. Eur Urol. 2014;66:529–541. doi: 10.1016/j.eururo.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Kikuchi E, Horiguchi Y, Nakashima J, Hatakeyama N, Matsumoto M, Nishiyama T, et al. Lymphovascular invasion independently predicts increased disease specific survival in patients with transitional cell carcinoma of the upper urinary tract. J Urol. 2005;174:2120–2123. doi: 10.1097/01.ju.0000181801.22474.8b. [DOI] [PubMed] [Google Scholar]
- 9.Kim DS, Lee YH, Cho KS, Cho NH, Chung BH, Hong SJ. Lymphovascular invasion and pT stage are prognostic factors in patients treated with radical nephroureterectomy for localized upper urinary tract transitional cell carcinoma. Urology. 2010;75:328–332. doi: 10.1016/j.urology.2009.07.1350. [DOI] [PubMed] [Google Scholar]
- 10.Novara G, Matsumoto K, Kassouf W, Walton TJ, Fritsche HM, Bastian PJ, et al. Prognostic role of lymphovascular invasion in patients with urothelial carcinoma of the upper urinary tract: an international validation study. Eur Urol. 2010;57:1064–1071. doi: 10.1016/j.eururo.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 11.Hurel S, Roupret M, Ouzzane A, Rozet F, Xylinas E, Zerbib M, et al. Impact of lymphovascular invasion on oncological outcomes in patients with upper tract urothelial carcinoma after radical nephroureterectomy. BJU Int. 2013;111:1199–1207. doi: 10.1111/bju.12116. [DOI] [PubMed] [Google Scholar]
- 12.Hong B, Park S, Hong JH, Kim CS, Ro JY, Ahn H. Prognostic value of lymphovascular invasion in transitional cell carcinoma of upper urinary tract. Urology. 2005;65:692–696. doi: 10.1016/j.urology.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Kuroda K, Asakuma J, Horiguchi A, Tasaki S, Yoshii H, Sato A, et al. Prognostic factors for upper urinary tract urothelial carcinoma after nephroureterectomy. Urol Int. 2012;88:225–231. doi: 10.1159/000335274. [DOI] [PubMed] [Google Scholar]
- 14.Lee SE, Hong SK, Han BK, Yu JH, Han JH, Jeong SJ, et al. Prognostic significance of tumor necrosis in primary transitional cell carcinoma of upper urinary tract. Jpn J Clin Oncol. 2007;37:49–55. doi: 10.1093/jjco/hyl123. [DOI] [PubMed] [Google Scholar]
- 15.Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester R, Burger M, et al. European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol. 2013;63:1059–1071. doi: 10.1016/j.eururo.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 16.Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998;22:1435–1448. doi: 10.1097/00000478-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 18.Hasui Y, Nishi S, Kitada S, Osada Y, Asada Y. The prognostic significance of vascular invasion in upper urinary tract transitional cell carcinoma. J Urol. 1992;148:1783–1785. doi: 10.1016/s0022-5347(17)37028-3. [DOI] [PubMed] [Google Scholar]
- 19.Bolenz C, Shariat SF, Fernandez MI, Margulis V, Lotan Y, Karakiewicz P, et al. Risk stratification of patients with nodal involvement in upper tract urothelial carcinoma: value of lymphnode density. BJU Int. 2009;103:302–306. doi: 10.1111/j.1464-410X.2008.07988.x. [DOI] [PubMed] [Google Scholar]


