Abstract
Purpose
Polycystic ovary syndrome (PCOS) is an endocrine-metabolic disorder that leads to lower natural reproductive potential and presents a challenge for assisted reproductive medicine because patients may exhibit immature oocyte retrieval and a higher risk of ovarian hyper stimulation syndrome during in vitro fertilization (IVF) treatment. This study aimed to identify potential lipid biomarkers for women with PCOS and a hyper response to controlled ovarian stimulation.
Methods
Follicular fluid samples were collected from patients who underwent IVF, including normal responder women who became pregnant (control group, n = 11), women with PCOS and a hyper response to gonadotropins (PCOS group, n = 7) and women with only hyper response to gonadotropins (HR group, n = 7). A lipidomic analysis was performed by electrospray ionization mass spectrometry, and candidate biomarkers were analyzed by tandem mass spectrometry experiment.
Results
The lipid profiles indicated particularities related to differences in phosphatidylcholine (PCOS and HR), phosphatidylserine, phosphatydilinositol and phosphatidylglycerol (control), sphingolipids (PCOS) and phosphatidylethanolamine (control and HR).
Conclusions
These findings contribute to the understanding of the molecular mechanisms associated with lipid metabolism in the PCOS-related hyper response, and strongly suggest that these lipids may be useful as biomarkers, leading to the development of more individualized treatment for pregnancy outcome.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-014-0375-0) contains supplementary material, which is available to authorized users.
Keywords: Polycystic ovary syndrome, Hyper response, Follicular fluid, Lipids, ESI-MS
Introduction
Polycystic ovary syndrome (PCOS) is a metabolic endocrine disorder that affects approximately 10 % of women according to Rotterdam criteria [1]. This disorder is characterized as a heterogeneous syndrome and current guidelines recommend that a PCOS diagnosis should be made with the occurrence of 2 out of 3 factors: oligo or anovulation, clinical or biochemical hyperandrogenism and the presence of polycystic ovaries. In addition, disorders that mimic the clinical presentation of PCOS, such as congenital adrenal hyperplasia, Cushing’s syndrome and androgen-secreting tumors, must be excluded [2, 3].
In addition to the presence of low oocyte quality in women with PCOS, when these patients undergo in vitro fertilization (IVF) treatment, they possess a higher risk for developing ovarian hyper stimulation syndrome [4, 5]. During IVF cycles, an appropriate manner to analyze oocytes and the molecular mechanisms involved in diseases related to female infertility include follicular fluid (FF) analysis because it provides the oocyte development microenvironment [6]. Moreover, analysis of FF components may generate information about metabolic changes in blood plasma, which is similar to the circulating biochemical environment that may be reflected in the composition of the follicular fluid [7].
It is becoming increasingly clear that deregulated lipid metabolism plays an important role in many human diseases because lipid homeostasis is fundamental to health maintenance [8]. With progress in the lipidomics field, knowledge of lipids has improved, particularly related to the role of lipids in cell signaling pathways, which can indicate changes in the cellular microenvironment, such as steroidogenesis processes, cell proliferation and apoptosis [9–11]. Moreover, prospective lipid biomarkers have been related to female fertility, such as phospholipids changes in cumulus cells have been related to cell differentiation. Furthermore, some studies reported a correlation between the fatty acid profile and oocyte maturity and embryonic development [12, 13].
To improve the characterization of biomarkers related to human fertility, the use of modern analytical platforms based on mass spectrometry (MS) has improved the efforts used to discover the lipid fingerprinting of some type of sample and in a certain condition in addition to find potential biomarkers, which allow for the understanding of lipids and their interaction with other molecules while considering different physiological conditions on a system-level scale [8].
The most widespread ionization source in MS is electrospray ionization (ESI), which enables creating and transferring ions from a broad range of molecules in solution directly to the gas phase in a gentle and efficient way, leading to the characterization of gaseous ions in a quick, sensitive and selective manner even for ions with high structural complexity [14, 15].
This study aimed to identify novel prospective candidate biomarkers in FF from patients presenting PCOS and HR during the superovulation IVF procedure. Moreover, we provide a lipid structural identification to better relate the potential markers with pathophysiological mechanisms related to PCOS and HR, leading to diagnosis improvement and individualized treatment for these patients in the future.
Materials and method
Study design
A prospective case-controlled study was conducted using FF from women treated at the UNIFESP Human Reproduction Section for IVF of São Paulo Federal University. This study received Institutional Review Board approval from the São Paulo Federal University Research Ethics Committee, and all patients participated in this study after signing an informed consent form.
Each patient was clinically and experimentally assessed to determine their infertility factor, whereas PCOS diagnoses were established according to Rotterdam criteria [3], which also guided the study direction. Therefore, patients diagnosed with PCOS primarily presented anovulation and clinical and/or biochemical hyperandrogenism or polycystic ovaries at ultrasound. The exclusion criteria for all groups were as follows: patients with a history of endometriosis, cancer, premature ovarian failure or other gynecologic factors leading to infertility that could affect the folliculogenesis or oocyte quality.
A total of 25 patients were included in this study. The control group was composed of women with a normal response to ovarian stimulation (5–15 oocytes) and the presence of only a tubal infertility factor. Control group patients received follicle-stimulating hormone (FSH) between 3 and 9 μg . mL−1 on the third day of the IVF cycle and achieve pregnancy after IVF treatment (n = 11). The PCOS group included women diagnosed with PCOS according to Rotterdam criteria (n = 7). The HR group included women without a PCOS diagnosis but who presented a hyper response (>15 oocytes) after gonadotropin administration and secondary characteristics of PCOS, such as higher LH levels and inversion of FSH/LH rates (n = 7).
For all patients, controlled ovarian stimulation was achieved using exogenous recombinant gonadotropins (225 IU/day of rFSH - Gonal-F, Merck-Serono, Darmstadt, Germany) starting on cycle day 2. When the leading follicle reached 13 mm in diameter, endogenous LH release was suppressed with a gonadotropin-releasing hormone (GnRH) antagonist analog (250 μg/day of cetrorelix acetate [Cetrotide®]; Merck-Serono, Darmstadt, Germany). When the leading follicle reached 17 mm in diameter, 250 mg hCG (Ovidrel®; Merck-Serono, Darmstadt, Germany) was administered. Ultrasound-guided transvaginal oocyte retrieval was performed 35 h after hCG administration using a 16-gauge needle. The oocytes were isolated from the follicular fluid for evaluation and culture.
The oocytes were incubated in culture medium (SSM Ivine Scientific—Santa Ana, CA) for three hours until the intracytoplasmic sperm injection procedure (ICSI) in which sperm was injected into the oocyte cytoplasm. The inseminated oocytes were further cultured, and after 18 h, fertilization was evaluated considering the presence of 2 polar bodies and 2 pronuclei. Embryo cleavage was observed 48 h after sperm injection. Seventy-two hours after the sperm injection, the quality of the embryos was rated, and embryos with better morphology scores were transferred to the patient uterus. For all patients, the beta subunit of human chorionic gonadotropin (βhCG) was measured 15 days after the transfer of embryos and for the patients with more than 25 mIU . mL−1 of βhCG, the implantation was assessed 15 days after by ultrasound observation.
Sample preparation
The FF collected from each patient was pooled, centrifuged for 10 min at 800 × g to separate the portion of interest from erythrocytes and leukocytes. The samples were stored at −20 °C until lipid extraction.
The Bligh-Dyer method was performed for FF lipid extraction [16]. Briefly, 50 μL FF, 50 μL water, 125 μL chloroform and 250 μL methanol were placed in a 1.5 mL microcentrifuge tube. The homogeneous mixture was vortexed for 30 s. The polar and apolar phases where separated by the addition of 100 μL water and 125 μL chloroform. The lower phase containing the lipids was recovered by pipetting and transferring to a clean microcentrifuge tube, which was maintained closed at −20 °C until analysis. For the ESI-MS experiment, samples were dissolved in 300 μL of methanol.
Electrospray mass spectrometry
The ESI-MS assay was performed in the positive mode using a hybrid Micromass mass spectrometer quadrupole time-of-flight (Q-TOF) (Manchester, UK) with high resolution with the following conditions: source temperature: 100 °C, desolvation temperature: 70–100 °C, capillary voltage ranging from 2.5 to 4 kV and cone voltage ranging from 10 to 40 V. The samples were injected with an infusion pump (Harvard Apparatus) at a flow rate of 10 μL min−1. The mass/charge range acquired was 700–1,200 m/z.
Statistical analysis and ions structural elucidation
Statistical data processing was based on the average ion intensity. Data are presented as the means ± standard deviation (SD). All data were normally distributed (P < 0.05, Kolmogorov- Smirnov’s test) after z score normalization. One-way measures analysis of variance (ANOVA) was used to perform comparisons among groups for continuous variables. Statistical significance was set at P < 0.05. LSD was applied for post hoc comparison. The incidence of categorical variables was compared using bootstrap resampling and the chi-square (x2) test using PASW 18.0 software (SPSS Chicago, IL).
MassLynx 4.1 software was used for mass spectra analysis (Waters, Manchester, UK), and data were pre-processed by MarkerLynx 4.1 software (Waters, Manchester, UK) to identify potential markers.
The intensity values were normalized by PARETO scaling. PCA and PLS-DA were applied to the dataset using MetaboAnalyst 2.0 software (http://www.metaboanalyst.ca). From these analyses, a list of potential biomarker ions was obtained, and the lipid categories for these ions were identified.
From the m/z values differentially expressed in each group, a fragmentation profile analysis was individually performed by the ESI-MS/MS experiment, which allowed the structural elucidation of differentially expressed ions through a CID experiment. The collision was performed using argon with the energy source ranging from 4 to 40 eV. In each case, the collision energy was optimized to produce extensive dissociation with minimal lost of the ion of interest. For ions whose intensities were too low to detect, it was not possible to identify the lipid through MS/MS. Thus, these lipids were identified using SimLipid software (PREMIER Biosoft International,CA, USA) adopting a mass tolerance of 0.1 Da with an accuracy of 50 ppm.
Results
Comparisons among the groups regarding clinical data demonstrated that PCOS patients present a lower age average; the LH and LH/FSH rate are significantly higher compared to the other groups. Regarding the number of follicles, oocytes and oocytes in metaphase II, the control group presented significantly lower averages for these variables (Table 1).
Table 1.
Clinical characteristics of patients from the control, PCOS and HR groups who underwent IVF treatment (ANOVA test)
| Control group mean; SD | PCOS group mean; SD | HR group mean; SD | p <0.05 | |
|---|---|---|---|---|
| Age | 34.9; 2.62b | 29.5; 4.07a | 34.0; 4.20b | 0.015 |
| BMI | 24.6; 2.74 | 25.5; 5.67 | 23.1; 1.10 | 0.592 |
| LH | 3.47; 1.96a | 6.5; 2.76b | 4.94; 2.19ab | 0.045 |
| FSH | 5.65; 2.36 | 5.12; 1.61 | 6.25; 2.40 | 0.637 |
| LF/FSH | 0.57; 0.34a | 1.34; 0.73b | 0.90; 0.56ab | 0.029 |
| Follicles (n) | 15,36; 8,74a | 34,0; 22,29b | 41,17; 22,47b | 0.004 |
| Oocytes (n) | 8.64; 4.05a | 14.29; 8.93b | 17.43; 7.50b | 0.033 |
| MII (n) | 6.27; 3.71a | 11.57; 9.69b | 16.14; 8.11b | 0.027 |
p < 0,05 values are indicated in bold. (n): total number. BMI body mass index, LH luteinizing hormone, FSH follicle-stimulating hormone, MII metaphasis II
ab different letters on the same line show the differences among the groups
Considering embryo quality, PCOS and HR groups differed from the control group and presented a higher percentage of embryo with good quality in the third day of culture, previously to the transfer (Control = 36.4 % PCOS and HR = 100.0 %). In relation to βhCG and Implantation rates, all patients from control group were pregnant after IVF. For all patients from PCOS group, the βhCG was negative 15 days after embryos transfer and for the HR group, one patient was pregnant after IVF treatment.
With regards to the PCOS-specific characteristics defined by Rotterdam criteria, anovulation was present in 6 of the patients, hyperandrogenism was present in 3 patients and the presence of polycystic ovaries upon ultrasound was observed in 5 patients.
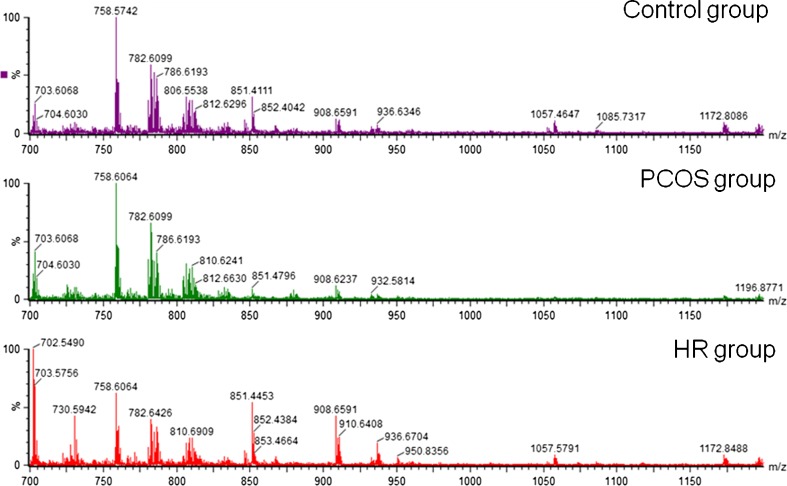
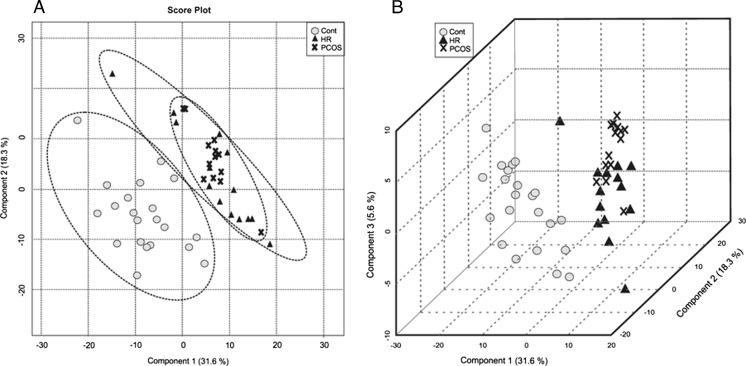
Representative mass spectra for each group are shown in Fig. 1. Initially, 368 ions were analyzed by principal component analysis (PCA), which explained the maximum variance of all ions. It should be noted that this analysis does not take into consideration the group divisions but rather the characteristics of each individual according to a score plot chart (Fig. 2a).
Fig. 1.
Representative ESI-MS data for the control, PCOS and HR groups. The y-axis shows relative abundances, whereas the x-axis shows m/z values.
Fig. 2.
a PCA 2D score plot: variance among groups according to Principal Component 1 without considering group division. b PLS-DA 3D shows the individual scores considering the groups division
The first principal component explained 53.5 % of the data variance, while the other lower level principal components were responsible for describing the remaining variation. The first 5 components explained 89.0 % of the variance in the data among the control, PCOS and HR groups.
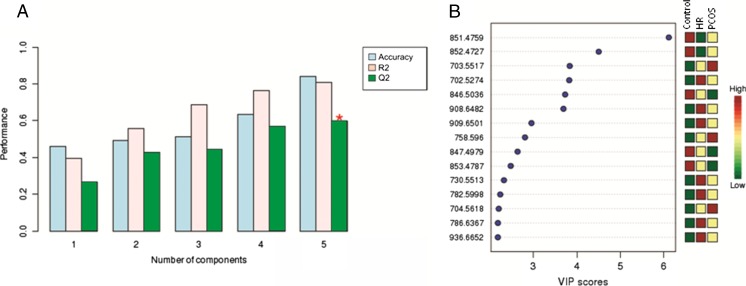
After PCA, partial least squares discriminant analysis (PLS-DA) was performed to determine the variance among the maximum number of ions associated with each group within each component (Fig. 2b). PLS-DA cross-validation reinforced the PCA results, indicating that only the first five components were considered for constructing the model, and component 5 was identified as the best indicator of the linear regression model prediction (Q2 = 59.0 %) (Fig. 3a).
Fig. 3.
a Cross validation chart. Blue bars: accuracy of the model; rose bars: R2 (variations); green bars: Q2 (prediction of the model). (*) Indicates a component with a higher power of projection for differentiation among the groups. b Ions with a higher contribution for the difference among the groups in component 5. The red color indicates a higher relative intensity in the respective group, while green indicates a lower relative intensity
Fifteen ions were selected considering their importance for the model prediction within component 5 (variable importance in the projection, VIP scores chart; Fig. 3b). These ions were chosen for an MS/MS experiment that makes possible the identification of lipid subclasses given their fragmentation pattern in a collision-induced dissociation (CID) (Supplementary data). After the MS/MS experiment, ions that could not be evaluated by MS/MS were identified with SimLipid software (PREMIER Biosoft International, CA, USA).
According to each lipid mass-to-charge ratio (m/z), five lipid classes were more represented in the control group, including phosphatidyletanolamine (PE, 846.5036 m/z), phosphatidylglycerol (PG, 847.4979 m/z), phosphatidylglycerol phosphate (PGP, 851.3683 m/z), phosphatidylserine (PS, 852.4727 m/z) and phosphatidylinositol (PI, 853.4787 m/z) (Table 2).
Table 2.
Ions identified by PLS-DA analysis in the groups
| Group | Mass (m/z) | Lipid Subclass | Formula | Adduct | Error (ppm) | Identification |
|---|---|---|---|---|---|---|
| control | 851.3683* | not identified | ||||
| 852.4727* | PS(18:3(6Z,9Z,12Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | C46H72NO10P | M + Na | 7,5691 | SimLipid | |
| PS(18:3(9Z,12Z,15Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | ||||||
| PS(20:4(5Z,8Z,11Z,14Z)/20:5(5Z,8Z,11Z,14Z,17Z)) | ||||||
| PS(20:5(5Z,8Z,11Z,14Z,17Z)/20:4(5Z,8Z,11Z,14Z)) | ||||||
| PS(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/18:3(6Z,9Z,12Z)) | ||||||
| PS(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/18:3(9Z,12Z,15Z)) | ||||||
| 846.5036* | PE(18:0/22:6(4Z,7Z,10Z,12E,16Z,19Z)(14OH)) | C45H78NO9P | M + K | 1,8048 | SimLipid | |
| 847.4979* | PG(17:2(9Z,12Z)/22:4(7Z,10Z,13Z,16Z)) | C45H77O10P | M + K | 10,3324 | SimLipid | |
| PG(19:1(9Z)/20:5(5Z,8Z,11Z,14Z,17Z)) | ||||||
| PG(20:5(5Z,8Z,11Z,14Z,17Z)/19:1(9Z)) | ||||||
| PG(22:4(7Z,10Z,13Z,16Z)/17:2(9Z,12Z)) | ||||||
| PG(22:6(4Z,7Z,10Z,13Z,16Z,19Z)/17:0) | ||||||
| PG(17:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | ||||||
| 853.4787* | PI(12:0/22:4(7Z,10Z,13Z,16Z)) | C43H75O13P | M + Na | 6,5584 | SimLipid | |
| PI(14:1(9Z)/20:3(8Z,11Z,14Z)) | ||||||
| PI(16:1(9Z)/18:3(6Z,9Z,12Z)) | ||||||
| PI(16:1(9Z)/18:3(9Z,12Z,15Z)) | ||||||
| PI(18:3(6Z,9Z,12Z)/16:1(9Z)) | ||||||
| PI(18:3(9Z,12Z,15Z)/16:1(9Z)) | ||||||
| PI(18:4(6Z,9Z,12Z,15Z)/16:0) | ||||||
| PI(20:3(8Z,11Z,14Z)/14:1(9Z)) | ||||||
| PI(20:4(5Z,8Z,11Z,14Z)/14:0) | ||||||
| PI(22:4(7Z,10Z,13Z,16Z)/12:0) | ||||||
| PI(17:2(9Z,12Z)/17:2(9Z,12Z)) | ||||||
| PI(16:0/18:4(6Z,9Z,12Z,15Z)) | ||||||
| PI(14:0/20:4(5Z,8Z,11Z,14Z)) | ||||||
| PCOS | 703.5517 | Sphingolipid | C39H79N2O6P | M + H | MS/MS | |
| 704.5618* | PC(O-16:0/15:1(9Z)) | C39H78NO7P | M + H | 3,3834 | SimLipid | |
| PC(P-16:0/15:0) | ||||||
| PC(P-18:0/13:0) | ||||||
| 758.4481a | PC | C40H72NO8P | M + CH3OH + H | MS/MS | ||
| HR | 702.5274 | PC | C38H72NO8P | M + H | MS/MS | |
| 909.6501 | PC | C52H94NO8P | M + NH4 | MS/MS | ||
| 782.5998 | PC | C44H80NO8P | M + H | MS/MS | ||
| 908.5482 | PC | C50H96NO8P | M + K | MS/MS | ||
| 730.5513a | PE | C38H68NO8P | M + CH3OH + H | MS/MS | ||
| 936.5073a | PC | C52H100NO8P | M + K | MS/MS | ||
| 786.6367* | PE(O-18:0/22:2(13Z,16Z)) | C45H88NO7P | M + H | 0,5313 | SimLipid | |
| PE(O-20:0/20:2(11Z,14Z)) | ||||||
| PE(P-18:0/22:1(11Z)) | ||||||
| PE(P-20:0/20:1(11Z)) |
* molecule identified by SimLipid, considering an error of mass less than 50 ppm
a molecule with mass changes due to the MS/MS experiment
PS phosphatidylserine, PE phosphatidyletanolamine, PG phosphatidylglycerol, PI phosphatidylinositol, PC phosphatidylcholines
For the PCOS group, 3 ions were identified. A sphingolipid (703.5517 m/z) and two PCs (704.5618, 758.4481) were overrepresented (Table 2).
Seven ions were identified in the HR group, including 5 PC ions (702.5274, 782.5998, 908.5482, 909.6501, and 936.5073 m/z) and two phosphatidyletanolamine (PE, 730.5513 and 786.6367 m/z) ions (Table 2).
Discussion
A certain degree of ovarian hyper stimulation is desirable during IVF cycles. However, an exaggerated response poses a life-threatening risk for the patient and may decrease oocyte quality, which adversely affects the assisted reproduction treatment [17]. Some women without a PCOS diagnosis have secondary characteristics of the syndrome and a hyper response when they undergo ovarian stimulation. Based on this fact, this study found potential lipid biomarkers related to PCOS and HR in patients who underwent IVF.
One of the factors related to HR is PCOS, which is a major cause of endocrine disorders in women with a prevalence ranging from 6 to 15 %, depending on the criteria used [18]. Previous studies have associated PCOS with an exacerbated growth in the number of follicles in the early stages of folliculogenesis [19] in addition to its features and metabolic implications, such as chronic anovulation, changes in LH levels, insulin resistance and obesity [20]. Considering all the aspects involved in this syndrome, the variability in its diagnosis may be related to its controversial etiology and heterogeneity in its phenotype [18].
Some studies have shown an increase in serum LH levels in PCOS patients [20, 21]. In this study, the LH level increase in the plasma was significant for the PCOS group. However, the Rotterdam consensus does not consider changes in serum LH levels as a factor for PCOS diagnosis, although the consensus suggests that more research needs to be conducted to further elucidate the clinical relevance of LH levels in this syndrome.
The numbers of follicles and oocytes increased in the PCOS and HR groups when compared with controls. A recent study made a comparison and suggested that the presence of polycystic ovaries upon ultrasound is not a condition that can be considered normal even when it is not associated with this syndrome [22]. In this study, both HR and PCOS patients had a larger number of follicles after hormonal stimulation than the control group, which appears to not be a physiological condition.
The PCOS and HR groups were scored with better embryo morphologic quality than the control group most likely because of the higher number of embryos. However, the PCOS and HR groups had low rates of implantation, which suggest that the embryo viability from these patients was compromised. Similar to this study, better morphological embryo quality was observed in women with PCOS compared with normal responders although both groups had shown equivalent implantation rates. Nonetheless, the rate of miscarriages was higher in the PCOS group even though it was not statistically significant [23].
Currently, one of the main objectives of MS-based lipidomic platforms is the characterization and elucidation of viable biomarkers for disease diagnosis and the elucidation of altered lipid pathways related to specific conditions [8]. Regarding the lipid content in cells, phospholipids comprise a fundamental subclass whose roles include the structure of the cell membrane and control of several cellular functions, such as enzymatic regulation, the process of transcription, and signal transduction and transport [24].
Phosphatidylcholines (PCs) represent the most important phospholipid subclass, are present in eukaryotic cells, and play an important role in the structure of membranes and cell signaling. PC has been related to changes in cell proliferation and differentiation conditions [25]. In contrast, changes in PC homeostasis may lead to the cell death process. The underlying mechanism in this process can be caused by defective enzymes involved in its path or by the increased consumption of PC [26]. In this study, different species of PC were found in the study group, which could be explained by the cell proliferation present in the exaggerated ovarian follicular activity in the PCOS and HR groups.
A previous study from our group described an increase in PC abundance in lipid profiles obtained from cumulus cells (CC) from pregnant patients undergoing IVF, which related this lipid subclass to the expansion of CC that occurs due to the induction of an LH surge by the administration of human chorionic gonadotropin (hCG) [12]. It is important to mention that lipids representation corresponds to a balance among the compared groups. Because the PCOS and HR groups had higher serum LH levels compared with the control group, our results suggest that LH may stimulate PC production. Thus, it can be hypothesized that in addition to the acute stimulation produced by the LH surge, elevated chronic LH levels induced the production of different types of PCs in all of the PCOS and HR groups.
The control group demonstrated the lipid phosphatidylglycerol (847.4979 m/z) was overrepresented. This lipid is synthesized from phosphatidylglycerol phosphate (PGP) through a dephosphorylation reaction with the enzyme phosphatidylglycerol phosphate phosphatase. Consumption and reduction of PGP with a consequent increase in PG is related to increased oxidative stress in animal tissues [27]. Thus, the overrepresentation of PG may be indirectly related to apoptosis. In another study from our group, it was possible to infer that PG may be related to the balance in the follicular microenvironment, protecting oocytes against damaging molecular processes and playing a role in ovarian functioning [28].
Phosphatidylserine with a 852.4727 m/z was more abundant in the control group. The lipids belonging to this subclass are associated with apoptosis [29]. It has been demonstrated that cells labeled to induce and signal cell death exhibit a redistribution of PS on their membranes during the initial stages of this process when PS is externalized. Moreover, the exposure of PS on the surface of activated platelets initiates the blood clotting cascade [30]. Despite the fact that our study evaluated only the presence of this lipid without considering its localization, it is suggested that a higher abundance of PS in the lipid profiles of the control group may be related to an appropriate response to the induction of the final follicle maturation and ovulation induced by hCG administration.
Phosphatidylinositol was overrepresented in the control group. PI molecules are considered important because of their role as a source of secondary messengers in signal transduction [31]. The synthesis of PI occurs from myo-inositol (MI) and diacylglycerol moieties, and the importance of this lipid is usually associated with its MI portion.
Hyperinsulinemia, which is often present in PCOS, is most likely related to hyperandrogenism, which occurs via two distinct mechanisms: a) androgen stimulation by the ovary and b) reduction in the liver secretion of the testosterone transporter. In addition, hyperinsulinemia plays a role in anovulation in PCOS due to an increase in ovarian androgen production, affecting follicular development. Myo-inositol has been related to changes in PCOS characteristics through its insulin-sensitizing effects. Supplementation of MI for women with PCOS demonstrated an improvement in hormonal parameters in these patients, and MI was related to the reestablishment of ovarian activity, considering spontaneous menstrual cyclicity, ovulation, better oocyte quality and fertility [32, 33].
The PCOS group was differentiated from the others by the presence of sphingolipid (703.5517 m/z). These lipids are important in signal transduction and cell recognition [34]. Changes in the metabolism of sphingolipids have been described in several biological conditions, including Alzheimer’s disease, cancer and insulin resistance [35–37].
Sphingolipids play multiple roles in the synthesis of steroid hormones, they participate in transcription, act as second messengers, and are involved in extracellular and nuclear regulation. Furthermore, these lipids are important for granulosa cell steroidogenesis, which is also associated with inflammatory cytokines and cellular growth factor production [38, 39].
At high levels, ceramides are responsible for changing the appropriate steroid hormone secretion. The exact molecular mechanism comprising the roles played by ceramides have not been well established, and many genes, transcription factors and signaling molecules are involved in this process. However, a recent study reported that down-regulation of acid ceramidase (N-acylsphingosine amidohydrolase - ASAH1) causes a shift in sphingolipid metabolism toward ceramide, sphingomielin, lactosylceramide and other lipid subspecies, concluding that ASAH1 is a mediator of steroidogenic gene expression and adrenocortical steroidogenesis [38, 40].
In this study, the presence of sphingolipid was not related to the hormone levels described. However, the presence of sphingolipids in this group infers that this lipid subclass can be considered a potential biomarker for PCOS. It was also observed that the HR group presented an intermediate frequency of sphingolipids, according to the VIP score graph (Fig. 3b). These data suggest that even if the lipids are not overrepresented in this group, there may be a potential evolution from hyper responder patients to patients diagnosed with polycystic ovary syndrome.
The phosphatidylethanolamine subclass was differentially represented in the control and HR groups. Considering the potential PE functions, it was found that PE tends to form a non-lamellar membrane structure and modulates the membrane curvature [41]. According to this finding, it was suggested that PE plays an important role in contractile ring disassembly at the cleavage furrow during cytokinesis in mammalian cells [42].
PE also comprises approximately 25 % of mammalian phospholipids and despite its wide presence in the brain, this lipid is particularly enriched in the inner membranes of mitochondria compared with other membranes [43]. Thus, this lipid subclass has recently been related to autophagy in a way that the PE from mitochondria is used for autophagosome formation [44].
The relationship between low PC intensity and high PE intensity in cumulus cells was described in follicular fluid, indicating that PE may be involved in cumulus cell apoptosis in non-pregnant women [12]. Regarding our study, the presence of PE in the control group may be related to physiological control of cell proliferation, which can also occurs via apoptosis. In contrast, with regards to the presence of PE in the HR group, two hypotheses can be suggested: the presence of these molecules may be involved in the final processes of cell division due to high proliferation in response to hormonal stimulation and an attempt to control high cell proliferation and oocyte development, which may be also in response to ovarian stimulation.
Despite the limited number of samples, strict criteria were used for statistical analysis because the groups were well defined to reduce possible biases in interpretation.
This study noted the lipid profiles of women with PCOS and a hyper response to gonadotropins, which were different from the lipid profiles found for normal responder patients who achieved pregnancy. Furthermore, the potential lipid markers found in follicular fluid, highlighted by the relative increase in PS and PGP in the control group and increase in PC in the PCOS and PE in HR groups, contributed to improving the understanding of the molecular mechanisms involved in polycystic ovary syndrome and the hyper response to ovarian stimulation in women without PCOS, since the study of these biomarkers have demonstrated the lipids are related to molecular processes in PCOS, such as apoptosis failure and intensive follicle proliferation during IVF, which may also be associated to OHSS risk.
Therefore, follicular fluid lipid profile analysis is an important tool for identifying a panel of potential biomarkers, because it reflects the ovarian microenvironment. Because of follicular fluid is an exudate of blood plasma, a quantitative validation of the potential biomarkers found in our study could be performed in this type of sample before the ovarian stimulation, which would assist in identify lipids related to the risk of hyper response to gonadotropins. Furthermore, the study of these biomarkers may support the development of more individualized treatments for these patients.
Electronic supplementary material
(DOC 264 kb)
Acknowledgments
This work was supported by CNPq (National Council for Scientific and Technological Development), Brazil and by grant 2012/06389-4, São Paulo Research Foundation (FAPESP).
Conflicts of interest
All authors declare that there are no conflicts of interest.
Footnotes
Capsule Follicular fluid lipid profile by ESI/MS.
References
- 1.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;24:1223–36. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 2.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 3.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 4.Vieira RC, Barcelos ID, Ferreira EM, de Araújo MC, dos Reis RM, Ferriani RA, et al. Evaluation of meiotic abnormalities of oocytes from policystic ovary syndrome patients submitted to ovarian stimulation. Rev Bras Ginecol Obstet. 2008;30:241–7. doi: 10.1590/S0100-29452008000100044. [DOI] [PubMed] [Google Scholar]
- 5.MacDougall MJ, Tan SL, Balen A, Jacobs HS. A controlled study comparing patients with and without polycystic ovaries undergoing in-vitro fertilization. Hum Reprod. 1993;8:233–7. doi: 10.1093/oxfordjournals.humrep.a138029. [DOI] [PubMed] [Google Scholar]
- 6.Fortune JE. Ovarian follicular growth and development in mammals. Biol Reprod. 1994;50:225–32. doi: 10.1095/biolreprod50.2.225. [DOI] [PubMed] [Google Scholar]
- 7.Leroy JL, Vanholder T, Delanghe JR, Opsomer G, Van Soom A, Bols PE, et al. Metabolic changes in follicular fluid of the dominant follicle in high-yielding dairy cows early post partum. Theriog. 2004;15:1131–43. doi: 10.1016/j.theriogenology.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Lam SM, Shui G. Lipidomics as a principal tool for advancing biomedical research. J Genet Genomics. 2013;20:375–90. doi: 10.1016/j.jgg.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Zehethofer N, Pinto DM. Recent developments in tandem mass spectrometry for lipidomic analysis. Anal Chim Acta. 2008;3:62–70. doi: 10.1016/j.aca.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 10.Roberts LD, McCombie G, Titman CM, Griffin JL. A matter of fat: an introduction to lipidomic profiling methods. J Chromatogr B Anal Technol Biomed Life Sci. 2008;15:174–81. doi: 10.1016/j.jchromb.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Lucki NC, Sewer MB. Multiple roles for sphingolipids in steroid hormone biosynthesis. Subcell Biochem. 2008;49:387–412. doi: 10.1007/978-1-4020-8831-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montani DA, Cordeiro FB, Regiani T, Victorino AB, Pilau EJ, Gozzo FC, et al. The follicular microenviroment as a predictor of pregnancy: MALDI-TOF MS lipid profile in cumulus cells. J Assist Reprod Genet. 2012;29:1289–97. doi: 10.1007/s10815-012-9859-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaaker M, Rahimipour A, Nouri M, Khanaki K, Darabi M, Farzadi L, et al. Fatty acid composition of human follicular fluid phospholipids and fertilization rate in assisted reproductive techniques. Iran Biomed J. 2012;16:162–8. doi: 10.6091/ibj.1081.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989;6:64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki Y, Nakano Y, Mochizuki K, Nomoto M, Takahashi Y, Ito R, et al. A new strategy for ionization enhancement by derivatization for mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2011;15:1159–65. doi: 10.1016/j.jchromb.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 17.Nastri CO, Ferriani RA, Rocha IA, Martins WP. Ovarian hyperstimulation syndrome: pathophysiology and prevention. J Assist Reprod Genet. 2010;27:121–8. doi: 10.1007/s10815-010-9387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belosi C, Selvaggi L, Apa R, Guido M, Romualdi D, Fulghesu AM, et al. Is the PCOS diagnosis solved by ESHRE/ASRM 2003 consensus or could it include ultrasound examination of the ovarian stroma? Hum Reprod. 2006;21:3108–15. doi: 10.1093/humrep/del306. [DOI] [PubMed] [Google Scholar]
- 19.Webber LJ, Stubbs S, Stark J, Trew GH, Margara R, Hardy K, et al. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;27:1017–21. doi: 10.1016/S0140-6736(03)14410-8. [DOI] [PubMed] [Google Scholar]
- 20.Fauser BC, Pache TD, Lamberts SW, Hop WC, de Jong FH, Dahl KD. Serum bioactive and immunoreactive luteinizing hormone and follicle-stimulating hormone levels in women with cycle abnormalities, with or without polycystic ovarian disease. J Clin Endocrinol Metab. 1991;73:811–7. doi: 10.1210/jcem-73-4-811. [DOI] [PubMed] [Google Scholar]
- 21.Taylor AE, McCourt B, Martin KA, Anderson EJ, Adams JM, Schoenfeld D, et al. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1997;82:2248–56. doi: 10.1210/jcem.82.7.4105. [DOI] [PubMed] [Google Scholar]
- 22.Catteau-Jonard S, Bancquart J, Poncelet E, Lefebvre-Maunoury C, Robin G, Dewailly D. Polycystic ovaries at ultrasound: normal variant or silent polycystic ovary syndrome? Ultrasound Obstet Gynecol. 2012;40:223–9. doi: 10.1002/uog.11202. [DOI] [PubMed] [Google Scholar]
- 23.Kdous M, Chaker A, Zhioua A, Zhioua F. Oocyte and embryo quality and outcome of ICSI cycles in patients with polycystic ovary syndrome (PCOS) versus normo-ovulatory. J Gynecol Obstet Biol Reprod. 2009;38:133–43. doi: 10.1016/j.jgyn.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Lykidis A. Comparative genomics and evolution of eukaryotic phospholipid biosynthesis. Prog Lipid Res. 2007;46:171–99. doi: 10.1016/j.plipres.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Verhoven B, Schlegel RA, Williamson P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med. 1995;1:1597–601. doi: 10.1084/jem.182.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui Z, Houweling M. Phosphatidylcholine and cell death. Biochim Biophys Acta. 2002;30:87–96. doi: 10.1016/S1388-1981(02)00328-1. [DOI] [PubMed] [Google Scholar]
- 27.Kawasaki K, Kuge O, Chang SC, Heacock PN, Rho M, Suzuki K, et al. Isolation of a chinese hamster ovary (CHO) cDNA encoding phosphatidylglycerophosphate (PGP) synthase, expression of which corrects the mitochondrial abnormalities of a PGP synthase-defective mutant of CHO-K1 cells. J Biol Chem. 1999;15:1828–34. doi: 10.1074/jbc.274.3.1828. [DOI] [PubMed] [Google Scholar]
- 28.Cataldi T, Cordeiro FB, Costa L do V, Pilau EJ, Ferreira CR, Gozzo FC, Eberlin MN, Bertolla RP, Cedenho AP, Turco EG. Lipid profiling of follicular fluid from women undergoing IVF: young poor ovarian responders versus normal responders. Hum Fertil. 2013; 16:269–77. [DOI] [PubMed]
- 29.Balasubramanian K, Mirnikjoo B, Schroit AJ. Regulated externalization of phosphatidylserine at the cell surface: implications for apoptosis. J Biol Chem. 2007;282:18357–64. doi: 10.1074/jbc.M700202200. [DOI] [PubMed] [Google Scholar]
- 30.Schroit AJ, Zwaal RF. Transbilayer movement of phospholipids in red cell and platelet membranes. Biochim Biophys Acta. 1991;13:313–29. doi: 10.1016/0304-4157(91)90019-S. [DOI] [PubMed] [Google Scholar]
- 31.Balla T. Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol Rev. 2013;93:1019–137. doi: 10.1152/physrev.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Croze ML, Soulage CO. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie. 2013;95:811–27. doi: 10.1016/j.biochi.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Ciotta L, Stracquadanio M, Pagano I, Carbonaro A, Palumbo M, Gulino F. Effects of myo-inositol supplementation on oocyte’s quality in PCOS patients: a double blind trial. Eur Rev Med Pharmacol Sci. 2011;15:509–14. [PubMed] [Google Scholar]
- 34.Bou Khalil M, Hou W, Zhou H, Elisma F, Swayne LA, Blanchard AP, et al. Lipidomics era: accomplishments and challenges. Mass Spectrom Rev. 2010;29:877–929. doi: 10.1002/mas.20294. [DOI] [PubMed] [Google Scholar]
- 35.van Echten-Deckert G, Walter J. Sphingolipids: critical players in Alzheimer’s disease. Prog Lipid Res. 2012;51:378–93. doi: 10.1016/j.plipres.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J. 2012;279:2610–23. doi: 10.1111/j.1742-4658.2012.08644.x. [DOI] [PubMed] [Google Scholar]
- 37.Straczkowski M, Kowalska I. The role of skeletal muscle sphingolipids in the development of insulin resistance. Rev Diabet Stud. 2008;5:13–24. doi: 10.1900/RDS.2008.5.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucki NC, Sewer MB. The interplay between bioactive sphingolipids and steroid hormones. Steroids. 2010;75:390–9. doi: 10.1016/j.steroids.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santana P, Llanes L, Hernandez I, Gonzalez-Robayna I, Tabraue C, Gonzalez-Reyes J, et al. Interleukin-1 beta stimulates sphingomyelin hydrolysis in cultured granulose cells: evidence for a regulatory role of ceramide on progesterone and prostaglandin biosynthesis. Endocrinol. 1996;137:2480–9. doi: 10.1210/endo.137.6.8641202. [DOI] [PubMed] [Google Scholar]
- 40.Lucki NC, Li D, Bandyopadhyay S, Wang E, Merrill AH, Sewer MB. Acid ceramidase (ASAH1) represses steroidogenic factor 1-dependent gene transcription in H295R human adrenocortical cells by binding to the receptor. Mol Cell Biol. 2012;32:4419–31. doi: 10.1128/MCB.00378-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–56. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Emoto K, Umeda M. An essential role for a membrane lipid in cytokinesis. Regulation of contractile ring disassembly by redistribution of phosphatidylethanolamine. T J Cell Biol. 2000;12:1215–24. doi: 10.1083/jcb.149.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steenbergen R, Nanowski TS, Beigneux A, Kulinski A, Young SG, Vance JE. Disruption of the phosphatidylserine decarboxylase gene in mice causes embryonic lethality and mitochondrial defects. J Biol Chem. 2005;2:40032–40. doi: 10.1074/jbc.M506510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, Kim PK, et al. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell. 2010;14:656–67. doi: 10.1016/j.cell.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 264 kb)