Abstract
Purpose
The purpose of this study was to carry out a meta-analysis for a comprehensive understanding and estimation of the association between sperm DNA Fragmentation Index (DFI) and pregnancy outcome after in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) treatment.
Methods
Studies concerning the link of DFI with pregnancy outcome were included after literature search of database PUBMED, EMBASE, MEDLINE. Related information was extracted from the eligible studies by two independent authors and a meta-analysis was conducted by using STATA 12.0 software. Pregnancy outcomes consisted of biochemical pregnancy (BP), clinical pregnancy (CP) and pregnancy loss (PL). The studies included for meta-analysis were divided into three groups according to the DFI threshold value (DFI >27 %, 15–27 %, ≤15 % group). The odds ratio (OR ) and their 95 % confidence intervals (95 % CIs) were used to evaluate the association between DFI and pregnancy outcome.
Results
Twenty articles were included in our meta-analysis. The results indicated that infertile couples were more likely to get pregnant if DFI was less than threshold value (For threshold value > 27 % and 15–27 % group, combined overall OR (95 % CI) = 1.437 (1.186–1.742), 1.639 (1.093–2.459) respectively). However, when stratified by DFI detection methods, using sperm chromatin structure assay (SCSA) as the DFI test method, the results indicated a similar CP rate between groups with a high DFI or a lower DFI value (SCSA, For threshold value >27 % and 15–27 % group, combined overall OR (95 % CI) = 1.242(0.978–1.577), 1.480(0.921–2.377) respectively). The meta-analysis based on BP (overall OR (95 % CI) = 0.952 (0.697–1.302)) and PL((For DFI >27 %, 15–27 %, ≤15 % group, OR (95 % CI) = 0.786 (0.491–1.258), 1.509 (0.655–3.476), (0.538 (0.264–1.097) respectively) outcome yielded nonsignificant results.
Conclusions
The predication value of DFI for IVF or ICSI outcome is not confirmed in our meta-analysis. Further better designed studies with larger subjects involved are needed to better address this issue.
Keywords: DNA fragmentation index, Pregnancy, Meta-analysis, In vitro fertilization, Intracytoplasmic sperm injection
Introduction
Over the past decades, an increasing number of infertile couples seek medical assistance by assisted reproductive technology (ART) [1]. However, the pregnancy outcome after ART procedures was unpredictable because several possible factors were involved in the process. Moreover, routine semen parameters, like semen concentration, motility and the percentage of normal sperm morphology were not sufficient to predict pregnancy outcome after ART procedures.
DFI, known as sperm DNA fragmentation index, was established to evaluate sperm chromatin integrity, and has gained increasing application for its diagnostic capabilities of male fertility potential and pregnancy outcome [2, 3].
Several clinical studies have been conducted to assess the association between the sperm DNA integrity and fertility after in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) treatment. Some studies have established different threshold levels with regard to the prediction of fertility and pregnancy outcomes. Notably, two meta-analyses by Evenson [4] and Collins [5] have appeared. However, over the past several years, a number of substantial newly published studies concerning the association between DFI and pregnancy outcome after fertilization were performed. Therefore, we conducted a new meta-analysis with more studies included to provide a more precise and comprehensive estimation on the link between DFI and pregnancy outcome and determined to establish a DFI threshold value for a more precise diagnostic and prognostic estimation of pregnancy outcome.
Materials and methods
Literature search
We conducted electronic searches in the database PUBMED, EMBASE, MEDLINE up to June 1, 2014, using the MeSH terms “DNA fragmentation index,” “in vitro fertilization,” “intracytoplasmic sperm injection,” “pregnancy”.
Inclusion criteria
Study design. Clinical trials evaluating the effect of DFI or sperm DNA damage on IVF or ICSI outcome were included in our meta-analysis.
Type of participants. Couples who attended the fertility clinic for ART (IVF or ICSI) program.
Type of outcome measures. Biochemical pregnancy (BP) was defined as a plasma β-HCG level of >10 IU/L 12 days after embryo transfer. Clinical pregnancy (CP) was defined as ultrasound detection of fetal heartbeat 3 weeks after embryo transfer. Pregnancy loss (PL) was defined as a spontaneous abortion before 12 weeks after fertilization.
Evidence quality assessment
Two reviewers (Zheng Zhang, Leilei Zhu) independently used the Newcastle-Ottawa Scale (NOS) to assess the quality of the included studies [6]. In the 9-score system, studies with an overall quality score of more than 5 were considered high-quality.
Data extraction
Two investigators (Zhang Zheng, Zhu Lei-lei) independently assessed articles for possible enrollment and extracted related information: study characteristics, the first author,s name, year of publication, DFI cutoff value, methods to evaluate sperm DNA damage (terminal deoxynucleotidyl transferase-mediated dUDP nick-end labeling (TUNEL), sperm chromatin structure assay (SCSA), neutral comet assay (comet), acridine orange test (AOT)), number of people underwent CP, BP, PL in cases and controls respectively. Any discrepancy was consulted by discussion between the two investigators.
Statistical analysis
This systematic review and meta-analysis was performed using the STATA software (version 12.0; Stata corporation, College Station, TX, USA). Heterogeneity among the articles was measured using Q test or I2 value. The heterogeneity was considered significant If P < 0.1 or I2 > 40 %. The random effect model was adopted if there was heterogeneity among the studies. Otherwise we selected the fixed-effects model. Odds Ratio (OR) and their corresponding 95 % confidence intervals (95 % CIs) were calculated for outcomes. For a comparison of DFI value in pregnant group versus non-pregnant group, we used standardized mean difference (SMD) and their 95 % confidence intervals (95 % CIs) accordingly for outcomes. Publication bias was estimated using Begg,s funnel plots and Egger,s test. A value of “Pr > |z|” above 0.05 for Begg,s funnel plots or a value of “P > |t|” above 0.05 Egger,s test was considered negative publication bias.
Subgroup analysis
To elucidate the relationship between DFI and pregnancy outcome, we divided the included studies into several groups according to DFI cutoff values or threshold levels (>27 %, 15–27 %, ≤15 %). Subgroup analysis was performed by the type of fertilization (IVF or ICSI) and DFI detection methods (SCSA, Tunel, comet and AOT) . The related data was pooled together within each subgroup. Outcome measures included BP, CP and PL.
Results
Twenty studies [7–26] were indentified for our meta-analysis. The flow chart of the process for the identification of the studies were shown in Fig. 1. The characteristics of the selected studies were summarized in Table 1.
Fig. 1.
the flow gram of the identification and selection of the studies
Table 1.
Related information of the selected studies
| Study(publication year) | Country or region | DFI% cutoff value | DFI assay | Type of ART | Outcome measurement | Quality score |
|---|---|---|---|---|---|---|
| Benchaib(2007) | France | 15 | Tunel | IVF ICSI | CP PL | 7 |
| Boe-Hansen(2006) | Denmark | 27 | SCSA | IVF ICSI | BP CP | 7 |
| Bungum(2004) | Denmark | 27 | SCSA | IVF ICSI | BP CP | 9 |
| Bungum(2007) | Denmark | 30 | SCSA | IUI IVF ICSI | BP CP PL | 9 |
| Bungum (2008) | Denmark | – | SCSA | IVF ICSI | CP | 9 |
| Chi(2011) | Korea | 14 | comet | IVF ICSI | CP PL | 8 |
| Gandini(2004) | Italy | – | SCSA | IVF ICSI | CP | 8 |
| Kennedy(2010) | USA | – | SCSA | IVF ICSI | CP | 6 |
| Lin(2008) | China | 9 27 |
SCSA | IVF ICSI | CP PL | 9 |
| Micinski(2008) | East eur | 15 | SCSA | ICSI | CP | 8 |
| Niu(2011) | China | 27 | SCSA | IVF | BP CP PL | 8 |
| Payne(2005) | USA | – | SCSA | IVF ICSI | CP | 9 |
| Henkel(2003) | Germany | 36.5 24.3 |
Tunnel AOT | IVF ICSI | CP | 6 |
| Henkel(2004) | Germany | 36.5 12 |
Tunnel AOT | IVF | CP | 6 |
| Zhang(2008) | China | 10 | AOT | IVF | CP | 5 |
| Zini(2005) | Canada | 15 30 | SCSA | ICSI | CP PL | 8 |
| Dar(2013) | Canada | 15 50 | SCSA | ICSI | CP | 8 |
| Speyer(2010) | UK | 19 30 | SCSA | IVF ICSI | CP PL | 9 |
| Jiang(2011) | China | 30 | SCSA | IVF ICSI | CP | 8 |
| Yang(2013) | China | 25 | SCSA | ICSI | BP CP PL | 9 |
The results of meta-analysis
DFI and CP
A total of 15 studies with CP as the outcome measurement was included in the meta-analysis [7, 8, 10, 12, 15–17, 19–26]. These studies were divided into 3 groups according to the DFI cutoff value (>27 %, 15–27 %, ≤15 %) as described previously. Within each group, subgroup analysis was performed by the type of fertilization (IVF or ICSI) and DFI detection methods.
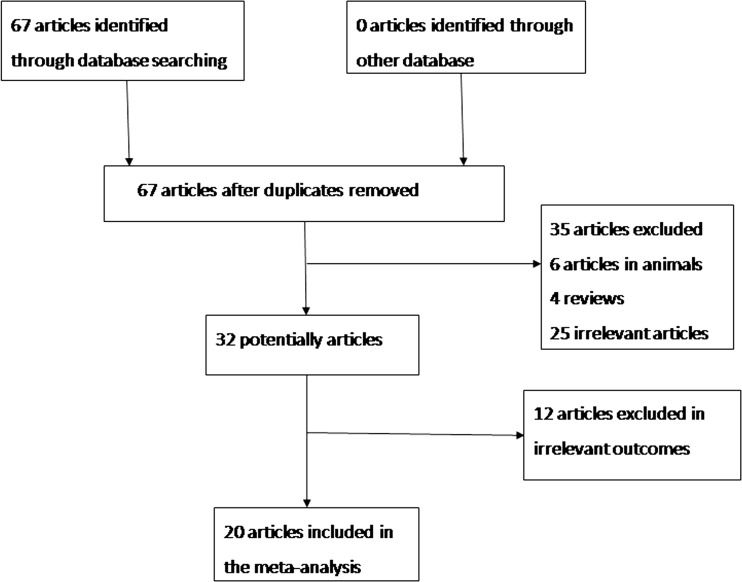
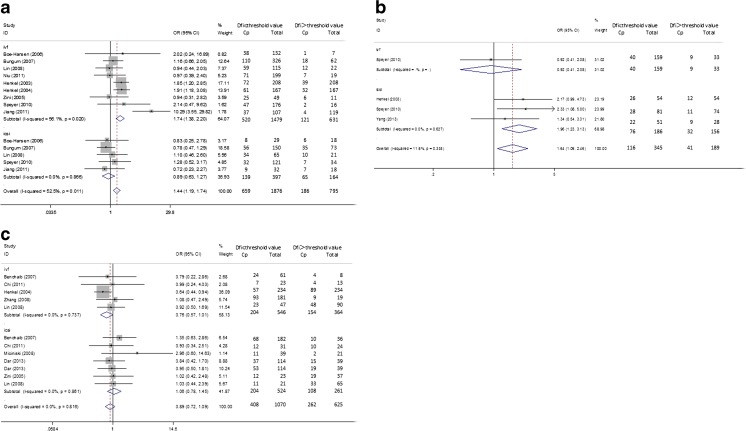
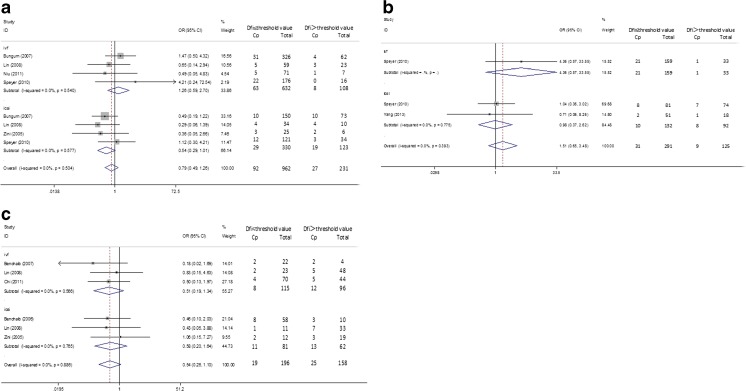
When the studies with a DFI cutoff value >27 % were pooled into the meta-analysis [8, 10, 15, 17, 19, 20, 22, 24, 25], the pooled OR demonstrated that the couples were more likely to achieve CP if the DFI was <27 % (OR (95%CI) = 1.437 (1.186–1.742), p = 0.000). As for the heterogeneity test, the I2 value between the studies was 52.5 %, indicated a moderate heterogeneity. Subgroup analysis was conducted by the type of fertilization (IVF or ICSI). When IVF was the type of fertilization [8, 10, 15, 17, 19, 20, 22, 24, 25], the pooled OR indicated a similar result (OR (95%CI) = 1.742 (1.382–2.195), p = 0.000). The I2 of the included studies was 56.1 %. But the result was not statistically significant (OR (95%CI) = 0.895 (0.629–1.273), p = 0.537) in the ICSI subgroup [8, 10, 15, 24, 25] with no heterogeneity existed (I squared = 0 % ). Detailed results were shown in Fig. 2a and Table 2. Separate analysis by detection methods (SCSA and Tunel) revealed a higher chance to get CP if DFI < 27 % in Tunel subgroup (OR (95 % CI) = 1.87 (1.36–2.58), p = 0.000), while DFI was not associated with CP in SCSA subgroup (OR (95 % CI) = 1.24(0.98–1.58), p = 0.076) (see Fig. 3a and Table 2).
Fig. 2.
Forest plots for the overall and subgroup association (IVF or ICSI) of CP and DFI. a DFI threshold level > 27 % group; b DFI threshold level 15–27 % group c DFI ≤ 15 % group
Table 2.
Main results of the meta-analysis
| Group | Subgroup | I2 (%) | OR(95%CI) | P value | begg(Pr > |z|) | Egger(P > |t|) | |
|---|---|---|---|---|---|---|---|
| CP | dfi(>27 %) | ivf | 56.1 | 1.742(1.382–2.195) | 0 | 0.913 | 0.974 |
| icsi | 0 | 0.895(0.629–1.273) | 0.537 | ||||
| SCSA | 49.0 | 1.242(0.978–1.577) | 0.076 | ||||
| Tunel | 0 | 1.873(1.358–2.584) | 0.000 | ||||
| overall | 52.5 | 1.437(1.186–1.742) | 0 | ||||
| dfi(15–27 %) | ivf | – | 0.922(0.409–2.083) | 0.846 | 0.308 | 0.427 | |
| icsi | 0 | 1.961(1.230–3.127) | 0.005 | ||||
| SCSA | 25.3 | 1.480(0.921–2.377) | 0.105 | ||||
| AOT | – | 2.167(0.992–4.732) | 0.052 | ||||
| overall | 11.6 | 1.639(1.093–2.459) | 0.017 | ||||
| dfi(≤15 %) | ivf | 0 | 0.759(0.571–1.008) | 0.057 | 0.451 | 0.006 | |
| icsi | 0 | 1.062(0.780–1.446) | 0.703 | ||||
| Tunel | 0 | 1.183(0.619–2.261) | 0.611 | ||||
| comet | 0 | 0.949(0.422–2.135) | 0.889 | ||||
| AOT | 21.7 | 0.701(0.498–0.987) | 0.042 | ||||
| SCSA | 0 | 0.993(0.728–1.354) | 0.963 | ||||
| overall | 0 | 0.886(0.719–1.090) | 0.252 | ||||
| BP | dfi(>27 %) | ivf | 0 | 1.178(0.752–1.845) | 0.473 | 0.462 | 0.28 |
| icsi | 0 | 0.766(0.491–1.194) | 0.239 | ||||
| SCSA | 0 | 0.952(0.697–1.302) | 0.759 | ||||
| overall | 0 | 0.952(0.697–1.302) | 0.759 | ||||
| dfi(15–27 %) | icsi | – | 1.318(0.552–3.145) | 0.534 | – | – | |
| PL | dfi(>27 %) | ivf | 0 | 1.262(0.589–2.704) | 0.549 | 1 | 0.866 |
| icsi | 0 | 0.542(0.290–1.013) | 0.055 | ||||
| SCSA | 0 | 0.786(0.491–1.258) | 0.316 | ||||
| overall | 0 | 0.786(0.491–1.258) | 0.316 | ||||
| dfi(15–27 %) | ivf | – | 4.358(0.566–33.547) | 0.157 | 1 | 0.775 | |
| icsi | 0 | 0.985(0.370–2.619) | 0.976 | ||||
| SCSA | 0 | 1.509(0.655–3.476) | 0.334 | ||||
| overall | 0 | 1.509(0.655–3.476) | 0.334 | ||||
| dfi(≤15 %) | ivf | 0 | 0.506(0.191–1.341) | 0.171 | 0.707 | 0.665 | |
| icsi | 0 | 0.577(0.203–1.641) | 0.303 | ||||
| Tunel | 0 | 0.349(0.103–1.175) | 0.089 | ||||
| SCSA | 0 | 0.739(0.248–2.195) | 0.586 | ||||
| comet | – | 0.503(0.128–1.975) | 0.325 | ||||
| overall | 0 | 0.538(0.264–1.097) | 0.088 | ||||
| Pregnant group vs not pregnant group | – | SMD(95%CI) | |||||
| ivf | 79.4 | 0.502(−0.356–1.359) | 0.251 | 0.764 | 0.17 | ||
| icsi | 87.2 | 0.259(−0.421–0.939) | 0.455 | ||||
| SCSA | 83.5 | 0.345(−0.110–0.799) | 0.137 | ||||
| overall | 83.5 | 0.345(−0.110–0.799) | 0.137 |
Fig. 3.
Forest plots for the overall and subgroup association (DFI test methods) of CP and DFI. a DFI threshold level > 27 % group; b DFI threshold level 15–27 % group c DFI ≤ 15 % group
Accordingly, when we enrolled the studies with a DFI cutoff value 15–27 % [19, 24, 26], the overall effect indicated a higher possibility to get a pregnancy when DFI was less than the cutoff value (15–27 %) (OR (95%CI) = 1.639 (1.093–2.459), p = 0.017). The result of ICSI subgroup [19, 24, 26] was similar (OR (95%CI) = 1.961 (1.230–3.127), p = 0.005), while it was not for the IVF subgroup with only one article selected [24] for the analysis (OR (95%CI) = 0.922 (0.409–2.083), p = 0.846). No heterogeneity was detected between the enrolled studies within each subgroup analyzed (I squared ranged from 0 to 11.6 %). Results were presented in Fig. 2b and Table 2. Moreover, when stratified by DFI detection methods (SCSA and AOT), the results indicated that there was no significant association with CP and DFI (SCSA, OR(95 % CI) = 1.480(0.921–2.377), p = 0.105; AOT, OR(95 % CI) = 2.167(0.992–4.732), p = 0.052) (Fig. 3b and Table 2).
As for the articles included if the DFI threshold value was less than 15 % [7, 12, 15, 16, 20–23], the pooled OR of all studies (OR (95%CI) = 0.886 (0.719–1.090), p = 0.252) and IVF subgroup (OR (95%CI) = 0.759 (0.571–1.008), p = 0.057) [7, 12, 20, 15, 21] and ICSI (OR (95%CI) = 1.062 (0.780–1.446), p = 0.703) subgroup [7, 12, 15, 16, 22, 23] showed that DFI was not associated with CP. We found no heterogeneity existed with I squared = 0 % in the overall and IVF and ICSI subgroup (see Fig. 2c and Table 2). Subgroup analysis stratified by DEI test methods (SCSA, AOT, Tunel and comet) showed similar results (Tunel, OR(95 % CI) = 1.183(0.619–2.261)p = 0.611; comet, OR(95 % CI) = 0.949(0.422–2.135)p = 0.889; AOT, OR(95 % CI) = 0.701(0.498–0.987)p = 0.042; SCSA, OR(95 % CI) = 0.993(0.728–1.354)p = 0.963) (Fig. 3c and Table 2).
DFI and BP
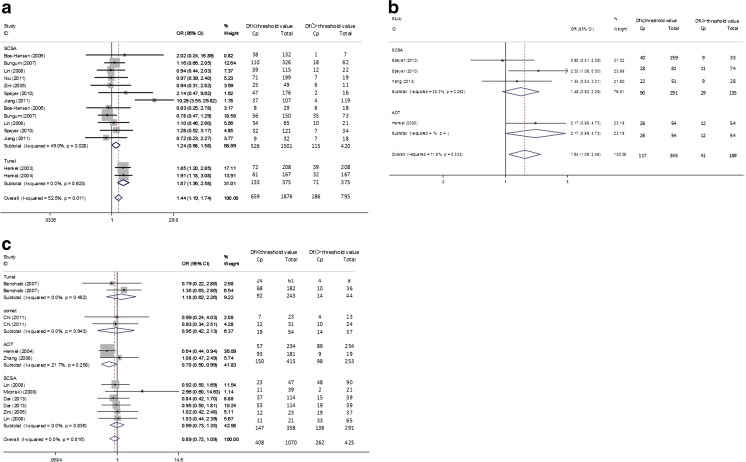
We enrolled 4 studies that evaluated the association between DFI and BP [8, 10, 17, 26].. Three studies with a DFI cutoff value >27 % were included in the meta-analysis [8, 10, 17]. The results indicated that there was no significant difference both for the overall effect (OR (95%CI) = 0.952 (0.697–1.302), p = 0.759) and IVF (OR (95%CI) = 1.178 (0.752–1.845), p = 0.473) and ICSI (OR (95%CI) = 0.766 (0.491–1.194), p = 0.239) subgroup. Only one article [26] had a DFI cutoff value between 15 to 27 % and was analyzed. The result showed a similar trend (OR (95%CI) = 1.318 (0.552–3.145), p = 0.534). The overall and subgroup heterogeneity was not found (I squared = 0 %). The results of the meta-analysis were presented in Fig. 4 and Table 2. The DFI test method adopted in the enrolled 5 studies was SCSA, and the result of the meta-analysis was not significant (DFI > 27 %, SCSA, OR(95 % CI) = 0.952(0.697–1.302) p = 0.759) (See Table 2).
Fig. 4.
Forest plots for the overall and subgroup association(IVF or ICSI) of BP and DFI
DFI and PL
A total of eight studies evaluated the association between DFI and PL were included [7, 10, 12, 15, 17, 22, 24, 26]. As for the results of the meta-analysis of the studies with a DFI cutoff value above 27 % [10, 15, 17, 22, 24], the overall effect (OR (95%CI) = 0.786 (0.491–1.258), p = 0.326) and IVF (OR (95%CI) = 1.262 (0.589–2.704), p = 0.549) and ICSI (OR (95%CI) = 0.542 (0.290–1.013), p = 0.055) group yielded nonsignificant results (Fig. 5a). The similar trend was found in the meta-analysis that enrolled the articles with a DFI cutoff value 15 to 27 % [24, 26] (Fig. 5b) (overall (OR (95%CI) = 1.509 (0.655–3.476), p = 0.334), IVF (OR (95%CI) = 4.358 (0.566–33.547), p = 0.157), ICSI (OR (95%CI) = 0.542 (0.290–1.013), p = 0.976)) or a DFI cutoff value less than 15 % [7, 12, 15, 22] (Fig. 5c) (overall (OR (95%CI) = 0.538(0.264–1.097), p = 0.088), IVF (OR (95%CI) = 0.506 (0.191–1.341), p = 0.171), ICSI (OR (95%CI) = 0.577 (0.203–1.641), p = 0.303). No heterogeneity was found within subgroup and overall analysis between the studies. The details of the results of the meta-analysis were shown in Fig. 5 and Table 2. By contrast, subgroup analysis by DFI detection methods produced nonsignificant associations between DFI and PL for all the groups (DFI >27 %, SCSA, OR (95%CI) = 0.786 (0.492–1.258), p = 0.316; DFI 15–27 %, SCSA, OR (95%CI) = 1.509(0.655–3.476), p = 0.334; DFI ≤ 15 % group, SCSA, OR (95%CI) = 0.739(0.248–2.195) p = 0.586, Tunel OR (95%CI) = 0.349(0.103–1.175)p = 0.089, COMET OR (95%CI) = 0.503(0.128–1.975) p = 0.325) (Table 2).
Fig. 5.
Forest plots for the overall and subgroup association(IVF or ICSI) of PL and DFI. a DFI threshold level > 27 % group; b DFI threshold level 15–27 % group c DFI ≤ 15 % group
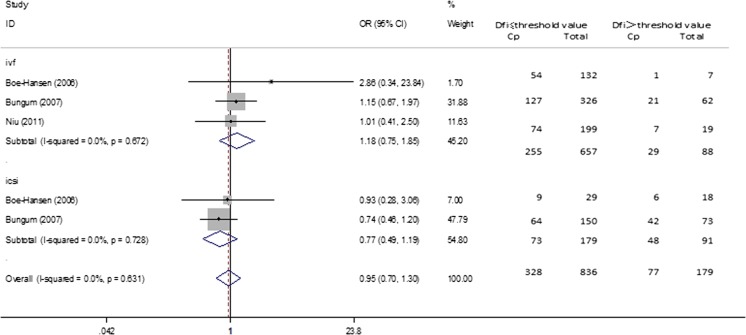
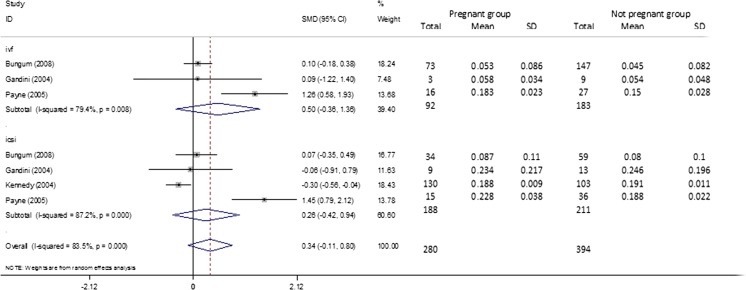
Pregnant group versus non-pregnant group
Four studies [11, 13, 14, 18], compared the DFI value between the pregnancy group with not pregnancy group and were included in the meta-analysis. Subgroup analysis was conducted by IVF and ICSI. The overall results and subgroup results showed no statistical difference between the two groups (Overall, OR (95%CI) = 0.345 (−0.110–0.799), p = 0.137; IVF, OR (95%CI) = 0.502 (−0.356–1.359), p = 0.251; ICSI, OR (95%CI) = 0.259 (−0.421–0.939), p = 0.455). The I squared of heterogeneity varied from 79.4 to 87.2 % indicated severe heterogeneity and a random effect model was used (Fig. 6 and Table 2). As for the SCSA subgroup, the results were similar (SCSA, OR(95 % CI) = 0.345(−0.110–0.799, p = 0.137) (Table 2).
Fig. 6.
Forest plots for the overall and subgroup analysis(IVF or ICSI) of pregnant group versus non-pregnant group
Assessment of publication bias
For the aim to assess the publication bias between the studies, Begg,s funnel plots and Egger,s test were conducted. The main results of the tests were presented in Table 2. In the DFI ≤ 15 % group of CP group, , Begg,s test indicated no publication bias (Pr > |z| = 0.451), but Egger,s test found publication bias (P > |t| = 0.006). Publication bias was not found in the remaining results of the meta-analysis.
Discussion
Two meta-analysis performed by Evenson [4] and Collins [5] evaluated the association of DFI and pregnancy outcome after ART procedures. Evenson found that infertile couples were more likely to achieve a pregnancy after IVF treatment if DFI detected using SCSA method was less than 30 % (OR (95%CI) = 2.0 (1.02–2.84). However, the results for ICSI showed a non-significant trend (OR (95 % CI) = 1.6(0.92–2.94). On the other hand, Collins did not confirm its positive role of sperm DNA integrity testing in the evaluation of pregnancy outcome in IVF or ICSI cycles in the meta-analysis. In the present meta-analysis, several newer studies were involved [7, 11, 12, 14–17, 21, 23–26]. In total, we added 12 additional newer studies with 1,526 subjects included and this makes the results of a pooled quantitative assessment more robust and reliable.
Several DFI threshold values corresponding to the DFI detection methods have been applied clinically with which to predict fertility outcome. There were several methods used to evaluate sperm DNA integrity: SCSA, TUNEL, COMET and AOT assay. For the SCSA method mentioned in the included studies, the authors adopted 15, 27, 30 % to be the threshold values. In a clinical study conducted in 2011 [27], the value of 30.28 % was picked as the threshold level to differ the infertile group from the fertile controls. Moreover, in another trial using neutral comet assay, DFI presented as the percentage of double strand DNA breaks ranged from 15 to 25 % in native sperm [28]. Furthermore, Henkel identified 36.5 % as the cutoff value using TUNEL assay by performing ROC analysis [19]. Reasons for the difference of the threshold value among the studies may include: different DFI detection methods, different study populations, different type of fertilization (IVF or ICSI), sperm preparation method (raw semen or obtained by density gradient) for ART procedure, whether or not underwent semen quality control (sperm count, motility, morphology) and whether a selection criteria was set for couples underwent ART (female partner age, body mass index (BMI) etc.).
In our analysis involving 15 studies, the CP outcome after IVF or ICSI was associated with DFI at some point. Infertile couples were more likely to achieve pregnant if DFI was less than cutoff value (cutoff value > 27 % and 15–27 % group). This trend was not all consistent with subgroup analysis. For DFI >27 % group, the pooled OR for achieving a pregnancy using IVF was 1.742 and showed a similar trend. In contrast, a analysis involved 5 studies in cases of ICSI subgroup reported a non-significant effect of DFI on CP; For DFI 15–27 % group, a subgroup analysis of only three articles included using the ICSI procedure did not differ from the overall effect which indicated a significant relationship of DFI and CP outcome. However, the analysis of IVF subgroup with only one article included found no relationship between DFI and CP outcome, yet we cannot reach definite conclusions based on the limited data (only one article included and the sample size was relatively small).
On the other hand, when the DFI threshold value was less than 15 %, the OR indicated a non-significant effect of DFI on pregnancy outcome. Furthermore, the results of meta-analysis of the link of DFI with BP and PL identified negative relationship. However, when stratified by DFI detection methods, the results demonstrated that DFI was not associated with pregnancy outcome for SCSA in all groups. The discrepancy between the results of subgroup analyses indicated that the link between DFI and pregnancy outcome is not fully established.
Notably, in a guideline issued by American Society for Reproductive Medicine (ASRM), the authors did not establish its positive role of predicting value for ART outcomes of assessing sperm DNA integrity. The results of our meta-analysis were partially consistent with the pervious study. However, Unlike the previous study, we divided the included studies into several groups (DFI ≤ 15 %, 15–27 %, >27 %) and determined to set a DFI threshold level with which the prediction of pregnancy outcome more accurate. Although subgroup analyses by the type of fertilization (IVF or ICSI) showed that DFI was likely to influence pregnancy outcome if DFI exceeded 15 % in IVF or ICSI, the analyses based on DFI test methods produced mainly nonsignificant results. The overall conclusion from the present meta-analysis is that the value of DFI test before ART to predict pregnancy outcome is not confirmed.
Several limitations should paid attention to our meta-analysis. First, the results of our meta-analysis indicated that the correlation of sperm DNA damage with BP, PL was not statistically significant. Besides, the analysis of the comparison of DFI value between the two groups was of no significance in spite of heterogeneity of the studies. These findings remind us that the results of the this meta-analysis should be interpreted with caution and better designed studies with larger sample size are needed to elucidate the possible relationship. Second, subgroup analysis was conducted by the type of fertilization (IVF or ICSI) and DFI test methods, however, because some of the data was not available, we were not capable of performing subgroup analysis on several important factors which was likely to influence the outcome, such as the semen preparation methods,.
There are some advantages of our meta-analysis that should take consideration. First, additional newer studies were added to the present study and this makes the results more reliable. Second, we performed subgroup analysis on DFI detection methods. Although the results revealed that the DFI threshold value of SCSA is not associated with pregnancy outcome, yet a meaningful value corresponded to the different DFI test methods (SCSA, AOT, Tunel and comet) is warranted. Third, we performed a meta-analysis to explore the link between pregnancy loss and DFI as well. This was a complement to the pervious findings in spite of the limited studies and data.
In summary, the present meta-analysis suggests that DNA integrity test before ART is not sufficient to be a predicative index for infertile men.. However, more studies concerning this topic are needed to further illustrate the issue.
Acknowledgments
The study was supported by the National Natural Science Foundation of China (No. 81170563 and 81270694).
Conflict of interest
All authors declare no competing financial interests.
Footnotes
Capsule DFI is an important value to assess human sperm quality, but this meta-analysis found no association between DFI and pregnancy outcome after IVF or ICSI.
Contributor Information
Zheng Zhang, Email: 13770864680@163.com.
Yutian Dai, Email: 13913957628@163.com.
References
- 1.Katz P, Nachtigall R, Showstack J. The economic impact of the assisted reproductive technologies. Nat Cell Biol. 2002;4:29–32. doi: 10.1038/ncb-nm-fertilityS29. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal A, Allamaneni SS. The effect of sperm DNA damage on assisted reproduction outcomes. A review. Minerva Ginecol. 2004;56:235–45. [PubMed] [Google Scholar]
- 3.Shamsi MB, Kumar R, Dada R. Evaluation of nuclear DNA damage in human spermatozoa in men opting for assisted reproduction. Indian J Med Res. 2008;127:115–23. [PubMed] [Google Scholar]
- 4.Evenson D, Wixon R. Meta-analysis of sperm DNA fragmentation using the sperm chromatin structure assay. Reprod Biomed Online. 2006;12:466–72. doi: 10.1016/S1472-6483(10)62000-7. [DOI] [PubMed] [Google Scholar]
- 5.Collins JA, Barnhart KT, Schlegel PN. Do sperm DNA integrity tests predict pregnancy with in vitro fertilization? Fertil Steril. 2008;89:823–31. doi: 10.1016/j.fertnstert.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 6.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 7.Benchaib M, Lornage J, Mazoyer C, Lejeune H, Salle B, François GJ. Sperm deoxyribonucleic acid fragmentation as a prognostic indicator of assisted reproductive technology outcome. Fertil Steril. 2007;87:93–100. doi: 10.1016/j.fertnstert.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 8.Boe-Hansen GB, Fedder J, Ersbøll AK, Christensen P. The sperm chromatin structure assay as a diagnostic tool in the human fertility clinic. Hum Reprod. 2006;21:1576–82. doi: 10.1093/humrep/del019. [DOI] [PubMed] [Google Scholar]
- 9.Bungum M, Humaidan P, Spano M, Jepson K, Bungum L, Giwercman A. The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF and ICSI. Hum Reprod. 2004;19:1401–8. doi: 10.1093/humrep/deh280. [DOI] [PubMed] [Google Scholar]
- 10.Bungum M, Humaidan P, Axmon A, Spano M, Bungum L, Erenpreiss J, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod. 2007;22:174–9. doi: 10.1093/humrep/del326. [DOI] [PubMed] [Google Scholar]
- 11.Bungum M, Spanò M, Humaidan P, Eleuteri P, Rescia M, Giwercman A. Sperm chromatin structure assay parameters measured after density gradient centrifugation are not predictive for the outcome of ART. Hum Reprod. 2008;23:4–10. doi: 10.1093/humrep/dem353. [DOI] [PubMed] [Google Scholar]
- 12.Chi HJ, Chung DY, Choi SY, Kim JH, Kim GY, Lee JS, et al. Integrity of human sperm DNA assessed by the neutral comet assay and its relationship to semen parameters and clinical outcomes for the IVF-ET program. Clin Exp Reprod Med. 2011;38:10–7. doi: 10.5653/cerm.2011.38.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gandini L, Lombardo F, Paoli D, Caruso F, Eleuteri P, Leter G, et al. Full-term pregnancies achieved with ICSI despite high levels of sperm chromatin damage. Hum Reprod. 2004;19:1409–17. doi: 10.1093/humrep/deh233. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy C, Ahlering P, Rodriguez H, Levy S, Sutovsky P. Sperm chromatin structure correlates with spontaneous abortion and multiple pregnancy rates in assisted reproduction. Reprod Biomed Online. 2011;22:272–6. doi: 10.1016/j.rbmo.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Lin MH, Kuo-Kuang Lee R, Li SH, Lu CH, Sun FJ, et al. Sperm chromatin structure assay parameters are not related to fertilization rates, embryo quality, and pregnancy rates in in vitro fertilization and intracytoplasmic sperm injection, but might be related to spontaneous abortion rates. Fertil Steril. 2008;90:352–9. doi: 10.1016/j.fertnstert.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Miciński P, Pawlicki K, Wielgus E, Bochenek M, Tworkowska I. The sperm chromatin structure assay (SCSA) as prognostic factor in IVF/ICSI program. Reprod Biol. 2009;9:65–70. doi: 10.1016/S1642-431X(12)60095-3. [DOI] [PubMed] [Google Scholar]
- 17.Niu ZH, Shi HJ, Zhang HQ, Zhang AJ, Sun YJ, Feng Y. Sperm chromatin structure assay results after swim-up are related only to embryo quality but not to fertilization and pregnancy rates following IVF. s. 2011;13:862–6. doi: 10.1038/aja.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Payne JF, Raburn DJ, Couchman GM, Price TM, Jamison MG, Walmer DK. Redefining the relationship between sperm deoxyribonucleic acid fragmentation as measured by the sperm chromatin structure assay and outcomes of assisted reproductive techniques. Fertil Steril. 2005;84:356–64. doi: 10.1016/j.fertnstert.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 19.Henkel R, Kierspel E, Hajimohammad M, Stalf T, Hoogendijk C, Mehnert C, et al. DNA fragmentation of spermatozoa and assisted reproduction technology. Reprod Biomed Online. 2003;7:477–84. doi: 10.1016/S1472-6483(10)61893-7. [DOI] [PubMed] [Google Scholar]
- 20.Henkel R, Hajimohammad M, Stalf T, Hoogendijk C, Mehnert C, Menkveld R, et al. Influence of deoxyribonucleic acid damage on fertilization and pregnancy. Fertil Steril. 2004;81:965–72. doi: 10.1016/j.fertnstert.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Wang H, Wang L, Zhou Z, Sha J, Mao Y, et al. The clinical significance of sperm DNA damage detection combined with routine semen testing in assisted reproduction. Mol Med Rep. 2008;1:617–24. doi: 10.3892/mmr_00000002. [DOI] [PubMed] [Google Scholar]
- 22.Zini A, Meriano J, Kader K, Jarvi K, Laskin CA, Cadesky K. Potential adverse effect of sperm DNA damage on embryo quality after ICSI. Hum Reprod. 2005;20:3476–80. doi: 10.1093/humrep/dei266. [DOI] [PubMed] [Google Scholar]
- 23.Dar S, Grover SA, Moskovtsev SI, Swanson S, Baratz A, Librach CL. In vitro fertilization-intracytoplasmic sperm injection outcome in patients with a markedly high DNA fragmentation index (>50 %) Fertil Steril. 2013;100:75–80. doi: 10.1016/j.fertnstert.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Speyer BE, Pizzey AR, Ranieri M, Joshi R, Delhanty JD, Serhal P. Fall in implantation rates following ICSI with sperm with high DNA fragmentation. Hum Reprod. 2010;25:1609–18. doi: 10.1093/humrep/deq116. [DOI] [PubMed] [Google Scholar]
- 25.Jiang HH, He XJ, Song B, Cao YX. Sperm chromatin integrity test for predicting the outcomes of IVF and ICSI. Zhonghua Nan Ke Xue. 2011;17:1083–6. [PubMed] [Google Scholar]
- 26.Yang XY, Wang LL, Chen P, Zhang Y, Zhang W, Cui YG, et al. Impact of sperm DNA fragmentation index and sperm malformation rate on the clinical outcome of ICSI. Zhonghua Nan Ke Xue. 2013;19:1082–6. [PubMed] [Google Scholar]
- 27.Venkatesh S, Singh A, Shamsi MB, Thilagavathi J, Kumar R, Mitra DK, et al. Clinical significance of sperm DNA damage threshold value in the assessment of male infertility. Reprod Sci. 2011;18:1005–13. doi: 10.1177/1933719111401662. [DOI] [PubMed] [Google Scholar]
- 28.Van Kooij RJ, de Boer P, De Vreeden-Elbertse JM, Ganga NA, Singh N, Te Velde ER. The neutral comet assay detects double strand DNA damage in selected and unselected human spermatozoa of normospermic donors. Int J Androl. 2004;27:140–6. doi: 10.1111/j.1365-2605.2004.00463.x. [DOI] [PubMed] [Google Scholar]