Abstract
Purpose
Patients with a karyotype of 45,X (monosomy X) normally display a female phenotype. However, in some rare cases, monosomy X is associated with maleness. Here we describe a case of a male with a 45,X karyotype and primary infertility, which prompted molecular investigation of the sex-determination gene SRY.
Methods
Karyotyping was performed by GTG-banded chromosome analysis. The presence and location of SRY was investigated using PCR and FISH, respectively.
Results
PCR confirmed the presence of the SRY gene while FISH analysis demonstrated its location on the p arm of chromosome 13. These findings demonstrate that autosomal retention of SRY can be sub-microscopic and emphasize the importance of PCR and FISH in the genetic workup of the monosomic X male.
Keywords: Monosomy X, Azoospermia, Infertility, Y chromosome, Cryptic translocation, Sex determination
Introduction
The 45,X karyotype (monosomy X) is often seen in Turner syndrome, the patients of which are female with short stature, gonadal dysfunction and redundant nuchal skin [1]. However, in rare cases, monosomy X patients are male, usually due to an unbalanced Y-autosome translocation resulting in retention of the p arm of the Y chromosome harboring the sex determination gene SRY. Such patients often show infertility as a result of losing the spermatogenesis gene on Yq [2]. Here we describe a 45,X male with an SRY-bearing chromosome 13. The latter finding, obtained using polymerase chain reaction (PCR) and fluorescence in-situ hybridization (FISH) analysis was helpful for genetic counseling of this unusual case.
Case report
The patient was a 25-year-old male with a normal phenotype and primary infertility, born to a 32-year-old father and a 34-year-old mother. A diagnosis of azoospermia was made following semen analyses, performed in triplicate according to the World Health Organization guidelines [3]. Physical examination revealed normal penis and pubic hair and no signs of gynecomastia. Testicular volume was 12 ml on the left side and 16 ml on the right side and the testicular texture was normal. Reproductive hormone levels were normal for prolactin (0.29 nmol/L; normal range 0.18–0.69 nmol/L), luteinizing hormone (5.26 U/L; normal range 1.7–8.6 U/L), testosterone (14.93 nmol/L; normal range 9.9–27.8 nmol/L), and estradiol (147.7 pmol/L; normal range 27.96–155.92 pmol/L), but the level of follicle-stimulating hormone was higher than normal (17.11 U/L; normal range 1.5–12.4 U/L).
Materials and methods
Chromosomal analysis was performed on PHA-stimulated peripheral blood lymphocytes using colcemid as a mitotic arrestant after a 3-day cell culture in RPMI Medium 1640 (GIBCO, Invitrogen Carlsbad, CA, USA). Metaphase chromosomes were studied by standard GTG-banding procedures [3]. PCR amplification of the sequence-tagged site (STS) marker sY14, which maps to the SRY locus, was used on peripheral blood DNA to demonstrate the presence of the SRY gene (forward primer: GAATATTCCCGCTCTCCGGA; reverse primer: GCTGGTGCTCCATTCTTGAG; Shanghai Sangon Biotech, Shanghai, China). FISH was performed on 50 metaphase chromosome spreads using dual-color probe combinations for chromosome X and SRY (GLP SRY Spectrum Red and CEP X Spectrum Green; Beijing Cyto Test Biotechnology, Beijing, China), and chromosomes 13 and 21 (GLP 13 Spectrum Green and GLP 21 Spectrum Red; Beijing GP Medical Technologies, Beijing, China).
Results
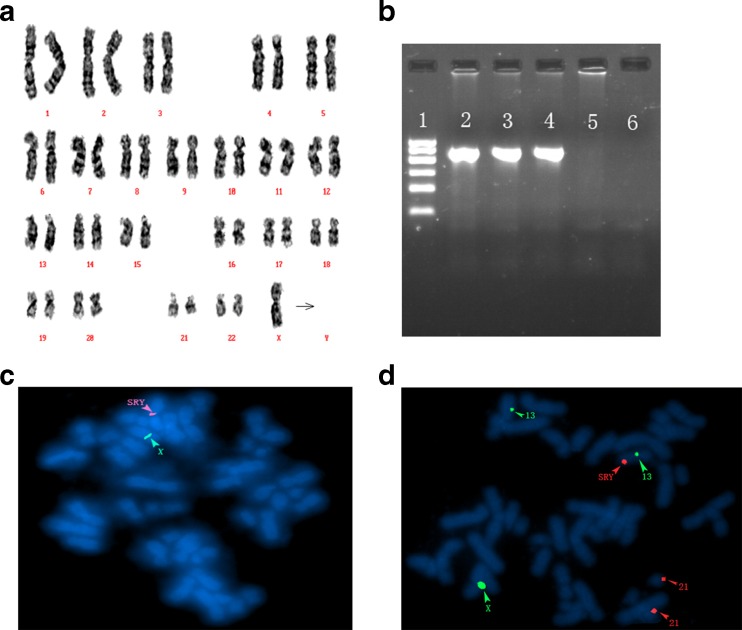
Cytogenetic analysis of 50 metaphase spreads from peripheral blood lymphocyte cultures showed a 45,X karyotype (Fig. 1a). PCR of peripheral blood DNA using primers for an STS within SRY produced a product of the expected size, confirming the presence of the SRY gene (Fig. 1b). Application of FISH probes for SRY (red) and the X chromosome centromere (green) produced a red signal at the p-terminus of a D-group autosome (Fig. 1c). Further FISH analysis with a mixture probes for chromosomes 13 and 21 determined that the SRY gene was on the chromosome 13 (Fig. 1d).
Fig. 1.
a GTG-banding analysis. Arrow shows the absence of the chromosome Y; b PCR amplification of the SRY gene(Line1: Marker; Line2: Normal male; Line3: Azoospermia male with normal karyotype; Line4: The patient in case; Line5: Female; Line6: Blank); c FISH with probes of the chromosome X (green) and the SRY gene (red); d FISH with probes of the chromosome X (green), the chromosome 13 (green), chromosome 21 (red) and the SRY gene (red)
Discussion
The SRY gene, which is usually located at Yp, encodes the testis determining factor protein required for the development of male genitalia [4]. Translocation of SRY to an autosome, most commonly to the short arm of an acrocentric (D or a G group) chromosome, has been reported in males lacking an obvious Y chromosome [5]. In the present case, the SRY gene was mapped to chromosome 13 by FISH analysis.
To date, five cases of monosomy X males with an SRY-bearing chromosome 13 have been reported (Table 1) [6–10]. In all the latter cases, the presence of Y chromosome material on chromosome 13 was visible at the resolution of G-band analysis. In contrast, karyotype analysis of the present case showed an XO sex chromosome complement with no additional cytogenetic aberrations. On further investigation, however, an SRY FISH signal was observed on the p arm of chromosome 13. The translocated region of the Y chromosome was thus too short to be observed by GTG-banding. A combination of conventional chromosome analysis and FISH is therefore recommended for genetic investigation of monosomy X males.
Table 1.
The reported cases of 45,X male with a SRY-bearing chromosome 13
| Case | Authors | Karyotype | Age | Clinical findings |
|---|---|---|---|---|
| 1 | Mohamed Boutouil et al. 1996 | 45,X,t(Y;13) | 13 | Psychomotor retardation |
| 2 | Alan Shanske et al. 1999 | 45,X,dic(Y;13)(p1?;p1?) | 10 | Short stature |
| 3 | Jean Pierre SiVroi et al. 2001 | 45,X,−13,−Y, +psudic(Y),t(Y;13)(q12;p11.2) | 20 | Azoospermia |
| 4 | Cla’udiaAlves et al. 2002 | 45,X,dic(Y;13)(p1?;p12) | 32 | Tesis abnormal, oligozoospermia |
| 5 | Cui Ying-xia et al. 2008 | 45,X,der(Y)t(Y;13)(q11.1;q12),-13 | 25 | Azoospermia, tesis soft and small |
| 6 | Present study | 45,X,t(Y;13) | 25 | Azoospermia, high FSH level |
As with the present case, all four reported adult patients with t(Y;13) were azoospermic, as shown in Table 1. This is likely to be due to absence of the genes involved in spermatogenesis, which are normally located on the long arm of the Y chromosome.
The patient reported by Shanske et al. was short in stature [7]. The authors concluded that haploinsufficiency of the SHOX gene may have been responsible for the growth deficiency of their patient. SHOX is located at Yp 11.3, and has been shown to cause short stature and delayed puberty when lost or mutated [11]. In contrast, the patient we describe is of normal stature, which suggests that the SHOX gene may be retained in this case.
In the report by Boutouil et al., the patient had psychomotor retardation and mild facial asymmetry, while other family members were normal [6]. FISH studies showed that the telomere of the Y chromosome had fused with the short arm of chromosome 13, resulting in loss of material from 13p. It is therefore possible that the developmental delay and phenotypic abnormalities observed in this patient are attributable to the loss of chromosome 13 sequences rather than deletion or rearrangement of Y. Indeed, deletion of the proximal part of 13p has been associated with mild or moderate mental and growth retardation and mild deformity [12]. The absence of psychomotor retardation in the other reported cases of t(Y;13), and in the present case, would support this theory.
In conclusion, we have described the clinical and genetic findings in a monosomy X male with an SRY-bearing chromosome 13. Translocation of SRY to chromosome 13 was detected by FISH. Our study highlights importance of augmenting conventional chromosome analysis of the monosomy X male with molecular methods to investigate the presence of a cryptic Y-autosome translocation, especially when no additional cytogenetic aberrations are apparent.
Acknowledgments
We express our deepest gratitude to all the staff of the Genetics and Andrology Laboratories for their excellent contribution. This work was supported by the National Population and Family Planning Commission of China (no. 2011-GJKJS-07).
Conflict of interest
The authors declare that the commercial party related to the subject and the authors of this manuscript have no conflict of interest.
Footnotes
Capsule For monosomy X male, it is necessary to map the SRY gene. Our study highlights importance of traditional chromosome analysis combining with FISH to determine the translocation of SRY, especially when no additional cytogenetic aberrations are apparent.
References
- 1.Sybert VP, McCauley E. Turner’s syndrome. N Engl J Med. 2004;351(12):1227–38. doi: 10.1056/NEJMra030360. [DOI] [PubMed] [Google Scholar]
- 2.Nataf V, Senat MV, Albert M, Bidat L, de Mazancourt P, Roume J, et al. Prenatal diagnosis of a 45, X male with a SRY-bearing chromosome 21. Prenat Diagn. 2002;22:675–80. doi: 10.1002/pd.376. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 5. Geneva: World Health Organization; 2010. [Google Scholar]
- 4.Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, et al. A gene from the human sex determining region encodes a protein with homology to a conserved DNA binding motif. Nature. 1990;346:240–4. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- 5.Hsu LYF. Phenotype/karyotype correlations of Y chromosome aneuploidy with emphasis on structural abberations in postnatally diagnosed cases. Am J Med Genet. 1994;53:108–40. doi: 10.1002/ajmg.1320530204. [DOI] [PubMed] [Google Scholar]
- 6.Boutouil M, Fetni R, Qu J, et al. Fragile site and interstitial telomererepeat sequences at the fusion point of a de novo (Y; 13) translocation. Hum Genet. 1996;98(3):323–7. doi: 10.1007/s004390050216. [DOI] [PubMed] [Google Scholar]
- 7.Shanske A, Ellison J, Vuguin P, et al. Deletion of the pseudoautosomal region in a male with a unique Y; 13 translocation and short stature. Am J Med Genet. 1999;82(1):34–9. doi: 10.1002/(SICI)1096-8628(19990101)82:1<34::AID-AJMG7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 8.Siffroi JP, Benzacken B, Angelopoulou R, et al. Alternative centromeric inactivation in a pseudodicentrict (Y; 13)(q12; p11. 2) translocation chromosome associated with extreme oligozoospermia. J Med Genet. 2001;38(11):802–6. doi: 10.1136/jmg.38.11.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alves C, Carvalho F, Cremades N, et al. Unique (Y; 13) translocation in a male with oligozoospermia: cytogenetic and molecular studies. Eur J Hum Genet EJHG. 2002;10(8):467–74. doi: 10.1038/sj.ejhg.5200835. [DOI] [PubMed] [Google Scholar]
- 10.Ying-xia CUI, et al. An infertile 45, X male carrying an unbalanced (Y, 13) translocation: a clinical cytogenetic and molecular study. J Med Postgrad. 2008;21(11):1168–71.
- 11.Stuppia L, et al. Loss of the SHOX gene associated with Leri-Weill dyschondrosteosis in a 45, X male. J Med Genet. 1999;36:711–3. [PMC free article] [PubMed] [Google Scholar]
- 12.Jones KJ, et al. Smith’s recognizable patterns of human malformation. Elsevier-Health Sciences Division; 2013. 9.