Abstract
Purpose
Embryonic poly(A)-binding protein (EPAB) and poly(A)-binding protein, cytoplasmic 1 (PABPC1) bind poly(A) tails of mRNAs and mediate their translational regulation in germ cells and early preimplantation embryos. Although expression patterns and possible functions of the Epab and Pabpc1 genes have been examined in vertebrate germ cells and early embryos, their expression levels and cellular localizations in the postnatal mouse ovaries remained elusive.
Methods
In the present study, we first aimed to characterize expression levels of the Epab and Pabpc1 genes in the prepubertal (1-, 2-, and 3-week old), pubertal (4-, 5-, and 6-week old), postpubertal (16-week and 18-week old), and aged (52-, 60-, and 72-week old) mouse ovaries by using quantitative real-time polymerase chain reaction (qRT-PCR).
Results
Epab mRNA was predominantly expressed in the prepubertal ovaries when compared to later developmental periods. However, Pabpc1 transcript was highly generated in the prepubertal and pubertal mouse ovaries except for 1-week old ovary than those of other developmental terms. In the prepubertal mouse ovaries, RNA in situ hybridization localized both Epab and Pabpc1 transcripts in the cytoplasm of oocytes and granulosa cells of all follicular stages. Consistently, Epab and Pabpc1 gene expression were detected in the cumulus cells and MII oocytes obtained from cumulus oocyte complexes (COCs). Ovarian follicle counting in the postnatal ovaries revealed that total number of follicles was higher in the prepubertal ovaries in comparison with later stages of development.
Conclusion
As a result, Epab and Pabpc1 expression exhibit differences at postnatal ovary development stages and both genes are transcribed in the granulosa cells and oocytes. These findings suggest that EPAB may predominantly play roles in translational regulation of the mRNAs during early oogenesis and folliculogenesis, but PABPC1 most likely perform these roles in the later terms of ovarian development along with EPAB protein.
Keywords: Epab, Pabpc1, Ovary, Oocyte, Granulosa cell
Introduction
Embryonic poly(A)-binding protein (EPAB; also known as PABPc1-like and ePABP) essentially play key roles in translational induction, cytoplasmic polyadenylation and in preventing deadenylation of maternally stored mRNAs until embryonic genome activation (EGA) occurs [1–3]. Following EGA, the poly(A)-binding protein, cytoplasmic 1 (PABPC1; also known as PABP1) largely carries out these functions in place of EPAB [2]. Both proteins have highly similar structures: RNA-recognition motifs (RRMs), the PABP C-terminal domain (PABC), and a proline-rich region between fourth RRM motif and PABC domain. While the RRM motifs provide EPAB or PABPC1 proteins binding to specific sequences of the mRNAs such as poly(A) tails, the PABC domain facilitates interactions between PABP and other cytoplasmic proteins. However, potential roles of the proline-rich region that structurally differs from other PABP domains remained elusive.
The expression patterns of the Epab and Pabpc1 genes have been assessed in mouse [4] and human [5] ovaries, oocytes and early embryos, and in the postnatal mouse testes [6]. Although Pabpc1 is transcribed in the mouse tissues such as liver, kidney, heart, spleen, small intestine, testis and ovary, Epab is expressed only in the mouse ovary and testis tissues [4]. In the mouse ovary, the Epab mRNA is localized to only oocyte cytoplasm of the growing follicles by using RNA in situ hybridization [4]. In the postnatal mouse testes, Epab is exclusively transcribed in the male germ cells at the ages from 6-day to 88-day old, whereas Pabpc1 is produced in the intertubuler cells as well as germinal epithelial cells of the postnatal testes tested here [6]. A recently published study showed that Epab-knockout (Epab−/−) female mice were infertile, but both Epab−/− and Epab+/− males, and Epab+/− females were found to be fertile [7]. Epab−/− female mice was not enable to produce metaphase II (MII) oocytes or embryos in vivo or in vitro, and could not activate translation of the maternally-derived mRNAs such as Ccnb1 (Cyclin B1) and Dazl (Deleted in azoospermia-like). Interestingly, microinjection of Epab mRNA into Epab−/− germinal vesicle (GV) stage oocytes did not rescue the oocyte maturation, suggesting that EPAB is most likely required for earlier stages of oogenesis [7]. Also, increased number of secondary follicles, decreased diameters of oocytes within antral follicles and antral follicles, a 8-fold decreased ovulation rates, and cumulus expansion impairment were reported in Epab−/− female mice. Consequently, these findings show that EPAB is essential for female fertility, oocyte maturation, and folliculogenesis [7].
Briefly, folliculogenesis defines follicular development from primordial to antral follicles, is started at early postnatal development, and continues until late term of life span [8–10]. Initially, a subset group of primordial follicles composed of flattened granulosa cells surrounding a diplotene-arrested oocyte is stimulated to form primary follicles, including one layer of cuboidal granulosa cells [11]. Then, they are transformed into secondary follicles that consist of two or more layers of granulosa cells, which will later be enclosed by a theca cell layer. With further growth, secondary follicles become preantral follicles (also known as early antral follicles) with small spaces between granulosa cells [8, 9]. When a female mouse reaches sexual maturity, around 4 weeks of development, the fully matured antral follicles with one big antrum, ovulate oocyte at metaphase II stage of the second meiotic division (reviewed in [12]). It is noteworthy that oocytes residing in the antral follicles are surrounded by specialized granulosa cells, called cumulus cells. These granulosa cells serve different functions from that of the granulosa cells lining the follicle wall, called mural granulosa cells [13]. Following ovulation, both mural granulosa cells and theca cells undergo the process of luteinization and terminally differentiate into an endocrine structure, known as corpus luteum (CL). If pregnancy does not occur, the corpus luteum will commence to gradually regress in size. Finally, it transforms into a corpus albicans structure, which is largely composed of fibrous connective tissue [14, 15].
As a result, follicular development begins at the early term of postnatal life, and during folliculogenesis granulosa cells and oocytes undergo key morphological and physiological changes (reviewed in [16, 17]). Maternal mRNAs in the growing oocytes, being used during oocyte maturation, fertilization and early embryo development are transcribed concomitantly with the beginning of early folliculogenesis in the early postnatal life, and it continues during later developmental periods [18, 19]. However, in aged ovaries the number of ovulating oocytes and follicles remarkably decreases, and conversely atretic follicle numbers predominantly increase [20, 21].
In the present study, we have characterized expression profiles of the Epab and Pabpc1 genes in the postnatal mouse ovaries at the terms of prepubertal (1-, 2-, and 3-week), pubertal (4-, 5-, and 6-week), postpubertal (16- and 18-week) and aged (52-, 60-, and 72-week). Additionally, we evaluated localizations of the Epab and Pabpc1 mRNA in the prepubertal mouse ovaries, counted all classes of follicles in the postnatal ovaries, and analyzed expression levels of these genes in the cumulus cells. As a result, our findings suggest that Epab and Pabpc1 are differently expressed during postnatal developmental stages, and they most probably play central roles in oogenesis and folliculogenesis.
Material and methods
Animals and collection of postnatal mouse ovaries
Postnatal mouse ovaries were collected from female BALB/c mice at the ages of 1-, 2-, 3-, 4-, 5-, 6-, 16-, 18-, 52-, 60-, and 72-week. All mice used in the current work were housed with free access to food and water, and they were kept under a 12 h light-dark cycle in the Experimental Animal Care and Production Unit of Akdeniz University, School of Medicine. All experimental protocols were approved by the Akdeniz University Institutional Animal Care and Use Committee (Protocol No. 2011.09.08).
Three female mice from each week were sacrificed with cervical dislocation after inhalation of ether vapor for 30 s. Then, ovaries were obtained under sterile conditions. For each mouse, one ovary was used for qRT-PCR, and the other one was taken for routine paraffin embedding.
qRT-PCR reaction of the Epab and Pabpc1 mRNA
Total RNA isolation from postnatal mouse ovaries was carried out using Trizol reagent (Life Technologies, Darmstadt, Germany) according to instructions of the manufacturer. The concentration of the isolated RNA was calculated by measuring the absorbance at 260 and 280 nm. The 10 μg of the RNA was subjected to DNase I (Ambion, Austin, Texas, USA) to eliminate genomic DNA contamination. Then, reverse transcription reaction was performed with a RETROscript kit (Ambion, Austin, TX, USA) in two steps according to the manufacturer’s instructions: first, equal amounts of DNase-treated RNA (2 μg) and random decamers were incubated at 85 °C for 3 min to unwind any secondary structure therein. Second, other reverse transcription reaction components composed of 2 μl of 10X RT buffer, 4 μl of 1.25 mM dNTP mix, 1 μl of RNase inhibitor (10 units/μl), and 1 μl of MMLV-RT (100 units/μl) were added and then incubated at 44 °C for 1 h. Finally, the MMLV-RT enzyme was inactivated at 92 °C for 10 min.
The expression levels of Epab and Pabpc1 mRNA in the postnatal mouse ovaries at the ages of 1-, 2-, 3-, 4-, 5-, 6-, 16-, 18-, 52-, 60-, and 72-week were assessed in triplicate by using qRT-PCR. The qRT-PCR reaction was set up in a reaction volume of 25 μl containing 12.5 μl of 2X SYBR green supermix (Qiagen, Valencia, CA, USA), 0.4 μM of each primer and 1 μl of cDNA template, and PCR cycling was performed on a Rotor-Gene (Corbett Research, Sydney, Australia). The sequences and localization of the Epab, Pabpc1, and β-actin primers are given in Table 1. The β-actin expression was used as an internal control, against which Epab and Pabpc1 gene expression were normalized. Then, the relative Epab and Pabpc1 gene expression levels were calculated by using 2-ΔΔCt (cycle threshold) method and reported as fold changes in the postnatal ovaries. Note that the specifity of Epab, Pabpc1 and β-actin qRT-PCR products were confirmed by a melting curve analysis at the end of each reaction.
Table 1.
The sequences and localization of the primers for the embryonic poly (A)-binding protein (Epab), poly(A)-binding protein, cytoplasmic 1 (Pabpc1) and Actb (β-actin) genes used in the current work
| Gene | Localization | Primer (5′→3′) | Product size (bp) |
|---|---|---|---|
| Epab | Exon 8F | ACGAAACCGCTCTATGTGGCCC | 246 |
| Exon 9R | CCTGGGGTCAGGTTGCATTGG | ||
| Pabpc1 | Exon 1F | CCCAGCGCCCCCAGCTAC | 196 |
| Exon 2R | CACGTTCCGCGTCCGCC | ||
| Actb | Exon 3F | TGCGTGACATCAAAGAGAAG | 244 |
| Exon 4R | CGGATGTCAACGTCACACTT |
The relative abundance of follicles in the postnatal ovaries
The postnatal ovaries obtained from female mice at the ages of 1-, 2-, 3-, 4-, 5-, 6-, 16-, 18-, 52-, 60-, and 72-week were fixed in Bouin’s fixative for 12 h at 4 °C, and then dehydrated in increasing concentrations of ethanol. Following dehydration, ovaries were cleared in xylene and embedded in paraffin. Serial sections at 5 μm thickness were cut from paraffin-embedded ovarian blocks using a rotary microtome (Leica, Nussloch, Germany).
To determine the relative abundance of follicles from primordial to antral follicles in the postnatal ovaries, hematoxylin and eosin (HE) staining was performed on the serial sections. For HE staining, the sections were initially incubated at 60 °C for 1 h, treated twice with fresh xylene for 10 min each time, and then rehydrated in decreasing ethanol series for 5 min each time. After sections were washed with tap water for 10 min, the hematoxylin followed by eosin staining was performed. After additional washes in distilled water, sections were dehydrated in increasing ethanol series, and cleared in fresh xylene. Finally, HE-stained postnatal ovary sections were analyzed under a bright-field microscope (Carl Zeiss Inc. Thornwood, NY, USA).
Histological structures of the postnatal ovaries were evaluated, and all follicles from primordial to antral follicles in the every seventh section of the postnatal ovaries were counted. Follicular classification was performed as primordial, primary, secondary, early antral, and antral follicles as defined previously [22]. In brief, primordial follicles had an oocyte surrounded by a single layer of flattened pregranulosa cells; however, primary follicles were identified as an oocyte enclosed by a layer of cuboidal granulosa cells. Secondary follicles possessed two or three layers of cuboidal granulosa cells surrounding the oocyte, but there was no visible antral cavity among granulosa cells. In the preantral follicles (also known as early antral follicles), small follicular spaces between granulosa cells began to form and these follicles were composed of four or more layers of granulosa cells. The most prominent characteristics of the antral follicles were presence of a big antrum filled with follicular fluid, and their oocytes were surrounded by specialized granulosa cells known as cumulus cells. Based on distinguishing morphological features of the follicles, all follicles from primordial to antral follicles were counted in the HE-stained postnatal ovary sections, and number of each follicle per section was calculated.
RNA in situ hybridization (RNA ISH)
Localization of the Epab and Pabpc1 mRNA in the prepubertal ovaries (1-, 2-, and 3-week old) was evaluated with RNA in situ hybridization. To this end, we have first created Epab and Pabpc1 DNA probes with PCR technique by using cDNA synthesized from mature mouse ovaries. Amplifications were performed for 35 cycles of PCR as used for qRT-PCR reaction, with the exception of 1 min extension steps in a volume of 50 μl reaction mixture as mentioned above including 0.02 mM Dig-UTP (Roche, Indianapolis, IN, USA). The PCR products were fractionated on a 1.5 % agarose gel and purified using QIAEX II gel extraction kit (Qiagen, Valencia, CA, USA). The efficiency of labeling and amount of the produced Epab or Pabpc1 probes were confirmed in comparison with a labeled control DNA in a dot blot according to the manufacturer’s instructions (Roche, Indianapolis, IN, USA). The labeled probes for the Epab or Pabpc1 mRNA were stored at −20 °C until use.
The RNA ISH method applied in the present study was modified from our previous work [6]. Briefly, paraffin sections at 5 μm thickness taken from prepubertal mouse ovaries were incubated at 60 °C for 1 h. After that, sections were cleared twice with fresh xylene and rehydrated in decreasing concentrations of ethanol followed by rinsing in distilled water. Permeabilization with 5 μg/ml proteinase K (Roche, Indianapolis, USA) in PBS with 0.1 % Tween 20 (PBST) at 37 °C for 30 min was followed by postfixation in 4 % paraformaldehyde in PBS for 5 min at room temperature. After washing with PBST, sections were incubated in prehybridization buffer [50 % formamide, 5 mM EDTA, 4X SSC, 0.1 % Tween 20, 1 % blocking reagent (Roche, Indianapolis, IN, USA), 100 μg/ml yeast tRNA (Ambion, Austin, Texas, USA), 0.5 % CHAPS (Sigma-Aldrich, St. Louis, MO, USA)] at 37 °C for 2 h. Hybridization with 300 ng/ml of digoxigenin-labeled Epab or Pabpc1 probes was carried out at 42 °C overnight. It is important to note that for negative control, certain sections were hybridized with digoxigenin-unlabeled Epab or Pabpc1 probes instead of labeled probes in each experiment. After hybridization, sections were washed twice in 2X SSC, and then twice in 0.1X SSC for 20 min at 65 °C, and twice in MABT [0.1 M maleic acid (Sigma-Aldrich, St. Louis, MO, USA), 0.15 M NaCl, pH 7.5, 0.1 % Tween 20] for 10 min at room temperature. Blocking was conducted in 1 % (wt/vol) blocking reagent (Roche, Indianapolis, IN, USA) in MAB [0.1 M maleic acid (Sigma-Aldrich, St. Louis, MO, USA)/0.15 M NaCl] for 30 min at room temperature. After that sections were incubated in anti-digoxigenin antibody conjugated to alkaline phosphatase (1:800; Roche, Indianapolis, IN, USA) for 1 h at room temperature. After six times 10-min washes with MABT, sections were equilibrated in NTMT buffer [0.1 M NaCl, 0.1 M Tris-HCl, 50 mM MgCl2 (Sigma-Aldrich, St. Louis, MO, USA), 0.1 % Tween 20] and incubated with 5-bromo-4-chloro-3-indolyl phosphate (BCIP)/nitroblue tetrazolium (NBT) in NTMT (Roche, Indianapolis, IN, USA) for approximately 16 h. Color development was stopped with several washes in PBST, and sections were mounted with Entellan (Merck Millipore, Massachusetts, USA) using cover glasses after dehydration in increasing ethanol series and cleared in xylene (Thermo Shandon, Pittsburgh, PA, USA). Finally, sections were analyzed under a bright-field microscope (Carl Zeiss Inc. Thornwood, NY, USA).
RT-PCR reaction for the cumulus cells
To confirm RNA in situ hybridization findings on presence of the Epab mRNA expression in the granulosa cells of growing ovarian follicles, we isolated cumulus-oocyte complexes (COCs). For this purpose, 6-week old BALB/c female mice were superovulated with 5 IU pregnant mare serum gonadotropin (PMSG), and 48 h later 5 IU human chronic gonadotropin (hCG) were injected intraperitoneally. After 14 h of hCG injection, the COCs were collected from oviducts by using a mouth-controlled pipette under a dissecting microscope (Zeiss, Oberkochen, Germany). By incubating the COCs with a 1 mg/ml hyaluronidase solution (Sigma-Aldrich, St. Louis, MO, USA), the cumulus cells and MII oocytes were enzymatically separated.
Total RNA isolation from cumulus cells and MII oocytes were carried out using RNAqueous Microkit (Ambion, Austin, Texas, USA) according to instructions of the manufacturer. The DNase treatment and reverse transcription reaction steps of the extracted RNA were performed as described above. PCRs were performed on cDNAs using Taq DNA polymerase (Qiagen, Valencia, CA, USA). Amplifications were carried out with 35 cycles of PCR, in which the initial 5 min denaturation at 95 °C was followed by a “touch-down” program for 10 cycles of 92 °C for 30 s, 65 °C for 30 s (−1 °C per cycle), and 72 °C for 30 s, and final extension at 72 °C for 10 min, in a volume of 25 μl reaction mixture containing 10X PCR buffer (Qiagen, Valencia, CA, USA), 0.125 mM of each dNTP (Roche, Indianapolis, IN, USA), 0.5 μM of each primer (Keck Facility, CT, USA), and 2 units of Taq DNA polymerase (Qiagen, Valencia, CA, USA). All PCR products were separated on 1.5 % agarose/TBE gels and visualized by ethidium bromide staining. All primers used here are given in Table 1.
Statistical analysis
The one-way ANOVA test was used to test the differences among postnatal mouse ovaries. Statistical calculations were carried out using SigmaStat for Windows, version 3.0. The value of P < 0.05 was considered to be statistically significant.
Results
In the present study, expression levels and localization of the Epab and Pabpc1 genes were first evaluated in the postnatal mouse ovaries. Findings revealed that these genes were differentially expressed during postnatal ovary developmental periods.
Epab and Pabpc1 gene expression differ in the postnatal mouse ovaries
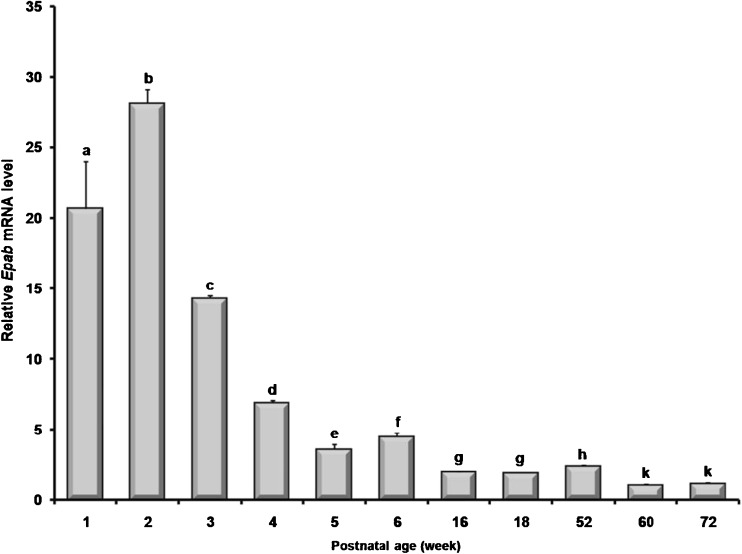
When expression levels of the Epab mRNA in the prepubertal (1-, 2-, and 3-week), pubertal (4-, 5-, and 6-week), postpubertal (16- and 18-week), and aged ovaries (52-, 60-, and 72-week) were analyzed, it was observed a significant higher level in the prepubertal ovaries (P < 0.05; Fig. 1). In the pubertal and postpubertal ovaries, the Epab mRNA levels gradually declined from 4-week to 18-week, except for the 6-week ovary that exhibited a significant increase in the Epab expression compared with the 5-week ovary. It is important to note that the 60- and 72-week old ovaries transcribed the Epab mRNA at the very lowest levels when compared to other postnatal ages (P < 0.05; Fig. 1). Similarly, there was slightly, but significantly increased Epab expression in the 52-week ovary than those of postpubertal ovaries (P < 0.05; Fig. 1).
Fig. 1.
Expression levels of the Epab mRNA in the postnatal mouse ovaries. The Epab gene expression was normalized to β-actin levels, and the relative expression profiles of the target gene were calculated by using the 2-ΔΔCt (cycle threshold) method. The 60-week was used as a calibrator, and its values were set to 1. The findings revealed that prepubertal mouse ovaries had significantly higher Epab gene expression when compared to later developmental terms. In general, Epab gene expression was gradually decreased during later developmental periods and reached its lowest levels in the aged mouse ovaries. The qRT-PCR results were given as mean ± SD (standard deviation). The P value of <0.05 was considered significant. Different letters depict statistical difference
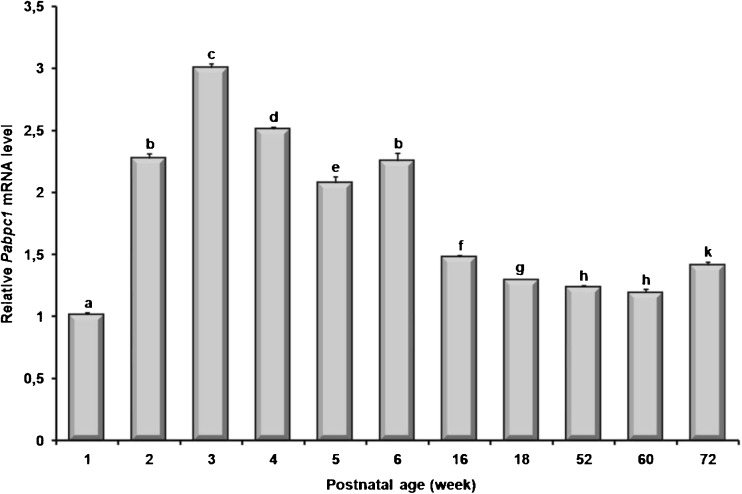
The Pabpc1 mRNA expression was found to be at higher levels in both prepubertal and pubertal ovaries and it was reasonably decreased in the postpubertal and aged ovaries (P < 0.05; Fig. 2). Interestingly, the 1-week ovary had the lowest Pabpc1 mRNA expression when compared to other ovarian developmental stages (P < 0.05; Fig. 2).
Fig. 2.
Expression levels of the Pabpc1 mRNA in the postnatal mouse ovaries. The Pabpc1 gene expression was normalized to β-actin levels, and the relative expression profiles of the target gene in the postnatal ovaries were calculated by using the 2-ΔΔCt (cycle threshold) method. The 1-week was used as a calibrator, and was set to 1. The findings revealed that both prepubertal and pubertal mouse ovaries possessed significantly higher Pabpc1 gene expression, except for 1-week ovary. Generally, Pabpc1 gene expression was gradually decreased during postpubertal development periods. The most interesting finding of this analysis was that the 1-week ovary had the lowest Pabpc1 expression among postnatal ovaries tested here. The results were given as mean ± SD (standard deviation). The P value of <0.05 was considered significant. Different letters depict statistical difference
Epab and Pabpc1 mRNA localization in the prepubertal mouse ovaries
Since Epab mRNA was highly expressed in the prepubertal ovaries at the ages of 1-, 2-, and 3-week of development, we examined cellular and subcellular localizations of the Epab and Pabpc1 mRNA in the prepubertal ovaries. Thus, the spatial and temporal expression pattern of the Epab and its relationship with Pabpc1 was first assayed in the early ovaries.
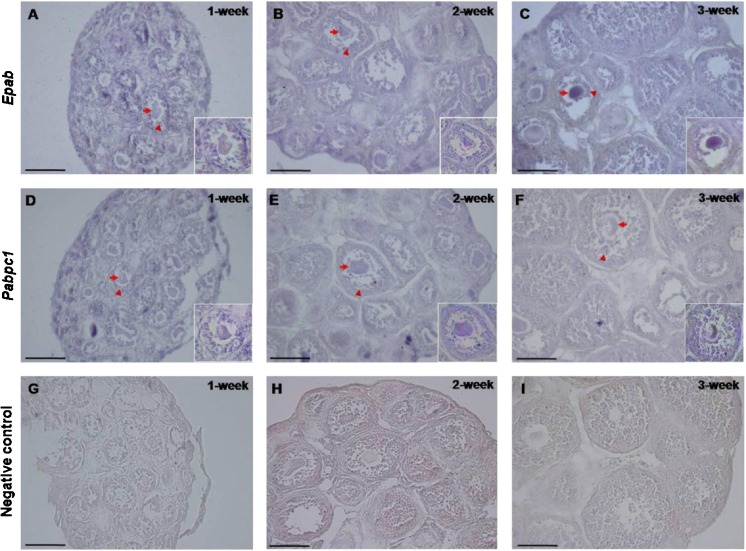
RNA in situ hybridization localized the Epab mRNA in the follicles from primordial to preantral follicles, but there was no expression present in the stromal cells of the prepubertal ovaries tested here (Fig. 3). Notably, as antral follicles were not found in the prepubertal mouse ovaries, they were not analyzed for Epab mRNA content. In all follicles, both Epab and Pabpc1 mRNA were cytoplasmically resided in the oocytes and granulosa cells. Since PABPC1 functions as a general cytoplasmic poly(A)-binding protein in somatic and germ cells, it was also transcribed in the stromal cells of prepubertal ovaries as expected (Fig. 3).
Fig. 3.
Localizations of the Epab (A, B and C) and Pabpc1 (D, E and F) mRNA in the prepubertal mouse ovaries. Having found higher Epab expression in the prepubertal mouse ovaries at the ages of 1-, 2-, and 3-week, localizations of the Epab and Pabpc1 mRNA were analyzed. RNA in situ hybridization demonstrated that the Epab mRNA was localized to cytoplasm of oocytes and granulosa cells of the follicles from primordial to preantral stages. Similarly, all follicles and also stromal cells had the Pabpc1 mRNA expression. It is important to note that there was no reaction in the negative control sections (G, H, and I) established for each experiment. The arrows depict oocytes including Epab or Pabpc1 mRNA, and the arrowheads demonstrate granulosa cells expressing these genes. Note that all pictures were taken at X400 original magnification, and inserts were at X600 magnification. The scale bar represents 50 μm
Follicles were counted in the postnatal mouse ovaries
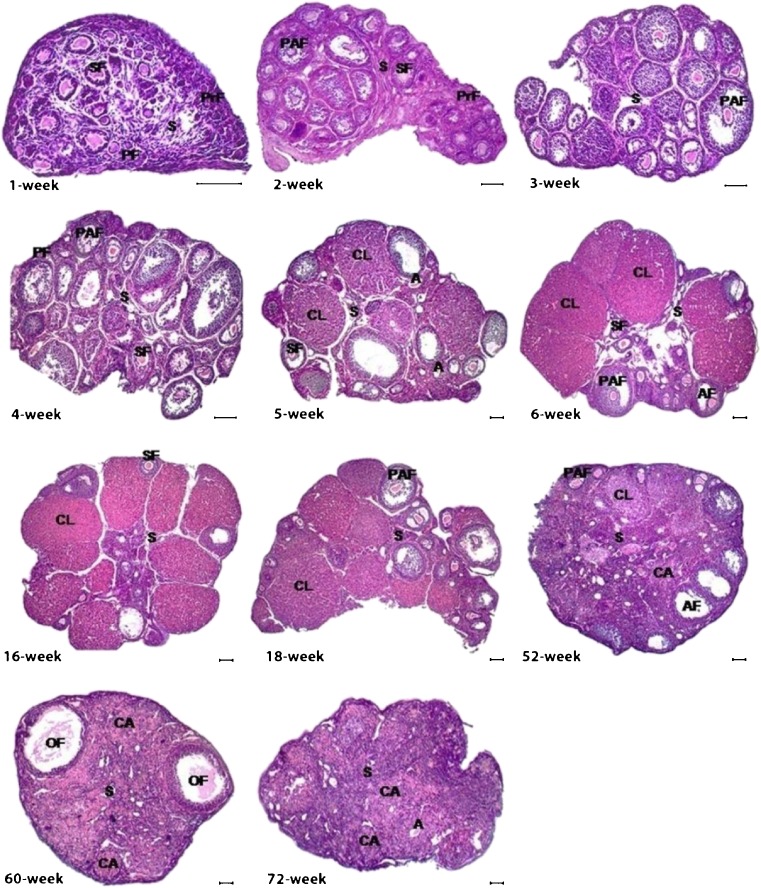
The HE-stained sections of the postnatal mouse ovaries were analyzed to evaluate their histological structures and to count the follicles from primordial to antral follicles. Since RNA in situ hybridization localized the Epab mRNA in all follicles from primordial to antral follicles, we have counted all follicle stages to assess the relationship between Epab mRNA expression and follicle numbers. In the 1-week ovary, histological development continued and only certain follicles such as primordial, primary and secondary follicles were observed at this stage of early development. However, in addition to the early follicles, preantral follicles began to appear, but there were no antral follicles in the 2- and 3-week ovaries (Fig. 4). The antral follicles first occurred in the 4-week ovary as well as early stage follicles, and ovarian development was largely complete at this age (Fig. 4). Based on our ovarian development time points, corpus luteum formation first appeared in the 5-week ovary (Fig. 4). Similarly, during later ovarian development periods at the 6-, 16- and 18-week, all follicular stages and corpus luteum formation were also monitored. However, remarkable changes were observed in the aged ovaries from 52-, 60-, and 72-week old mice (Fig. 4). The number of follicles and corpus luteum development in the aged ovaries predominantly fell to minimal levels, and ovarian stromal area was regressed and partially destroyed (Fig. 4). Moreover, number of both atretic follicles and corpus albicans reached relatively large amounts in comparison with former postnatal ovarian stages (Fig. 4).
Fig. 4.
Hematoxylin-eosin (HE) stained postnatal mouse ovaries. Histological structures of the postnatal ovaries were analyzed herein. All follicles from primordial to antral follicles began to occur based on the time point of ovarian development, and corpus luteum formation was first observed in 5-week ovary onward. However, in the aged ovaries number of follicles and corpus luteum remarkably decreased, and many atretic follicles existed in these stages. PrF, Primordial follicle; PF, Primary follicle; SF, Secondary follicle; PAF, Preantral follicle; OF, Ovulated follicle; A, Atretic follicle; CL, Corpus luteum; CA, Corpus albicans; S, Stroma. The scale bar represents 50 μm
We counted all follicles from primordial to antral follicles and found that populations of the follicles in the postnatal ovaries exhibited dramatic differences during aging. As expected, the total number of follicles was in the highest levels in the prepubertal ovaries (Table 2). Total follicle numbers slightly decreased in the 4-, 5-, and 6-week old ovaries, further declined in the 16-, 18-, and 52-week ovaries, and it reached the lowest levels in the 60- and 72-week ovaries (Table 2).
Table 2.
Follicle count in the postnatal ovaries
| Age of female mice | Primordial follicle | Primary follicle | Secondary follicle | Preantral follicle | Antral follicle | Total follicle |
|---|---|---|---|---|---|---|
| 1-week | 51.8 ± 16.7 | 11.6 ± 3.2 | 17.7 ± 3.4 | 0.0 ± 0.0 | 0.0 ± 0.0 | 81.1 |
| 2-week | 15.7 ± 4.1 | 5.6 ± 1.5 | 18.6 ± 4.9 | 4.8 ± 1.9 | 0.0 ± 0.0 | 44.7 |
| 3-week | 25.2 ± 7.6 | 2.3 ± 0.9 | 12.2 ± 3.9 | 16.0 ± 3.0 | 0.0 ± 0.0 | 55.7 |
| 4-week | 13.3 ± 3.1 | 4.3 ± 1.5 | 11.5 ± 4.5 | 10.3 ± 3.1 | 0.5 ± 0.7 | 39.9 |
| 5-week | 16.1 ± 5.5 | 6.7 ± 1.4 | 6.6 ± 1.7 | 7.6 ± 3.3 | 1.4 ± 0.7 | 38.4 |
| 6-week | 9.8 ± 2.6 | 9.2 ± 3.0 | 13.3 ± 4.6 | 8.3 ± 2.8 | 0.5 ± 0.5 | 41.1 |
| 16-week | 2.7 ± 1.1 | 6.7 ± 3.1 | 5.3 ± 3.7 | 5.3 ± 1.9 | 1.3 ± 1.5 | 21.3 |
| 18-week | 3.1 ± 1.9 | 4.0 ± 2.2 | 5.1 ± 2.7 | 7.6 ± 3.2 | 1.5 ± 1.3 | 21.3 |
| 52-week | 2.6 ± 1.4 | 4.6 ± 2.1 | 5.4 ± 1.2 | 4.7 ± 2.3 | 3.1 ± 1.2 | 20.4 |
| 60-week | 1.1 ± 0.7 | 3.3 ± 1.6 | 2.3 ± 1.5 | 0.4 ± 0.7 | 0.7 ± 0.8 | 7.8 |
| 72-week | 1.6 ± 0.8 | 3.6 ± 0.8 | 1.8 ± 0.8 | 0.8 ± 0.8 | 0.5 ± 0.5 | 8.3 |
Follicle counts were performed on the HE-stained serial sections of three ovaries from each week, and number of each follicle per section was given in the table. The prepubertal mouse ovaries had higher number of total follicles than that of later developmental terms. During later stages of development, total number of follicles remarkably decreased at 16-week ovary and reached its lowest levels in 60-week and 72-week old ovary
Cumulus cells expressed both Epab and Pabpc1 mRNA
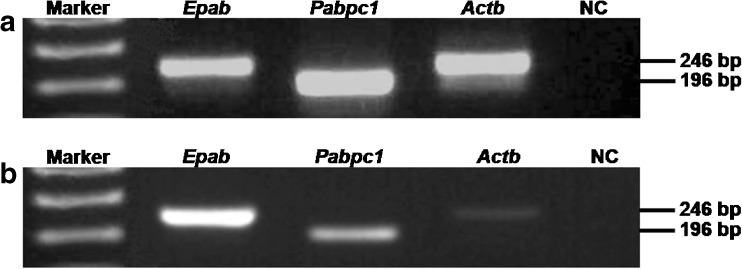
RT-PCR results revealed that cumulus cells expressed both Epab and Pabpc1 mRNA (Fig. 5). Since cumulus cells were originated from granulosa cells of the antral follicles, presence of the Epab and Pabpc1 expression in the cumulus cells suggested that granulosa cells of the antral follicles transcribed the Epab and Pabpc1 genes. As expected, MII oocytes obtained from COCs also expressed both Epab and Pabpc1 mRNA (Fig. 5).
Fig. 5.
RT-PCR analysis in cumulus cells (a) and MII oocytes (b). RNA in situ hybridization results prompted us to examine expression levels of the Epab and Pabpc1 genes in the cumulus cells and MII oocytes. Following cumulus cells and metaphase II oocytes isolation from cumulus oocyte complexes, RNA isolation and cDNA synthesis were carried out to establish RT-PCR reaction. We found that both cumulus cells and MII oocytes expressed the Epab and Pabpc1 genes. These findings confirmed that cumulus cells originated from granulosa cells transcribed the Epab mRNA as well as Pabpc1 mRNA. Epab: 246 bp in length; Pabpc1: 196 bp in length; Actb (β-actin): 244 bp in length. The 1 kb plus DNA ladder (Invitrogen, NY, USA) was used to estimate molecular mass of PCR fragments of the target genes
Discussion
In the present study, we first showed that the Epab and Pabpc1 mRNA were differently expressed in the postnatal mouse ovaries at different ages of development. Also, both Epab and Pabpc1 mRNA were cytoplasmically localized in the granulosa cells and oocytes of all follicles from primordial to preantral follicle.
Since follicular development in mammals starts early in ovarian development, synthesis and storage of maternal mRNAs in the oocytes are carried out concomitantly. Therefore, we propose that EPAB may function in stabilization and translational control of these mRNAs generated during early oogenesis. Consistent with this, Epab expression remarkably increased in the prepubertal mouse ovaries, which most probably derives from presence of higher number of follicles in these stages than that of later terms of the mouse ovaries. Similarly, slightly increased Epab and Papbc1 mRNA expression in the 6-week old ovary when compared to 5-week old ovary may result from higher content of the follicles where both Epab and Pabpc1 mRNA were found to be resided. In parallel, Epab expression gradually decreased in pubertal and postpubertal ovaries, and reached its lowest levels at ages 60- and 72-week old ovaries, in which predominant changes in the follicular content occurred.
PABPC1 is ubiquitously expressed poly(A)-binding protein and functions in stabilization and promotion of translation in somatic cells or germ cells. For proper follicular development, transcribed mRNAs in the granulosa cells and oocytes must be translated based on the time table. We think that the PABPC1 protein plays central roles in posttranscriptional regulation of the mRNAs throughout folliculogenesis and oocyte maturation. As the follicular contents of postnatal ovaries change during developmental stages, remarkable fluctuations were observed for the Pabpc1 expression in the postnatal ovaries. For example, the Papbc1 mRNA expression was lowest levels at 1-week ovaries, even lower than in aged mice ovaries. Most probably, this may derive from the expression level of the Papbc1 gene progressively increased during follicular development since the 1-week ovary had higher number of primordial follicles than other follicle types. However, this should be proved by analyzing the expression levels of the Pabpc1 gene in the isolated follicles from primordial to antral stages. Moreover, throughout ovarian development the number and gene expression characteristics of these cells probably exhibit alterations, which might lead to fluctuation in the Pabpc1 gene expression as seen in the 1-week ovary.
Studies on the Xenopus laevis [2], mouse [4], and human [5] oocytes and early embryos showed that there was a robust correlation between Epab and Pabpc1 gene expression patterns. In the present study, Epab and Pabpc1 gene exhibited different expression patterns in the postnatal mouse ovaries, indicating no prominent association between these two genes. It is important to keep in mind that the expected correlation for the Epab and Pabpc1 genes may not emerge in the total ovary evaluations, thus granulosa cells and oocytes obtained from each follicular type should be analyzed separately.
The localization of Epab mRNA in mouse ovary was first documented in a previous study [4]. In that study, Epab mRNA was detected only in oocytes of the growing follicles. In our study, we found the Epab mRNA in both granulosa cells and oocytes of the follicles in the prepubertal mouse ovaries. The remarkable differences in these two studies may arise from use of distinct probes specifically hybridized to different parts of the Epab mRNA. The RNA probe created by Seli et al. was specific to the exon 11 of the Epab gene, whereas our DNA probe was designed to specifically hybridize to the exon 8/exon 9 of the Epab mRNA. Also, the use of different mouse strains, CD1 in the previous study and BALB/c in the present study, may implicate in the different findings. However, presence of the Epab mRNA expression in the cumulus cells increases the possibility that granulosa cells of the follicles express the Epab transcript. Additionally, impaired folliculogenesis in the Epab-deficient female mice such as increased number of secondary follicles and expansion defects in the cumulus cells [7] suggests that EPAB may play critical roles in the granulosa cells throughout folliculogenesis.
The Epab-null oocytes also exhibited abnormal spindle formation and impaired chromosome alignment at the metaphase plate [7]. As is well known, meiosis in mouse oocytes begins at around embryonic day 13.5 and it is arrested at diplotene stage of meiosis I [23, 24]. These findings suggest that EPAB may play roles in spindle formation and chromosomal organization during prenatal development as well as in the postnatal developmental stages. In the current work, we found Epab mRNA expression in the cumulus cells isolated from mature mice; however, Guzeloglu-Kayisli et al. (2012) observed quite low Epab mRNA expression in the cumulus cells [7]. This difference may result from use of different techniques and use of distinct primers to amplify the Epab mRNA, since there are several Epab mRNA isoforms identified before [4, 5, 25].
Up to now, there is no antibody specific to mammalian EPAB protein; however, previously generated antibody for the Xenopus EPAB protein provided researchers to characterize the interaction between EPAB and translation related complexes. They found that EPAB associates with the CPEB1-SYMPK-CPSF [3] and DAZL-Pumilio complexes [26], both of which function in translational regulation of the maternal mRNAs. Additionally, a recently published study revealed that ePAB protein is undergone a dynamic phosphorylation at four residue cluster in Xenopus laevis oocytes, which is required for cytoplasmic polyadenylation but not for translation activation [27]. As a result the absence of a specific antibody for the mammalian EPAB protein restricts us to examine whether there is similar phosphorylation events and translation initiation complexes interacting with the EPAB protein in the mammalian oocytes and early embryos.
In conclusion, our results show that Epab and Pabpc1 gene expression differ in the postnatal mouse ovaries. This difference most probably results from dramatic changes of the follicles during postnatal ovary development periods. This suggests that these PABP proteins may play critical roles in regulation of certain mRNAs and proteins in the oocytes and granulosa cells, which should be evaluated in the future studies.
Acknowledgments
This study was supported by TUBITAK Fund (Grant No. 111S333). The authors thank to Onur Bakiner, PhD for corrections to this article.
Footnotes
Capsule Understanding importance of the PABP proteins during postnatal ovarian development would provide us to create new treatments for various types of ovarian diseases.
References
- 1.Wilkie GS, Gautier P, Lawson D, Gray NK. Embryonic poly(A)-binding protein stimulates translation in germ cells. Mol Cell Biol. 2005;25:2060–71. doi: 10.1128/MCB.25.5.2060-2071.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voeltz GK, Ongkasuwan J, Standart N, Steitz JA. A novel embryonic poly(A) binding protein, ePAB, regulates mRNA deadenylation in Xenopus egg extracts. Genes Dev. 2001;15:774–88. doi: 10.1101/gad.872201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JH, Richter JD. RINGO/cdk1 and CPEB mediate poly(A) tail stabilization and translational regulation by ePAB. Genes Dev. 2007;21:2571–9. doi: 10.1101/gad.1593007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seli E, Lalioti MD, Flaherty SM, Sakkas D, Terzi N, Steitz JA. An embryonic poly(A)-binding protein (ePAB) is expressed in mouse oocytes and early preimplantation embryos. Proc Natl Acad Sci U S A. 2005;102:367–72. doi: 10.1073/pnas.0408378102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guzeloglu-Kayisli O, Pauli S, Demir H, Lalioti MD, Sakkas D, Seli E. Identification and characterization of human embryonic poly(A) binding protein (EPAB) Mol Hum Reprod. 2008;14:581–8. doi: 10.1093/molehr/gan047. [DOI] [PubMed] [Google Scholar]
- 6.Ozturk S, Guzeloglu-Kayisli O, Demir N, Sozen B, Ilbay O, Lalioti MD, et al. Epab and Pabpc1 are differentially expressed during male germ cell development. Reprod Sci. 2012;19:911–22. doi: 10.1177/1933719112446086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzeloglu-Kayisli O, Lalioti MD, Aydiner F, Sasson I, Ilbay O, Sakkas D, et al. Embryonic poly(A)-binding protein (EPAB) is required for oocyte maturation and female fertility in mice. Biochem J. 2012;446:47–58. doi: 10.1042/BJ20120467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben-Or S. Morphological and functional development of the ovary of the mouse. I. Morphology and histochemistry of the developing ovary in normal conditions and after FSH treatment. J Embryol Exp Morpholog. 1963;11:1–11. [PubMed] [Google Scholar]
- 9.Peters H. The development of the mouse ovary from birth to maturity. Acta Endocrinol (Copenh) 1969;62:98–116. doi: 10.1530/acta.0.0620098. [DOI] [PubMed] [Google Scholar]
- 10.Oktem O, Urman B. Understanding follicle growth in vivo. Hum Reprod. 2010;25:2944–54. doi: 10.1093/humrep/deq275. [DOI] [PubMed] [Google Scholar]
- 11.Rankin T, Familari M, Lee E, Ginsberg A, Dwyer N, Blanchette-Mackie J, et al. Mice homozygous for an insertional mutation in the Zp3 gene lack a zona pellucida and are infertile. Development. 1996;122:2903–10. doi: 10.1242/dev.122.9.2903. [DOI] [PubMed] [Google Scholar]
- 12.Russell DL, Robker RL. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update. 2007;13:289–312. doi: 10.1093/humupd/dml062. [DOI] [PubMed] [Google Scholar]
- 13.Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci. 2007;120:1330–40. doi: 10.1242/jcs.000968. [DOI] [PubMed] [Google Scholar]
- 14.Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev. 2007;28:117–49. doi: 10.1210/er.2006-0022. [DOI] [PubMed] [Google Scholar]
- 15.Bachelot A, Binart N. Corpus luteum development: lessons from genetic models in mice. Curr Top Dev Biol. 2005;68:49–84. doi: 10.1016/S0070-2153(05)68003-9. [DOI] [PubMed] [Google Scholar]
- 16.Hutt KJ, Albertini DF. An oocentric view of folliculogenesis and embryogenesis. Reprod BioMed Online. 2007;14:758–64. doi: 10.1016/S1472-6483(10)60679-7. [DOI] [PubMed] [Google Scholar]
- 17.Choi Y, Rajkovic A. Genetics of early mammalian folliculogenesis. Cell Mol Life Sci. 2006;63:579–90. doi: 10.1007/s00018-005-5394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bachvarova R. Gene expression during oogenesis and oocyte development in mammals.Dev Biol (N Y 1985). 1985;1:453–524. [DOI] [PubMed]
- 19.Song JL, Wessel GM. How to make an egg: transcriptional regulation in oocytes. Differentiation. 2005;73:1–17. doi: 10.1111/j.1432-0436.2005.07301005.x. [DOI] [PubMed] [Google Scholar]
- 20.Danilovich N, Ram SM. Recent female mouse models displaying advanced reproductive aging. Exp Gerontol. 2006;41:117–22. doi: 10.1016/j.exger.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30:465–93. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- 22.Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, et al. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science. 2004;306:1947–50. doi: 10.1126/science.1103974. [DOI] [PubMed] [Google Scholar]
- 23.Pepling ME. From primordial germ cell to primordial follicle: mammalian female germ cell development. Genesis. 2006;44:622–32. doi: 10.1002/dvg.20258. [DOI] [PubMed] [Google Scholar]
- 24.Brook M, Smith JW, Gray NK. The DAZL and PABP families: RNA-binding proteins with interrelated roles in translational control in oocytes. Reproduction. 2009;137:595–617. doi: 10.1530/REP-08-0524. [DOI] [PubMed] [Google Scholar]
- 25.Guzeloglu-Kayisli O, Lalioti MD, Babayev E, Torrealday S, Karakaya C Seli E. Human embryonic poly(A)-binding protein (EPAB) alternative splicing is differentially regulated in human oocytes and embryos. Mol Hum Reprod. 2013 [DOI] [PubMed]
- 26.Padmanabhan K, Richter JD. Regulated Pumilio-2 binding controls RINGO/Spy mRNA translation and CPEB activation. Genes Dev. 2006;20:199–209. doi: 10.1101/gad.1383106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friend K, Brook M, Bezirci FB, Sheets MD, Gray NK, Seli E. Embryonic poly(A)-binding protein (ePAB) phosphorylation is required for Xenopus oocyte maturation. Biochem J. 2012;445:93–100. doi: 10.1042/BJ20120304. [DOI] [PMC free article] [PubMed] [Google Scholar]