Abstract
Background
Animal models have long been considered an important modality for studying ACL injuries. However, to our knowledge, the value of these preclinical models to study sex-related phenomena associated with ACL injury and recovery has not been evaluated.
Questions/purposes
We asked whether (1) prominent anatomic and (2) biomechanical factors differ between female and male porcine knees, particularly those known to increase the risk of ACL injury.
Methods
Eighteen intact minipig knees (nine males, nine females) underwent MRI to determine the femoral bicondylar width, intercondylar notch size (width, area and index), medial and lateral tibial slope, ACL size (length, cross-sectional area, and volume), and medial compartment tibiofemoral cartilage thickness. AP knee laxity at 30°, 60°, and 90° flexion and ACL tensile structural properties were measured using custom-designed loading fixtures in a universal tensile testing apparatus. Comparisons between males and females were performed for all anatomic and biomechanical measures. The findings then were compared with published data from human knees.
Results
Female pigs had smaller bicondylar widths (2.9 mm, ratio = 0.93, effect size = −1.5) and intercondylar notches (width: 2.0 mm, ratio = 0.79, effect size = −2.8; area: 30.8 mm2, ratio = 0.76, effect size = −2.1; index: 0.4, ratio = 0.84, effect size = −2.0), steeper lateral tibial slope (4.3°, ratio = 1.13, effect size = 1.1), smaller ACL (length: 2.7 mm, ratio = 0.91, effect size = −1.1; area: 6.8 mm2, ratio = 0.74, effect size = −1.5; volume: 266.2 mm3, ratio = 0.68, effect size = −1.5), thinner medial femoral cartilage (0.4 mm, ratio = 0.8, effect size = −1.1), lower ACL yield load (275 N, ratio = 0.81, effect size = −1.1), and greater AP knee laxity at 30° (0.7 mm, ratio = 1.32, effect size = 1.1) and 90° (0.5 mm, ratio = 1.24, effect size = 1.1) flexion compared with their male counterparts. These differences were significant for all parameters (p ≤ 0.04). Observed sex-related differences were similar to those reported for the human knee.
Conclusions
Significant differences exist between knees of male and female pigs with respect to prominent anatomic and biomechanical factors. Our findings strongly agreed with published data regarding human knees.
Clinical Relevance
The findings highlight the use of the porcine large animal model to study the role of sex on ACL injuries and surgical outcome. This validated preclinical model may facilitate the development of novel, sex-specific interventions to prevent and treat ACL injuries for male and female patients.
Introduction
The knee is distinguished by its complex geometry and multibody articulations. Joint stability during functional activities is provided by anatomic structures including the ligaments, menisci, and articular cartilage. Among those, the ACL plays a crucial role in guiding knee motion while maintaining knee stability in multiple planes [49]. ACL injuries are common, particularly in young, active individuals participating in sports [29]. ACL injuries also are associated with long-term clinical sequelae, which include meniscal tears, chondral lesions, and an increased risk of early onset posttraumatic osteoarthritis [14, 30, 33, 42, 57]. In addition to pain, instability, and the associated long-term sequelae, an ACL injury may affect a patient’s quality of life economically and socially [35].
The risk of ACL injury has been shown to be dependent on sex, with women being at a two- to 10-fold greater risk than men when playing the same sport [4, 5, 23, 31, 36, 41, 50, 54]. The high risk of injury in women along with their increased rate of sports participation during the last three decades has led to a rapid increase in ACL injuries among women. The study of sex disparities in ACL injury has emerged as an important topic for research, especially after the Institute of Medicine report in 2001 [27] and the 2004 combined American Academy of Orthopaedic Surgeons and National Institutes of Health workshop [55].
Animal models have been long used to study ACL injuries, treatments, and associated complications [6, 7, 17, 19, 20, 22, 26, 44, 45]. Animal models provide researchers with the ability to perform invasive procedures and to obtain samples at specific times that are not possible in human trials. Precise measures of ligament structure, composition, and biomechanics often are possible only in animal models because these methods require destructive assessment. In addition, translational work through animal models typically is required to comply with regulatory requirements before proceeding to human trials. Large animal models including pigs, sheep, goats, dogs, and rabbits have been used as surrogates to study the effects of surgical intervention after ACL injury [7, 17, 19, 20, 22, 26, 44]. Among those, the porcine model has been shown to be the closest to the human based on the size and anatomy of the knee [48], functional dependence on the ACL [10], gait biomechanics [60], and similarity of hematology and wound healing characteristics [15, 38, 40]. However, it is unknown if the porcine model also could be used to study sex-related phenomena associated with ACL injury.
We hypothesized that important anatomic and biomechanical differences exist between male and female porcine knees and that these differences mimic those observed in human knees. We therefore asked whether (1) prominent anatomic and (2) biomechanical factors differ between female and male porcine knees, particularly those known to increase the risk of ACL injury.
Materials and Methods
Eighteen normal (nine males, nine females) late adolescent skeletally mature Yucatan minipigs (Coyote CCI, Douglas, MA, USA; age, 27 ± 1 months; weight, 63 ± 7 kg) were euthanized. Yucatan minipigs have been reported to reach sexual and skeletal maturity at the age of 7 to 10 months [52] and 26 to 30 months [9, 34], respectively. These animals had undergone surgery on the left knee, for which the biomechanical data were reported [39]. The right, intact limbs that did not have any surgery were used in this study. All procedures had approval of the Institutional Animal Care and Use Committee. The right limbs were harvested and imaged using a surface knee coil and a 3-T MRI scanner (TIM Trio; Siemens, Erlangen, Germany). A T2*-weighted, three-dimensional (3-D) constructive interference in steady state sequence (TR/TE/FA, 12.9 ms/6.5 ms/35°; field of view, 160 mm; matrix, 512 × 512; slice length/gap, 0.8 mm/0; average, 1) was selected [8]. This sequence produces high contrast between the soft tissues, bony geometry, and joint fluid, which facilitates manual segmentation and anatomic index measurements [37]. Specimens then were stored at −20° C for subsequent mechanical testing.
Anatomic Indices Measurement
Established anatomic risk factors for human ACL injury were measured using the MR image stack, including ACL size (length, cross-sectional area and volume) [13, 18, 32], medial femoral and tibial cartilage thickness [11, 12], posterior slope of the tibial plateau in the medial and lateral compartments [24, 25], and intercondylar notch size (width, area, and index) [3]. The bicondylar width also was quantified as a measure of overall knee size [58]. All the dimensions were measured using Onis™ Viewer 2.5 (DigitalCore, Tokyo, Japan) and Mimics® 15 software (Materialise; Ann Arbor, MI, USA), and were reported in millimeters or degrees.
ACL (Length, Cross-sectional Area, Volume)
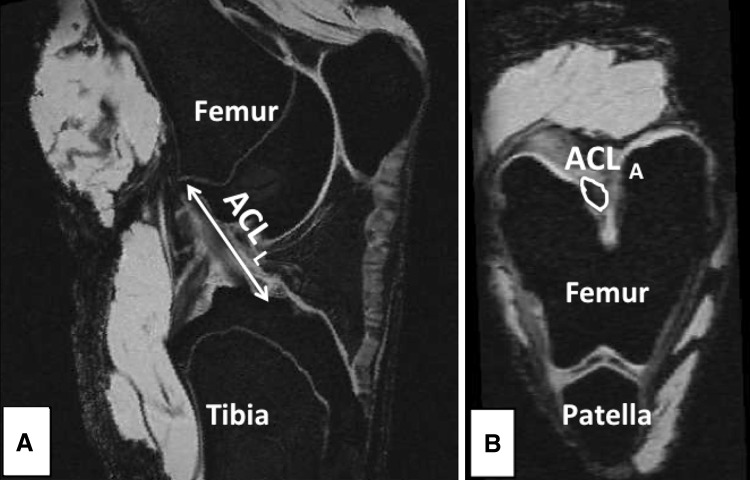
The ACL length was measured from multiple MR slices in an oblique-sagittal plane, which included the intercondylar notch and ligament insertion points. ACL length was defined as the maximum length of the line parallel to the ligament longitudinal axis connecting the center of the insertion points (Fig. 1). The ACL cross-sectional area was measured by outlining the ACL boundary on an oblique-axial view of the ligament, which was perpendicular to the oblique-sagittal plane (Fig. 1). A similar technique has been used reliably to quantify ACL cross-sectional area [32]. The area measurement was conducted at the distal 30% of the ACL ligament length, consistent with the known location of the minimum ACL cross-sectional area [32]. Ligament volume was estimated to be equal to (ACL cross-sectional area) × (ACL length) to avoid technical challenges in precise calculation of ACL volume owing to its nonuniform geometry.
Fig. 1A–B.
Oblique-sagittal and oblique-axial views of the knee were used to measure ACL (A) length and (B) cross-sectional area from MR images.
Articular Cartilage Thickness
The tibiofemoral articular cartilage from the medial compartment of each knee was segmented manually in the sagittal plane using Mimics® software. Once segmented, the cartilage was reconstructed, and a 3-D voxel model of each structure was created. The voxel models were wrapped with a triangular mesh to create virtual solid models [11]. Mean cartilage thickness measurements were measured in the weightbearing regions of interest of the medial femoral condyle and medial tibial plateau as previously described [11]. The regions of interests were defined using an established technique [11] to assess morphologic features of the cartilage in humans and pigs [12]. The mean cartilage thickness for each region of interest was calculated with a closest point algorithm using MATLAB® (The Mathworks Inc, Natick, MA, USA) [11]. This technique has been shown to have high reliability (mean coefficient of variability, 4%) and accuracy (mean absolute error, 4%) in quantifying tibiofemoral cartilage thickness [11].
Tibial Slope
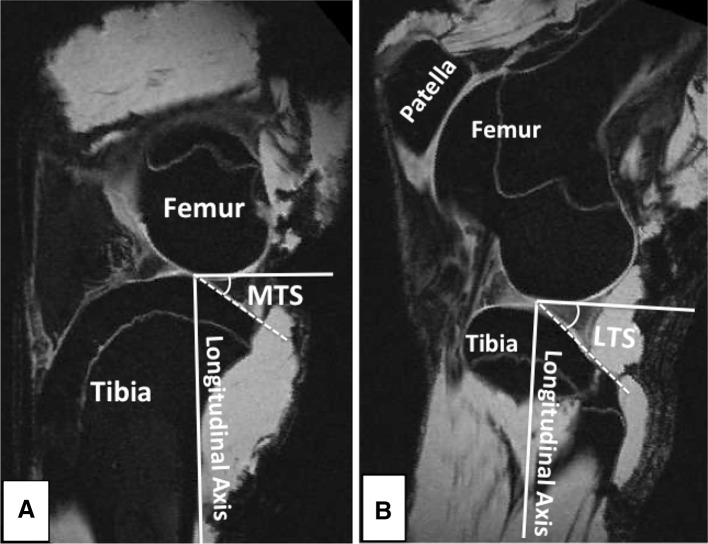
The anterior to posterior slopes of the medial and lateral tibial plateau were measured using the technique described by Hashemi et al. [24]. Briefly, an axial plane slice of the tibiofemoral joint showing the dorsal aspect of the tibial plateau was selected. Sagittal plane images from the midline of the medial and lateral tibial plateau were used to perform the tibial slope measurements. The longitudinal (diaphyseal) axis of the tibia was established by connecting the midpoints of the two horizontal lines in the sagittal plane across the midshaft of the tibia. Finally, the posterior slope of the tibial plateau across each compartment was measured as the angle between a line connecting the peak points on the anterior and posterior aspects of the plateau and the line perpendicular to the longitudinal axis (Fig. 2). This method has been shown to be able to quantify the tibial slope with a sensitivity of 1° [24].
Fig. 2A–B.
Sagittal plane MR images were used to measure the posterior slope of the tibial plateau across the (A) medial and (B) lateral compartments. MTS = medial tibial slope; LTS = lateral tibial slope.
Femoral Condyle (Bicondylar Width, Intercondylar Notch Size)
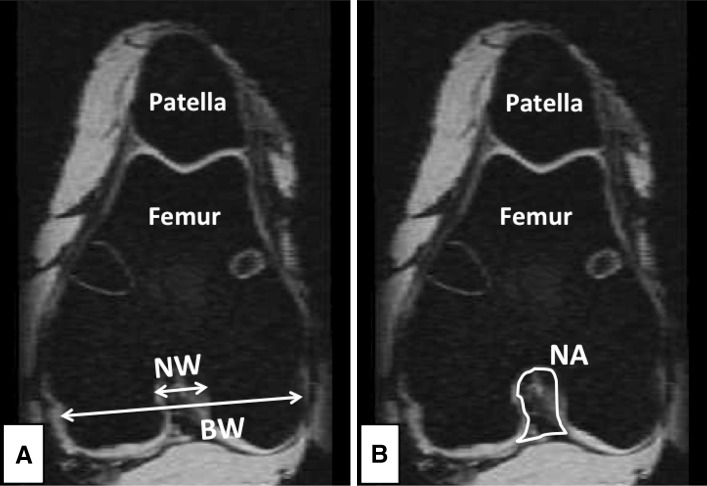
The sagittal plane images were used to identify the ideal axial plane slice to measure femoral bicondylar width, intercondylar notch width, and area. Blumensaat’s line was marked for each knee and the beginning (anterodistal) side of the line was used as the reference to select the axial plane image [3]. The oblique-axial view of the femoral condyle was used for these measurements (Fig. 3). The bicondylar and intercondylar notch widths were measured at the level of popliteal recess [3, 32] on a line perpendicular to the notch depth and parallel to the line connecting the ventral articular surfaces of the medial and the lateral femoral condyle (Fig. 3). The intercondylar notch area was measured by outlining the outer boundary of the notch space using the oblique-axial view as described by Dienst et al. [18] (Fig. 3). The intercondylar notch width index was defined as the ratio between the notch width and bicondylar width [3].
Fig. 3A–B.
(A) Femoral bicondylar width, intercondylar notch width, and (B) intercondylar notch area were measured in the oblique-axial view of the femoral condyle obtained from MR images. BW = bicondylar width; NW = notch width; NA = notch area.
Biomechanical Testing
The knees were thawed to room temperature 24 hours before testing. Specimens were sectioned at the proximal femur and distal tibia with all soft tissues external to the joint capsule removed. The distal tibia and proximal femur then were potted in polyvinyl chloride tubes with epoxy (SmoothCast® 300; Smooth-On Inc, Easton, PA, USA) for rigid attachment to the testing frame (MTS 810; Material Testing Systems, Prairie Eden, MN, USA) [22]. The joints were wrapped in towels saturated with physiologic saline to prevent dehydration. Investigators were blinded to the sex of the animal specimens during preparation and testing.
AP Knee Laxity
AP knee laxity values were measured at 30°, 60°, and 90° flexion angles [21]. The knees were locked at each flexion angle with axial tibial rotation constrained in neutral position, whereas tibial translation and rotation were unconstrained in the coronal plane [22]. The knees were subjected to 12 cycles of ± 40 N AP shear load at each specific flexion angle using the testing frame while the AP displacements were measured. The first three cycles were used to precondition the knees, while the data from the remaining nine cycles were used and averaged for final measurements. AP knee laxity was defined as the overall translational motion of the femur with respect to the tibia in AP shear load limits of ± 30 N [21, 22].
ACL Structural Properties
After the laxity assessment, all remaining soft tissues were dissected from the joint leaving the ACL intact. The femur-ACL-tibia constructs then were secured in a custom-designed tensile testing fixture so that the mechanical axis of the ACL was collinear with the load axis of the test frame [22]. The femoral rotation was unconstrained and the tibia was connected to the test frame through a sliding X-Y table helping the specimen to seek its own physiologic position under tensile loading. Specimens were loaded in tension to failure at 20 mm/minute. The rate of 20 mm/minute was adapted from established protocols for testing the tensile properties of bone-ligament-bone constructs in minipig and canine models [22, 26, 28, 59]. The recorded load-displacement data were used to quantify ACL linear stiffness, yield, and maximum load [28].
Statistical Analysis
The differences for all measured outcomes between female and male pigs were compared statistically using an independent sample t-test. Measurements for each sex were further normalized to the pooled male and female values and compared between the sexes to better characterize the role of the sex on quantified parameters. Comparisons were considered statistically significant for a probability of 0.05 or less. Cohen’s d test was used to measure the sex effect size on each of the quantified outcomes. The effect size (d) was classified as small (|d| ≤ 0.2), moderate (0.2 < |d| < 0.5), or large (|d| ≥ 0.5) [16]. The ratio between females and males for each variable was defined using the following equation to compare the sex-related variations in quantified anatomic and biomechanical factors with published data on human knees:
Results
Anatomic Indices
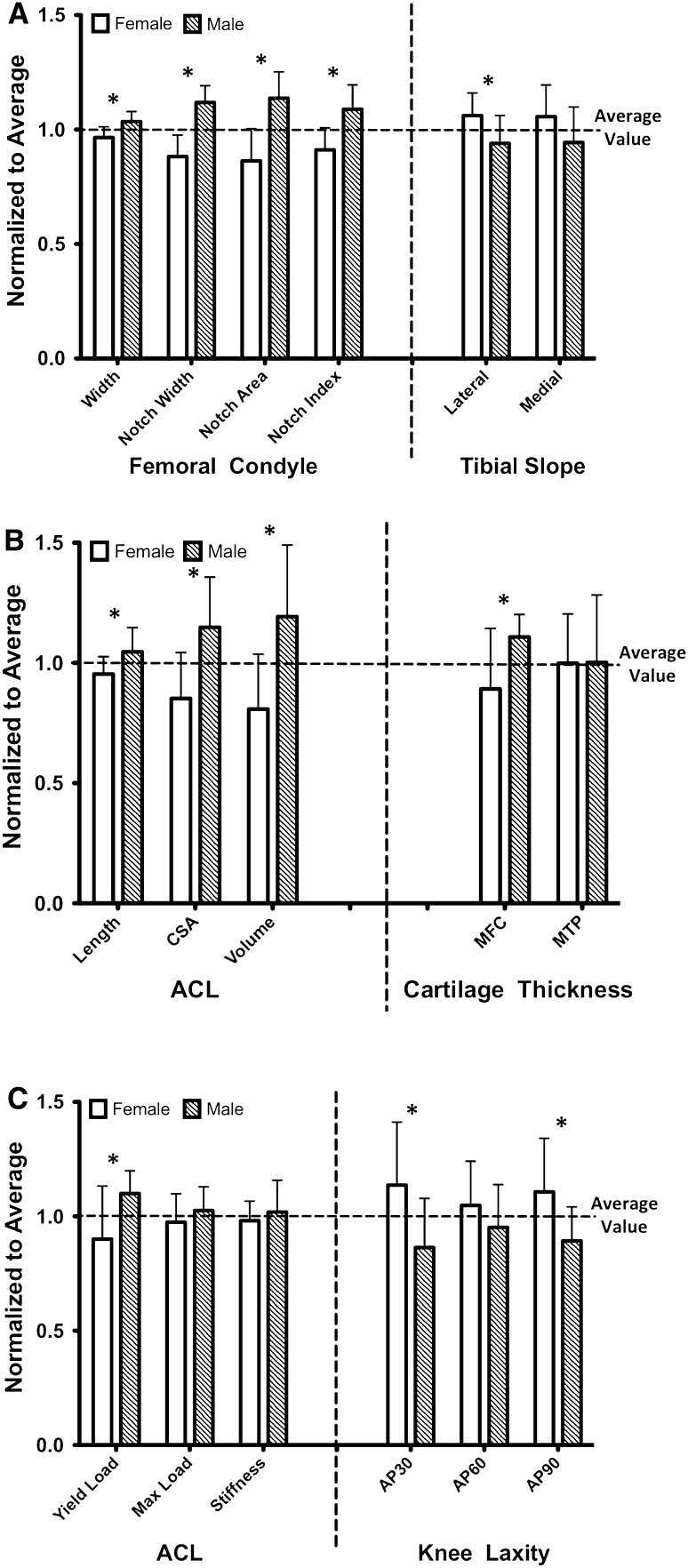
Sex was shown to have large effects on all the anatomic measured variables (effect size > 0.7) except for average cartilage thickness across the medial tibial plateau (Table 1). Males had larger bicondylar width (2.9 ± 0.9 mm; ratio, 0.93; effect size, −1.5; p = 0.005), intercondylar notch width (2.0 ± 0.3 mm; ratio, 0.79; effect size, −2.8; p < 0.001), area (30.8 ± 6.8 mm2; ratio, 0.76; effect size, −2.1; p < 0.001), and index (0.4 ± 0.0; ratio, 0.84; effect size, −2.0; p = 0.002) compared with females. Females had a steeper posterior tibial slope on the lateral side (4.3˚± 1.8˚; ratio, 1.13; effect size, 1.1; p = 0.034) than males. Medially, the mean value of the posterior tibial slope was not different between females and males (difference, 3.3˚± 2.0˚; ratio, 1.12; effect size, 0.8; p = 0.12). Females had thinner cartilage across the middle weightbearing region of the medial femoral condyle (0.4 ± 0.1 mm; ratio, 0.80; effect size, −1.1; p = 0.028). No differences were observed in the average articular cartilage thickness across the medial tibial plateau between the male and female pigs (difference, 0.0 ± 0.1 mm; ratio, 1.0; effect size, −0.1; p = 0.978). Males had a larger ACL than females with respect to length (2.7 ± 1.2 mm; ratio, 0.91; effect size, −1.1; p = 0.04), cross-sectional area (6.8 ± 2.2 mm2; ratio, 0.74; effect size, −1.5; p = 0.006), and volume (266.2 ± 86.7 mm3; ratio, 0.68; effect size, −1.5; p = 0.007). Overall, among anatomic factors, sex had the biggest effect size on intercondylar notch width and lowest effect on medial tibial cartilage thickness. Normalized bicondylar width, intercondylar notch size (width, area, and index), ACL size (length, cross-sectional area, and volume), and medial femoral cartilage thickness were smaller in females than males (bicondylar width, p = 0.005; notch size, p ≤ 0.002; ACL size, p ≤ 0.04; medial femoral cartilage thickness, p = 0.028). However, females had a steeper normalized lateral tibial slope than males (p = 0.034; Fig. 4).
Table 1.
Quantified anatomic and structural variables (mean ± SD)
| Measured factor | Mean ± SD | p value | Female:male ratio | Effect size (d) | ||
|---|---|---|---|---|---|---|
| Female (n = 9) | Male (n = 9) | Combined (n = 18) | ||||
| Bicondylar width (mm) | 41.5 ± 2.0 | 44.5 ± 1.9 | 42.9 ± 2.4 | 0.005 | 0.93 | −1.53 |
| Notch width (mm) | 7.5 ± 0.8 | 9.5 ± 0.6 | 8.5 ± 1.2 | < 0.001 | 0.79 | −2.78 |
| Notch area (mm2) | 96.9 ± 15.8 | 127.7 ± 12.9 | 112.3 ± 21.1 | < 0.001 | 0.76 | −2.13 |
| Notch width index | 0.18 ± 0.02 | 0.22 ± 0.02 | 0.20 ± 0.03 | 0.002 | 0.84 | −2.00 |
| Medial tibial slope (degrees) | 31.1 ± 4.1 | 27.8 ± 4.6 | 29.5 ± 4.5 | 0.124 | 1.12 | 0.77 |
| Lateral tibial slope (degrees) | 37.1 ± 3.5 | 32.8 ± 4.3 | 34.9 ± 4.4 | 0.034 | 1.13 | 1.09 |
| ACL length (mm) | 28.2 ± 2.1 | 30.9 ± 2.9 | 29.6 ± 2.9 | 0.04 | 0.91 | −1.05 |
| ACL area (mm2) | 19.6 ± 4.4 | 26.5 ± 4.9 | 23.0 ± 5.7 | 0.006 | 0.74 | −1.48 |
| ACL volume (mm3) | 558.5 ± 158.2 | 824.7 ± 206.3 | 691.6 ± 224.8 | 0.007 | 0.68 | −1.45 |
| Medial femoral cartilage thickness (mm) | 1.50 ± 0.42 | 1.86 ± 0.16 | 1.68 ± 0.36 | 0.028 | 0.80 | −1.13 |
| Medial tibial cartilage thickness (mm) | 1.06 ± 0.22 | 1.07 ± 0.30 | 1.06 ± 0.25 | 0.98 | 1.00 | −0.04 |
| ACL yield load (N) | 1244 ± 320 | 1519 ± 137 | 1381 ± 278 | 0.031 | 0.81 | −1.12 |
| ACL maximum load (N) | 1660 ± 192 | 1778 ± 193 | 1720 ± 196 | 0.215 | 0.93 | −0.61 |
| ACL stiffness (N/mm) | 257.5 ± 37.8 | 273.8 ± 18.8 | 265.6 ± 30.2 | 0.265 | 0.94 | −0.58 |
| AP laxity at 30° (mm) | 2.7 ± 0.6 | 2.0 ± 0.5 | 2.4 ± 0.7 | 0.033 | 1.32 | 1.10 |
| AP laxity at 60° (mm) | 3.2 ± 0.6 | 2.8 ± 0.6 | 3.0 ± 0.6 | 0.3 | 1.10 | 0.51 |
| AP laxity at 90° (mm) | 2.8 ± 0.6 | 2.2 ± 0.4 | 2.5 ± 0.5 | 0.034 | 1.24 | 1.11 |
Fig. 4A–C.
Measurements for each sex were normalized to the pooled male and female values (mean ± SD) to better characterize the role of the sex on quantified (A) bony geometry, (B) ACL and cartilage geometry, and (C) structural variables. Variables that are different (p < 0.05) between the males and females are indicated by an asterisk. CSA = cross-sectional area; MFC = medial femoral condyle; MTP = medial tibial plateau.
Biomechanical Factors
All the ACL ruptures occurred at the ligament midsubstance with no signs of bony avulsions. The ACLs from the male knees had a higher yield load than females (275 ± 116 N; ratio, 0.81; effect size, −1.1; p = 0.031) (Table 1). The mean values for the ACL maximum load (difference, 117 ± 91 N; ratio, 0.93; effect size, −0.6; p = 0.215) and linear stiffness (difference, −16.3 ± 14.1 N/mm; ratio, 0.94; effect size, −0.5; p = 0.265) were not different between males and females. Males showed lower AP knee laxity at 30° (0.7 ± 0.3 mm; ratio, 1.32; effect size, 1.1; p = 0.033) and 90° flexion (0.5 ± 0.2 mm; ratio, 1.24; effect size, 1.1; p = 0.034) compared with females. No differences in AP knee laxity at 60° flexion were observed between male and female knees (difference, 0.3 ± 0.3 mm; ratio, 1.10; effect size, 0.5; p = 0.3). Overall, for biomechanical properties, ACL yield load was the most influenced by sex, whereas the AP knee laxity at 60° flexion was the least affected factor by sex (Table 1). Females also had a smaller normalized ACL yield load (p = 0.031) and a greater normalized knee laxity at 30° (p = 0.033) and 90° (p = 0.034) flexion than males (Fig. 4).
Discussion
Disproportionately higher rates of ACL injuries in females than males has been the focus of multiple studies [4, 5, 23, 31, 36, 41, 50, 54]. Despite the well-described role of sex on ACL injury risk, the role of sex on ACL injury treatment outcomes has been a topic of considerable debate with inconclusive findings [1, 43, 47, 51]. The discrepancy in these results has been attributed mainly to the challenges associated with clinical studies of human cohorts, including relatively short followup and lack of outcome measures with sufficient sensitivity to detect sex-specific differences. Instead, the use of large animal models (ie, porcine) has been shown to be a plausible option in the study of ACL injuries and surgical treatments [21, 22, 34, 39, 48]. These preclinical models provide researchers with the ability to perform invasive procedures and to measure the relevant outcomes of interest with high accuracy that are challenging, if not impossible, in human trials. More importantly, porcine knees have posttraumatic osteoarthritis develop after ACL injury in a pattern similar to that reported in humans, but at a faster rate, with the findings at 6 or 12 months in the porcine model similar to those seen at 10 to 15 years after ACL reconstruction in humans [2, 39, 57]. However, little is known regarding the validity and clinical utility of such models in investigation of ACL-related complications regarding sex. Therefore, we examined the sensitivity of the porcine large animal model to sex-related differences in ACL-relevant anatomic and structural properties. In this study, the female knees were smaller with steeper lateral tibial slope, thinner medial femoral cartilage, lower ACL yield load, and greater laxity (at 30° and 90°) compared with their male counterparts. Findings agreed with those of previous published data on human knees (Table 2). These results support our hypotheses and further highlight the clinical use of the porcine knee as a plausible surrogate model for the human knee when studying phenomena related to anatomic and biomechanical sex-specific differences.
Table 2.
Sex-related differences in porcine large animal model compared with reported human data*
| Measured factor | Parameter | Porcine (mean ± SD) | Human (published data) | ||
|---|---|---|---|---|---|
| Mean ± SD | Number | Reference | |||
| Bicondylar width (mm) | Females | 41.5 ± 2.0 | 67.8 ± 4.3 | 10 | Lipps et al. [32] |
| Males | 44.5 ± 1.9 | 73.8 ± 3.9 | 10 | ||
| Ratio | 0.93 | 0.92 | |||
| Effect size | −1.53 | −1.46 | |||
| Notch width (mm) | Females | 7.5 ± 0.8 | 20.5† | 50 | Anderson et al. [3] |
| Males | 9.5 ± 0.6 | 23.7† | 50 | ||
| Ratio | 0.79 | 0.86 | |||
| Effect size | −2.78 | ǂ | |||
| Notch width index | Females | 0.18 ± 0.02 | 0.30† | 50 | Anderson et al. [3] |
| Males | 0.22 ± 0.02 | 0.31† | 50 | ||
| Ratio | 0.84 | 0.98 | |||
| Effect size | −2.00 | ǂ | |||
| Medial tibial slope (degrees) | Females | 31.1 ± 4.1 | 6.8 ± 3.6 | 27 | Hashemi et al. [25] |
| Males | 27.8 ± 4.6 | 5.6 ± 2.7 | 22 | ||
| Ratio | 1.12 | 1.15 | |||
| Effect size | 0.77 | 0.28 | |||
| Lateral tibial slope (degrees) | Females | 37.1 ± 3.5 | 8.4 ± 2.8 | 27 | Hashemi et al. [25] |
| Males | 32.8 ± 4.3 | 7.2 ± 2.7 | 22 | ||
| Ratio | 1.13 | 1.16 | |||
| Effect size | 1.09 | 0.44 | |||
| ACL length (mm2) | Females | 28.2 ± 2.1 | 27.0 ± 2.9 | 9 | Chandrashekar et al. [13] |
| Males | 30.9 ± 2.9 | 29.6 ± 2.7 | 8 | ||
| Ratio | 0.91 | 0.91 | |||
| Effect size | −1.05 | −0.91 | |||
| ACL area (mm2) | Females | 19.6 ± 4.4 | 57.3 ± 15.7 | 9 | Chandrashekar et al. [13] |
| Males | 26.5 ± 4.9 | 72.9 ± 18.9 | 8 | ||
| Ratio | 0.74 | 0.78 | |||
| Effect size | −1.48 | −0.89 | |||
| ACL volume (mm3) | Females | 558.5 ± 158.2 | 1996 ± 530 | 9 | Chandrashekar et al. [13] |
| Males | 824.7 ± 206.3 | 2722 ± 708 | 8 | ||
| Ratio | 0.68 | 0.73 | |||
| Effect size | −1.45 | −1.16 | |||
| Medial femoral cartilage thickness (mm) | Females | 1.50 ± 0.42 | 1.29 ± 0.28 | 40 | Otterness and Eckstein [46] |
| Males | 1.86 ± 0.16 | 1.71 ± 0.35 | 57 | ||
| Ratio | 0.80 | 0.75 | |||
| Effect size | −1.13 | −1.32 | |||
| Medial tibial cartilage thickness (mm) | Females | 1.06 ± 0.22 | 1.57 ± 0.25 | 40 | |
| Males | 1.07 ± 0.30 | 1.88 ± 0.26 | 57 | ||
| Ratio | 1.00 | 0.84 | |||
| Effect size | −0.04 | −1.22 | |||
| ACL yield load (N) | Females | 1244 ± 320 | 1266 ± 527 | 9 | Chandrashekar et al. [13] |
| Males | 1519 ± 137 | 1818 ± 699 | 8 | ||
| Ratio | 0.81 | 0.69 | |||
| Effect size | −1.12 | −0.89 | |||
| Knee AP laxity | Females | 2.7 ± 0.6 | 13.5 ± 3.6 | 10 | Shultz et al. [53] |
| Males | 2.0 ± 0.5 | 10.6 ± 1.2 | 10 | ||
| Ratio | 1.32 | 1.27 | |||
| Effect size | 1.10 | −1.08 | |||
* Female:male ratio and effect size values for humans are calculated based on reported data; †no SD was reported in the reference; ǂeffect size was not calculated because the SD was not reported in the reference.
There are potential shortcomings to our study. The pig model has some limitations common to all animal models of knee surgery. The pig is a quadruped and therefore does not fully represent the human condition. The investigations were conducted only on adolescent pigs (27 months old). Future studies are essential to warrant whether the current findings will translate to younger (skeletally immature) and older animals, because musculoskeletal development and maturity are affected by age. The sex-related variations have been investigated only on prominent structural factors. Further studies are required to explore whether the porcine is a valid large animal model to study knee sex-related disparities regarding biologic features (ie, genetic, hormonal, and inflammatory responses). Although the means for medial tibial slope, medial tibial cartilage thickness, ACL maximum load and linear stiffness, and AP laxity at 60º were not statistically significant between males and females, a larger sample size may be required; however, a post hoc power analysis with a nominal alpha of 0.05 indicated that the power for all anatomic and biomechanical factors was 0.9 or greater, except for ACL yield load and lateral tibial slope which were 0.75 or greater. Finally, the measurements conducted on pigs and reported data for human knees were not compared statistically because the human data were published only in means and standard deviations. However, we do not believe that any of these limitations would affect the reported findings qualitatively with respect to the role of sex on the investigated anatomic and biomechanical factors.
The anatomic differences observed in male and female porcine knees mimic those reported for human knees [3, 13, 25, 32, 46, 53]. In a cadaveric study of 20 age-, height-, and weight-matched human knees (10 females, 10 males), Lipps et al. [32] reported a significantly smaller femoral bicondylar width in females than males (ratio, 0.92). Our findings also showed a smaller bicondylar width in females with a ratio of 0.93. The bicondylar width measurements were obtained from coronal (Lipps et al.) and axial (current study) plane MR images at the popliteal recess level. Anderson et al. [3] reported a significantly smaller intercondylar notch size (width and index) in female subjects compared with males in 100 high school basketball players. They showed female:male ratios of 0.86 and 0.98, respectively for the intercondylar notch width and notch width index. Their findings correspond to the ratios of 0.79 (notch width) and 0.84 (notch width index) in our study. In both studies, intercondylar notch measurements were obtained from axial plane MR images at the popliteal recess level. For posterior tibial slope, Hashemi et al. [25] reported a significantly steeper posterior slope of the tibial plateau in the medial and lateral compartments in females compared with males, with a female:male ratio of 1.15 (medial) and 1.16 (lateral) in a case-control study of 104 subjects. Our findings followed those of Hashemi et al. with the female:male ratios of 1.12 (medial) and 1.13 (lateral) in pigs. A similar measurement approach using axial and sagittal MR images to quantify the tibial slope was used in both studies. Otterness and Eckstein [46] reported significantly thinner articular cartilage across the medial femoral condyle and tibial plateau in females when compared with males in 97 healthy adult individuals (40 females and 57 males). They reported female:male ratios of 0.75 and 0.84 for average cartilage thickness across the medial femoral condyle and medial tibial plateau, respectively. We also found a sex-related difference in cartilage thickness but only across the medial femoral condyle (female:male ratio = 0.80). No detectable difference was observed in the average cartilage thickness across the medial tibial plateau between male and female pigs (female:male ratio = 1.00). This may be partly attributable to the age range and skeletal maturity status in the porcine and human studies. Moreover, the thinner cartilage and smaller knee in the pig model compared with the human knee make it more challenging to detect sex-related differences in tibial articular cartilage using MRI. Considering that the medial femoral condyle is the primary site where cartilage damage first appears after ACL injury, the porcine model may be a valid surrogate model to study the effect of sex on osteoarthritis of the human knee. Image segmentation and 3-D reconstruction of the articular cartilage from the MR image stack were used in both studies to quantify the average cartilage thickness.
In a cadaveric study of human knees (n = 20), Chandrashekar et al. [13] reported significantly smaller ACL (length, cross-sectional area, and volume) in females than in their male counterparts. They reported female:male ratios of 0.91 (ACL length), 0.78 (ACL cross-sectional area), and 0.73 (ACL volume), which correspond to the values of 0.91 (length), 0.74 (cross-sectional area), and 0.68 (volume) in our study. They also reported a significantly lower ACL failure load in females compared with males, with a female:male ratio of 0.69. Our findings agree with those of Chandrashekar et al., with a female:male ratio of 0.81 in the pig ACL. The male ACL was significantly larger (length, cross-sectional area and volume) than the female ACL. However, two of the mechanical properties measured (maximum load and linear stiffness) were not statistically different between the sexes. This may partly attributable to the relatively small sample size of our study. However, even with this sample size, a significant difference in ACL yield load was detected. This parameter may be important as it is where the ACL starts to accrue irreversible damage. Chandrashekar et al. used a 3-D photographic scanning system and caliper measurements to quantify the ACL size (length, cross-sectional area, and volume), whereas we used a multiplanar MRI analysis technique. Mechanical testing on femur-ACL-tibia constructs was done in both studies but with different loading rates. The human ACLs were loaded to failure at a strain rate of 100%/second, which is substantially greater than the loading rate of 20 mm/minute used in the current study. This difference in testing method may be responsible for the slight variations in observed female:male ratio of ACL mechanical properties considering the rate-dependent characteristics of ACL tissue mechanical properties [56]. Finally, for AP laxity, in a study of 20 healthy young adults, Shultz et al. [53] reported significantly greater AP knee laxity in females than males, with a female:male ratio of 1.27. We also observed greater AP knee laxity at 30° in female pigs than in males, with a ratio of 1.32. Shultz et al. reported the AP laxity was measured at 25° (± 5°) flexion under a 133 N shear force using a knee arthrometer, whereas the porcine knees in our study were tested at 30° flexion under a 30 N shear force.
Significant differences between knees in male and female pigs with respect to prominent anatomic and biomechanical factors exist. The observed sex-specific differences were compared with reported data for human knees to better assess the reliability of the porcine large animal model for studying the effect of sex on human knee injuries, particularly ACL injuries (Table 2). Bicondylar width (ratio in porcine, 0.93; ratio in human, 0.92) [32], intercondylar notch width (ratio in porcine, 0.79; ratio in human, 0.86) and index (ratio in porcine, 0.84; ratio in human, 0.98) [3], posterior slope of the tibial plateau (ratio in porcine, 1.12; ratio in human, 1.15) [25], ACL length (ratio in porcine, 0.91; ratio in human, 0.91), cross-sectional area (ratio in porcine, 0.74; ratio in human, 0.78) and volume (ratio in porcine, 0.68; ratio in human, 0.73), ACL yield load (ratio in porcine, 0.81; ratio in human, 0.69) [13], medial femoral cartilage thickness (ratio in porcine, 0.80; ratio in human, 0.75) [46], and AP knee laxity (ratio in porcine, 1.32; ratio in human, 1.27) [53] showed similar differences between human and porcine male and female knees. To our knowledge, it has not yet been reported that it is possible that these anatomic differences may influence how males and females respond differently to surgical treatments of ACL injury, including ACL reconstruction. A validated preclinical model provides the opportunity to study complex wound healing, cartilage response, and neuromuscular changes after ACL injury and surgical treatment, which are not possible with in vitro, ex vivo, or clinical models. Use of this valid, sex-specific model can help researchers and clinicians develop novel interventions and treatments to better fit each sex instead of a “one-fits-all” approach. This may lead to improved surgical outcomes after ACL injury and a decreased risk of posttraumatic osteoarthritis after ACL injury and reconstruction, especially among females.
Acknowledgments
We thank Patrick Vavken MD (Department of Orthopaedic Surgery, Boston Children’s Hospital, Harvard Medical School) and Benedikt Proffen MD (Department of Orthopaedic Surgery, Boston Children’s Hospital, Harvard Medical School) for helping with surgical procedures, Alison Biercevics BS (Department of Orthopaedics, Warren Alpert Medical School of Brown University and Rhode Island Hospital) for assistance with MRI, and David Paller MS (Rhode Island Hospital Orthopaedic Foundation), Sarath Koruprolu BS (Rhode Island Hospital Orthopaedic Foundation), and Ryan Rich BS (Rhode Island Hospital Orthopaedic Foundation) for assistance with mechanical testing.
Footnotes
Funding for this study was (MMM and BCF) received from the National Institutes of Health (RO1-AR054099 [MMM], AR056834 [BCF and MMM], and P20 GM104937 [BCF] [Bioengineering Core]) and the Lucy Lippitt Endowment.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Sports Medicine Research Laboratory, Department of Orthopaedic Surgery, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA, and the Department of Orthopaedics, Rhode Island Hospital, Providence, RI, USA.
References
- 1.Ageberg E, Forssblad M, Herbertsson P, Roos EM. Sex differences in patient-reported outcomes after anterior cruciate ligament reconstruction: data from the Swedish knee ligament register. Am J Sports Med. 2010;38:1334–1342. doi: 10.1177/0363546510361218. [DOI] [PubMed] [Google Scholar]
- 2.Ajuied A, Wong F, Smith C, Norris M, Earnshaw P, Back D, Davies A. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2014;42:2242–2252. doi: 10.1177/0363546513508376. [DOI] [PubMed] [Google Scholar]
- 3.Anderson AF, Dome DC, Gautam S, Awh MH, Rennirt GW. Correlation of anthropometric measurements, strength, anterior cruciate ligament size, and intercondylar notch characteristics to sex differences in anterior cruciate ligament tear rates. Am J Sports Med. 2001;29:58–66. doi: 10.1177/03635465010290011501. [DOI] [PubMed] [Google Scholar]
- 4.Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer: NCAA data and review of literature. Am J Sports Med. 1995;23:694–701. doi: 10.1177/036354659502300611. [DOI] [PubMed] [Google Scholar]
- 5.Arendt EA, Agel J, Dick R. Anterior cruciate ligament injury patterns among collegiate men and women. J Athl Train. 1999;34:86–92. [PMC free article] [PubMed] [Google Scholar]
- 6.Attia E, Brown H, Henshaw R, George S, Hannafin JA. Patterns of gene expression in a rabbit partial anterior cruciate ligament transection model: the potential role of mechanical forces. Am J Sports Med. 2010;38:348–356. doi: 10.1177/0363546509348052. [DOI] [PubMed] [Google Scholar]
- 7.Beynnon BD, Johnson RJ, Tohyama H, Renstrom PA, Arms SW, Fischer RA. The relationship between anterior-posterior knee laxity and the structural properties of the patellar tendon graft: a study in canines. Am J Sports Med. 1994;22:812–820. doi: 10.1177/036354659402200613. [DOI] [PubMed] [Google Scholar]
- 8.Biercevicz AM, Miranda DL, Machan JT, Murray MM, Fleming BC. In situ, noninvasive, T2*-weighted MRI-derived parameters predict ex vivo structural properties of an anterior cruciate ligament reconstruction or bioenhanced primary repair in a porcine model. Am J Sports Med. 2013;41:560–566. doi: 10.1177/0363546512472978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bode G, Clausing P, Gervais F, Loegsted J, Luft J, Nogues V, Sims J, Steering Group of the RETHINK Project The utility of the minipig as an animal model in regulatory toxicology. J Pharmacol Toxicol Methods. 2010;62:196–220. doi: 10.1016/j.vascn.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Boguszewski DV, Shearn JT, Wagner CT, Butler DL. Investigating the effects of anterior tibial translation on anterior knee force in the porcine model: is the porcine knee ACL dependent? J Orthop Res. 2011;29:641–646. doi: 10.1002/jor.21298. [DOI] [PubMed] [Google Scholar]
- 11.Bowers ME, Trinh N, Tung GA, Crisco JJ, Kimia BB, Fleming BC. Quantitative MR imaging using “LiveWire” to measure tibiofemoral articular cartilage thickness. Osteoarthritis Cartilage. 2008;16:1167–1173. doi: 10.1016/j.joca.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowers ME, Tung GA, Trinh N, Leventhal E, Crisco JJ, Kimia B, Fleming BC. Effects of ACL interference screws on articular cartilage volume and thickness measurements with 1.5 T and 3 T MRI. Osteoarthritis Cartilage. 2008;16:572–578. doi: 10.1016/j.joca.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandrashekar N, Mansouri H, Slauterbeck J, Hashemi J. Sex-based differences in the tensile properties of the human anterior cruciate ligament. J Biomech. 2006;39:2943–2950. doi: 10.1016/j.jbiomech.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 14.Chu CR, Beynnon BD, Buckwalter JA, Garrett WE, Jr, Katz JN, Rodeo SA, Spindler KP, Stanton RA. Closing the gap between bench and bedside research for early arthritis therapies (EARTH): report from the AOSSM/NIH U-13 Post-Joint Injury Osteoarthritis Conference II. Am J Sports Med. 2011;39:1569–1578. doi: 10.1177/0363546511411654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clemmons RM, Bliss EL, Dorsey-Lee MR, Seachord CL, Meyers KM. Platelet function, size and yield in whole blood and in platelet-rich plasma prepared using differing centrifugation force and time in domestic and food-producing animals. Thromb Haemost. 1983;50:838–843. [PubMed] [Google Scholar]
- 16.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 17.Cummings JF, Grood ES, Levy MS, Korvick DL, Wyatt R, Noyes FR. The effects of graft width and graft laxity on the outcome of caprine anterior cruciate ligament reconstruction. J Orthop Res. 2002;20:338–345. doi: 10.1016/S0736-0266(01)00119-X. [DOI] [PubMed] [Google Scholar]
- 18.Dienst M, Schneider G, Altmeyer K, Voelkering K, Georg T, Kramann B, Kohn D. Correlation of intercondylar notch cross sections to the ACL size: a high resolution MR tomographic in vivo analysis. Arch Orthop Trauma Surg. 2007;127:253–260. doi: 10.1007/s00402-006-0177-7. [DOI] [PubMed] [Google Scholar]
- 19.Fan H, Liu H, Toh SL, Goh JC. Anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold in large animal model. Biomaterials. 2009;30:4967–4977. doi: 10.1016/j.biomaterials.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 20.Fisher MB, Liang R, Jung HJ, Kim KE, Zamarra G, Almarza AJ, McMahon PJ, Woo SL. Potential of healing a transected anterior cruciate ligament with genetically modified extracellular matrix bioscaffolds in a goat model. Knee Surg Sports Traumatol Arthrosc. 2012;20:1357–1365. doi: 10.1007/s00167-011-1800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleming BC, Carey JL, Spindler KP, Murray MM. Can suture repair of ACL transection restore normal anteroposterior laxity of the knee? An ex vivo study. J Orthop Res. 2008;26:1500–1505. doi: 10.1002/jor.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleming BC, Spindler KP, Palmer MP, Magarian EM, Murray MM. Collagen-platelet composites improve the biomechanical properties of healing anterior cruciate ligament grafts in a porcine model. Am J Sports Med. 2009;37:1554–1563. doi: 10.1177/0363546509332257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gwinn DE, Wilckens JH, McDevitt ER, Ross G, Kao TC. The relative incidence of anterior cruciate ligament injury in men and women at the United States Naval Academy. Am J Sports Med. 2000;28:98–102. doi: 10.1177/03635465000280012901. [DOI] [PubMed] [Google Scholar]
- 24.Hashemi J, Chandrashekar N, Gill B, Beynnon BD, Slauterbeck JR, Schutt RC, Jr, Mansouri H, Dabezies E. The geometry of the tibial plateau and its influence on the biomechanics of the tibiofemoral joint. J Bone Joint Surg Am. 2008;90:2724–2734. doi: 10.2106/JBJS.G.01358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hashemi J, Chandrashekar N, Mansouri H, Gill B, Slauterbeck JR, Schutt RC, Jr, Dabezies E, Beynnon BD. Shallow medial tibial plateau and steep medial and lateral tibial slopes: new risk factors for anterior cruciate ligament injuries. Am J Sports Med. 2010;38:54–62. doi: 10.1177/0363546509349055. [DOI] [PubMed] [Google Scholar]
- 26.Hunt P, Scheffler SU, Unterhauser FN, Weiler A. A model of soft-tissue graft anterior cruciate ligament reconstruction in sheep. Arch Orthop Trauma Surg. 2005;125:238–248. doi: 10.1007/s00402-004-0643-z. [DOI] [PubMed] [Google Scholar]
- 27.Institute of Medicine Exploring the biological contributions to human health: does sex matter. J Womens Health Gend Based Med. 2001;10:433–439. doi: 10.1089/152460901300233902. [DOI] [PubMed] [Google Scholar]
- 28.Katsuragi R, Yasuda K, Tsujino J, Keira M, Kaneda K. The effect of nonphysiologically high initial tension on the mechanical properties of in situ frozen anterior cruciate ligament in a canine model. Am J Sports Med. 2000;28:47–56. doi: 10.1177/03635465000280012001. [DOI] [PubMed] [Google Scholar]
- 29.Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93:994–1000. doi: 10.2106/JBJS.I.01618. [DOI] [PubMed] [Google Scholar]
- 30.Levine JW, Kiapour AM, Quatman CE, Wordeman SC, Goel VK, Hewett TE, Demetropoulos CK. Clinically relevant injury patterns after an anterior cruciate ligament injury provide insight into injury mechanisms. Am J Sports Med. 2013;41:385–395. doi: 10.1177/0363546512465167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindenfeld TN, Schmitt DJ, Hendy MP, Mangine RE, Noyes FR. Incidence of injury in indoor soccer. Am J Sports Med. 1994;22:364–371. doi: 10.1177/036354659402200312. [DOI] [PubMed] [Google Scholar]
- 32.Lipps DB, Oh YK, Ashton-Miller JA, Wojtys EM. Morphologic characteristics help explain the gender difference in peak anterior cruciate ligament strain during a simulated pivot landing. Am J Sports Med. 2012;40:32–40. doi: 10.1177/0363546511422325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50:3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 34.Mastrangelo AN, Magarian EM, Palmer MP, Vavken P, Murray MM. The effect of skeletal maturity on the regenerative function of intrinsic ACL cells. J Orthop Res. 2010;28:644–651. doi: 10.1002/jor.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mather RC, 3rd, Koenig L, Kocher MS, Dall TM, Gallo P, Scott DJ, Bach BR, Jr, Spindler JP, MOON Knee Group Societal and economic impact of anterior cruciate ligament tears. J Bone Joint Surg Am. 2013;95:1751–1759. doi: 10.2106/JBJS.L.01705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Messina DF, Farney WC, DeLee JC. The incidence of injury in Texas high school basketball: a prospective study among male and female athletes. Am J Sports Med. 1999;27:294–299. doi: 10.1177/03635465990270030401. [DOI] [PubMed] [Google Scholar]
- 37.Miller TT. MR imaging of the knee. Sports Med Arthrosc. 2009;17:56–67. doi: 10.1097/JSA.0b013e3181974353. [DOI] [PubMed] [Google Scholar]
- 38.Mueller XM, Tevaearai HT, Jegger D, Tucker O, von Segesser LK. Are standard human coagulation tests suitable in pigs and calves during extracorporeal circulation? Artif Organs. 2001;25:579–584. doi: 10.1046/j.1525-1594.2001.025007579.x. [DOI] [PubMed] [Google Scholar]
- 39.Murray MM, Fleming BC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41:1762–1770. doi: 10.1177/0363546513483446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murray MM, Spindler KP, Abreu E, Muller JA, Nedder A, Kelly M, Frino J, Zurakowski D, Valenza M, Snyder BD, Connolly SA. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 41.Myklebust G, Maehlum S, Holm I, Bahr R. A prospective cohort study of anterior cruciate ligament injuries in elite Norwegian team handball. Scand J Med Sci Sports. 1998;8:149–153. doi: 10.1111/j.1600-0838.1998.tb00185.x. [DOI] [PubMed] [Google Scholar]
- 42.Nebelung W, Wuschech H. Thirty-five years of follow-up of anterior cruciate ligament-deficient knees in high-level athletes. Arthroscopy. 2005;21:696–702. doi: 10.1016/j.arthro.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Noojin FK, Barrett GR, Hartzog CW, Nash CR. Clinical comparison of intraarticular anterior cruciate ligament reconstruction using autogenous semitendinosus and gracilis tendons in men versus women. Am J Sports Med. 2000;28:783–789. doi: 10.1177/03635465000280060301. [DOI] [PubMed] [Google Scholar]
- 44.O’Donoghue DH, Rockwood CA, Jr, Frank GR, Jack SC, Kenyon R. Repair of the anterior cruciate ligament in dogs. J Bone Joint Surg Am. 1966;48:503–519. [PubMed] [Google Scholar]
- 45.Oe K, Kushida T, Okamoto N, Umeda M, Nakamura T, Ikehara S, Iida H. New strategies for anterior cruciate ligament partial rupture using bone marrow transplantation in rats. Stem Cells Dev. 2011;20:671–679. doi: 10.1089/scd.2010.0182. [DOI] [PubMed] [Google Scholar]
- 46.Otterness IG, Eckstein F. Women have thinner cartilage and smaller joint surfaces than men after adjustment for body height and weight. Osteoarthritis Cartilage. 2007;15:666–672. doi: 10.1016/j.joca.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Paterno MV, Weed AM, Hewett TE. A between sex comparison of anterior-posterior knee laxity after anterior cruciate ligament reconstruction with patellar tendon or hamstrings autograft: a systematic review. Sports Med. 2012;42:135–152. doi: 10.2165/11596940-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Proffen BL, McElfresh M, Fleming BC, Murray MM. A comparative anatomical study of the human knee and six animal species. Knee. 2012;19:493–499. doi: 10.1016/j.knee.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quatman CE, Kiapour AM, Demetropoulos CK, Kiapour A, Wordeman SC, Levine JW, Goel VK, Hewett TE. Preferential loading of the ACL compared with the MCL during landing: a novel in sim approach yields the multiplanar mechanism of dynamic valgus during ACL injuries. Am J Sports Med. 2014;42:177–186. doi: 10.1177/0363546513506558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Renstrom P, Ljungqvist A, Arendt E, Beynnon B, Fukubayashi T, Garrett W, Georgoulis T, Hewett TE, Johnson R, Krosshaug T, Mandelbaum B, Micheli L, Myklebust G, Roos E, Roos H, Schamasch P, Shultz S, Werner S, Wojtys E, Engebretsen L. Non-contact ACL injuries in female athletes: an International Olympic Committee current concepts statement. Br J Sports Med. 2008;42:394–412. doi: 10.1136/bjsm.2008.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ryan J, Magnussen RA, Cox CL, Hurbanek JG, Flanigan DC, Kaeding CC. ACL reconstruction: do outcomes differ by sex? A systematic review. J Bone Joint Surg Am. 2014;96:507–512. doi: 10.2106/JBJS.M.00299. [DOI] [PubMed] [Google Scholar]
- 52.Sebert SP, Lecannu G, Kozlowski F, Siliart B, Bard JM, Krempf M, Champ MM. Childhood obesity and insulin resistance in a Yucatan mini-piglet model: putative roles of IGF-1 and muscle PPARs in adipose tissue activity and development. Int J Obes (Lond). 2005;29:324–333. doi: 10.1038/sj.ijo.0802823. [DOI] [PubMed] [Google Scholar]
- 53.Shultz SJ, Shimokochi Y, Nguyen AD, Schmitz RJ, Beynnon BD, Perrin DH. Measurement of varus-valgus and internal-external rotational knee laxities in vivo: Part II: relationship with anterior-posterior and general joint laxity in males and females. J Orthop Res. 2007;25:989–996. doi: 10.1002/jor.20398. [DOI] [PubMed] [Google Scholar]
- 54.Stevenson H, Webster J, Johnson R, Beynnon B. Gender differences in knee injury epidemiology among competitive alpine ski racers. Iowa Orthop J. 1998;18:64–66. [PMC free article] [PubMed] [Google Scholar]
- 55.Tosi LL, Boyan BD, Boskey AL. Does sex matter in musculoskeletal health? The influence of sex and gender on musculoskeletal health. J Bone Joint Surg Am. 2005;87:1631–1647. doi: 10.2106/JBJS.E.00218. [DOI] [PubMed] [Google Scholar]
- 56.van Dommelen JA, Jolandan MM, Ivarsson BJ, Millington SA, Raut M, Kerrigan JR, Crandall JR, Diduch DR. Pedestrian injuries: viscoelastic properties of human knee ligaments at high loading rates. Traffic Inj Prev. 2005;6:278–287. doi: 10.1080/15389580590969436. [DOI] [PubMed] [Google Scholar]
- 57.Von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63:269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vrooijink SH, Wolters F, Van Eck CF, Fu FH. Measurements of knee morphometrics using MRI and arthroscopy: a comparative study between ACL-injured and non-injured subjects. Knee Surg Sports Traumatol Arthrosc. 2011;19(suppl 1):S12–S16. doi: 10.1007/s00167-011-1502-4. [DOI] [PubMed] [Google Scholar]
- 59.Woo SL, Gomez MA, Seguchi Y, Endo CM, Akeson WH. Measurement of mechanical properties of ligament substance from a bone-ligament-bone preparation. J Orthop Res. 1983;1:22–29. doi: 10.1002/jor.1100010104. [DOI] [PubMed] [Google Scholar]
- 60.Xerogeanes JW, Fox RJ, Takeda Y, Hyoung-Soo K, Ishibashi Y, Carlin GJ, Woo SL-Y. A functional comparison of animal anterior cruciate ligament models to the human anterior cruciate ligament. Ann Biomed Engin. 1998;26:345–352. doi: 10.1114/1.91. [DOI] [PubMed] [Google Scholar]