Abstract
Background
Shoulder arthroplasty provides reliable pain relief and restoration of function. However, the effects of fatty infiltration and atrophy in the supraspinatus and infraspinatus muscles on functional outcomes are not well understood.
Questions/purposes
The purposes of this study were to (1) compare preoperative with postoperative fatty infiltration and atrophy of the supraspinatus and infraspinatus muscles after primary shoulder arthroplasty; and (2) identify any associations between these variables and outcome measures.
Methods
A retrospective analysis was undertaken of 62 patients with a mean age of 67 years (range, 34–90 years) who underwent shoulder arthroplasty. CT scans were conducted preoperatively and at 12 months postoperatively. Outcome variables included the degree of supraspinatus and infraspinatus fatty infiltration (percent fatty infiltration and Goutallier grade), muscle area (percent muscle area and Warner atrophy grade), shoulder strength, and the Western Ontario Osteoarthritis Score (WOOS), American Shoulder and Elbow Surgeons score, and Constant outcome score.
Results
Preoperatively, the mean percent fatty infiltration (FI) within the supraspinatus and infraspinatus was identical at 14%. One year after shoulder arthroplasty, both muscles had less fatty infiltration (6% and 7%, respectively; p < 0.001). Similarly, the Goutallier grade significantly improved postoperatively for the supraspinatus (p = 0.0037) and infraspinatus (p = 0.0007). Conversely, measures of muscle atrophy remained unchanged postoperatively (p > 0.251). Preoperatively, greater supraspinatus percent FI was negatively associated with preoperative shoulder strength (r = 0.37, p = 0.001) and Constant score (r = 0.38, p = 0.001). Postoperative infraspinatus percent FI was negatively associated with postoperative strength (r = 0.3, p = 0.021) and Constant score (r = 0.3, p = 0.04). Multivariable regression analysis of possible predictive factors demonstrated that preoperative supraspinatus percent muscle area (p = 0.016) and the diagnosis of osteoarthritis (p = 0.017) were associated with better followup WOOS scores, and preoperative supraspinatus strength was associated with postoperative strength (p = 0.0024). Higher degrees of preoperative percent FI were not associated with worse patient-reported outcomes postoperatively.
Conclusions
Supraspinatus and infraspinatus fatty infiltration improves after shoulder arthroplasty, whereas muscle area remains unchanged. Although further study of these variables is required, the negative associations identified between preoperative supraspinatus atrophy and the diagnosis of rheumatoid arthritis and postoperative quality-of-life outcome scores may aid the clinician in selecting the best treatment option for glenohumeral arthrosis and in the management of patient expectations.
Level of Evidence
Level III, prognostic study.
Introduction
Shoulder arthroplasty provides reliable pain relief and restoration of function [3, 5, 19]. The majority of patients maintain their improvements over the long term; however, complications and failures do occur, and these have been associated with component malposition, glenoid failure, and glenohumeral malalignment [4]. Additionally, complications such as prosthetic loosening, periprosthetic fractures, rotator cuff tears, and instability have an adverse effect on pain and function [12, 21].
Rotator cuff fatty infiltration and muscle atrophy have been shown to adversely affect outcome in the treatment of rotator cuff tears [9]. However, the degree of fatty infiltration and atrophy in the supraspinatus and infraspinatus muscles in the arthritic shoulder preoperatively, whether progression occurs after shoulder arthroplasty, and whether these factors have an effect on functional outcomes are not well understood. Anecdotally, it has been suggested that a higher degrees of fatty infiltration preoperatively may be a relative indication for reverse shoulder arthroplasty over an anatomic implant.
The purposes of this study were to (1) compare preoperative with postoperative fatty infiltration and atrophy of the supraspinatus and infraspinatus muscles after primary shoulder arthroplasty; and (2) identify any associations between these variables and outcome measures.
Patients and Methods
Patients
A retrospective analysis was undertaken in patients who were part of a larger multicenter, prospective, randomized clinical trial that assessed functional outcomes after lesser tuberosity osteotomy as compared with subscapularis peel [13]. Research ethics board approval was obtained.
Patients were recruited for inclusion through outpatient specialty clinics by the study coordinator or research assistants. The target population was men and women of any age with advanced arthritis of the glenohumeral joint who were considered by the treating surgeon to be a candidate for shoulder replacement after failure of standard nonsurgical management. The arthritis must have been amenable to treatment with a standard total shoulder arthroplasty. Preoperative CT scans are carried out routinely at both centers. For the purpose of the larger randomized trial, CT scans were obtained 12 months postoperatively.
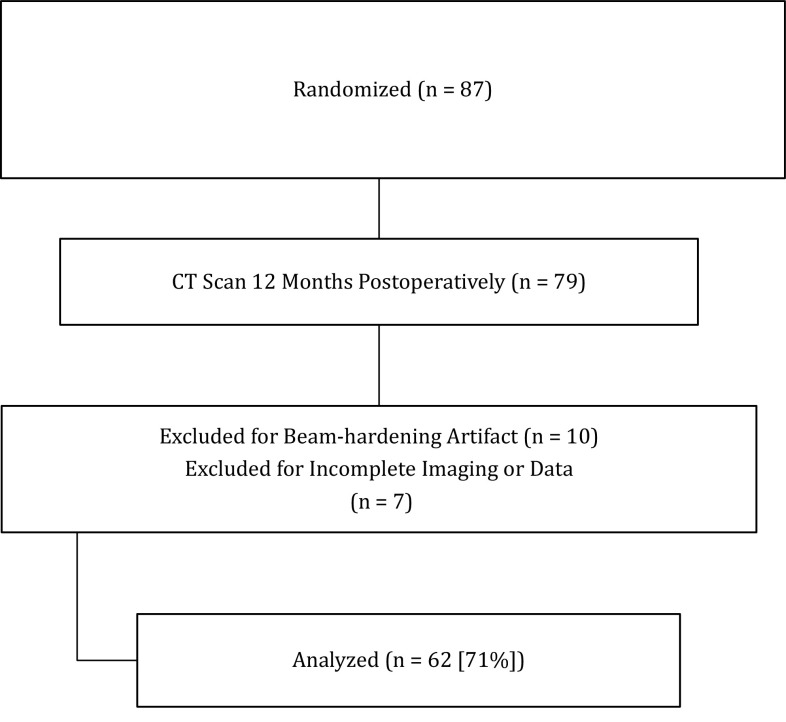
Patients were excluded if it was not possible to reliably interpret the postoperative CT scans as a result of beam-hardening metal artifact. Between November 2006 and June 2009, 87 patients were enrolled and randomized in the larger study at both centers. Of these, 79 patients underwent CT scan imaging 12 months after the index procedure and were included in the study. Ten patients had beam-hardening artifact precluding analysis of the images, and seven patients had incomplete imaging. These patients were excluded from the analysis leaving a study cohort of 62 patients (71%) (Fig. 1) with a diagnosis of osteoarthritis (57) or rheumatoid arthritis (five) consisting of 28 men and 34 women with a combined mean age of 67 years (SD 11 years). Surgery was carried out on the right shoulder in 35 cases and in all cases, the rotator cuff was intact at the time of surgery.
Fig. 1.
This flow-through diagram demonstrates the number of patients enrolled in the study, excluded, and analyzed.
All patients underwent total shoulder arthroplasty with a press-fit humeral stem and a cemented, keeled glenoid component (Tornier SAS, Saint-Ismier Cedex, France). After surgery, patients received 24 hours of prophylactic antibiotic coverage. The postoperative care regimen has been previously described [13]. A shoulder sling was worn for the first 6 weeks. Self-assisted forward elevation was initiated the first postoperative day to a maximum of 90°, and self-assisted external rotation was limited to neutral for the first 6 weeks. The self-assisted exercises were carried out in the supine position every 2 hours with the contralateral arm while keeping the operative side in a relaxed state. Patients were generally discharged by Day 2 and continued their rehabilitation program as outpatients. Physiotherapy was initiated at 6 weeks postoperatively followed by active ROM at 6 weeks. Gentle strengthening exercises were initiated at 12 weeks.
Outcome Variables
Clinical outcome measures included supraspinatus strength as measured by a handheld dynamometer in the tangent position, Constant score, Western Ontario Osteoarthritis Score [15], and American Shoulder and Elbow Surgeons score [18] determined at baseline (preoperatively) and at 12 months postoperatively. All clinical outcome instruments were administered by the same research coordinator in the clinic setting at both sites.
As noted, we included in this study only those patients with CT scans that were obtained both preoperatively and at 12 months after shoulder arthroplasty. Images were acquired in the axial plane, and multiplanar reformations were reconstructed in the axial, coronal, and sagittal planes with 1-mm thickness (with respect to the glenohumeral joint) in both bone and soft tissue algorithms. CT analysis and grading were carried out by a fellowship-trained shoulder surgeon (LJ) who was not involved with the surgery. Fatty infiltration was measured using two methods: a computed percent fatty infiltration was calculated using CT Digital Imaging and Communications in Medicine (DICOM) data and the traditional Goutallier grade was determined. For the computed percent fatty infiltration, the CT DICOM images were extracted and analyzed with Image J Version 1.46 (shareware), which calculated the surface areas in question. Fatty infiltration was measured on the sagittal-oblique images, at the level where the scapular spine was still in continuity with the body of the scapula (the Y-view) [22]. This method of computed fatty infiltration measurement has been previously validated [24]. Fat on CT was distinguished from muscle by characteristic attenuation differences with average attenuation values being 60 Hounsfield Units (HU) and 275 HU, respectively. Finally, a threshold was applied to isolate the intramuscular fat (areas of low signal intensity compared with surrounding muscle) using characteristic attenuation values. The total percent fatty infiltration was then calculated by dividing the cross-sectional area of intramuscular fat into the total area of muscle.
For the traditional Goutallier fatty infiltration grades [9], the 5-point scale was used with zero indicating no fat within muscle, Grade 1 indicating the presence of fatty streaks, Grade 2 indicating the presence of less than 50% fat within muscle, Grade 3 denoting an equal ratio of fat to muscle, and Grade 4 designating greater fat content than muscle [9].
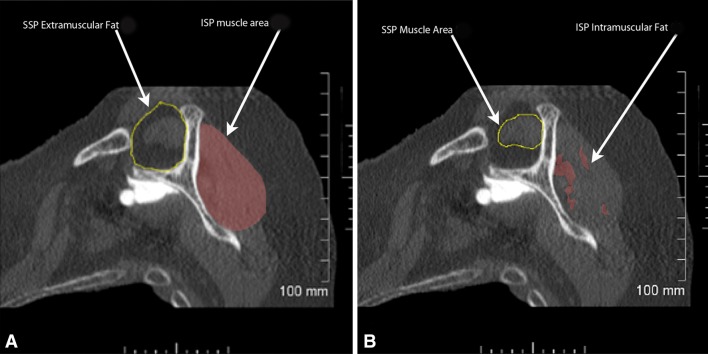
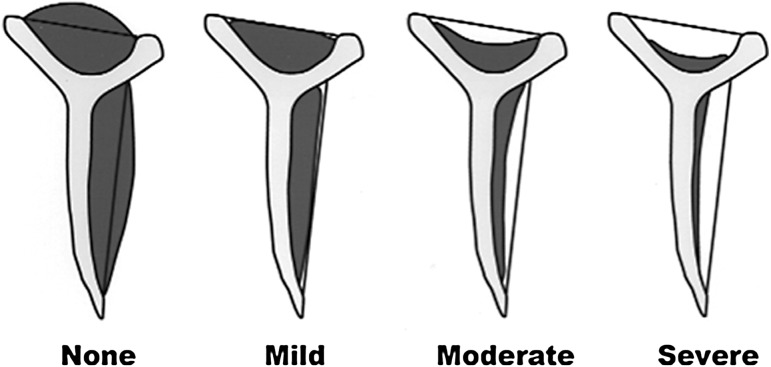
Rotator cuff muscle area, a measure of atrophy, was also documented in two ways: percent muscle area was computed and the Warner atrophy grade was determined [26]. Computed supraspinatus muscle area was determined as an occupation area percentage (Fig. 2), which was calculated by dividing the area of the supraspinatus muscle by the total area of supraspinatus extramuscular fat, which occupied the supraspinatus fossa. It was not possible to perform a similar calculation for the infraspinatus given that the outer boundaries of infraspinatus extramuscular fat are not well defined in the literature. The Warner atrophy grade is a four-category scale describing atrophy as none, mild, moderate, or severe [26]. “None” indicates supraspinatus muscle extending superior to a line extending between the superior tip of the coracoid and the superior tip of the scapular spine; “mild” indicates that the supraspinatus muscle extends to, but not beyond, this line; “moderate” and “severe” indicate higher degrees of muscle atrophy. Infraspinatus atrophy was graded using the same scale with respect to a line extending between the tip of the scapular spine and the inferior tip of the scapular body. These scales were converted to numerical scales (1 = none, 4 = severe) for the purposes of statistical analysis (Fig. 3).
Fig. 2A–B.
On the CT scan sagittal oblique view (“Y-view”), five cross-sectional area measurements are made: supraspinatus (SSP) extramuscular fat (A), supraspinatus muscle area (B), supraspinatus intramuscular fat (not shown in figure), infraspinatus (ISP) muscle area (A), and infraspinatus intramuscular fat (B).
Fig. 3.
The Warner atrophy grade is a four-category scale describing atrophy as none, mild, moderate, or severe [26]. This scale was converted to a numerical scale (1 = none, 4 = severe).
Statistical Methods
A paired t-test was used for comparing mean difference of preoperative to postoperative computed fatty infiltration and computed atrophy changes as well as clinical outcome scores. The Pearson correlation coefficient was calculated to evaluate the correlation between the preoperative or postoperative computed fatty infiltration and occupation area percentages and the Constant, Western Ontario Osteoarthritis Score, American Shoulder Elbow Surgeons score, and supraspinatus strength scores. A multivariable regression analysis was carried out to determine correlation between computed preoperative supraspinatus fatty infiltration and occupation area (atrophy), infraspinatus fatty infiltration and occupation area (atrophy), and postoperative functional outcome measures while controlling for other possible confounders including age, sex, hand dominance, diagnosis (osteoarthritis or rheumatoid arthritis), and baseline strength. Differences between preoperative and postoperative Goutallier fatty infiltration grades and Warner atrophy grades were carried out with a nonparametric signed rank test. A Spearman correlation coefficient was used to evaluate the association between Goutallier fatty infiltration and Warner atrophy grades and functional outcomes preoperatively and postoperatively. All analyses were conducted using SAS Version 9.2 (SAS Institute Inc, Cary, NC, USA).
Results
Fatty Infiltration and Muscle Atrophy
Fatty infiltration appeared to decrease between the preoperative CT scans and the CT scans obtained 1 year after surgery. Preoperatively, the mean computed fatty infiltration for the supraspinatus muscle was 14% (95% confidence interval [CI], 11%–17%), which was found to improve 1 year after shoulder arthroplasty to 6% (95% CI, 4%–8%; p < 0.0001) (Table 1). The preoperative computed fatty infiltration for the infraspinatus muscle was a mean of 14% (95% CI, 11%–16%), which also improved after surgery to a mean of 7% (95% CI, 5%–9%; p < 0.0001). Analysis of the Goutallier fatty infiltration grades revealed similar improvements of 0.3 grades (95% CI, 0.1–0.5; p = 0.005) from the preoperative to the postoperative status for the supraspinatus (Table 2). The infraspinatus improved a mean of 0.3 grades (95% CI, 0.1–0.4; p < 0.001) (Table 3).
Table 1.
Results of imaging analysis of fatty infiltration and occupation volume (atrophy) for the supraspinatus and infraspinatus muscles
| Muscle | Mean preoperative fat (%) (SD) | Mean postoperative fat (%) (SD) | p value | Mean preoperative area (%) (SD) | Mean post-operative area (%) (SD) | p value |
|---|---|---|---|---|---|---|
| SSP | 14 (12) | 6 (7) | < 0.001 | 77 (19) | 71 (53) | 0.3223 |
| ISP | 14 (10) | 7 (7) | < 0.001 | – | – | – |
Table 2.
Results of supraspinatus fatty infiltration grading
| Fatty infiltration grade | Preoperative frequency | Percent | Postoperative frequency | Percent |
|---|---|---|---|---|
| 0 | 4 | 6 | 11 | 17 |
| 1 | 38 | 58 | 45 | 69 |
| 2 | 21 | 32 | 9 | 14 |
| 3 | 2 | 3 |
| Preoperative mean grade (95% CI) | Postoperative mean grade (95% CI) | Difference (95% CI) | p value |
|---|---|---|---|
| 1.3 (0.1–0.5) | 1.0 (0.8–1.1) | −0.3 (−0.4 to −0.1) | 0.005 |
CI = confidence interval.
Table 3.
Results of infraspinatus fatty infiltration grading
| Fatty infiltration grade | Preoperative frequency | Percent | Postoperative frequency | Percent |
|---|---|---|---|---|
| 0 | 3 | 5 | 12 | 21 |
| 1 | 39 | 67 | 35 | 61 |
| 2 | 16 | 28 | 10 | 18 |
| Preoperative mean grade (95% CI) | Postoperative mean grade (95% CI) | Difference (95% CI) | p value |
|---|---|---|---|
| 1.2 (1.1–1.3) | 0.9 (0.8–1.1) | −0.3 (−0.4 to −0.1) | < 0.001 |
CI = confidence interval.
Atrophy did not appear to change from the preoperative CT and the CT scans obtained 1 year after surgery. The supraspinatus mean occupational area was 77% (95% CI, 73–82) preoperatively and it was not statistically different from the postoperative mean of 71% (95% CI, 58–85; p = 0.3223) (Table 1). Similarly, no statistical differences were observed in the preoperative versus postoperative mean Warner atrophy grades for either the supraspinatus (1.8; 95% CI, 1.5–2.0; p = 0.241) (Table 4) or the infraspinatus (−0.03; 95% CI, −0.1 to 0.03; p = 0.322) (Table 5).
Table 4.
Results of supraspinatus atrophy grades
| Atrophy grade | Preoperative frequency | Percent | Postoperative frequency | Percent |
|---|---|---|---|---|
| 1 | 26 | 45 | 28 | 49 |
| 2 | 20 | 34 | 24 | 42 |
| 3 | 11 | 19 | 5 | 9 |
| 4 | 1 | 2 |
| Preoperative mean grade (95% CI) | Postoperative mean grade (95% CI) | Difference (95% CI) | p value |
|---|---|---|---|
| 1.8 (1.5–2.0) | 1.6 (1.4–1.7) | 0.14 (−0.4 to 0.1) | 0.241 |
CI = confidence interval.
Table 5.
Results of infraspinatus atrophy grades
| Atrophy grade | Preoperative frequency | Percent | Postoperative frequency | Percent |
|---|---|---|---|---|
| 1 | 56 | 96 | 53 | 98 |
| 2 | 1 | 2 | 1 | 2 |
| 3 | 1 | 2 | 0 | 0 |
| Preoperative mean grade (95% CI) | Postoperative mean grade (95% CI) | Difference (95% CI) | p value |
|---|---|---|---|
| 1.0 (0.9–1.1) | 1.0 (0.9–1.0) | −0.03 (−0.1 to 0.03) | 0.322 |
CI = confidence interval.
Associations Between Muscle Appearance and Outcome Scores
On the preoperative CT scans, associations were observed between fatty infiltration and functional outcome scores as well as between atrophy and functional outcome scores. Greater supraspinatus percent fatty infiltration was associated with lower preoperative supraspinatus strength (r = 0.37, p = 0.001) and a lower preoperative Constant score (r = 0.38, p = 0.001). Similarly, a higher preoperative supraspinatus Goutallier grade was associated with lower American Shoulder and Elbow Surgeons scores (p = 0.014, r = 0.3), Constant scores (p = 0.002, r = 0.4), and supraspinatus strength (p = 0.0007, r = 0.4). Likewise, a higher preoperative infraspinatus Goutallier grade was associated with lower Constant scores (p = 0.027, r = 0.3). Additionally, a higher (more severe) supraspinatus Warner atrophy grade was associated with lower preoperative Constant scores (p = 0.021, r = 0.3). However, no associations were observed between infraspinatus atrophy grades and functional outcomes.
On the postoperative CT scans, associations were observed between fatty infiltration and postoperative outcome scores as well as between atrophy and postoperative outcome scores. Postoperative infraspinatus percent fatty infiltration was associated with lower postoperative supraspinatus strength (r = 0.30, p = 0.021) and a lower Constant score (r = 0.30, p = 0.04). A higher postoperative supraspinatus Goutallier grade was associated with lower postoperative Constant score (r = 0.3, p = 0.016) and supraspinatus strength (r = 0.3, p = 0.002). Greater postoperative supraspinatus atrophy (Warner grade) was associated with poorer postoperative Constant score (r = 0.3, p = 0.025) and less supraspinatus strength (r = 0.3, p = 0.027). Greater postoperative infraspinatus fatty infiltration (Goutallier grade) was associated with poorer postoperative Constant score (r = 0.3, p = 0.006) and supraspinatus strength (r = 0.3, p = 0.030). No such associations were observed for postoperative infraspinatus atrophy and functional outcome measures (p > 0.05). No postoperative (p > 0.05) associations existed for the other comparisons.
After controlling for possible confounding variables using a multivariable regression analysis, we found that preoperative supraspinatus atrophy, the diagnosis of rheumatoid arthritis, and preoperative supraspinatus strength were all associated with postoperative functional and quality-of-life outcome measures. An increase of 1% in preoperative supraspinatus occupation area was associated with an increase of 0.27 (95% CI, 0.06–0.47; p = 0.013) in the final Western Ontario Osteoarthritis Score. A diagnosis of rheumatoid arthritis was associated with a lower Western Ontario Osteoarthritis Score by 16.8 points (95% CI, 3.2–30.3; p = 0.017). An increase in baseline supraspinatus strength by 1 lb was associated with an increase in 0.36 lb (95% CI, 0.16–0.56; p < 0.001) in the 1-year supraspinatus strength. The presence of higher preoperative supraspinatus and infraspinatus percent fatty infiltration was not associated with poorer patient-rated outcomes with anatomic shoulder arthroplasty at followup.
Discussion
The association between fatty infiltration and atrophy on functional outcomes after surgical repair of the rotator cuff has been studied extensively [6–9, 14, 16, 20]. The effects of cuff muscle status on the functional and quality-of-life outcomes of the arthritic shoulder treated with anatomic shoulder arthroplasty are less well understood. Our findings demonstrate that preoperative supraspinatus and infraspinatus muscle area (a measure of atrophy) did not substantially change after shoulder arthroplasty. However, we observed a major improvement in supraspinatus and infraspinatus percent fatty infiltration after shoulder arthroplasty. Associations among the preoperative supraspinatus fatty infiltration, strength, and Constant scores were observed. As well, associations between the postoperative infraspinatus fatty infiltration and the postoperative strength and Constant scores occurred. Finally, both preoperative supraspinatus atrophy and the diagnosis of rheumatoid arthritis were negatively associated with the postoperative Western Ontario Osteoarthritis Score scores, and preoperative supraspinatus strength was associated with postoperative supraspinatus strength, demonstrating these variables as potentially useful prognostic factors for functional and quality-of-life outcomes and strength outcome in primary shoulder arthroplasty.
There are certain limitations to the current study, including those that are inherent to a study design involving retrospective analysis of data. One possible limitation of the study is selection bias, which was minimized given that the patients in this study were part of a larger, prospective randomized clinical trial [13]. Although it is possible that transfer bias may have affected the results, 91% of patients in the larger study returned for their postoperative CT scan and therefore it is unlikely that this played a significant role. Assessment bias was minimized given that the same research coordinators administered all of the functional outcome measures at both sites. Trudel et al. [24] demonstrated a gradient of increasing fatty infiltration from medial to lateral in an animal model of rotator cuff pathology. It is possible that tension is increased in the muscle-tendon unit after shoulder arthroplasty with lateralization of the joint line after restoration of humeral head diameter and resurfacing of the glenoid; this may have resulted in a slightly more medial sagittal oblique cut being studied postoperatively. If this gradient occurs in humans, it is possible that the postoperative fatty infiltration may have been underestimated. Another possible limitation is the use of a two-dimensional CT scan to study a three-dimensional muscle. However, similar limitations may also occur during assessment of rotator cuff fatty infiltration after tendon repair. Postoperative beam-hardening artifact was observed in some cases despite the metal artifact reduction sequences, which may have had an effect on the interpretation of the scans. However, patients in whom this artifact was visible were excluded from the analysis to minimize this issue. A further limitation may exist in that external rotation strength was not assessed as a separate entity from abduction strength. However, this should not diminish the validity of supraspinatus strength assessment. Finally, CT scans without athrograms are not an ideal modality to determine rotator cuff tendon integrity. Given that no formal study was carried out by ultrasound postoperatively to assess the rotator cuff tendons, it is possible that secondary rotator cuff tears may have been present and that the results may have been affected as a result.
Preoperatively, the degree of computed fatty infiltration of the supraspinatus was similar to that of the infraspinatus. Our results would support that these changes may be partially reversible when function is restored and pain is relieved. In the current study, the supraspinatus fatty infiltration was 14% preoperatively and decreased to 6% postoperatively, whereas the infraspinatus fatty infiltration was 14% preoperatively and decreased substantially to 7% 1 year postoperatively. To our knowledge, changes in the degree of fatty infiltration and atrophy have not been studied in the setting of shoulder arthroplasty. After rotator cuff repair, Goutallier et al. [9] have documented partial reversal of muscle atrophy and fatty infiltration. However, whether atrophy and fatty infiltration are reversible still remains controversial. Previous reports have suggested that fatty infiltration in patients with rotator cuff tears is irreversible, although surgical repair may prevent its progression [7, 8, 14, 16, 20]. In Gerber et al. [6] in a series of patients with both intact and failed rotator cuff repairs, no differences in fatty infiltration of the infraspinatus and subscapularis muscles was found with the numbers they had available. Once healing of the repaired tendon occurred, supraspinatus muscle atrophy did not progress. In another study, however, fatty infiltration of the rotator cuff muscles as measured on MRI increased despite successful tendon repair [1]. It is possible that fatty infiltration and atrophy of the rotator cuff resulting from loss of the tendon insertion may occur by a different pathophysiologic mechanism than fatty infiltration and atrophy in the setting arthritis resulting from inactivity, pain, and reduced ROM. Therefore, the aforementioned study findings may not translate to patients with osteoarthritis managed with shoulder arthroplasty.
In the current study, the diagnosis of rheumatoid arthritis and preoperative supraspinatus atrophy both appear to be negative prognostic factors in shoulder arthroplasty, because they were adversely associated with postoperative functional and quality-of-life outcomes. The best predictor of postoperative supraspinatus strength was its preoperative strength. Interestingly, supraspinatus fatty infiltration was not observed to have associations with postoperative shoulder function. Preoperative supraspinatus fatty infiltration, supraspinatus atrophy, and infraspinatus fatty infiltration were moderately well associated with baseline functional outcome measures. Postoperative supraspinatus fatty infiltration, supraspinatus atrophy, and infraspinatus fatty infiltration were moderately well associated with postoperative functional outcome measures. The fact that stronger associations were not observed in this study is expected given that muscle status is only one element among many determinants of outcome after shoulder arthroplasty. Edwards et al. [2] observed an association between preoperative increasing degrees of infraspinatus fatty infiltration and poorer Constant score results in a large series of patients treated with shoulder arthroplasty. The authors also noted that while controlling for age and gender, full-thickness tears of the supraspinatus were negatively associated with the postoperative strength score. Moineau et al. [17] found that patients with posttraumatic humeral head collapse or necrosis with fatty infiltration of the rotator cuff muscles treated with anatomic shoulder arthroplasty had poorer functional outcomes. A previous investigation demonstrated that preoperative fatty infiltration of the infraspinatus muscle and superior glenoid tilt on the immediate postoperative films were major predictors of the development of secondary rotator cuff dysfunction in primary arthroplasty [27]. Secondary cuff dysfunction was in turn associated with poorer Constant scores. Although we did not investigate the effect of glenoid tilt in the current study, the association between infraspinatus fatty infiltration and poorer functional outcomes was not borne out. For reasons that are not fully understood, supraspinatus atrophy, and not fatty infiltration, was found to correlate with functional outcomes. It is possible that the degree of supraspinatus atrophy is of greater importance to shoulder function because it may reflect a greater degree of dysfunction in the arthritic shoulder. van de Sande et al. [25] observed that a major relationship exists between the Larsen score and fatty degeneration of the rotator cuff in the rheumatoid shoulder. Greiner et al. [10] found postoperative fatty infiltration of the cuff was associated with lower clinical scores after hemiarthroplasty for proximal humeral fractures. Fatty degeneration is a well-established poor prognostic factor in rotator cuff repair [8, 9]. Previous studies have observed substantial effects of infraspinatus fatty degeneration on function [2, 8, 9] and, in addition, an ineffective infraspinatus, either through rupture or muscle degeneration, can substantially offset the biomechanics of the glenohumeral joint [11, 23].
In conclusion, a reduction in supraspinatus and infraspinatus fatty infiltration was observed after shoulder arthroplasty. This was an unexpected finding that should be investigated further with longer term followup. Associations were identified between supraspinatus fatty infiltration, infraspinatus fatty infiltration, and supraspinatus atrophy and poorer supraspinatus strength and Constant scores. In addition, an association was identified among preoperative supraspinatus atrophy, the diagnosis of rheumatoid arthritis, and poorer quality-of-life outcome scores and between preoperative supraspinatus strength and its postoperative strength. These factors should be considered in the treatment algorithm, in conjunction with other patient factors, when selecting the most appropriate prosthesis for the management of glenohumeral arthritis and in managing patient expectations.
Acknowledgments
We thank Ms Kimberly Paquin for her contributions in collection of patient outcome measures and data management.
Footnotes
One of the authors (GSA) received research funding support from Tornier Orthopedics Inc, Mississauga, Ontario, Canada.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the Division of Orthopedics, Ottawa Hospital Research Institute, University of Ottawa, Ottawa, Ontario, Canada; and St Joseph’s Health Care, Hand and Upper Limb Centre, University of Western Ontario, London, Ontario, Canada.
References
- 1.Di Schino M, Augereau B, Nich C. Does open repair of anterosuperior rotator cuff tear prevent muscular atrophy and fatty infiltration? Clin Orthop Relat Res. 2012;470:2776–2784. doi: 10.1007/s11999-012-2443-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards TB, Boulahia A, Kempf JF, Boileau P, Nemoz C, Walch G. The influence of rotator cuff disease on the results of shoulder arthroplasty for primary osteoarthritis: results of a multicenter study. J Bone Joint Surg Am. 2002;84:2240–2248. doi: 10.2106/00004623-200212000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Fevang BT, Lygre SH, Bertelsen G, Skredderstuen A, Havelin LI, Furnes O. Good function after shoulder arthroplasty. Acta Orthop. 2012;83:467–473. doi: 10.3109/17453674.2012.720118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franta AK, Lenters TR, Mounce D, Neradilek B, Matsen FA., 3rd The complex characteristics of 282 unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2007;16:555–562. doi: 10.1016/j.jse.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Gartsman GM, Roddey TS, Hammerman SM. Shoulder arthroplasty with or without resurfacing of the glenoid in patients who have osteoarthritis. J Bone Joint Surg Am. 2000;82:26–34. doi: 10.2106/00004623-200001000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82:505–515. doi: 10.2106/00004623-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004;86:1973–1982. doi: 10.1302/0301-620X.86B6.14577. [DOI] [PubMed] [Google Scholar]
- 8.Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med. 2007;35:719–728. doi: 10.1177/0363546506297539. [DOI] [PubMed] [Google Scholar]
- 9.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;304:78–83. [PubMed] [Google Scholar]
- 10.Greiner SH, Diederichs G, Kroning I, Scheibel M, Perka C. Tuberosity position correlates with fatty infiltration of the rotator cuff after hemiarthroplasty for proximal humeral fractures. J Shoulder Elbow Surg. 2009;18:431–436. doi: 10.1016/j.jse.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Jost B, Pfirrmann CW, Gerber C, Switzerland Z. Clinical outcome after structural failure of rotator cuff repairs. J Bone Joint Surg Am. 2000;82:304–314. doi: 10.1302/0301-620X.82B2.10931. [DOI] [PubMed] [Google Scholar]
- 12.Khan A, Bunker TD, Kitson JB. Clinical and radiological follow-up of the Aequalis third-generation cemented total shoulder replacement: a minimum ten-year study. J Bone Joint Surg Br. 2009;91:1594–1600. doi: 10.1302/0301-620X.91B12.22139. [DOI] [PubMed] [Google Scholar]
- 13.Lapner PL, Sabri E, Rakhra K, Bell K, Athwal GS. Comparison of lesser tuberosity osteotomy to subscapularis peel in shoulder arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2012;94:2239–2246. doi: 10.2106/JBJS.K.01365. [DOI] [PubMed] [Google Scholar]
- 14.Liem D, Lichtenberg S, Magosch P, Habermeyer P. Magnetic resonance imaging of arthroscopic supraspinatus tendon repair. J Bone Joint Surg Am. 2007;89:1770–1776. doi: 10.2106/JBJS.F.00749. [DOI] [PubMed] [Google Scholar]
- 15.Lo IK, Griffin S, Kirkley A. The development of a disease-specific quality of life measurement tool for osteoarthritis of the shoulder: the Western Ontario Osteoarthritis of the Shoulder (WOOS) index. Osteoarthritis Cartilage. 2001;9:771–778. doi: 10.1053/joca.2001.0474. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto F, Uhthoff HK, Trudel G, Loehr JF. Delayed tendon reattachment does not reverse atrophy and fat accumulation of the supraspinatus—an experimental study in rabbits. J Orthop Res. 2002;20:357–363. doi: 10.1016/S0736-0266(01)00093-6. [DOI] [PubMed] [Google Scholar]
- 17.Moineau G, McClelland WB, Jr, Trojani C, Rumian A, Walch G, Boileau P. Prognostic factors and limitations of anatomic shoulder arthroplasty for the treatment of posttraumatic cephalic collapse or necrosis (type-1 proximal humeral fracture sequelae) J Bone Joint Surg Am. 2012;94:2186–2194. doi: 10.2106/JBJS.J.00412. [DOI] [PubMed] [Google Scholar]
- 18.Richards RR, Kai-Nan A, Bigliani LU, Friedman RJ, Gartsman GM, Gristina AG, Iannotti JP, Mow VC, Sidles JA, Zuckerman JD. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3:347–352. doi: 10.1016/S1058-2746(09)80019-0. [DOI] [PubMed] [Google Scholar]
- 19.Sears BW, Johnston PS, Ramsey ML, Williams GR. Glenoid bone loss in primary total shoulder arthroplasty: evaluation and management. J Am Acad Orthop Surg. 2012;20:604–613. doi: 10.5435/JAAOS-20-09-604. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu T, Itoi E, Minagawa H, Pradhan RL, Wakabayashi I, Sato K. Atrophy of the rotator cuff muscles and site of cuff tears. Acta Orthop Scand. 2002;73:40–43. doi: 10.1080/000164702317281387. [DOI] [PubMed] [Google Scholar]
- 21.Sperling JW, Cofield RH, Rowland CM. Neer hemiarthroplasty and Neer total shoulder arthroplasty in patients fifty years old or less. Long-term results. J Bone Joint Surg Am. 1998;80:464–473. doi: 10.2106/00004623-199804000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Tae SK, Oh JH, Kim SH, Chung SW, Yang JY, Back YW. Evaluation of fatty degeneration of the supraspinatus muscle using a new measuring tool and its correlation between multidetector computed tomography and magnetic resonance imaging. Am J Sports Med. 2011;39:599–606. doi: 10.1177/0363546510384791. [DOI] [PubMed] [Google Scholar]
- 23.Thompson WO, Debski RE, Boardman ND, 3rd, Taskiran E, Warner JJ, Fu FH, Woo SL. A biomechanical analysis of rotator cuff deficiency in a cadaveric model. Am J Sports Med. 1996;24:286–292. doi: 10.1177/036354659602400307. [DOI] [PubMed] [Google Scholar]
- 24.Trudel G, Ryan SE, Rakhra K, Uhthoff HK. Extra- and intramuscular fat accumulation early after rabbit supraspinatus tendon division: depiction with CT. Radiology. 2010;255:434–441. doi: 10.1148/radiol.10091377. [DOI] [PubMed] [Google Scholar]
- 25.van de Sande MA, de Groot JH, Rozing PM. Clinical implications of rotator cuff degeneration in the rheumatic shoulder. Arthritis Rheum. 2008;59:317–324. doi: 10.1002/art.23330. [DOI] [PubMed] [Google Scholar]
- 26.Warner JJ, Higgins L, Parsons IMT, Dowdy P. Diagnosis and treatment of anterosuperior rotator cuff tears. J Shoulder Elbow Surg. 2001;10:37–46. doi: 10.1067/mse.2001.112022. [DOI] [PubMed] [Google Scholar]
- 27.Young AA, Walch G, Pape G, Gohlke F, Favard L. Secondary rotator cuff dysfunction following total shoulder arthroplasty for primary glenohumeral osteoarthritis: results of a multicenter study with more than five years of follow-up. J Bone Joint Surg Am. 2012;94:685–693. doi: 10.2106/JBJS.J.00727. [DOI] [PubMed] [Google Scholar]