Abstract
Background
The diagnosis of periprosthetic joint infection (PJI) in patients with failed metal-on-metal (MoM) bearings and corrosion reactions in hip arthroplasties can be particularly difficult, because the clinical presentation of adverse local tissue reactions may mimic that of PJI, because it can also occur concurrently with PJI, and because common laboratory tests used to diagnose PJI may be elevated in patients with MoM THAs.
Questions/purposes
We sought to determine the test properties of the serum erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), synovial fluid white blood cell (WBC) count, and synovial fluid differential (percent polymorphonuclear cells [PMNs]) in diagnosing PJI in either MoM hips undergoing revision for a variety of indications or in non-MoM hips undergoing revision for either corrosion reaction or full-thickness wear. Additionally, we sought to describe how MoM bearings, metal debris, and corrosion reactions can confound the analysis of the synovial fluid WBC count and affect its diagnostic use for PJI.
Methods
We reviewed 150 revision hips meeting specified inclusion criteria (92 MoM total hips, 19 MoM hip resurfacings, 30 non-MoM bearings with corrosion, and nine full-thickness bearing surface wear with metallosis). In our review, we diagnosed 19 patients as infected using Musculoskeletal Infection Society (MSIS) criteria. Mean laboratory values were compared between infected and not infected patients and receiver operator characteristic curves were generated with an area under the curve (AUC) to determine test performance and optimal cutoffs.
Results
After excluding the inaccurate synovial fluid samples, the synovial fluid WBC count (performed accurately in 102 patients) was the best test for the diagnosis of PJI (AUC = 98%, optimal cutoff 4350 WBC/μL) followed by the differential (performed accurately in 102 patients; AUC = 90%, optimal cutoff 85% PMN). The ESR (performed in 131 patients) and CRP (performed in 129 patients) both had good sensitivity (83% and 94%, respectively). Patients meeting MSIS criteria for PJI had higher mean serum ESR, CRP, synovial fluid WBC count, and differential than those not meeting MSIS criteria (p < 0.05 for all). An observer blinded to the MSIS diagnosis of the patient assessed the synovial fluid samples for inaccuracy secondary to metal or cellular debris. Synovial fluid sample “inaccuracy” was defined as the laboratory technician noting the presence of metal or amorpous material, fragmented cells, or clots, or the sample having some defect preventing an automated cell count from being performed. Of the 141 patients who had a synovial fluid sample initially available for review, 47 (33%) had a synovial fluid sample deemed to be inaccurate. A synovial fluid WBC count was still reported; however, 35 of these 47 hips (75%) and 11 of these 35 (31%) were falsely positive for infection.
Conclusions
The diagnosis of PJI is extremely difficult in patients with MoM bearings or corrosion and the synovial fluid WBC count can frequently be falsely positive and should be relied on only if a manual count is done and if a differential can be performed. A more aggressive approach to preoperative evaluation for PJI is recommended in these patients to allow for careful evaluation of the synovial fluid specimen, the integration of synovial fluid culture results, and repeat aspiration if necessary.
Level of Evidence
Level III, diagnostic study. See Guidelines for Authors for a complete description of levels of evidence.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-014-3902-5) contains supplementary material, which is available to authorized users.
Introduction
Periprosthetic joint infection (PJI) is a rare but devastating complication after THA that is associated with substantial morbidity, mortality, and cost [2, 4, 15, 16, 27]. Currently accounting for 15% of all revision THAs [3], the burden of PJI is projected to increase dramatically well into 2030 [14]. The increasing incidence of PJI and the resulting burden placed on patients and the economy alike necessitate timely and accurate methods to diagnose a deep infection.
Adverse local tissue reactions (ALTRs) are being increasingly encountered secondary to failed metal-on-metal (MoM) bearings [8, 17, 18, 34, 35] as well as in metal-on-polyethylene (MoP) bearing THAs secondary to taper corrosion reactions [6, 7]. The diagnosis of PJI in these patients can be particularly difficult, because the clinical presentation of ALTR may mimic that of PJI with purulent-appearing fluid often seen at the time of revision [21]. ALTR can also occur concurrently with PJI [13, 33]. Furthermore, the standard laboratory tests used to diagnose PJI (serum erythrocyte sedimentation rate [ESR], C-reactive protein [CRP], and synovial fluid white blood cell [WBC] count and differential) have been shown to be elevated in small series of noninfected MoM THAs [10, 13, 21, 34] and MoP THAs with corrosion [6, 7].
The purposes of the present study were (1) to determine the test properties of the serum ESR and CRP and the synovial fluid WBC count and polymorphonuclear cell differential (%PMN) in diagnosing PJI in either MoM hips undergoing revision for a variety of indications or in non-MoM hips undergoing revision for either corrosion reaction or full-thickness wear; and (2) to describe how MoM bearings, metal debris, and corrosion reactions can confound the analysis of synovial fluid WBC count and, specifically, how this issue can change the test’s diagnostic use for PJI.
Patients and Methods
After institutional review board approval was obtained, we identified 165 consecutive failed hip arthroplasties with a MoM bearing (THA or hip resurfacing) or a non-MoM bearing revised specifically for corrosion at a modular junction or full-thickness bearing wear noted in the operative report to have produced metallic debris within the joint. All revisions were performed between May 1, 2004, and August 1, 2014. Fifteen hips (9%) were excluded from our analysis because of prior surgery within 6 weeks before the revision (nine hips) [1, 36], oral or intravenous antibiotics given within 2 weeks before laboratory test draw (three hips), and a history suggestive of acute hematogenous infection (three hips). Neither inflammatory arthropathy [5] nor the administration of prophylactic antibiotics before incision during revision surgery [30] was used as an exclusion criteria. Thus, 150 hips of the original 165 (91%) remained for analysis, including those undergoing revision of THA with a MoM bearing (92 hips [61%]), a MoM hip resurfacing (19 hips [13%]), a non-MoM THA with corrosion at a modular junction (30 hips [20%]), or non-MoM THA with full bearing surface wear (nine hips; eight with a polyethylene liner and one with a broken ceramic liner [6%]). The mean age at the time of surgery was 59 ± 11 years old (range, 33–84 years), and the cohort consisted of 78 women (52%) and 72 men (48%). The mean time from the index procedure to reoperation was 56 ± 51 months (range, 4–271 months).
Each patient’s data were reviewed, focusing on the preoperative serum ESR and CRP and either a preoperative or intraoperative synovial fluid WBC count and %PMN. Synovial fluid samples were judged to be inaccurate if the laboratory technician noted the presence of any of the following: metal debris, amorphous material, fragmented or degenerating cells, or the presence of clots; or if the sample had some defect, eg, excessive viscosity, that prevented an automated cell count from being performed. The person determining whether a synovial fluid sample was “inaccurate” was blinded to the Musculoskeletal Infection Society (MSIS) diagnosis of the patient.
The upper limits of normal for serum ESR and CRP in our institution’s laboratory are 27 mm/hr and 8.0 mg/L, respectively. Intraoperatively, a minimum of three deep culture specimens was obtained along with multiple periprosthetic soft tissue samples for intraoperative frozen section and permanent histopathological examination.
Nineteen patients were retrospectively diagnosed as infected as determined by three observers in consensus using MSIS criteria; five of these patients (26%) were culture-negative but met MSIS criteria for PJI (Table 1) [26]. The most common reasons for aseptic revision were aseptic component loosening and corrosion at a modular junction (Table 2).
Table 1.
Organisms cultured in periprosthetic joint infections
| Organism | Number of hips* |
|---|---|
| Peptostreptococcus species | 3 |
| Coagulase-negative Staphylococcus | 2 |
| Staphylococcus aureus † | 1 |
| Streptococcus agalactiae | 1 |
| Streptococcus intermedius | 1 |
| Nutritionally dependent Streptococcus | 1 |
| Escherichia coli | 1 |
| Multiple organisms | 3 |
| Culture-negative | 5 |
* One patient did not have cultures available for review; †methicillin-sensitive.
Table 2.
Reasons for aseptic revision
| Reason for revision | Number of hips (n = 131) |
|---|---|
| Aseptic loosening | 48 (37%) |
| Corrosion at a modular junction* | 40 (31%) |
| Problems related to MoM bearing | 23 (18%) |
| Retroverted acetabular component with soft tissue impingement | 5 (4%) |
| Liner dissociation | 4 (3%) |
| Instability | 4 (3%) |
| Full-thickness bearing surface wear | 3 (2%) |
| Fractured ceramic liner | 1 (0.8%) |
| Stem fracture | 1 (0.8%) |
| Leg length discrepancy | 1 (0.8%) |
| Removal of painful cable | 1 (0.8%) |
* Includes both MoM (15) and MoP (25) bearing surfaces; MoM = metal-on-metal; MoP = metal-on-polyethylene.
Student’s t-tests were used to compare normally distributed univariate data. Logistic regression models for the prediction of infection were created to evaluate the diagnostic variables. To assess the fit and clinical applicability of the predictive logistic regression models, receiver operating characteristic curves and their associated area under the curve (AUC) measures were generated. Optimal diagnostic cutoff values were determined using Youden’s J statistic, which selects the highest possible combination of sensitivity and specificity; clinically acceptable levels of sensitivity and specificity informed final judgment. Combinations of diagnostic variables were assessed in the predictive logistic models to identify any incremental use. The data for patients with failed MoM THA and hip resurfacings together, failed MoM THA alone, and non-MoM THA with corrosion were analyzed separately to determine if any differences existed among these groups. It should be noted that 75 of the 165 patients originally screened for inclusion in this study were previously included in a study of 871 consecutive revision THAs and revision TKAs evaluated for the diagnostic use of serological laboratory tests for PJI in patients with inflammatory arthritis [5]. All analysis was performed using SAS® Version 9.1.3 software (SAS Institute Inc, Cary, NC, USA).
Results
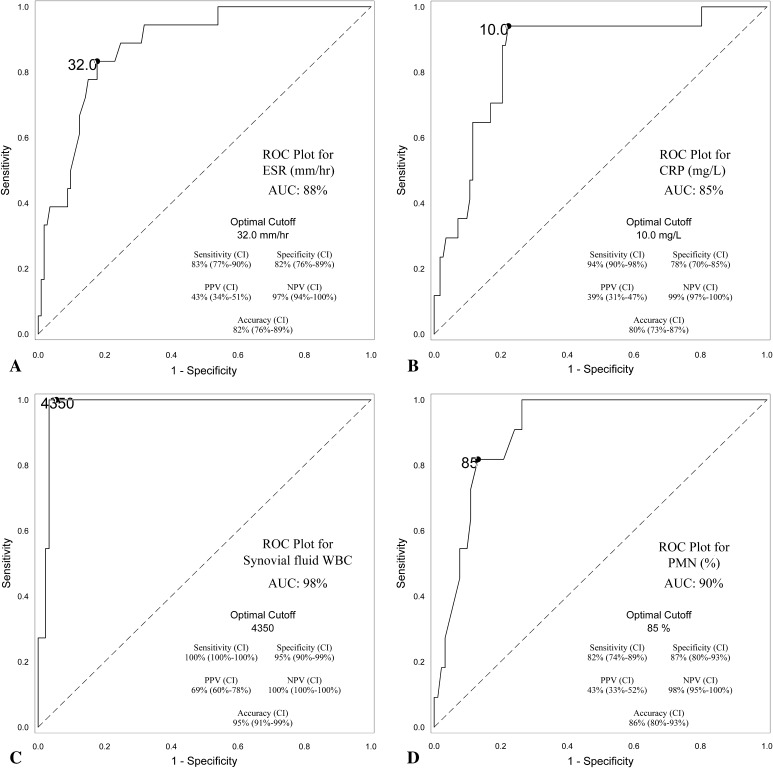
After excluding the samples that were judged to be inaccurate, the synovial fluid WBC count and differential were both excellent tests for diagnosis of PJI; the synovial fluid WBC count was the best diagnostic test with an AUC of 98% (optimal cutoff = 4350 WBC/μL), whereas the differential had an AUC of 90% (optimal cutoff = 85% PMN). The ESR (AUC = 88%, optimal cutoff = 32 mm/hr) and CRP (AUC = 85%, optimal cutoff = 10.0 mg/L) both had good sensitivity (83% and 94%, respectively) (Fig. 1A–D; Table 3). All four laboratory tests’ values were higher in patients meeting MSIS criteria for infection than those not meeting MSIS criteria for infection (Table 4). Diagnostic performance was similar when analyzing MoM bearings (THA and resurfacings) alone, MoM THA alone, and non-MoM THA with corrosion alone (Appendix 1 [Supplemental materials are available with the online version of CORR.®]).
Fig. 1A–D.
(A) A receiver operating characteristic (ROC) curve for the serum ESR is shown with an AUC of 88%. A cutoff value of 32.0 mm/hr demonstrates 83% sensitivity, 82% specificity, 43% positive predictive value (PPV), 97% negative predictive value (NPV), and 82% accuracy. (B) A ROC curve for the serum CRP is shown with an AUC of 85%. A cutoff value of 10.0 mg/L demonstrates 94% sensitivity, 78% specificity, 39% PPV, 99% NPV, and 80% accuracy. (C) A ROC curve for the synovial fluid WBC count is shown with an AUC of 98%. A cutoff value of 4350 cells/μL demonstrates 100% sensitivity, 95% specificity, 69% PPV, 100% NPV, and 95% accuracy. (D) A ROC curve for the %PMN is shown with an AUC of 90%. A cutoff value of 85% PMNs demonstrates 82% sensitivity, 87% specificity, 43% PPV, 98% NPV, and 86% accuracy. CI = confidence interval.
Table 3.
Optimal cutoff values and performance of diagnostic measures
| Diagnostic measure | Optimal cutoff values | Sensitivity* | Specificity* | PPV* | NPV* | Accuracy* | AUC | p value for AUC |
|---|---|---|---|---|---|---|---|---|
| ESR (n = 131) | 32 mm/hr | 83% (77%–90%) | 82% (76%–89%) | 43% (34%–51%) | 97% (94%–100%) | 82% (76%–89%) | 88% | N/A |
| CRP (n = 129) | 10 mg/L | 94% (90%–98%) | 78% (70%–85%) | 39% (31%–47%) | 99% (97%–100%) | 80% (73%–87%) | 85% | N/A |
| Synovial fluid WBC count | 0.1013 | |||||||
| Before exclusion of inaccurate samples (n = 141) | 3111 cells/μL | 94% (90%–98%) | 86% (80%–92%) | 50% (41%–59%) | 99% (97%–100%) | 87% (81%–93%) | 89% | |
| After exclusion of inaccurate samples (n = 102)† | 4350 cells/μL | 100% (100%–100%) | 95% (90%–99%) | 69% (60%–78%) | 100% (100%–100%) | 95% (91%–99%) | 98% | |
| %PMN | 0.8826 | |||||||
| Before exclusion of inaccurate samples (n = 112) | 85% | 85% (78%–91%) | 86% (80%–93%) | 46% (36%–55%) | 98% (95%–100%) | 86% (80%–93%) | 90% | |
| After exclusion of inaccurate samples (n = 102)‡ | 85% | 82% (74%–89%) | 87% (80%–93%) | 43% (33%–52%) | 98% (95%–100%) | 86% (79%–93%) | 90% | |
* Ninety-five percent confidence intervals in parentheses; †n reflects 39 synovial fluid samples excluded (from 141 samples originally available for review) for inaccuracy secondary to metal debris, fragmented cells, etc, as described in Materials and Methods; ‡n reflects 10 synovial fluid samples excluded (from 112 samples originally available for review with a differential) for inaccuracy secondary to metal debris, fragmented, cells, etc, as described in Materials and Methods; PPV = positive predictive value; NPV = negative predictive value; AUC = area under the receiver operator curve; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; WBC = white blood cell; %PMN = percentage polymorphonuclear cells; N/A = not applicable.
Table 4.
Mean values of diagnostic measures between infected and not infected hips
| Diagnostic measure | Infected | Not infected | p value |
|---|---|---|---|
| ESR (mm/hr) (n = 131; 18 infected) | 50 ± 25 (10–108) | 18 ± 17 (1–99) | < 0.001* |
| CRP (mg/L) (n = 129; 17 infected) | 65 ± 94 (4–340) | 13 ± 25 (0.03–199.5) | 0.0432* |
| Synovial fluid WBC count (cells/μL) (n = 102†; 11 infected) | 25,547 ± 29,064 (6800–106,129) | 1720 ± 3260 (27–21,300) | 0.0432* |
| %PMN (n = 102‡; 11 infected) | 89 ± 8 (74–100) | 52 ± 29 (1–97) | < 0.001* |
Values are expressed as mean ± SD with range in parentheses; controlled for error inflation using the stepdown Bonferroni method; *significantly different at the 0.05 level with the stepdown Bonferroni correction method; †n reflects 39 synovial fluid samples excluded (from 141 samples originally available for review) for inaccuracy as described in Materials and Methods; ‡n reflects 10 synovial fluid samples excluded (from 112 samples originally available for review with a differential) for inaccuracy described in Materials and Methods; ESR = erythrocyte sedimentation rate; CRP = C-reactive protein; Synovial WBC = synovial fluid WBC count; %PMN = percentage polymorphonuclear cells.
One hundred forty-one of the 150 revision THAs with a MoM bearing or corrosion reaction had synovial fluid available for analysis; the remaining nine did not have synovial fluid aspiration data in the patient record, either because they had a dry aspiration or the surgeon did not obtain the laboratory value. Of the 141 samples, 47 (33%) had an automated synovial fluid WBC count that was deemed inaccurate. These 47 samples came from 17 of the 85 MoM THAs with a synovial fluid sample (20%), seven of the 18 MoM hip resurfacings (39%), 19 of the 29 non-MoM hips with corrosion (66%), and four of the nine hips with full bearing surface wear (44%). Forty-one of the 47 (87%) inaccurate synovial fluid samples were obtained in aseptic failures. Despite the sample being inaccurate in these aseptic cases, the technician reported a synovial fluid WBC count for 35 of the 41 aseptic samples with a mean WBC count of 19,705 WBC/μL (range, 0–263,920 WBC/μL). Furthermore, 11 of these 35 (31%) were reported with a synovial fluid WBC > 3000/μL (a commonly used cutoff value for PJI), suggesting PJI when it was not present. A differential was generated from nine of the 41 inaccurate samples obtained from aseptic failures (22%) with a mean %PMN of 56% (range, 1%–100%); similar to the synovial fluid WBC count; three of these nine samples (33%) had false-positive differential values (> 80% PMNs), suggesting PJI when it was not present. When considering all of the synovial fluid samples that had a synovial fluid WBC and differential reported (not excluding those that were deemed inaccurate), and assuming a cutoff value of 3000 WBC/μL and 80%, respectively, the synovial fluid WBC count and differential had false-positive rates of 9% (12 of 141 samples) and 8% (nine of 112 samples), respectively. Of the 47 inaccurate synovial fluid samples (including both septic and aseptic failures), four had a subsequently successful manual count (of 27 attempts) and an additional four had successful reaspiration with an accurate synovial fluid sample. In total 39 of the 47 hips (83%) with initially inaccurate samples were excluded from a secondary analysis of the test performance of the synovial fluid WBC count; similarly, 10 of the 47 (21%) initially inaccurate synovial fluid samples were excluded from final analysis of the synovial fluid differential. When considering all of the synovial fluid samples (including the inaccurate samples), the synovial fluid WBC count had an AUC of 89% (optimal cutoff = 3111 cells/μL), whereas the differential had an AUC of 90% (optimal cutoff = 85%) (Table 3).
Discussion
The diagnosis of PJI can be difficult, particularly in failed hips with a MoM bearing or corrosion [34]. Aseptically failed hips with a MoM bearing can present with symptoms and signs that mimic infection such as pain, limited ROM, and swelling around the hip [10, 13, 21, 34]. Furthermore, purulent-appearing fluid is frequently observed intraoperatively in association with a failed MoM bearing or corrosion and prior reports suggest that the synovial fluid WBC count, a commonly used test for diagnosing PJI, can be falsely positive secondary to cellular debris in the joint [35]. Finally, ALTR can present concurrently with PJI in hips with a MoM bearing [13, 33] and with modular junction corrosion [6]. Hence, it is important to have accurate, objective methods to diagnose PJI in hips with a MoM bearing or corrosion. We thus sought to determine the test properties of serum ESR, CRP, and synovial fluid WBC count and differential in diagnosing PJI in either MoM hips undergoing revision for a variety of indications or in non-MoM hips undergoing revision for either corrosion reaction or full-thickness wear. Furthermore, we sought to describe how MoM bearings, metal debris, and corrosion reactions can confound the analysis of synovial fluid WBC count; more specifically, we sought to assess how this issue can change the test’s diagnostic use for PJI.
There are several important limitations to the present study. First, although we used well-accepted criteria to diagnose PJI [26], patients may nevertheless have been wrongly categorized as infected or not infected, which would affect our reported results; this is particularly important when considering that several of the infected cases were culture-negative. Second, these patients were evaluated by multiple different surgeons, who evaluated these patients in a similar but not identical manner. For example, not all patients underwent synovial fluid WBC counts and not all had a differential performed. However, the multisurgeon design may allow for better generalizability of our findings. Third, it is unclear how the etiology of failure affects inflammatory markers such as the ESR, CRP, and synovial fluid WBC count and whether differing cutoff values may be needed depending on the mode of failure. Specifically, in patients with ALTR, these tests may be less accurate; however, in this report, we did not correlate the histopathologic findings with these diagnostic tests. Nevertheless, we believe that our study population captures the spectrum of MoM bearing and corrosion-related reactions that the orthopaedic surgeon will encounter in practice. Fourth, we did not perform automated and manual counts on every sample, and we are unable to comment specifically on the use of one method as opposed to the other. However, we believe a manual count can be more helpful because it assists with identifying potentially unreliable samples. Fifth, of the 165 original patients reviewed for potential study inclusion, only 112 had a complete set of viable laboratory values for the serum ESR, CRP, and synovial fluid WBC count and differential (although greater than this number had at least some of these laboratory values); because this was a retrospective review, the complete set of laboratory values were not obtained in every patient. Finally, we grouped together MoM THAs with MoM hip resurfacings as well as those with MoP bearings that were revised for corrosion at a modular junction and those with full bearing surface wear with metallosis. We felt that grouping these samples together was reasonable, however, given the likely final common pathway of metal debris causing clinical problems such as ALTR.
Serum ESR and CRP are useful screening tools for the diagnosis of PJI after TKA and THA in the chronic [9, 11, 12, 20, 22, 24, 25, 28, 29, 31, 32] and acute settings [1, 36] as well as in the presence of inflammatory arthritis [5]. However, the use of these tests in the presence of ALTR and corrosion is unclear with concerns over falsely positive results [6, 7, 10, 13, 21, 34]. Wyles et al. [34] previously reported sensitivity and specificity of 75% and 68%, respectively, for both the serum ESR and CRP at thresholds of 8.0 mg/L and 22 mm/hr, respectively. Our results demonstrated similar sensitivity and specificity of 83% and 82%, respectively, for serum ESR (optimal cutoff of 32 mm/hr) and 94% and 78% sensitivity and specificity, respectively, for serum CRP (optimal cutoff of 10 mg/L). Our optimal thresholds are similar to values determined by previous studies [9, 12, 28, 31, 32], which makes obtaining a serum ESR and CRP as an initial screen for PJI seem like a reasonable first step in evaluating the potentially infected patient with a MoM bearing or corrosion reaction, consistent with previous guidelines [23].
The synovial fluid WBC count and differential have excellent sensitivity and specificity and are thus useful tests to diagnose acute PJI [1, 36] as well as chronic PJI in patients with [5] or without [9, 11, 12, 20, 22, 24, 25, 28, 29, 31, 32] inflammatory arthritis. In hips with a MoM bearing or corrosion, however, there is a risk of falsely positive synovial fluid WBC counts because of metal debris, degenerating cells, and other foreign material [6, 7] being erroneously counted as WBCs by an automated machine [19, 34]. When we excluded all synovial fluid samples that could potentially falsely elevate the synovial fluid WBC count in this manner, both the WBC count and differential were excellent diagnostic tests for PJI with optimal cutoff values consistent with those previously reported [9, 11, 12, 20, 22, 24, 25, 28, 29, 31, 32]. Furthermore, when compared with a control group of revision THAs and TKAs without a MoM bearing or corrosion from a previously published cohort from our institution [5] (Appendix 2), the diagnostic use of all the laboratory values assessed in our study was not significantly different (as measured by AUC), including the synovial fluid WBC count and differential.
We found that the synovial fluid WBC count can be confounded by inaccurate samples secondary to metal debris and other foreign material as well as degenerating cells. More specifically, approximately one-third of the synovial fluid samples in our series were deemed unreliable with another one-third of the synovial fluid WBC counts and differential values being falsely positive for PJI. Our results concur with the recommendations of others [19, 34] that a manual synovial fluid WBC count should be requested when evaluating these samples Although a manual count will not necessarily lead to an accurate count (a manual count performed after an automated count generated a reliable sample in only four of 27 cases in our series), it can alert the technician and the ordering physician to a synovial fluid WBC count that may be unreliable. Similarly, if a differential cannot be generated from the sample, the diagnostic accuracy of the synovial fluid WBC count obtained should be suspect. In the only previous study [34] that has analyzed the use of synovial fluid samples in the diagnosis of PJI in MoM THAs, the authors reported that of 35 noninfected MoM THAs analyzed, 12 (34%) had synovial fluid WBC counts of 10,000 cells/μL or greater. Furthermore, the differential could not be performed in 16 hips in this series as a result of excessive turbidity of the samples, and hence their findings are similar to our own with approximately one-third (in our series) to one-half (in their series) of samples unable to have a WBC count and/or differential performed because of specimen quality. In our own practice, we have traditionally relied heavily on intraoperative synovial fluid WBC counts; however, in the setting of a failed MoM bearing or corrosion, we are more aggressive with preoperative aspiration to allow for the integration of culture results to help reconcile potentially falsely positive synovial fluid WBC counts and to allow the technician more time to examine the samples. Furthermore, this also allows for a second aspiration to assist with diagnosis if needed.
In conclusion, the diagnosis of PJI is extremely difficult in patients who have a failed THA with MoM bearings or modular junction corrosion, because the intraoperative appearance and the synovial fluid WBC count can both frequently be falsely positive. If there is any question of the accuracy of the synovial fluid sample resulting from metal debris, other foreign material, or fragmented or degenerating cells, the synovial fluid WBC count should be determined from a manual count and only relied on if no abnormalities are noted by the technician and if a differential can be performed on the sample. If these criteria are met, the synovial fluid WBC count has similar diagnostic use for hips with MoM bearings or corrosion as those determined in previous studies and when compared with a control group without MoM bearings or corrosion. Given the magnified difficulty in the diagnosis of PJI in this group of patients, surgeons should consider a more aggressive prerevision evaluation to allow for a thorough evaluation of the synovial fluid specimen and for synovial fluid culture results to assist with diagnosis.
Electronic supplementary material
Acknowledgments
We thank Laura Quigley for her tireless efforts in maintaining our institution’s total joint database.
Footnotes
One of the authors certifies that he (CJDV), or a member of his or her immediate family, has or may receive payments or benefits, during the study period, an amount of USD 10,000 to USD 100,000 from Biomet, Inc (Warsaw, IN, USA), an amount less than USD 10,000 from Convatec (Skillman, NJ, USA), an amount of USD 10,000 to USD 100,000 from DePuy (Warsaw, IN, USA), an amount of USD 10,000 to USD 100,000 from Smith & Nephew, Inc (Memphis, TN, USA), and an amount of USD 10,000 to USD 100,000 from CD Diagnostics (Wynnewood, PA, USA). One of the authors certifies that he (CJDV), or a member of his or her immediate family, has or may receive funding, during the study period, an amount USD 10,000 to USD 100,000 from Stryker (Kalamazoo, MI, USA). One of the authors certifies that he (BRL), or a member of his or her immediate family, has or may receive payments or benefits, during the study period, an amount less than USD 10,000 from CONMED Linvatec (San Mateo, CA, USA), an amount of USD 10,000 to USD 100,000 from DePuy, an amount of USD 10,000 to USD 100,000 from Biomet, Inc, an amount of USD 10,000 to 100,000, from Zimmer, Inc (Warsaw, IN, USA), an amount of less than USD 10,000 from Janssen Pharmaceuticals (Beerse, Belgium), and an amount of less than USD 10,000 from Orthoview (Jacksonville, FL, USA). One of the authors certifies that he (SMS), or a member of his or her immediate family, has or may receive payments or benefits, during the study period, an amount of USD 10,000 to 100,000, from Zimmer, Inc, an amount of USD 10,000 to 100,00 from Cadence Health (Winfield, IL, USA), an amount of USD 10,000 to USD 100,000 from Smith & Nephew, Inc, and an amount of less than USD 10,000 from SLACK Incorporated (Thorofare, NJ, USA). One of the authors (WGP) certifies that he has received or may receive payments or benefits, during the study period, an amount of USD 100,001–USD 1,000,000, from Zimmer, Inc, an amount of USD 100,001 to USD 1,000,000, from Stryker, an amount of USD 100,001 to USD 1,000,000, from DePuy, an amount of less than USD 10,000 from Medtronic (Minneapolis, MN, USA), and an amount of USD 10,000 to USD 1,000 from Lippincott (Philadelphia, PA, USA). One of the authors (JJJ) certifies that he has received or may receive payments or benefits, during the study period, an amount of USD 10,000 to USD 100,000 from Zimmer, Inc, an amount of USD 10,000 to USD 100,000 from Medtronic Sofamor Danek (Memphis, TN, USA), an amount of USD 10,000 to USD 100,000 from Nuvasive (San Diego, CA, USA), and an amount less than USD 10,000 from Implant Protection (Tel Aviv, Israel).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Rush University Medical Center, Chicago, IL, USA.
References
- 1.Bedair H, Ting N, Jacovides C, Saxena A, Moric M, Parvizi J, Della Valle CJ. The Mark Coventry Award: diagnosis of early postoperative TKA infection using synovial fluid analysis. Clin Orthop Relat Res. 2011;469:34–40. doi: 10.1007/s11999-010-1433-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berend KR, Lombardi AV, Jr, Morris MJ, Bergeson AG, Adams JB, Sneller MA. Two-stage treatment of hip periprosthetic joint infection is associated with a high rate of infection control but high mortality. Clin Orthop Relat Res. 2013;471:510–518. doi: 10.1007/s11999-012-2595-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:128–133. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- 4.Bozic KJ, Ries MD. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg Am. 2005;87:1746–1751. doi: 10.2106/JBJS.D.02937. [DOI] [PubMed] [Google Scholar]
- 5.Cipriano CA, Brown NM, Michael AM, Moric M, Sporer SM, Della Valle CJ. Serum and synovial fluid analysis for diagnosing chronic periprosthetic infection in patients with inflammatory arthritis. J Bone Joint Surg Am. 2012;94:594–600. doi: 10.2106/JBJS.J.01318. [DOI] [PubMed] [Google Scholar]
- 6.Cooper HJ, Della Valle CJ, Berger RA, Tetreault M, Paprosky WG, Sporer SM, Jacobs JJ. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg Am. 2012;94:1655–1661. doi: 10.2106/JBJS.K.01352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper HJ, Urban RM, Wixson RL, Meneghini RM, Jacobs JJ. Adverse local tissue reaction arising from corrosion at the femoral neck-body junction in a dual-taper stem with a cobalt-chromium modular neck. J Bone Joint Surg Am. 2013;95:865–872. doi: 10.2106/JBJS.L.01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies AP, Willert HG, Campbell PA, Learmonth ID, Case CP. An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements. J Bone Joint Surg Am. 2005;87:18–27. doi: 10.2106/JBJS.C.00949. [DOI] [PubMed] [Google Scholar]
- 9.Della Valle CJ, Sporer SM, Jacobs JJ, Berger RA, Rosenberg AG, Paprosky WG. Preoperative testing for sepsis before revision total knee arthroplasty. J Arthroplasty. 2007;22:90–93. doi: 10.1016/j.arth.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Earll MD, Earll PG, Rougeux RS. Wound drainage after metal-on-metal hip arthroplasty secondary to presumed delayed hypersensitivity reaction. J Arthroplasty. 2011;26(338):e5–e7. doi: 10.1016/j.arth.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Ghanem E, Antoci V, Jr, Pulido L, Joshi A, Hozack W, Parvizi J. The use of receiver operating characteristics analysis in determining erythrocyte sedimentation rate and C-reactive protein levels in diagnosing periprosthetic infection prior to revision total hip arthroplasty. Int J Infect Dis. 2009;13:e444–e449. doi: 10.1016/j.ijid.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Ghanem E, Parvizi J, Burnett RSJ, Sharkey PF, Keshavarzi N, Aggarwal A, Barrack RL. Cell count and differential of aspirated fluid in the diagnosis of infection at the site of total knee arthroplasty. J Bone Joint Surg Am. 2008;90:1637–1643. doi: 10.2106/JBJS.G.00470. [DOI] [PubMed] [Google Scholar]
- 13.Judd KT, Noiseux N. Concomitant infection and local metal reaction in patients undergoing revision of metal on metal total hip arthroplasty. Iowa Orthop J. 2011;31:59–63. [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 15.Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23:984–991. doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 16.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(61–65):e1. doi: 10.1016/j.arth.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AVF. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg Br. 2010;92:38–46. doi: 10.1302/0301-620X.92B1.22770. [DOI] [PubMed] [Google Scholar]
- 18.Langton DJ, Joyce TJ, Jameson SS, Lord J, Van Orsouw M, Holland JP, Nargol AVF, De Smet KA. Adverse reaction to metal debris following hip resurfacing: the influence of component type, orientation and volumetric wear. J Bone Joint Surg Br. 2011;93:164–171. doi: 10.1302/0301-620X.93B2.25099. [DOI] [PubMed] [Google Scholar]
- 19.Lombardi AV, Jr, Barrack RL, Berend KR, Cuckler JM, Jacobs JJ, Mont MA, Schmalzried TP. The Hip Society: algorithmic approach to diagnosis and management of metal-on-metal arthroplasty. J Bone Joint Surg Br. 2012;94:14–18. doi: 10.1302/0301-620X.94B11.30680. [DOI] [PubMed] [Google Scholar]
- 20.Mason JB, Fehring TK, Odum SM, Griffin WL, Nussman DS. The value of white blood cell counts before revision total knee arthroplasty. J Arthroplasty. 2003;18:1038–1043. doi: 10.1016/S0883-5403(03)00448-0. [DOI] [PubMed] [Google Scholar]
- 21.Mikhael MM, Hanssen AD, Sierra RJ. Failure of metal-on-metal total hip arthroplasty mimicking hip infection. A report of two cases. J Bone Joint Surg Am. 2009;91:443–446. doi: 10.2106/JBJS.H.00603. [DOI] [PubMed] [Google Scholar]
- 22.Nilsdotter-Augustinsson A, Briheim G, Herder A, Ljunghusen O, Wahlström O, Ohman L. Inflammatory response in 85 patients with loosened hip prostheses: a prospective study comparing inflammatory markers in patients with aseptic and septic prosthetic loosening. Acta Orthop. 2007;78:629–639. doi: 10.1080/17453670710014329. [DOI] [PubMed] [Google Scholar]
- 23.Parvizi J, Della Valle CJ. AAOS Clinical Practice Guideline: diagnosis and treatment of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18:771–772. doi: 10.5435/00124635-201012000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Parvizi J, Ghanem E, Menashe S, Barrack RL, Bauer TW. Periprosthetic infection: what are the diagnostic challenges? J Bone Joint Surg Am. 2006;88(Suppl 4):138–147. doi: 10.2106/JBJS.F.00609. [DOI] [PubMed] [Google Scholar]
- 25.Parvizi J, Ghanem E, Sharkey P, Aggarwal A, Burnett RSJ, Barrack RL. Diagnosis of infected total knee: findings of a multicenter database. Clin Orthop Relat Res. 2008;466:2628–2633. doi: 10.1007/s11999-008-0471-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;392:15–23. doi: 10.1097/00003086-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Schinsky MF, Della Valle CJ, Sporer SM, Paprosky WG. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty. J Bone Joint Surg Am. 2008;90:1869–1875. doi: 10.2106/JBJS.G.01255. [DOI] [PubMed] [Google Scholar]
- 29.Spangehl MJ, Masri BA, O’Connell JX, Duncan CP. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg Am. 1999;81:672–683. doi: 10.2106/00004623-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Tetreault MW, Wetters NG, Aggarwal V, Mont M, Parvizi J, Della Valle CJ. The Chitranjan Ranawat Award: Should prophylactic antibiotics be withheld before revision surgery to obtain appropriate cultures? Clin Orthop Relat Res. 2014;472:52–56. doi: 10.1007/s11999-013-3016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med. 2004;117:556–562. doi: 10.1016/j.amjmed.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 32.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357:654–663. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 33.Watters TS, Eward WC, Hallows RK, Dodd LG, Wellman SS, Bolognesi MP. Pseudotumor with superimposed periprosthetic infection following metal-on-metal total hip arthroplasty: a case report. J Bone Joint Surg Am. 2010;92:1666–1669. doi: 10.2106/JBJS.I.01208. [DOI] [PubMed] [Google Scholar]
- 34.Wyles CC, Larson DR, Houdek MT, Sierra RJ, Trousdale RT. Utility of synovial fluid aspirations in failed metal-on-metal total hip arthroplasty. J Arthroplasty. 2013;28:818–823. doi: 10.1016/j.arth.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Wyles CC, Van Demark RE, 3rd, Sierra RJ, Trousdale RT. High rate of infection after aseptic revision of failed metal-on-metal total hip arthroplasty. Clin Orthop Relat Res. 2014;472:509–516. doi: 10.1007/s11999-013-3157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yi PH, Cross MB, Moric M, Sporer SM, Berger RA, Della Valle CJ. The 2013 Frank Stinchfield Award: Diagnosis of infection in the early postoperative period after total hip arthroplasty. Clin Orthop Relat Res. 2014;472:424–429. doi: 10.1007/s11999-013-3089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.