Abstract
Background
If revision of a failed anatomic hemiarthroplasty or total shoulder arthroplasty is uncertain to preserve or restore satisfactory rotator cuff function, conversion to a reverse total shoulder arthroplasty has become the preferred treatment, at least for elderly patients. However, revision of a well-fixed humeral stem has the potential risk of loss of humeral bone stock, nerve injury, periprosthetic fracture, and malunion or nonunion of a humeral osteotomy with later humeral component loosening.
Questions/purposes
The purposes of this study were to determine whether preservation of a modular stem is associated with (1) less blood loss and operative time; (2) fewer perioperative and postoperative complications, including reoperations and revisions; and/or (3) improved Constant and Murley scores and subjective shoulder values for conversion to a reverse total shoulder arthroplasty compared with stem revision.
Methods
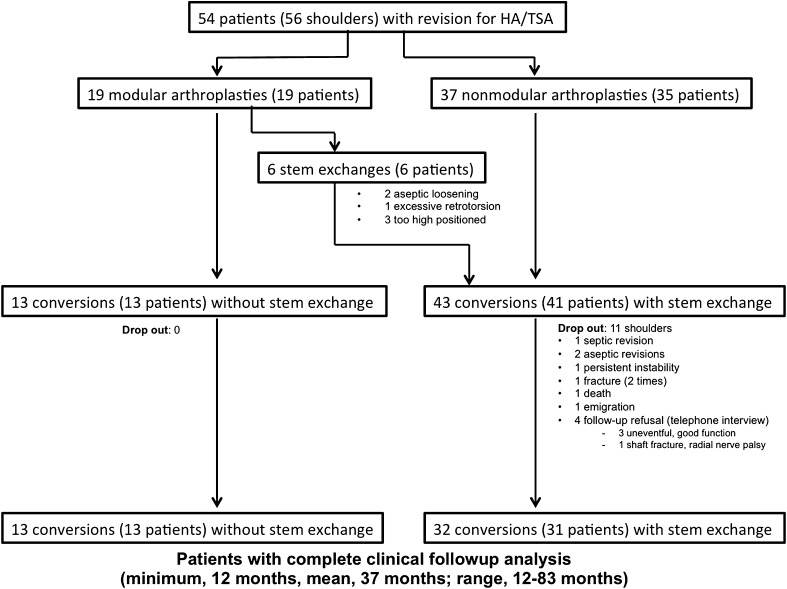
Between 2005 and 2011, 48 hemiarthroplasties and eight total shoulder arthroplasties (total = 56 shoulders; 54 patients) were converted to an Anatomical™ reverse total shoulder arthroplasty system without (n = 13) or with (n = 43) stem exchange. Complications and revisions for all patients were tallied through review of medical and surgical records. The outcomes scores included the Constant and Murley score and the subjective shoulder value. Complete clinical followup was available on 80% of shoulders (43 patients; 45 of 56 procedures, 32 with and 13 without stem exchange) at a minimum of 12 months (mean, 37 months; range, 12–83 months).
Results
Blood loss averaged 485 mL (range, 300–700 mL; SD, 151 mL) and surgical time averaged 118 minutes (range, 90–160 minutes; SD, 21 minutes) without stem exchange and 831 mL (range, 350–2000 mL; SD, 400 mL) and 176 minutes (range, 120–300 minutes; SD, 42 minutes) with stem exchange (p = 0.001). Intraoperative complications (8% versus 30%; odds ratio [OR], 5.2) and reinterventions (8% versus 14%; OR, 1.9) were substantially fewer in patients without stem exchange. The complication rate leading to dropout from the study was substantial in the stem revision group (six patients; 43 shoulders [14%]), but there were no complication-related dropouts in the stem-retaining group. If, however, such complications could be avoided, with the numbers available we detected no difference in the functional outcome between the two groups.
Conclusions
Patients undergoing revision of stemmed hemiarthroplasty or total to reverse total shoulder arthroplasty without stem exchange had less intraoperative blood loss and operative time, fewer intraoperative complications, and fewer revisions than did patients whose index revision procedures included a full stem exchange. Therefore modularity of a shoulder arthroplasty system has substantial advantages if conversion to reverse total shoulder arthroplasty becomes necessary and should be considered as prerequisite for stemmed shoulder arthroplasty systems.
Level of Evidence
Level III, therapeutic study.
Introduction
With substantial improvements in prosthetic design, materials, and surgical technique, hemiarthroplasties and total shoulder arthroplasties have become well-established treatment options for glenohumeral osteoarthritis [3, 10, 11, 22] and complex fractures of the proximal humerus [1, 4, 28]. If a prosthesis fails, however, because of loosening, infection, instability, glenoid wear, or rotator cuff dysfunction, serious challenges can arise [1, 4, 11, 16, 22, 24, 28, 31]. If revision is uncertain to preserve or restore clinically satisfactory cuff function, conversion to reverse total shoulder arthroplasty has become the preferred treatment, especially for elderly patients [8, 20, 23, 33].
In the majority of patients, revision is performed to treat cuff failure with associated instability [2, 19, 30, 34] or glenoid erosion [3]. With instability, secondary cuff failures, or glenoid erosion, the humeral component often is well fixed in the humeral shaft. Revision of a stem has the potential risk of loss of humeral bone stock, nerve injury, periprosthetic fracture, and malunion or nonunion of a humeral osteotomy with later humeral component loosening [8, 12, 32]. Newer modular stemmed shoulder arthroplasty systems theoretically allow conversion to reverse total shoulder arthroplasty without stem exchange.
The purposes of this study were to determine whether preservation of a modular stem for revision of an arthroplasty using an Anatomical Shoulder™ System (Zimmer, Warsaw, IN, USA) to a reverse total shoulder arthroplasty is associated with (1) less blood loss and operative time; (2) fewer perioperative and postoperative complications, including reoperations and revisions; and/or (3) improved Constant and Murley scores and subjective shoulder values.
Patients and Methods
All patients identified in our prospectively collected, comprehensive database as having undergone aseptic single-stage revision of a stemmed hemiarthroplasty or total to a reverse total shoulder arthroplasty between January 2005 and December 2011 were retrospectively reviewed. Fifty-four patients (38 women, 16 men) (56 shoulders: 28 left, 28 right; of those, two were bilateral) were identified and had complete perioperative and early postoperative documentation. Eleven of the patients (11 shoulders), however, were not available for final clinical followup. Six patients (all in the group with stem exchange) had complications requiring major rerevision surgery. These patients were not analyzed further and their surgeries were considered failures. Five additional patients could not be traced. Clinical and radiographic followup for the remaining 44 patients (45 shoulders, 32 with and 13 without stem exchange, one bilateral) was at a minimum of 12 months (mean, 37 months; range, 12–83 months) (Fig. 1). There were 14 patients (15 shoulders) with more than 1 year, six patients (six shoulders) with more than 2 years, nine patients (nine shoulders) with more than 3 years, and 15 patients (15 shoulders) with more than 5 years followup. The demographic data of the two study groups were not found to be statistically significantly different (Table 1). Forty-eight shoulders were converted from hemiarthroplasties and eight from total shoulder arthroplasties. The mean age of the patients at the time of index surgery was 64 years (range, 41–85 years) and the mean age at revision to reverse total shoulder arthroplasty was 67 years (range, 44–87 years). The mean time between index surgery and conversion to the reverse total shoulder arthroplasty was 38 months (range, 0–147 months). Before the index shoulder arthroplasty, the mean number of previous surgeries was 1 (range, 0–7). The mean number of operations before conversion to reverse total shoulder arthroplasty was 2.2 (range, 1–8 operations). The main indications for primary hemiarthroplasty and total shoulder arthroplasty were humeral head fractures and primary osteoarthritis, respectively (Table 2). Most conversions to reverse total shoulder arthroplasties were performed because of secondary rotator cuff failure (Table 3).
Fig. 1.
The flow diagram shows enrollment, dropout, and loss to followup of the study cohort. HA = hemiarthroplasty; TSA = total shoulder arthroplasty.
Table 1.
Demographic data for the study groups
| Parameter | With stem exchange (n = 41 patients [43 procedures]) | Without stem exchange (n = 13 patients [13 procedures]) | p value |
|---|---|---|---|
| Age at index surgery (years) | 63 (41–76) | 66 (43–85) | 0.27 |
| Age at conversion (years) | 67 (44–81) | 68 (44–87) | 0.48 |
| Months to conversion | 42 (0–147) | 24 (2–93) | 0.2 |
| Female:male | 30:13 | 10:3 | 0.59 |
| Primary – implant hemiarthroplasty: total | 38:5 | 10:3 | 0.39 |
| Interventions before conversion | 2.1 (1–8) | 2.6 (1–8) | 0.55 |
Values are mean with ranges in parentheses.
Table 2.
Indication for index surgery
| Indication | Hemiarthroplasty (number of procedures) | Total shoulder arthroplasty (number of procedures) |
|---|---|---|
| Humeral head fracture | 22 | 0 |
| Posttraumatic arthritis/necrosis | 12 | 1 |
| Primary osteoarthritis | 4 | 5 |
| Cuff tear arthropathy | 8 | 0 |
| Rheumatoid arthritis | 1 | 1 |
| Tumor | 1 | 1 |
| Total | 48 | 8 |
Table 3.
Indication for revision surgery
| Indication | Number of indications |
|---|---|
| Rotator cuff lesion with instability/loss of function | 29 |
| Aseptic stem loosening | 8 |
| Stem malposition with functional deficit | 8 |
| Failure of glenoid component after total shoulder arthroplasty | 6 |
| Glenoid erosion after hemiarthroplasty | 5 |
Surgical Technique
All revision procedures were performed with the patient in a beach chair position, through a deltopectoral approach, by the senior author (CG) or under his supervision. General anesthesia in combination with an interscalane block was used and all patients received perioperative intravenous antibiotic prophylaxis. If the subscapularis tendon was intact, it was elevated off the lesser tuberosity and reattached before wound closure. In all cases, the Anatomical Shoulder™ Inverse/Reverse System™ (Zimmer) was used as the revision implant on the humeral side. For patients with a modular Anatomical Shoulder™ System in place (n = 19), conversion to reverse total shoulder arthroplasty was performed without removal of the humeral stem whenever possible (n = 13, three total shoulder arthroplasties, 10 hemiarthroplasties) (Fig. 2). The prosthetic head was detached from the stem with the extraction instrument provided by the manufacturer. The humeral metaphysis was prepared with a flat reamer to allow seating of the reverse metaphysis on the humeral stem. Stem exchange despite previous use of a modular Anatomical Shoulder™ System was necessary in six patients: two uncemented stems were loose, three were implanted far too proximally, and one stem had been inserted with retrotorsion of approximately 50° leading to instability even with the reverse trial implant providing 20° correction toward antetorsion.
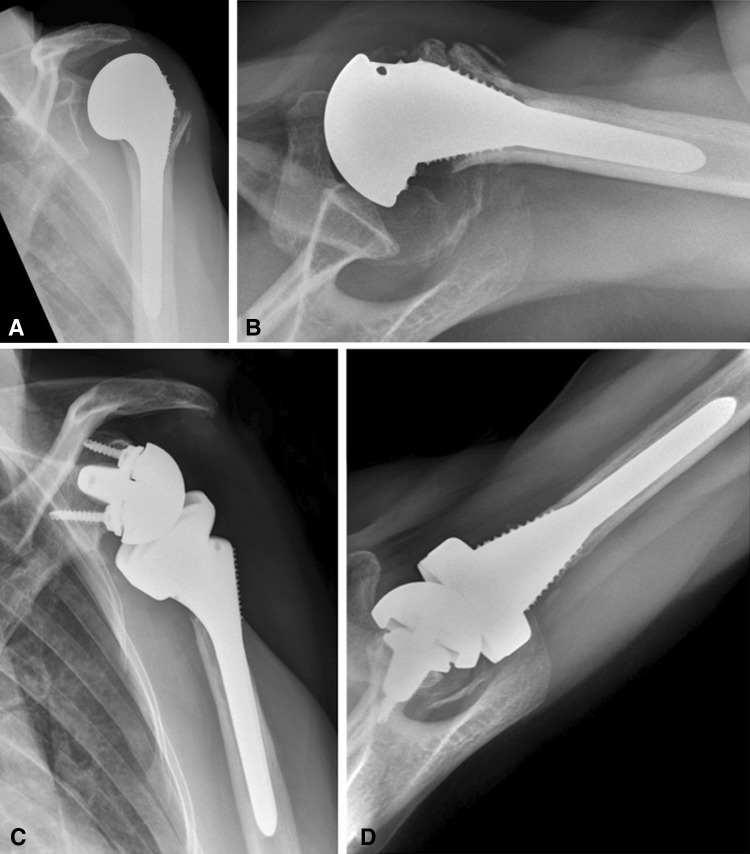
Fig. 2A–D.
A 55-year-old woman presented with severe a pseudoparalysis of her left arm 7 months after a hemiarthroplasty as a result of a four-part humeral head fracture. Her (A) AP and (B) axial radiographs show a well-fixed humeral stem with anterosuperior instability resulting from rotator cuff insufficiency. Two years after conversion to a reverse total shoulder arthroplasty, the patient had a pain-free overhead function with good component positioning without any signs of loosening observed on the (C) AP and (D) axial radiographs.
Of the remaining 37 shoulders without modular implants, four were malpositioned and eight were loose. The remaining 25 stems had to be exchanged to allow for conversion to reverse total shoulder arthroplasty, although they were well fixed and well positioned.
For well-fixed, nonconvertible stems, efforts were made to remove the prosthesis without splitting the humeral shaft. In 12 cases, an anterior longitudinal osteotomy of the humeral shaft was necessary to remove the stem and osteosynthesis was performed using cerclage wires. The new stem was cemented in the well-preserved cement mantle (n = 9) or in cases of complete cement extraction, cemented (n = 17) or press-fitted in the shaft (n = 5).
After preparation of the humeral stem, the glenoid was exposed. For conversions from hemiarthroplasty to reverse total shoulder arthroplasty, any remaining labrum was excised and capsule or scar tissue was released. If a glenoid component had to be extracted and cement removed, care was taken to preserve as much of the glenoid bone stock as possible. The tendon of the long head of the triceps was released from the inferior glenoid neck with protection of the axillary nerve. In the majority of procedures (n = 52), the Anatomical Shoulder™ System glenoid component was used as the standard implant. In four cases, a long-pegged glenoid base plate was used (two Aequalis Reversed II [Tornier, Amsterdam, The Netherlands]; one Delta III [DePuy International, Leeds, UK]; and one Trabecular metal [Zimmer]). The guide wire for the glenoid reamer was positioned so that the glenoid baseplate was as low as possible with a minimal inferior tilt. Locking screws were used to provide primary angular stability. In cases of marked glenoid bone loss, autograft (n = 8) or allograft (n = 3), bone grafting was performed to provide inherent stability and lateralization of the glenoid baseplate.
If the primary stem had been inserted in excessive retrotorsion, corrective metaphyseal implants of 10° or 20° retrotorsion or antetorsion are available. However, this was used in only one patient; all the other implants were standard 0° implants. The height of the polyethylene component was chosen based on the preoperatively assessed length of the arm and the soft tissue tension and stability of the joint after relocation. After reapproximation of the subscapularis, two suction drains were inserted for 24 to 48 hours. A sling was used for 4 to 6 weeks postoperatively.
Data Acquisition and Clinical Outcome Measurement
All operative notes and patient charts were reviewed for intraoperative and/or postoperative complications or reoperations. Exact surgical time and blood loss were recorded from the anesthesia charts. All patients undergoing arthroplasty in our unit are systematically reviewed clinically and radiographically at 6 weeks, 12 weeks, 1 year, and yearly thereafter. The outcomes scores included the Constant and Murley score [6] (which ranges from 0 to 100 points, with 100 being the best score) and the subjective shoulder value [10] (which ranges from 0 to 100 points, with 100 being the best score), which were measured preoperatively and yearly after surgery during regular postoperative followups, with documentation of the data in a prospective prosthesis database. The last available Constant and Murley score was used for followup analysis. If there was later revision surgery with exchange or removal of the converted implant, this was considered a failure and subsequently excluded from functional assessment. Patients who had undergone surgery other than removal of prosthetic components (eg, débridement without implant removal for early infection, arthroscopic acromioclavicular joint resection, or latissimus dorsi transfer) were included in the final functional analysis.
Based on a general permit issued by the responsible state agency, our institutional review board allows retrospective analysis of patient data relating to standard diagnostic or therapeutic procedures if the patients agree that their data are used in an anonymous fashion.
Patient Dropouts and Loss to Followup
Of the included 54 patients (56 shoulders), perioperative and early postoperative data for 11 patients were available for analysis, however clinical data for the patients were incomplete (Fig. 1): one patient with stem exchange had a late (chronic) infection, which was treated successfully with a two-stage revision and implant exchange 19 months after conversion to reverse total shoulder arthroplasty; and two patients (two shoulders) had revision surgery, one for humeral stem loosening after intraoperative shaft fracture (13 months) and one because of glenoid loosening (21 months). These three patients (all with stem exchange) were excluded from further clinical analysis.
One patient’s Anatomical Shoulder™ arthroplasty was converted to a reverse total shoulder arthroplasty for instability, however the instability persisted. Another patient fractured the acromion 6 months postoperatively and sustained an additional periprosthetic shaft fracture 30 months after conversion surgery. Both fractures were treated conservatively. No functional outcome scores were available for these two patients. One patient left the country 11 months after conversion surgery after an uneventful course and could not be contacted, and one patient died 4 months after the conversion surgery of unrelated causes. Four patients refused followup visits. Three of them were interviewed via telephone and reported an uneventful course with good function. The other patient sustained an intraoperative shaft fracture with radial nerve palsy and preferred further treatment in a specialized neurology department at another hospital. Therefore, there were six complication-related dropouts and five patients lost to followup without documented complications. All 11 patients were in the group in which the humeral stem had been exchanged during conversion to reverse total shoulder arthroplasty. Therefore, the number of patients with complete perioperative documentation and followup of at least 1 year was 44 (45 shoulders).
Statistical Analysis
For statistical analysis of intraoperative blood loss and surgical time, the Mann-Whitney U test was used. Comparisons of age, sex, primary implant, and interventions before conversion in the two groups were performed with an unpaired t-test. Comparisons of preoperative and postoperative scores were performed with the Wilcoxon signed-rank test. A p value less than 0.05 was considered significant. Odds ratios (OR) were calculated as crude OR and stratified by subgroup to allow identification of interacting factors. All statistical analyses were performed using IBM SPSS® statistics software (Version 20.0; Chicago, IL, USA).
Results
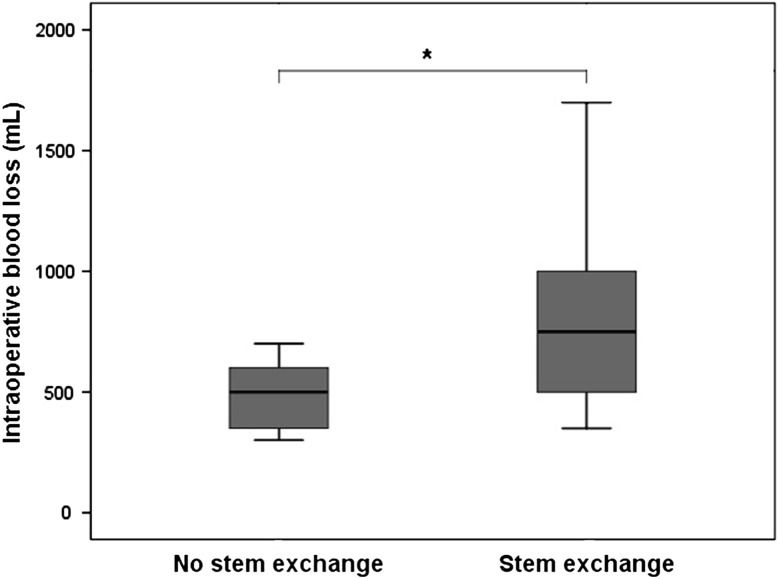
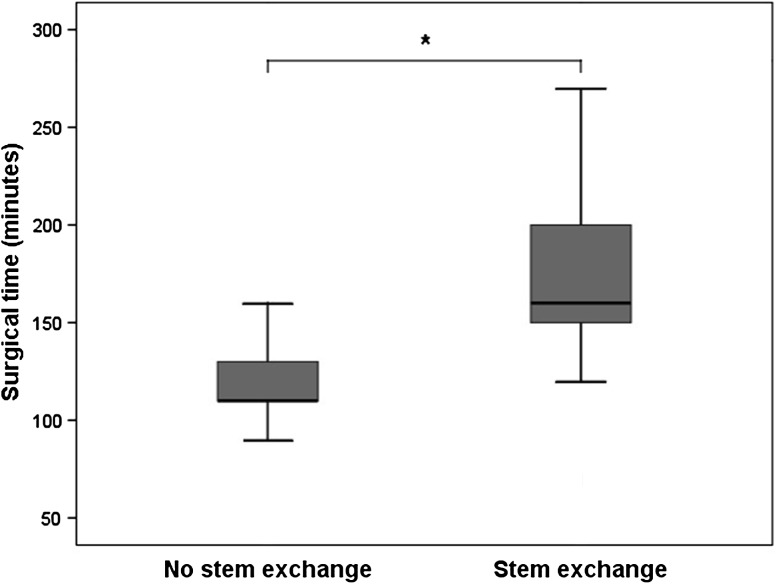
Patients who did not undergo complete stem revision had less intraoperative blood loss and operative time than those who underwent complete stem revision. Blood loss averaged 485 mL (range, 300–700; SD, 151 mL) without and 831 mL (range, 350–2000; SD, 400 mL) with stem exchange (p = 0.001) (Fig. 3). Surgical time averaged 118 minutes (range, 90–160; SD, 21 minutes) without stem exchange and 176 minutes (range, 120–300; SD, 42 minutes) with stem exchange (p = 0.0001) (Fig. 4).
Fig. 3.
The mean blood loss was reduced (p = 0.001) in patients without stem exchange (mean, 485 mL; range, 300–700 mL; SD, 151 mL) compared with the group with stem exchange (mean, 831 mL; range, 350–2000 mL; SD, 400 mL).
Fig. 4.
The surgical time could be decreased to 118 minutes (range, 90–160 minutes; SD, 21 minutes) when the stem could be left in place, which spared, on average, 58 minutes surgical time (p < 0.0001) compared with cases in which the stem had to be exchanged (mean, 176 minutes; range, 120–300 minutes; SD, 42 minutes).
Intraoperative complication and reoperation rates were greater in patients who needed stem exchange (Table 4). We identified 13 (30%) intraoperative and nine (seven patients [16%]) postoperative complications in the 43 shoulders in which the humeral component was exchanged compared with one (8%) intraoperative and two (15%) postoperative complications in the 13 shoulders in which the stem was left in place. The OR of sustaining an intraoperative complication for patients with stem exchange was 5.2 (95% CI, 0.6–44.3) and 1.1 (95% CI, 0.2–5.9) for postoperative complications if compared with patients who did not need stem exchange. The group with stem exchange had nine conversion-related reoperations (six patients [14%]), whereas one (8%) reoperation was performed in the 13 patients without stem removal (OR, 1.9; 95% CI, 0.2–17.8).
Table 4.
Complications and reoperations
| Complication | With stem exchange | Without stem exchange | ||||
|---|---|---|---|---|---|---|
| Intraoperative | Postoperative | Reoperation | Intraoperative | Postoperative | Reoperation | |
| Shaft fracture | 5 | 1* | 1 | |||
| Shaft fracture with radial nerve palsy | 1 | |||||
| Fracture of greater tuberosity | 2 | 1 | ||||
| Fracture of glenoid | 2 | |||||
| Radial nerve palsy | 1 | |||||
| Cement extrusion | 2 | 1 | ||||
| Fracture of acromion | 4* | 1 | 1 | |||
| Glenoid loosening | 1* | 1 | ||||
| Early infection | 1 | 1 | ||||
| Late (chronic) infection | 1 | 3 | ||||
| Wound healing problem | 1 | 2 | ||||
| Instability | 1 | |||||
| Total | 13 | 9 (7 patients) | 9 (6 patients) | 1 | 2 | 1 |
* Two patients with two postoperative complications (one with an acromial fracture and periprosthetic humeral shaft fracture; one with an acromial fracture and glenoid component loosening)
Thus, the complication rate leading to dropout with more or less functional compromise was substantial in the stem revision group of 41 patients (six patients; six of 43 shoulders [14%]), but there were no complication-related dropouts in the stem-retaining group. If, however, such complications could be avoided, with the numbers available, we detected no difference in the functional outcome between the two groups after a minimum followup of 12 months (Table 5). The 13 patients without stem exchange showed an improvement of the relative Constant and Murley score of 42% (range, 11%–88%) to 67% (range, 34%–100%) (p = 0.002), the mean absolute Constant and Murley score improved from 30 (range, 10–56 points) to 48 points (range, 29–69 points) (p = 0.002), and the mean subjective shoulder value increased from 27% (range, 0%–50%) to 55% (range, 20%–90%) (p = 0.002). The 32 shoulders with stem exchange showed an improvement of the relative Constant and Murley score of 32% (range, 6%–97%) to 61% (range, 28%–100%) (p = 0.0001), the mean absolute Constant and Murley score improved from 24 (range, 4–68 points) to 45 points (range, 18–80 points) (p = 0.0001), and the mean subjective shoulder value increased from 29% (range, 0%–75%) to 55% (range, 10%–100%) (p = 0.002).
Table 5.
Clinical results according to Constant and Murley score and subjective shoulder value*
| Clinical outcome parameter | With stem exchange (n = 32 shoulders) | Without stem exchange (n = 13 shoulders) | p value† | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Preoperative | Followup | Improvement | p value | Preoperative | Followup | Improvement | p value | ||
| Absolute Constant and Murley score | 24 (4–68) | 45 (18–80) | 21 (−11 to 64) | 0.0001 | 30 (10–56) | 48 (29–69) | 20 (6–52) | 0.002 | 0.48 |
| Adjusted Constant and Murley score | 32 (6–97) | 61 (28–100) | 29 (−15 to 64) | 0.0001 | 42 (11–88) | 67 (34–100) | 27 (8–72) | 0.002 | 0.34 |
| Pain (maximum 15) | 6 (0–15) | 11 (3–15) | 5 (−2 to 12) | 0.0001 | 6 (3–5) | 11 (4–15) | 5 (−1 to 11) | 0.002 | 0.9 |
| Activity (maximum 20) | 3 (0–7) | 6 (1–10) | 3 (−3 to 10) | 0.0001 | 3 (0–6) | 7 (3–10) | 4 (0–10) | 0.003 | 0.45 |
| ROM | |||||||||
| Flexion (°) | 61 (0–140) | 108 (30–170) | 47 (−60 to 120) | 0.0001 | 74 (0–150) | 112 (80–165) | 40 (−10 to 110) | 0.005 | 0.37 |
| Abduction (°) | 54 (0–150) | 94 (40–165) | 41 (−60 to 123) | 0.0001 | 66 (0–160) | 98 (50–150) | 33 (−35 to 90) | 0.02 | 0.66 |
| External rotation (°) | 19 (−10–70) | 13 (−20–60) | −6 (−40 to 35) | 0.09 | 24 (0–70) | 22 (−10–50) | −1 (−35 to 40) | 0.85 | 0.7 |
| Subjective shoulder value (maximum 100) | 29 (0–75) | 55 (10–100) | 27 (−25 to 80) | 0.0001 | 27 (0–50) | 55 (20–90) | 29 (5–70) | 0.002 | 0.66 |
* The data are mean with the range in parentheses; †comparison between groups.
Discussion
If revision of a failed hemiarthroplasty or total shoulder arthroplasty is uncertain to preserve or restore satisfactory rotator cuff function, conversion to a reverse total shoulder arthroplasty is the most reliable surgical option to restore overhead function [8, 20, 21, 23, 33] and therefore has become the preferred treatment, at least for elderly patients. Such revisions, however, are associated with the risk of intraoperative and/or postoperative complications [8, 12, 18, 20, 21, 23, 26, 27, 32, 33]. Our study showed that revision of hemiarthroplasties or total- to reverse total shoulder arthroplasties performed without stem removal reduced operative time, blood loss, intraoperative complications, and reoperations compared with stem exchange. With the numbers available, there were no statistically significant differences in postoperative complications or validated outcomes scores between the groups.
The main indications for conversion to reverse total shoulder arthroplasty were rotator cuff insufficiency, instability, and failure of the glenoid component in total shoulder arthroplasties or symptomatic glenoid wear in hemiarthroplasties. This is consistent with the literature, in which the most common reasons for failure of hemiarthroplasties or total shoulder arthroplasties include superior migration or subluxation of the humeral head secondary to rotator cuff dysfunction occurring in 17% to 54% of patients [2, 7, 13, 17, 19, 30, 34] with the likelihood of a higher incidence with longer followup [5, 34]. Our study showed that a modular stem designed for exchange has distinct advantages in the case of revision and that the quality of implantation (prosthetic height and torsion) at the index operation is important, because improper positioning can mandate stem exchange even in a system designed to allow stem preservation. As correct height of the prosthetic stem might be well achieved in most patients with primary osteoarthritis, this is a problem in patients with fractures. We therefore use the following references to obtain correct height of a fracture stem [9]: (1) the distance of the upper border of the pectoralis major tendon to the head [25]; (2), the distance of the tip of the fractured greater tuberosity to the articular side insertion of the rotator cuff, which helps to determine the desired height of the lateral aspect of the humeral head; and (3) we measure the height of the cartilage-free zone of the humeral head at the calcar, which determines the medial inferior border of the prosthetic head. To achieve correct humeral torsion at revision, design features (ie, modular head), which allow implantation of a primary anatomic stem in near neutral rotation, even in the presence of substantial retroversion of the humeral head, seems desirable. With the system we used, errors of humeral torsion of the primary stem can be compensated up to 20° in either direction. With the system used, revision always was possible if an anatomic system had been implanted correctly and the clinical results were at least as good as with exchanged stems, suggesting that there was no inordinate lengthening, tension, or malrotation of the arm.
There are limitations to our study. The sample size, especially in the group of patients without stem removal, is small, comprising variable indications for revision of hemiarthroplasties and total shoulder arthroplasties, which compromises the analysis of confounding factors. There was no selection bias owing to the retrospective study design, as the only factor deciding in which arm of the study the patients fell was not in our control because the type of previously implanted prosthesis determined whether stem removal was attempted, and a randomized trial is not possible because removal of a well-fixed stem, which allows conversion without removal, would be unreasonable and unethical. Although one observer (KW) analyzed the data, different physicians examined the patients during regular postoperative followups, documenting functional outcome scores in our prospective prosthesis database. Furthermore surgical time and blood loss were recorded retrospectively from the anesthesia charts. While surgical time can be measured, we are aware of the potential difficulties estimating perioperative blood loss. Although an assessment bias cannot be completely excluded, we do not believe that data acquisition was different between the study groups owing to the retrospective study design. Another potential bias of this investigation is that we included patients with less than 24 months followup (six patients [six of 13 shoulders without exchange]; eight patients [nine of 32 shoulders with stem exchange]) and have no clinical outcome data for 11 patients (six patients with complications, five patients lost to followup), in the group with stem revision. This and the limited sample size of patients without stem exchange might be the reasons for detecting no difference regarding patients’ satisfaction and clinical outcome between the study groups.
Despite these limitations the study was able to answer our primary research questions.
Increased intraoperative blood loss [15] and the use of a reverse implant design [14] have been identified as independent risk factors for postoperative blood transfusion after shoulder arthroplasty. The need or liberal use of such postoperative blood transfusions is associated with an increased postoperative (ie, infection and respiratory) complication rate [29]. Therefore the benefits of reduced surgical time and especially intraoperative blood loss, as achieved if conversion to reverse total shoulder arthroplasty without stem exchange was possible, cannot be questioned.
Numerous studies have shown that conversion to reverse total shoulder arthroplasty in patients with prior operations is associated with a substantial risk of intraoperative and postoperative complications and further revision is between 22% and 32% [20, 21, 23, 26, 33]. This is markedly higher than the complication rate for primary reverse total shoulder arthroplasty. In particular, the need for removal of a well-fixed humeral stem can be a challenge associated with a high risk for bone loss, malunion, or nonunion of a humeral osteotomy, periprosthetic fracture, and later humeral component loosening [8, 12, 32]. Therefore, there is an interest in the possibility for revision without stem exchange. This may be achievable primarily by using humeral head resurfacing or stemless humeral components, which, in case of failure, would provide an ideal way to avoid this complication and would be an attractive solution if they provide results as good as stemmed implants, which seems to be the case for certain indications [18, 27]. However, there are indications such as fractures for which stemmed implants most likely will continue to be preferred and their revision needs to be made safer and more effective. Solutions for stem retention also need to be investigated. In our series, the complication and reoperation rates for patients who have had revision of a hemiarthroplasty or total shoulder to reverse total shoulder arthroplasty without the need for stem exchange compare favorably not only with rates of our control group, but also with reported results [20, 21, 23, 26, 33].
Although there are some articles proposing the potential advantages of modular systems in anatomic prosthetic designs [7, 13, 17], we found only one case series that reported the outcome of 27 patients after they had conversion surgery from hemiarthroplasty or total to a reverse total shoulder arthroplasty without stem exchange using a different modular prosthetic design [5]. Although Castagna et al. [5] did not report a control group, there were no intraoperative or postoperative complications and the improvement of the Constant and Murley score was from 25 to 48 points, which is comparable to the results in our series.
Patients undergoing revision of stemmed hemiarthroplasty or total to reverse total shoulder arthroplasty without stem exchange had less intraoperative blood loss and operative time, fewer intraoperative complications, and fewer revisions than patients whose index revision procedures included stem exchange. Therefore modularity of a shoulder arthroplasty system has proven and substantial advantages if conversion to reverse total shoulder arthroplasty becomes necessary and might be considered as prerequisite for stemmed shoulder arthroplasty systems.
Footnotes
One of the authors certifies that he (CG) or a member of his or her immediate family, has or may receive payments or benefits, during the study period, an amount of more than USD 1,000,001 from Zimmer, Inc (Warsaw, IN, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Besch L, Daniels-Wredenhagen M, Mueller M, Varoga D, Hilgert RE, Seekamp A. Hemiarthroplasty of the shoulder after four-part fracture of the humeral head: a long-term analysis of 34 cases. J Trauma. 2009;66:211–214. doi: 10.1097/TA.0b013e31815d9649. [DOI] [PubMed] [Google Scholar]
- 2.Bohsali KI, Wirth MA, Rockwood CA., Jr Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88:2279–2292. doi: 10.2106/JBJS.F.00125. [DOI] [PubMed] [Google Scholar]
- 3.Bryant D, Litchfield R, Sandow M, Gartsman GM, Guyatt G, Kirkley A. A comparison of pain, strength, range of motion, and functional outcomes after hemiarthroplasty and total shoulder arthroplasty in patients with osteoarthritis of the shoulder: a systematic review and meta-analysis. J Bone Joint Surg Am. 2005;87:1947–1956. doi: 10.2106/JBJS.D.02854. [DOI] [PubMed] [Google Scholar]
- 4.Cadet ER, Ahmad CS. Hemiarthroplasty for three- and four-part proximal humerus fractures. J Am Acad Orthop Surg. 2012;20:17–27. doi: 10.5435/JAAOS-20-01-017. [DOI] [PubMed] [Google Scholar]
- 5.Castagna A, Delcogliano M, Caro F, Ziveri G, Borroni M, Gumina S, Postacchini F, Biase CF. Conversion of shoulder arthroplasty to reverse implants: clinical and radiological results using a modular system. Int Orthop. 2013;37:1297–1305. doi: 10.1007/s00264-013-1907-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;214:160–164. [PubMed] [Google Scholar]
- 7.Dines JS, Fealy S, Strauss EJ, Allen A, Craig EV, Warren RF, Dines DM. Outcomes analysis of revision total shoulder replacement. J Bone Joint Surg Am. 2006;88:1494–1500. doi: 10.2106/JBJS.D.02946. [DOI] [PubMed] [Google Scholar]
- 8.Flury MP, Frey P, Goldhahn J, Schwyzer HK, Simmen BR. Reverse shoulder arthroplasty as a salvage procedure for failed conventional shoulder replacement due to cuff failure: midterm results. Int Orthop. 2011;35:53–60. doi: 10.1007/s00264-010-0990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fucentese SF, Sutter R, Wolfensperger F, Jost B, Gerber C. Large metaphyseal volume hemiprostheses for complex fractures of the proximal humerus. J Shoulder Elbow Surg. 2014;23:427–433. doi: 10.1016/j.jse.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs B, Jost B, Gerber C. Posterior-inferior capsular shift for the treatment of recurrent, voluntary posterior subluxation of the shoulder. J Bone Joint Surg Am. 2000;82:16–25. doi: 10.2106/00004623-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Gartsman GM, Roddey TS, Hammerman SM. Shoulder arthroplasty with or without resurfacing of the glenoid in patients who have osteoarthritis. J Bone Joint Surg Am. 2000;82:26–34. doi: 10.2106/00004623-200001000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Gohlke F, Rolf O. [Revision of failed fracture hemiarthroplasties to reverse total shoulder prosthesis through the transhumeral approach: method incorporating a pectoralis-major-pedicled bone window][in German] Oper Orthop Traumatol. 2007;19:185–208. doi: 10.1007/s00064-007-1202-x. [DOI] [PubMed] [Google Scholar]
- 13.Groh GI, Wirth MA. Results of revision from hemiarthroplasty to total shoulder arthroplasty utilizing modular component systems. J Shoulder Elbow Surg. 2011;20:778–782. doi: 10.1016/j.jse.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Gruson KI, Accousti KJ, Parsons BO, Pillai G, Flatow EL. Transfusion after shoulder arthroplasty: an analysis of rates and risk factors. J Shoulder Elbow Surg. 2009;18:225–230. doi: 10.1016/j.jse.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Hardy JC, Hung M, Snow BJ, Martin CL, Tashjian RZ, Burks RT, Greis PE. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22:233–239. doi: 10.1016/j.jse.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Hasan SS, Leith JM, Campbell B, Kapil R, Smith KL, Matsen FA., 3rd Characteristics of unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2002;11:431–441. doi: 10.1067/mse.2002.125806. [DOI] [PubMed] [Google Scholar]
- 17.Hattrup SJ. Revision total shoulder arthroplasty for painful humeral head replacement with glenoid arthrosis. J Shoulder Elbow Surg. 2009;18:220–224. doi: 10.1016/j.jse.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Huguet D, DeClercq G, Rio B, Teissier J, Zipoli B, TESS Group Results of a new stemless shoulder prosthesis: Radiologic proof of maintained fixation and stability after a minimum of three years’ follow-up. J Shoulder Elbow Surg. 2010;19:847–852. doi: 10.1016/j.jse.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Khan A, Bunker TD, Kitson JB. Clinical and radiological follow-up of the Aequalis third-generation cemented total shoulder replacement: a minimum ten-year study. J Bone Joint Surg Br. 2009;91:1594–1600. doi: 10.1302/0301-620X.91B12.22139. [DOI] [PubMed] [Google Scholar]
- 20.Levy J, Frankle M, Mighell M, Pupello D. The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. J Bone Joint Surg Am. 2007;89:292–300. doi: 10.2106/JBJS.E.01310. [DOI] [PubMed] [Google Scholar]
- 21.Levy JC, Virani N, Pupello D, Frankle M. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89:189–195. doi: 10.1302/0301-620X.89B2.18161. [DOI] [PubMed] [Google Scholar]
- 22.Lo IK, Litchfield RB, Griffin S, Faber K, Patterson SD, Kirkley A. Quality-of-life outcome following hemiarthroplasty or total shoulder arthroplasty in patients with osteoarthritis: a prospective, randomized trial. J Bone Joint Surg Am. 2005;87:2178–2185. doi: 10.2106/JBJS.D.02198. [DOI] [PubMed] [Google Scholar]
- 23.Melis B, Bonnevialle N, Neyton L, Lévigne C, Favard L, Walch G, Boileau P. Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Shoulder Elbow Surg. 2012;21:342–349. doi: 10.1016/j.jse.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Mighell MA, Kolm GP, Collinge CA, Frankle MA. Outcomes of hemiarthroplasty for fractures of the proximal humerus. J Shoulder Elbow Surg. 2003;12:569–577. doi: 10.1016/S1058-2746(03)00213-1. [DOI] [PubMed] [Google Scholar]
- 25.Murachovsky J, Ikemoto RY, Nascimento LGP, Fujiki EN, Milani C, Warner JJ. Pectoralis major tendon reference (PMT): a new method for accurate restoration of humeral length with hemiarthroplasty for fracture. J Shoulder Elbow Surg. 2006;15:675–678. doi: 10.1016/j.jse.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Ortmaier R, Resch H, Matis N, Blocher M, Auffarth A, Mayer M, Hitzl W, Tauber M. Reverse shoulder arthroplasty in revision of failed shoulder arthroplasty: outcome and follow-up. Int Orthop. 2013;37:67–75. doi: 10.1007/s00264-012-1742-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22:206–214. doi: 10.1016/j.jse.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Robinson CM, Page RS, Hill RM, Sanders DL, Court-Brown CM, Wakefield AE. Primary hemiarthroplasty for treatment of proximal humeral fractures. J Bone Joint Surg Am. 2003;85:1215–1223. doi: 10.1302/0301-620X.85B7.13959. [DOI] [PubMed] [Google Scholar]
- 29.So-Osman C, Nelissen R, Brand R, Faber F, Slaa RT, Stiggelbout A, Brand A. The impact of a restrictive transfusion trigger on post-operative complication rate and well-being following elective orthopaedic surgery: a post-hoc analysis of a randomised study. Blood Transfus. 2013;11:289–295. doi: 10.2450/2013.0172-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sperling JW, Cofield RH, Rowland CM. Minimum fifteen-year follow-up of Neer hemiarthroplasty and total shoulder arthroplasty in patients aged fifty years or younger. J Shoulder Elbow Surg. 2004;13:604–613. doi: 10.1016/j.jse.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Sperling JW, Cofield RH. Revision total shoulder arthroplasty for the treatment of glenoid arthrosis. J Bone Joint Surg Am. 1998;80:860–867. doi: 10.2106/00004623-199806000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Sperling JW, Cofield RH. Humeral windows in revision shoulder arthroplasty. J Shoulder Elbow Surg. 2005;14:258–263. doi: 10.1016/j.jse.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Walker M, Willis MP, Brooks JP, Pupello D, Mulieri PJ, Frankle MA. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21:514–522. doi: 10.1016/j.jse.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Young AA, Walch G, Pape G, Gohlke F, Favard L. Secondary rotator cuff dysfunction following total shoulder arthroplasty for primary glenohumeral osteoarthritis: results of a multicenter study with more than five years of follow-up. J Bone Joint Surg Am. 2012;94:685–693. doi: 10.2106/JBJS.J.00727. [DOI] [PubMed] [Google Scholar]