Abstract
Background
Total hip arthroplasty (THA) for the treatment of posttraumatic osteoarthritis (OA) after acetabular fracture has been associated with a high likelihood of aseptic loosening, instability, and infection. Porous metal components may help to address the issue of loosening, but there are few data on the use of porous metal acetabular components for posttraumatic OA after acetabular fracture.
Questions/purposes
Using an institutional registry, we aimed to report (1) radiographic evidence of fixation; (2) survivorship free from revision; (3) Harris hip scores; and (4) complications and reoperations after THA with a porous metal acetabular component for posttraumatic OA in patients previously treated with open reduction and internal fixation (ORIF) of a displaced acetabular fracture.
Methods
Thirty primary THAs were performed with a porous metal acetabular component for the treatment of posttraumatic OA after ORIF of an acetabular fracture from 1999 through 2010; of these, 28 (93%) were available for followup at a minimum of 2 years. During that same time, 51 primary THAs were performed using other acetabular designs in patients who had previously undergone ORIF of the acetabulum. During the period in question, the general indications for use of porous metal in this setting included compromised acetabular bone stock or quality to the extent that the treating surgeon believed primary fixation with a titanium shell and screws may have been difficult to achieve. Mean age at the time of arthroplasty was 45 years (range, 23–75 years). Median time from ORIF to THA and from THA to last followup was 107 months (range, 4 months to 42 years) and 60 months (range, 25 months to 10 years), respectively. Radiographs were reviewed for this specific study to evaluate the components for evidence of osteointegration. Survivorship free from revision, hip scores, and complications were extracted from our institutional database and electronic medical record.
Results
No acetabular or femoral components were revised for aseptic loosening. Five-year survival with revision for any reason as the endpoint was 88% (95% confidence interval, 0.70–0.96). Harris hip scores improved from a median of 39 preoperatively (range, 3–87) to 82 at last followup (range, 21–100; p < 0.01). Three hips (11%) underwent resection for infection and all three had been treated with staged arthroplasty for concern of infection. Two patients (7%) experienced at least one dislocation postoperatively.
Conclusions
The short-term results of the use of porous metal acetabular components in THA for treatment of posttraumatic OA after acetabular fracture demonstrate low rates of mechanical failure. Although infection and instability remain major concerns in patients with this diagnosis seemingly regardless of the implant design used, porous metal components appear to offer a high likelihood of osseointegration in this clinical setting.
Level of Evidence
Level IV, therapeutic study. See Instructions for Authors for a complete description of levels of evidence.
Introduction
Despite advances in operative techniques in the treatment of displaced acetabular fractures, many patients develop posttraumatic osteoarthritis (OA) [9, 26]. Degenerative joint disease after an acetabular fracture may develop as a result of residual articular incongruity, damage to the articular cartilage at the time of the injury, or avascular necrosis of the femoral head [19, 26]. When posttraumatic OA develops, THA frequently is performed as a salvage procedure.
Early studies using cemented acetabular components found that many patients developed loosening and instability [22, 27]. Loosening was attributed to loss of acetabular bone stock, the presence of hardware, altered anatomy of the hemipelvis, and a relatively younger and more active patient population [27]. Infection and further compromised bone stock are especially a concern in those patients who have previously undergone open reduction and internal fixation (ORIF). More recent reports on the use of uncemented acetabular components have demonstrated a lower frequency of aseptic loosening [1, 2, 16, 17, 21, 28], and a recent study by Kamath et al. [14] confirmed these results in a small number of patients treated with THA using a porous metal cup after acetabular fracture. However, the majority of acetabular fractures in these studies had initially been managed nonoperatively, and thus the degree of bone loss and the environment for early osseointegration of the uncemented acetabular component to the host pelvis may not be comparable to the acetabulum that has already undergone ORIF [27]. Many acetabular fractures that are initially managed nonoperatively have minimal displacement and thus the resulting amount of bone loss may be less than those patients who required ORIF. Additionally, the presence of postoperative scarring and previously placed hardware present additional challenges for acetabular reconstruction at the time of THA [1]. The use of porous metals for acetabular reconstruction has shown excellent early clinical and radiographic results for a variety of clinical scenarios with reduced acetabular bone stock and quality [4, 13, 20, 23].
We therefore sought to use a single institution’s registry to report (1) radiographic evidence of fixation; (2) survivorship free from revision; (3) Harris hip scores; and (4) complications and reoperations after THA with a porous metal acetabular component for posttraumatic OA in patients previously treated with ORIF of a displaced acetabular fracture.
Patients and Methods
Using a computerized institutional database, all patients who underwent THA with a porous metal acetabular component for treatment of posttraumatic OA after acetabular fracture before 2010 were identified. Institutional review board approval was obtained for the study.
Participants were included in the study if they had undergone ORIF for treatment of an acetabular fracture before THA and had the THA performed with a porous metal acetabular component. Patients with posttraumatic OA resulting from a pathologic acetabular fracture resulting from tumor (primary or metastatic) or acetabular fractures which were managed nonoperatively were excluded from the analysis.
From 1999 through 2010, 30 patients (30 hips) met criteria for inclusion. Nine patients were female and 21 were male. Mean age at the time of ORIF and index arthroplasty was 37 years (range, 17–74 years) and 45 years (range, 23–75 years), respectively. The mean time from ORIF until THA was 9 years (range, 4 months to 42 years). Mean body mass index at the time of THA was 32 kg/m2 (range, 20–63 kg/m2). Of the 30 patients, two were lost to clinical followup at less than 2 years and an additional six hips were only evaluated clinically through letter or telephone questionnaire after 2 years. Thus, complete clinical and radiographic followup at a minimum of 2 years (mean, 5 years; range, 2–11 years) was available in 28 (93%) and 22 hips (73%), respectively.
During the study period, 51 primary THAs were performed using other acetabular components in patients who had previously undergone ORIF of the acetabulum. There was a significant bias toward use of a porous metal cup design in THA cases with more compromised bone stock or poor bone quality, regardless of individual surgeon preference for routine arthroplasty cases.
The original fracture pattern was of the elementary type in eight of 30 hips (27%, posterior wall fracture in six, transverse fracture in two) and associated type in 13 of 30 hips (43%, T-type fracture in five, transverse-posterior wall fracture in four, posterior column/posterior wall in three, and associated both column in one). The original fracture pattern was not known in nine of 30 hips. Before arthroplasty, nine of 30 hips (30%) had radiographic evidence of osteonecrosis of the femoral head, and six of those had confirmed traumatic dislocations at the time of their original injury. Partial sciatic nerve palsies were present in seven of 30 patients (23%) before THA. These represented sensory symptoms only in five of 30 patients and foot drop treated with an orthosis in two of 30 patients.
An anterolateral approach was used in 21 and a posterior approach in nine patients at the discretion of the treating surgeon. Sciatic nerve monitoring was not routinely used. All acetabular reconstructions were performed with a tantalum porous metal hemispherical shell. The acetabular component was a Trabecular Metal® revision shell (Zimmer, Warsaw, IN, USA) in 29 patients and a modular Trabecular Metal® shell (Zimmer) in one patient. In those hips with a revision shell, the acetabular component fixation was supplemented with screws in all cases (mean number of screws, 3; range, 2–9 screws); no screws were used in the hip with the modular component. An ultrahigh-molecular-weight polyethylene liner was used in all hips and in the 29 hips with a nonmodular revision shell, the liner was cemented in place. An elevated liner was used in eight of 30 patients to address intraoperative instability. Preexisting hardware was only removed as needed to allow press-fit of the acetabular component. Acetabular bone defects were addressed with allograft, autograft, or tantalum metal augments as necessary.
Twenty-seven of the 30 patients were treated with uncemented femoral components: 12 HA Proxilock (Zimmer), eight Summit (DePuy, Warsaw, IN, USA), three Trabecular Metal® (Zimmer), two Synergy HA (Smith & Nephew, Memphis, TN, USA), one AML (DePuy), and one Echelon (Smith & Nephew). The remaining three hips had a cemented femoral component (one Cobrex, Zimmer; one F-130 and one ODC plus stem; Osteonics, Mahwah, NJ, USA). Although the most common head sizes were 28 and 32 mm (12 hips each), 11 of the 12 28-mm heads were implanted before 2004. A 36-mm head was used in five hips and a 40-mm head in one hip. Cobalt-chrome heads were used in 28 hips, a 32-mm Oxinium (Smith & Nephew) head in one hip, and a 28-mm Zirconia (Zimmer) ceramic head in one hip. Head size was determined by surgeon preference and through an intraoperative assessment of stability.
According to the AAOS classification [6], bony acetabular defects were classified as Type I (segmental) in one of 30 hips, Type II (cavitary) in nine of 30 hips, Type III (combined) in one of 30 hips, and Type IV (pelvic discontinuity) in one of 30 hips. One patient with a Type IV defect was noted to have a transverse acetabular nonunion at the time of THA and was treated with femoral head autograft in the nonunion site at the time of THA. Bone grafting of the acetabulum was performed in 10 of 30 hips (33%). A particulate autologous femoral head graft was used to fill cavitary acetabular defects in seven hips, autologous femoral head graft was used as a structural graft for a segmental posterosuperior defect in one hip, and particulate cancellous allograft was used to fill contained cavitary defects in two patients. A tantalum augment was used to address medial bone loss in one hip.
Intraoperative blood loss averaged 956 mL, and 14 of 30 patients received allogeneic blood transfusions intraoperatively or postoperatively. In those patients who did receive a transfusion, the mean number of units transfused was three (range, 1–7 units).
Staged débridement with hardware removal followed by THA was undertaken in five of 30 patients with a concern for deep infection based on history of prior infection (three of five patients) or rapid joint destruction (two of five patients; Table 1). The femoral head was resected at the time of débridement, and all patients were treated with intravenous antibiotics. In one of five patients, the hardware could not be removed completely as a result of severe scarring around the posterior column and sciatic nerve. Cultures taken at the time of initial débridement and hardware removal showed no growth in three of five hips, methicillin-resistant Staphylococcus aureus in one of five hips, and Pseudomonas aeruginosa in one of five hips. THA was undertaken when inflammatory markers had returned to normal after an antibiotic holiday of at least 2 weeks. Intraoperative pathology was sent at the time of THA and was negative for acute inflammation in all patients. The mean time between débridement and THA was 12 months (range, 2–31 months).
Table 1.
Patients treated with staged débridement and THA
| Patient number | Age (years) | Sex | Medical comorbidities | Reason for staged treatment | Time between ORIF and débridement | Culture results at time of débridement | Months from débridement to THA | Recurrent infection? |
|---|---|---|---|---|---|---|---|---|
| 1 | 43 | M | DM 2, obesity, HTN | Rapid joint destruction | 11 months | No growth | 2 | Yes, Staphylococcus epidermidis 21 months after THA |
| 2 | 39 | F | Obesity | History of infection | 16 months | MRSA | 31 | Yes, P aeruginosa 45 months after THA |
| 3 | 49 | M | DVT, tobacco use, IVDU | History of infection | 33 years | Pseudomonas aeruginosa | 4 | Yes, P aeruginosa 12 months after THA |
| 4 | 36 | F | Schizophrenia | Rapid joint destruction | 40 months | No growth | 22 | No |
| 5 | 75 | F | HTN | History of infection | 36 years | No growth | 3 | No |
M = male; F = female; DM = diabetes mellitus; HTN = hypertension; DVT = deep vein thrombosis; IVDU = intravenous drug use; MRSA = methicillin-resistant Staphylococcus aureus.
Patients were routinely followed with clinical examination and radiographs at 1 year, 2 years, 5 years, and every 5 years thereafter. Patients who could not return for evaluation were asked to send hard-copy radiographs for review and completed a telephone interview or survey by mail. Preoperative and followup Harris hip scores (HHS) [12] were calculated by an independent observer (BJY) at each time point. Scores < 70 were defined as poor, 70 to 79 as fair, 80 to 89 as good, and 90 to 100 as excellent [15]. Radiographs were obtained preoperatively and at each postoperative clinical encounter and included an AP radiograph of the pelvis as well as an AP and lateral radiograph of the involved hip. Radiographs were evaluated with a computerized PACS system by an independent observer (BJY) and one of the senior authors (ADH). Radiolucent lines at the implant-bone interface were assessed according to DeLee and Charnley zones [7]. Acetabular components with evidence of streaming trabeculae in at least one zone without any evidence of loosening were considered osseointegrated. Migration of the acetabular component was evaluated according to Massin et al. [18]. Loosening of an uncemented acetabular component was defined as implant migration; a complete radiolucent line at the implant-bone interface or fixation screw breakage [11]. Femoral component loosening was assessed according to the zones described by Gruen et al. [10] and criteria of Engh et al. [8]. The presence of heterotopic ossification was classified according to Brooker et al. [5]. Complications were collected through review of the institutional database and confirmed through review of each patient’s medical record. Patients who do not return for clinical followup in person are questioned about specific complications (episodes of instability requiring a reduction, infection treated by a physician or surgeon, or reoperation for any reason) at the time of telephone interview or survey by mail [3].
The Mann-Whitney U test was used to compare the clinical results of patients with and without a history of infection. A p value of < 0.05 was considered significant. Kaplan-Meier analysis was used to determine survivorship free from revision at 5 years. Patients were censored at the time of death or at the time of last followup. Statistics were calculated using JMP software (SAS, Cary, NC, USA).
Results
One of three patients who developed an infection did show evidence of a complete radiolucent line > 2 mm and migration of the acetabular component at 21 months (Fig. 1). All remaining acetabular components were radiographically osseointegrated at last followup. Nonprogressive partial radiolucent lines of < 1 mm wide were present about the acetabular component in nine of 22 hips (41%), and no radiolucent lines were present in the remaining 12 of 22 cups (55%). No acetabular component had evidence of marginal, retroacetabular, or screw-related osteolysis. All femoral components were radiographically stable.
Fig. 1.
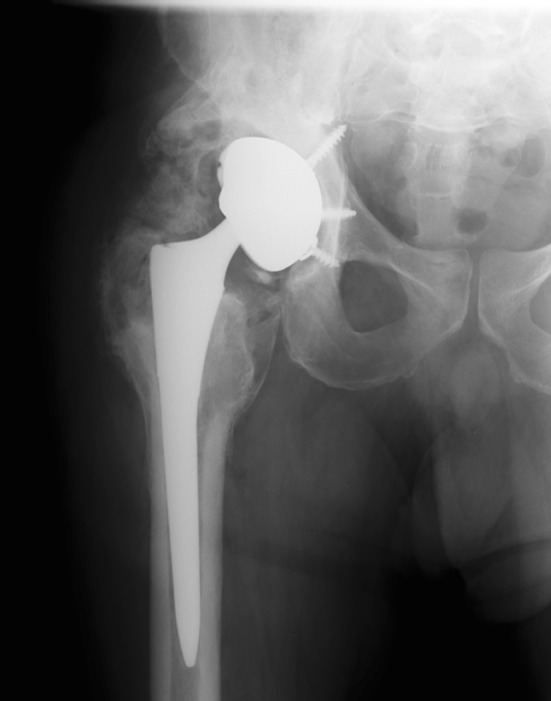
AP radiograph was obtained 21 months postoperatively on Patient 1. This patient was subsequently revised for deep periprosthetic infection and septic loosening.
Survivorship free of reoperation at 5 years was 88% (95% confidence interval, 0.70–0.96). The causes of revision were all related to infection, two patients with recurrent or recalcitrant infection with stable implants and another patient with septic loosening of the acetabular component. All of these hips had been treated with removal of hardware, Girdlestone, and staged THA for concern of infection. No reoperations were performed for instability or aseptic loosening.
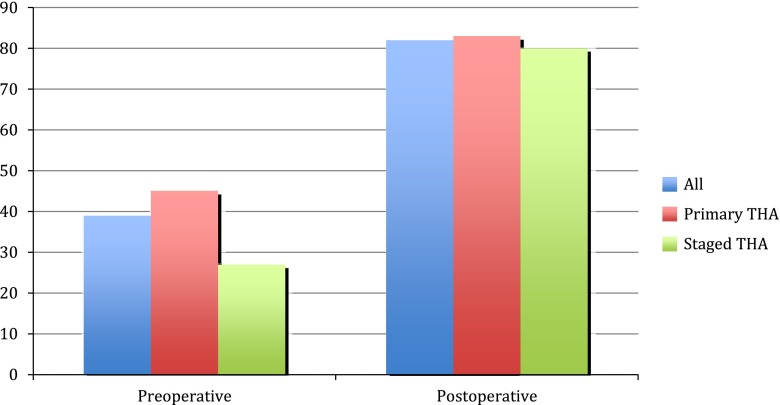
The median HHS improved from 39 preoperatively (range, 3–71 points) to 82 points (range, 21–100 points) at the time of last followup (p < 0.01; Fig. 2). Fifteen of 28 hips (54%) had a good or excellent result, three of 28 hips (11%) had a fair result, and 10 of 28 hips (35%) had a poor result.
Fig. 2.
Harris hip scores are shown.
Two of 28 patients (7%) experienced early postoperative posterior dislocations at 1 and 3 months after the index arthroplasty, respectively. Both hips had been implanted through a posterior approach and both had a 32-mm head. Each patient had a second posterior dislocation within 30 days of the initial closed reduction. Both patients were again successfully treated with closed reduction in addition to placement into a hip abduction brace. Neither patient had experienced another episode of instability at last followup. Three of 28 patients developed deep periprosthetic infection after THA (11%); all of them had been treated with staged débridement and arthroplasty for concern of infection (Table 1). Each of these patients was treated with repeat resection arthroplasty. No deep or superficial infectious complications were observed in any of the 23 patients who did not undergo a staged arthroplasty. One of 28 patients had an early greater trochanteric fracture 2 months after THA that was treated nonoperatively and went on to nonunion. At the time of last followup (50 months), the patient had a persistent radiographic nonunion and a Trendelenburg gait. Heterotopic ossification of Class III or IV was present in four of 30 hips (13%) preoperatively and five of 22 hips (23%) after THA [5]. Preoperatively, lesions were Brooker Class III in three of 30 patients and Class IV in one of 30 patients. Postoperatively the lesions were Class III in five of 22 patients and Class IV in no patients [5] (Fig. 3). No patient had new or progressive sciatic nerve symptoms after THA. Only one patient had a persistent foot drop at the time of last followup.
Fig. 3.
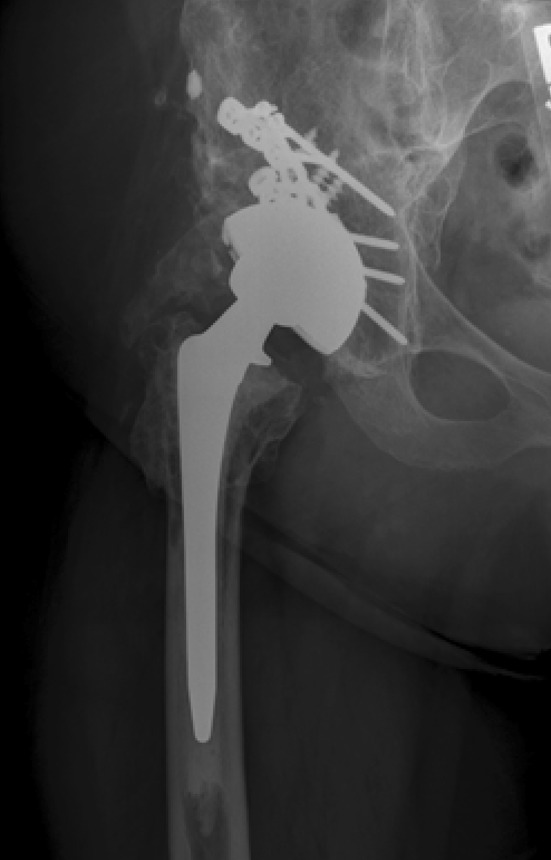
AP radiograph shows a patient with Class III heterotopic ossification 9 years after THA. Class III or IV heterotopic ossification was present in 23% of hips postoperatively.
Discussion
Despite advances in techniques for internal fixation, the frequency of posttraumatic OA after acetabular fracture has been shown to be as high as 21% to 30% [9, 26]. Acetabular reconstruction in the setting of prior acetabular fracture has historically been met with high rates of aseptic loosening, particularly in the era of cemented acetabular fixation [22, 27]. More recent reports with uncemented acetabular fixation have demonstrated reduced rates of mechanical failure [1, 2, 16, 17, 21, 28]. Despite these improvements, most of these reports included patients who were managed both with ORIF and nonoperatively at the time of acetabular fracture. Many patients who require ORIF have more initial fracture displacement and soft tissue destruction at the time of injury, and thus the environment for host bone integration to an uncemented acetabular component may not be comparable to those patients who were initially managed nonoperatively. In our experience, the presence of prior hardware, postoperative scarring, and bone loss after ORIF of an acetabular fracture presents unique challenges to uncemented acetabular reconstruction at the time of THA. We therefore sought to use a single institution’s registry to report (1) radiographic evidence of fixation; (2) survivorship free from revision; (3) HHSs; and (4) complications and reoperations after THA with a porous metal acetabular component for posttraumatic OA in patients previously treated with ORIF of a displaced acetabular fracture.
Limitations of the current study include its retrospective nature, variability in time from ORIF to THA, relatively short followup, and incomplete radiographic followup. Short and incomplete followup should cause one to interpret our findings as “best-case estimates” of the performance of this implant in this setting, because patients lost to followup and with incomplete followup may not be faring as well as those with complete followup. Because this was a retrospective study, a consistent set of indications for use of a porous metal acetabular component was not established a priori, introducing the possibility of significant selection bias. In general these components were used in cases with increasingly compromised acetabular bone stock and/or quality at the judgment of the treating surgeon. However, the current series represents a large number of patients treated in a relatively uniform fashion. Although there was a large range of time between ORIF and THA (4 months to 42 years), no patients were treated with arthroplasty in the acute period after their fracture and all patients had undergone prior operative ORIF. Similarly, the type of acetabular component was identical in 29 of 30 hips and only one monoblock tantalum component was used.
We found reliable osseointegration in this series, and no cups demonstrated signs of migration or loosening in the absence of infection. This compares favorably to multiple previous reports on the use of standard titanium uncemented acetabular components [1, 16, 17, 21, 28]. Rates of radiographic or clinical aseptic loosening in these studies ranged from 0% to 17% at similar short-term followup. Porous tantalum acetabular components have been shown to offer potential for improved biologic fixation to the host pelvis [4, 20, 23, 24]. As a result of concerns regarding compromised bone stock and unknown periacetabular bone viability, porous metal acetabular components offer a potential alternative in the setting of prior ORIF of acetabular fracture.
We are aware of only one other report on the use of a porous metal component for acetabular reconstruction in the setting of prior acetabular fracture. Kamath et al. [14] described the use of a porous tantalum acetabular component in 12 patients, seven of whom had undergone prior ORIF. Four patients had undergone index arthroplasty at less than 3 months after injury, and three patients had already undergone prior THA. There was only one hip (8%) with radiographic evidence of acetabular component loosening in a renal transplant patient with rheumatoid arthritis. All other components were radiographically stable at a minimum of 2 years. Whether the high rate of osseointegration after acetabular fracture demonstrated in this study and the previous study by Kamath et al. is superior to the previously described excellent results with standard uncemented fixation surfaces remains to be seen.
We found that survivorship at 5 years was 88%, and all of the reoperations in this small series were related to the treatment of infection. This is similar to previous reports on uncemented fixation after acetabular fracture. In a study of 32 patients with cementless acetabular reconstruction after acetabular fracture, Ranawat et al. [21] described a low rate of aseptic loosening (one of 32 patients [3%]) but two patients developed deep periprosthetic infections requiring explantation and both of those patients had been treated with staged débridement and removal of hardware, similar to our study.
Harris hip scores in our group were comparable to those seen in other similar series of patients who underwent THA after ORIF of the acetabulum [1, 2, 21]. These included mostly good to excellent outcomes with a subset of patients experiencing poor functional outcomes mainly related to the not insignificant rate of complications in this cohort.
Complications were frequent in this group as has been observed to be the case in other similar reports [1, 2, 14, 16, 17, 21]. Previous authors have described these complications and that they may be more prevalent in those patients who have previously undergone ORIF, because they experience a longer operative time with more blood loss and more intraoperative instability [1, 2, 21]. These patients frequently have a history of infection related to their original operation and thus experience a higher rate of deep periprosthetic infection after THA [21, 25]. In our study this risk remained elevated despite attempts to treat these patients in a staged fashion with débridement and removal of hardware before definitive THA [25].
In summary, THA in the setting of prior ORIF of acetabular fracture poses major challenges. Although infection and instability remain major concerns in patients with this diagnosis seemingly regardless of the implant design used, porous metal components appear to offer a high likelihood of osseointegration in this clinical setting. In this short-term followup study of THA for treatment of posttraumatic OA after operatively treated acetabular fracture, the use of a porous tantalum acetabular component appears to be a viable option.
Footnotes
One of the authors (DGL) certifies he has or may receive payments or benefits, in any one year, an amount in excess of USD 100,000 from a commercial entity (Zimmer, Warsaw, IN, USA) related to this work. The institution of one or more of the authors (DGL, ADH) has received funding from DePuy (Warsaw, IN, USA), Zimmer, Stryker (Mahwah, NJ, USA), and Biomet (Warsaw, IN, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Bellabarba C, Berger RA, Bentley CD, Quigley LR, Jacobs JJ, Rosenberg AG, Sheinkop MB, Galante JO. Cementless acetabular reconstruction after acetabular fracture. J Bone Joint Surg Am. 2001;83:868–876. doi: 10.1302/0301-620X.83B6.11649. [DOI] [PubMed] [Google Scholar]
- 2.Berry DJ, Halasy M. Uncemented acetabular components for arthritis after acetabular fracture. Clin Orthop Relat Res. 2002:164–167. [DOI] [PubMed]
- 3.Berry DJ, Kessler M, Morrey BF. Maintaining a hip registry for 25 years. Mayo Clinic experience. Clin Orthop Relat Res. 1997;344:61–68. doi: 10.1097/00003086-199711000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Bobyn JD, Poggie RA, Krygier JJ, Lewallen DG, Hanssen AD, Lewis RJ, Unger AS, O’Keefe TJ, Christie MJ, Nasser S, Wood JE, Stulberg SD, Tanzer M. Clinical validation of a structural porous tantalum biomaterial for adult reconstruction. J Bone Joint Surg Am. 2004;86(Suppl 2):123–129. doi: 10.2106/00004623-200412002-00017. [DOI] [PubMed] [Google Scholar]
- 5.Brooker AF, Bowerman JW, Robinson RA, Riley LH., Jr Ectopic ossification following total hip replacement. Incidence and a method of classification. J Bone Joint Surg Am. 1973;55:1629–1632. [PubMed] [Google Scholar]
- 6.D’Antonio JA, Capello WN, Borden LS, Bargar WL, Bierbaum BF, Boettcher WG, Steinberg ME, Stulberg SD, Wedge JH. Classification and management of acetabular abnormalities in total hip arthroplasty. Clin Orthop Relat Res. 1989;243:126–137. [PubMed] [Google Scholar]
- 7.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed] [Google Scholar]
- 8.Engh CA, Bobyn JD, Glassman AH. Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. J Bone Joint Surg Br. 1987;69:45–55. doi: 10.1302/0301-620X.69B1.3818732. [DOI] [PubMed] [Google Scholar]
- 9.Giannoudis PV, Grotz MR, Papakostidis C, Dinopoulos H. Operative treatment of displaced fractures of the acetabulum. A meta-analysis. J Bone Joint Surg Br. 2005;87:2–9. [PubMed] [Google Scholar]
- 10.Gruen TA, McNeice GM, Amstutz HC. ‘Modes of failure’ of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;141:17–27. [PubMed] [Google Scholar]
- 11.Haidukewych GJ, Jacofsky DJ, Hanssen AD, Lewallen DG. Intraoperative fractures of the acetabulum during primary total hip arthroplasty. J Bone Joint Surg Am. 2006;88:1952–1956. doi: 10.2106/JBJS.E.00890. [DOI] [PubMed] [Google Scholar]
- 12.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed] [Google Scholar]
- 13.Joglekar SB, Rose PS, Lewallen DG, Sim FH. Tantalum acetabular cups provide secure fixation in THA after pelvic irradiation at minimum 5-year followup. Clin Orthop Relat Res. 2012;470:3041–3047. doi: 10.1007/s11999-012-2382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamath AF, Evangelista PJ, Nelson CL. Total hip arthroplasty with porous metal cups following acetabular fracture. Hip Int. 2013;23:465–471. doi: 10.5301/hipint.5000037. [DOI] [PubMed] [Google Scholar]
- 15.Kavanagh BF, Fitzgerald RH., Jr Clinical and roentgenographic assessment of total hip arthroplasty. A new hip score. Clin Orthop Relat Res. 1985;193:133–140. [PubMed] [Google Scholar]
- 16.Lai O, Yang J, Shen B, Zhou Z, Kang P, Pei F. Midterm results of uncemented acetabular reconstruction for posttraumatic arthritis secondary to acetabular fracture. J Arthroplasty. 2011;26:1008–1013. doi: 10.1016/j.arth.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 17.Lizaur-Utrilla A, Sanz-Reig J, Serna-Berna R. Cementless acetabular reconstruction after acetabular fracture: a prospective, matched-cohort study. J Trauma Acute Care Surg. 2012;73:232–238. doi: 10.1097/TA.0b013e31824cf39e. [DOI] [PubMed] [Google Scholar]
- 18.Massin P, Schmidt L, Engh CA. Evaluation of cementless acetabular component migration. An experimental study. J Arthroplasty. 1989;4:245–251. doi: 10.1016/S0883-5403(89)80020-8. [DOI] [PubMed] [Google Scholar]
- 19.Matta JM. Fractures of the acetabulum: accuracy of reduction and clinical results in patients managed operatively within three weeks after the injury. J Bone Joint Surg Am. 1996;78:1632–1645. [PubMed] [Google Scholar]
- 20.Meneghini RM, Meyer C, Buckley CA, Hanssen AD, Lewallen DG. Mechanical stability of novel highly porous metal acetabular components in revision total hip arthroplasty. J Arthroplasty. 2010;25:337–341. doi: 10.1016/j.arth.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Ranawat A, Zelken J, Helfet D, Buly R. Total hip arthroplasty for posttraumatic arthritis after acetabular fracture. J Arthroplasty. 2009;24:759–767. doi: 10.1016/j.arth.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Romness DW, Lewallen DG. Total hip arthroplasty after fracture of the acetabulum. Long-term results. J Bone Joint Surg Br. 1990;72:761–764. doi: 10.1302/0301-620X.72B5.2211750. [DOI] [PubMed] [Google Scholar]
- 23.Sporer SM, Paprosky WG. Acetabular revision using a trabecular metal acetabular component for severe acetabular bone loss associated with a pelvic discontinuity. J Arthroplasty. 2006;21:87–90. doi: 10.1016/j.arth.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Sporer SM, Paprosky WG. The use of a trabecular metal acetabular component and trabecular metal augment for severe acetabular defects. J Arthroplasty. 2006;21:83–86. doi: 10.1016/j.arth.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Sterling RS, Krushinski EM, Pellegrini VD., Jr THA after acetabular fracture fixation: is frozen section necessary? Clin Orthop Relat Res. 2011;469:547–551. doi: 10.1007/s11999-010-1612-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tannast M, Najibi S, Matta JM. Two to twenty-year survivorship of the hip in 810 patients with operatively treated acetabular fractures. J Bone Joint Surg Am. 2012;94:1559–1567. doi: 10.2106/JBJS.K.00444. [DOI] [PubMed] [Google Scholar]
- 27.Weber M, Berry DJ, Harmsen WS. Total hip arthroplasty after operative treatment of an acetabular fracture. J Bone Joint Surg Am. 1998;80:1295–1305. doi: 10.2106/00004623-199809000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Zhou Y, Li Y, Xu H, Guo X. Total hip arthroplasty for failed treatment of acetabular fractures: a 5-year follow-up study. J Arthroplasty. 2011;26:1189–1193. doi: 10.1016/j.arth.2011.02.024. [DOI] [PubMed] [Google Scholar]