Abstract
Background
Recent studies have shown successful midterm outcomes after total hip arthroplasty (THA) in patients with human immunodeficiency virus (HIV). However, little data exist on the epidemiology, risk of perioperative complications, and length of stay in patients with HIV receiving THA.
Questions/purposes
The purposes of this study were to assess (1) the demographic trends of patients with HIV who underwent primary THA; (2) the differences in the risk of major and minor perioperative complications among patients with and without HIV; and (3) the differences in mean length of hospital stay among patients with and without HIV.
Methods
The Nationwide Inpatient Sample was used to compare patients with and without HIV who were admitted for THA between 1998 and 2010 in the United States. We extracted data on each admission’s age, sex, race, insurance, and comorbidities. The study population consisted of 2,656,696 patients without HIV and 9275 patients with HIV.
Results
Patients with HIV were more likely to be younger, be male, not pay with Medicare, and be of a nonwhite race. After controlling for confounding variables, patients with HIV were more likely to have major complications (2.9% [266 of 9275] versus 2.7% [71,952 of 2,656,696]; odds ratio [OR], 1.47; 95% confidence interval [CI], 1.08–2.00; p = 0.014) and minor complications (5.2% [483 of 9275] versus 4.8% [127,940 of 2,656,696]; OR, 1.61; 95% CI, 1.29–2.02; p < 0.001) compared with patients who did not have HIV. Patients undergoing THA who had HIV also had an increased length of hospital stay compared with patients without HIV (4.31 versus 3.83 days, p < 0.001).
Conclusions
Given these findings, we believe orthopaedic surgeons should be aware of the potential for longer and more complicated hospital stays after THA among patients with HIV. However, the modest increase in risk of adverse outcomes does not cause us to recommend against THA for patients with HIV who otherwise meet reasonable surgical indications. Future studies should explore the relationships between markers of HIV severity and risk of adverse outcomes after THA during the hospital stay and followup.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
HIV infection has been identified as a potential risk factor for hip osteonecrosis, which can lead to secondary arthritis. At least 33% of patients with HIV receiving THA are estimated to have osteonecrosis [11, 13, 17, 22]. Furthermore, patients with HIV have been found to have a higher risk for hip fracture [6]. Although many patients with HIV are candidates for surgery, the potential risk of multisystem postoperative complications and periprosthetic infections secondary to compromised immune function have been topics of debate [20, 21].
Studies performed before the widespread use of highly active antiretroviral therapy found that complication rates in patients with HIV approached 50% [10, 15]. However, a recent study found that complication rates were a modest 13% among 55 patients with HIV undergoing total hip, knee, or shoulder arthroplasties at a minimum followup of 2 years (mean, 6 years; range, 2–14 years) [7]. Previous studies of periprosthetic infection after THA have observed higher infection rates in patients with HIV (4%–7%) compared with patients without HIV (1%–2%) [11, 12]. However, these data may be biased by the confounding effect of hemophilia among historical cohorts of patients with HIV [9]. Moreover, existing data on infection and other complications in patients with HIV have been limited by small sample sizes with low statistical power [2, 9, 11, 12, 14, 21].
We therefore sought to use a large US database, the Nationwide Inpatient Sample (NIS), to address some of these controversies. We specifically assessed (1) the demographic trends of patients with HIV who underwent primary THA; (2) differences in the risk of major and minor perioperative complications among patients with and without HIV; and (3) the differences in mean length of hospital stay among patients with and without HIV.
Materials and Methods
The NIS contains a 20% representative sample of annual hospital admissions in the United States [19]. The NIS contains demographic and clinical variables for each admission, including International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnostic and procedure codes. In addition, discharge weights, which are based on the size and location of the hospital where each admission occurred, allow researchers to calculate nationwide estimates of patient discharges. The NIS is publicly available to researchers and contains deidentified data. Therefore, our institutional review boards determined that this study did not meet human subject research criteria.
Our study population included all patients between 1998 and 2010 who had a THA as their primary ICD-9 procedure code (81.51, 00.74, 00.75, 00.76, 00.77). We excluded patients with diagnosis codes indicating pathological fracture, malunion of fracture, traumatic femoral neck fracture, and long-term mechanical loosening associated with revisions, as has been done previously [11], because these admissions predominantly are nonelective. After exclusions, our study population of THA admissions consisted of 9275 patients with HIV and 2,656,696 patients without HIV.
We extracted data for each admission concerning length of stay, which was log-transformed in regression analyses as a result of its right skew. Perioperative complications were calculated using diagnosis codes as defined by a recent study of orthopaedic-related complications [11]. We defined a major complication as death, myocardial infarction, tachycardia, pulmonary embolism, pneumonia, acute renal failure, or stroke, and we defined a minor complication as wound hemorrhage, wound complication, deep vein thrombosis, wound infection, implant infection, irrigation and débridement, postoperative dislocation, sepsis, or urinary tract infection [16]. We used diagnosis codes to identify patients who had HIV (042, 795.71, V08) and designated all other THA admissions as not having HIV. We also used diagnosis codes to identify patients with osteonecrosis (733.4, 733.40, 733.42, 733.43, 733.49). For each admission, we extracted data on demographic variables including age (in years), sex, race categorization (white, black, Hispanic, other), and insurance (Medicare, Medicaid, private, other). We calculated the severity of comorbidities from diagnosis codes using Charlson and Deyo’s method [3], excluding HIV/AIDS from the patient’s total score, and distributed patients into score categories of 0, 1, and ≥ 2. We used logistic regression to calculate the odds ratios (ORs) of perioperative complications (any, major, or minor) during the hospital stay for patients with diagnosis codes for HIV infection as compared with patients who did not have diagnosis codes for HIV infection. We also used linear regression to calculate parameter estimates for mean length of stay. We interpreted the results of our linear regression as percent differences using the formula 100*(eb − 1), where b is the regression coefficient of a log-transformed outcome variable [5]. All of our regression models were adjusted for age, sex, race, insurance, and the Charlson and Deyo comorbidity score. Admissions with missing age (N = 3287), sex (N = 8786), race (N = 678,604), and insurance (N = 4569) could not be included in multivariable regression models. To rule out the possibility that the exclusion of this subset of admissions with missing race data biased our results, we also performed a sensitivity analysis on our data, in which we removed race from our regression model, thereby including all patients with missing race. We also used regression models with HIV (no, yes) as the outcome variable and age, sex, race, insurance, comorbidities, and osteonecrosis as independent predictor variables to assess the effect size of each demographic variable. We used weighting variables in all our analyses to simulate national US rates of THA admission.
All statistical analyses were performed using SAS Version 9.3 (SAS Institute Inc, Cary, NC, USA). All p values were two-tailed, and p < 0.05 was interpreted as being statistically significant. All figures were generated using Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA).
Results
Patients with HIV were younger (47 versus 65 years; OR, 0.92; 95% confidence interval [CI], 0.91–0.92; p < 0.001), were less likely to be female (17% [1616 of 9256] versus 56% [1,493,781 of 2,647,929]; OR, 0.16; 95% CI, 0.15–0.18; p < 0.001), were more likely to be black (32% [2406 of 7495] versus 6% [126,925 of 1,979,872]; OR, 8.05; 95% CI, 7.19–9.01; p < 0.001), Hispanic (10% [714 of 7495] versus 3% [56,497 of 1,979,872]; OR, 5.37; 95% CI, 4.50–6.41; p < 0.001), or other race (4% [276 of 7495] versus 3% [56,754 of 1,979,872]; OR, 2.07; 95% CI, 1.57–2.71; p < 0.001) and were more likely to pay with Medicaid (19% [1764 of 9257] versus 3% [80,286 of 2,652,145]; OR, 7.93; 95% CI, 6.99–9.00; p < 0.001) or other insurance (4% [377 of 9257] versus 3% [82,767 of 2,652,145]; OR, 1.65; 95% CI, 1.30–2.08; p < 0.001). We did not observe any differences between patients with and without HIV in the number of patients with a Charlson and Deyo score of 1 (23% [1976 of 9275] versus 21% [614,995 of 2,656,696]; p = 0.110) or ≥ 2 (10% [905 of 9275] versus 9% [226,174 of 2,656,696]; p = 0.109). Osteonecrosis was observed in 76% (7087 of 9275) of patients with HIV compared with 11% (286,824 of 2,656,696) of patients without HIV (OR, 26.76; 95% CI, 24.03–29.79; p < 0.001) (Table 1).
Table 1.
Patient characteristics according to HIV status
| Patient characteristic | No | Yes | OR (95% CI) | p value |
|---|---|---|---|---|
| Admissions (number) | 2,656,696 | 9275 | ||
| Mean age (years) | 65 | 47 | 0.92 (0.91–0.92)* | < 0.001 |
| Sex, % (number) | ||||
| Male | 44 (1,154,148) | 83 (7640) | Referent | |
| Female | 56 (1,493,781) | 17 (1616) | 0.16 (0.15–0.18) | < 0.001 |
| Missing | (8767) | (19) | ||
| Race, % (number) | ||||
| White | 88 (1,739,696) | 54 (4099) | Referent | |
| Black | 6 (126,925) | 32 (2406) | 8.05 (7.19–9.01) | < 0.001 |
| Hispanic | 3 (56,497) | 10 (714) | 5.37 (4.50–6.41) | < 0.001 |
| Other | 3 (56,754) | 4 (276) | 2.07 (1.57–2.71) | < 0.001 |
| Missing | (676,824) | (1780) | ||
| Deyo score, % (number) | ||||
| 0 | 68 (1,815,527) | 69 (6394) | Referent | |
| 1 | 23 (614,995) | 21 (1976) | 0.91 (0.82–1.02) | 0.110 |
| ≥ 2 | 9 (226,174) | 10 (905) | 1.14 (0.97–1.33) | 0.109 |
| Insurance, % (number) | ||||
| Medicare | 54 (1,429,774) | 43 (3960) | Referent | |
| Medicaid | 3 (80,286) | 19 (1764) | 7.93 (6.99–9.00) | < 0.001 |
| Private | 40 (1,059,318) | 34 (3156) | 1.08 (0.97–1.19) | 0.170 |
| Other | 3 (82,767) | 4 (377) | 1.65 (1.30–2.08) | < 0.001 |
| Missing | (4551) | (18) | ||
| Osteonecrosis, % (number) | ||||
| No | 89 (2,369,872) | 24 (2188) | Referent | |
| Yes | 11 (286,824) | 76 (7087) | 26.76 (24.03–29.79) | < 0.001 |
* Interpret as chance of having HIV for each 1-year increase in age; CI = confidence interval.
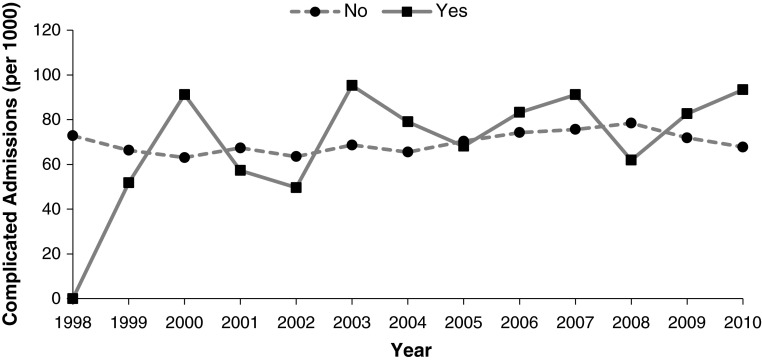
There was a greater risk of complications in patients with HIV after adjusting our models for age, sex, race, insurance, and comorbidities. This was true for any complication (7.4% [694 of 9275] versus 7.0% [186,337 of 2,656,696]; OR, 1.55; 95% CI, 1.27–1.88; p < 0.001), major complication (2.9% [266 of 9275] versus 2.7% [71,952 of 2,656,696]; OR, 1.47; 95% CI, 1.08–2.00; p = 0.014), or minor complication (5.2% [483 of 9275] versus 4.8% [127,940 of 2,656,696]; OR, 1.61; 95% CI, 1.29–2.02; p < 0.001) (Table 2). For specific complications, patients with HIV were more likely to have acute renal failure (1.6% [150 of 9275] versus 0.9% [24,455 of 2,656,696]; OR, 1.74; 95% CI, 1.14–2.65; p = 0.010), pneumonia (0.8% [73 of 9275] versus 0.6% [274 of 2,656,696]; OR, 1.81; 95% CI, 1.01–3.26; p = 0.048), wound hemorrhage (1.6% [148 of 9275] versus 1.1% [30,158 of 2,656,696]; OR, 1.63; 95% CI, 1.10–2.40; p = 0.015), and wound infection (0.7% [63 of 9275] versus 0.2% [6588 of 2,656,696]; OR, 2.38; 95% CI, 1.32–4.30; p = 0.004) compared with patients without HIV. The number of complicated admissions occurring annually stayed constant for patients with (mean, 2.01; r = 0.43, p = 0.144) and without (mean, 0.59; r = 0.48, p = 0.094) HIV (Fig. 1). In addition, the risk of any complication for patients with HIV from 1998 to 2003 (OR, 1.55; 95% CI, 1.05–2.29; p = 0.027) was not different from the risk from 2004 to 2010 (OR, 1.53; 95% CI, 1.22–1.91; p < 0.001). In our sensitivity analysis, the risk of any complication (OR, 1.58; 95% CI, 1.32–1.88; p < 0.001) was very similar to our full multivariable model, suggesting that the exclusion of patients with missing race did not bias our results.
Table 2.
Risk of perioperative complications and length of stay according to HIV status
| Outcome | No | Yes* | p value |
|---|---|---|---|
| Any complication OR | Referent | 1.55 (1.27–1.88) | < 0.001 |
| Major complication OR | Referent | 1.47 (1.08–2.00) | 0.014 |
| Minor complication OR | Referent | 1.61 (1.29–2.02) | < 0.001 |
| Length of stay estimate† | Referent | 9.05 (6.70–11.44) | < 0.001 |
* Models adjusted for age, sex, race, insurance, and Deyo comorbidity score; †interpret as percentage difference, under the formula 100(eb − 1), where b is the estimated standardized regression coefficient of a log-transformed outcome variable.
Fig. 1.
The annual number of admissions with complications per 1000 THA admissions, according to HIV status, is shown.
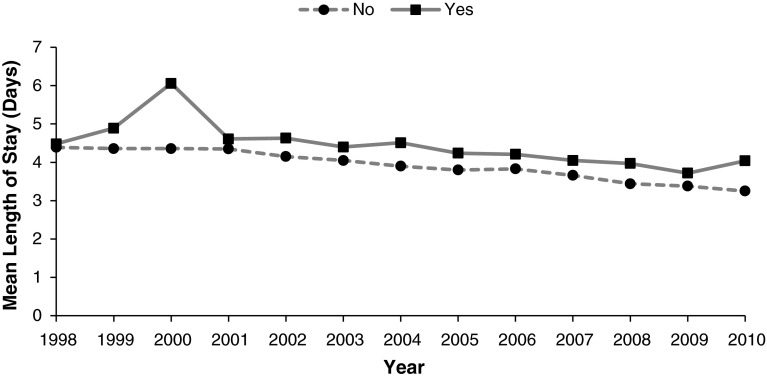
There was a longer mean length of stay among patients with HIV. The mean length of stay was 4.31 days (95% CI, 4.16–4.46) for patients with HIV and 3.83 days (95% CI, 3.82–3.84) for patients without HIV. Patients with HIV had a multivariate-adjusted mean length of stay that was 9% (95% CI, 6.70–11.44; p < 0.001) longer compared with patients without HIV (Table 2). We observed a negative linear trend in length of stay for both patients with (b = −0.12, p < 0.001) and without (b = −0.10, p < 0.001) HIV who were admitted for THA between 1998 and 2010 (Fig. 2).
Fig. 2.
The annual mean length of stay of THA admissions, according to HIV status, is shown.
Discussion
This study was designed to address the paucity of data on demographic trends, early complications, and length of stay after admission for THA among patients who had an HIV infection using a large US inpatient database. We found that after adjusting for important confounders including age, sex, race, insurance, and comorbidities, patients with HIV were at an increased risk for both major and minor perioperative complications compared with patients without HIV. Furthermore, patients with HIV had a longer mean length of stay.
There were several limitations of the present study. First, NIS data are restricted to diagnoses recorded during each patient’s hospital stay and provide no information on long-term outcomes such as readmission and arthroplasty revision. Furthermore, minor complications, including infections and implant dislocations, are more likely to appear during followup than during admission and could not be assessed using this inpatient database. Second, ICD-9 coding for diagnoses and procedures limits our ability to account for differences in implant type, severity of joint degeneration, and reason for surgery. Third, it is unlikely that all patients with HIV who underwent THA were correctly identified, which may be secondary to medical record coding procedures for comorbid diagnoses, undiagnosed HIV infection, or presumed negative status by medical personnel. Because we do not know any of the characteristics of these misclassified patients, it is impossible to predict the potential effect of their misclassification on our outcomes. Fourth, patients with HIV who are undergoing an elective surgery such as THA may be healthier than the average person with HIV. Our data, therefore, should be interpreted within the context of patients who are fit to undergo elective surgery. Fifth, our multivariable models excluded over 600,000 patients with missing race data, which may have biased our results. However, in our sensitivity analysis, there was no material difference in the risk of observed risk of complications in a model that included all patients with missing race as compared with the model that excluded these patients. Therefore, our data do not appear to be subject to this missing data bias. Lastly, one must note that our study is observational and we cannot rule out the effect of residual confounding our results.
Demographic data of patients with HIV undergoing THA have been described previously in small studies. Mean age has ranged from 45 to 50 years with the proportion of male patients ranging from 58% to 71% [2, 9, 12, 18]. Our study was consistent with these previous studies, observing an average age of 47 years with 83% of admissions being male. Furthermore, we were able to describe the racial (54% white, 32% black, 10% Hispanic, 4% other) and insurance (43% Medicare, 19% Medicaid, 34% private, 4% other) demographics of this population among a patient sample of more than 9000 patients with HIV undergoing THA.
Several recent studies have found that patients with HIV undergoing THA have a higher risk of developing perioperative complications [13, 18]. Although the etiology of this relationship is unclear, metabolic, cardiovascular, and pulmonary comorbidities as well as the possible side effects of antiretroviral medications have been proposed as potential risk factors [1, 4, 8]. Two small studies assessing postoperative complications among admitted patients with HIV found an incidence of 12% among 41 THAs [18] and 13% among 55 lower extremity total joint arthroplasties [7]. Our data support these smaller existing studies and demonstrate that although patients with HIV have a higher risk of both major and minor complications during admission compared with patients without HIV, the chances of a complication occurring are still relatively modest. Our findings, however, differ from a similar HIV-focused study by Lin et al. [12], which found that HIV infection was not an independent risk factor for complications after primary THA. This discrepancy may be the result of the fact that our regression models controlled for the confounding effects of age, sex, race, insurance, and comorbidities.
The relationship between THA and length of hospital stay in patients with HIV has not been evaluated in prior studies, to our knowledge. We observed that patients with HIV had a mean hospital stay that was 13% longer, but some caution should be used in interpreting these data. It is unclear whether this increased length of stay is secondary to (1) additional precautions and/or complications precipitated by the presence of HIV infection; (2) concomitant medical comorbidities that were present at a higher rate among patients with HIV; (3) some other unknown factor; or (4) a clinically insignificant difference.
In conclusion, we observed that patients with HIV were at higher risk of perioperative complications after primary THA and also had a longer hospital stay, although these increases were relatively modest. Orthopaedic surgeons should be aware of the potential for longer and more complicated hospital stays after THA in this patient population. Furthermore, surgical candidates with HIV should be informed that despite advancements in the medical management of their disease, they indeed appear to have a higher risk of complications compared with patients without HIV. However, this modest increase in risk does not cause us to recommend against THA for patients with HIV who otherwise meet reasonable surgical indications.
Acknowledgments
We thank the HCUP Data Partners who contribute annually to the NIS. A full listing of participants can be found at <http://www.hcup-us.ahrq.gov/hcupdatapartners.jsp>.
Footnotes
The institution of one or more of the authors has received or will receive, during the study period, funding from Stryker Orthopaedics (Mahwah, NJ, USA) (MAM), TissueGene (Rockville, MD, USA) (LCJ, MAM), Sage Products LLC (Cary, IL, USA) (MAM), Wright Medical Technology, Inc (Arlington, TN, USA) (MAM), Biocomposites Inc (Wilmington, NC, USA), Jannsen, Inc (Toronto, Ontario, Canada) (MAM), Joint Active Systems, Inc (Effingham, IL, USA) (MAM), Medtronic, Inc (Minneapolis, MN, USA) (MAM), Zimmer (Warsaw, IN, USA) (LCJ), and Johnson and Johnson (New Brunswick, NJ, USA) (LCJ).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
References
- 1.Blanco F, San Roman J, Vispo E, Lopez M, Salto A, Abad V, Soriano V. Management of metabolic complications and cardiovascular risk in HIV-infected patients. AIDS Rev. 2010;12:231–241. [PubMed] [Google Scholar]
- 2.Capogna BM, Lovy A, Blum Y, Kim SJ, Felsen UR, Geller DS. Infection rate following total joint arthroplasty in the HIV population. J Arthroplasty. 2013;28:1254–1258. doi: 10.1016/j.arth.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 3.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Gandhi RT, Sax PE, Grinspoon SK. Metabolic and cardiovascular complications in HIV-infected patients: new challenges for a new age. J Infect Dis. 2012;205(Suppl 3):S353–S354. doi: 10.1093/infdis/jis202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glidden DV, Shiboski SC, McCulloch CE. Linear Regression: Checking Model Assumptions and Fit. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. New York, NY, USA: Springer-Verlag; 2011.
- 6.Guerri-Fernandez R, Vestergaard P, Carbonell C, Knobel H, Aviles FF, Castro AS, Nogues X, Prieto-Alhambra D, Diez-Perez A. HIV infection is strongly associated with hip fracture risk, independently of age, gender, and comorbidities: a population-based cohort study. J Bone Miner Res. 2013;28:1259–1263. doi: 10.1002/jbmr.1874. [DOI] [PubMed] [Google Scholar]
- 7.Habermann B, Eberhardt C, Kurth AA. Total joint replacement in HIV positive patients. J Infect. 2008;57:41–46. doi: 10.1016/j.jinf.2008.01.045. [DOI] [PubMed] [Google Scholar]
- 8.Hester EK. HIV medications: an update and review of metabolic complications. Nutr Clin Pract. 2012;27:51–64. doi: 10.1177/0884533611431985. [DOI] [PubMed] [Google Scholar]
- 9.Issa K, Naziri Q, Rasquinha V, Maheshwari AV, Delanois RE, Mont MA. Outcomes of cementless primary THA for osteonecrosis in HIV-infected patients. J Bone Joint Surg Am. 2013;95:1845–1850. doi: 10.2106/JBJS.L.01583. [DOI] [PubMed] [Google Scholar]
- 10.Jellis JE. Viral infections: musculoskeletal infection in the human immunodeficiency virus (HIV) infected patient. Baillieres Clin Rheumatol. 1995;9:121–132. doi: 10.1016/s0950-3579(05)80149-9. [DOI] [PubMed] [Google Scholar]
- 11.Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95:1028–1036. doi: 10.2106/JBJS.L.00269. [DOI] [PubMed] [Google Scholar]
- 12.Lin CA, Takemoto S, Kandemir U, Kuo AC. Mid-term outcomes in HIV-positive patients after primary total hip or knee arthroplasty. J Arthroplasty. 2014;29:277–282. doi: 10.1016/j.arth.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Mahoney CR, Glesby MJ, DiCarlo EF, Peterson MG, Bostrom MP. Total hip arthroplasty in patients with human immunodeficiency virus infection: pathologic findings and surgical outcomes. Acta Orthop. 2005;76:198–203. doi: 10.1080/00016470510030571. [DOI] [PubMed] [Google Scholar]
- 14.Mohanty SS, Dash KK. Total knee arthroplasty in haemophilic arthropathy with severe flexion deformity and HIV-TB co-infection: a case report. Haemophilia. 2013;19:e259–e261. doi: 10.1111/hae.12157. [DOI] [PubMed] [Google Scholar]
- 15.Morrison E, McGill PE, Jellis JE. Viral infections: arthritis in the human immunodeficiency virus (HIV) infected patient. Baillieres Clin Rheumatol. 1995;9:133–144. doi: 10.1016/S0950-3579(05)80150-5. [DOI] [PubMed] [Google Scholar]
- 16.Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89:27–32. doi: 10.2106/JBJS.E.01443. [DOI] [PubMed] [Google Scholar]
- 17.Ries MD, Barcohana B, Davidson A, Jergesen HE, Paiement GD. Association between human immunodeficiency virus and osteonecrosis of the femoral head. J Arthroplasty. 2002;17:135–139. doi: 10.1054/arth.2002.28727. [DOI] [PubMed] [Google Scholar]
- 18.Snir N, Wolfson TS, Schwarzkopf R, Swensen S, Alvarado CM, Hamula M, Dayan AJ. Outcomes of total hip arthroplasty in human immunodeficiency virus-positive patients. J Arthroplasty. 2014;29:157–161. doi: 10.1016/j.arth.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 19.Steiner C, Elixhauser A, Schnaier J. The healthcare cost and utilization project: an overview. Eff Clin Pract. 2002;5:143–151. [PubMed] [Google Scholar]
- 20.Swensen S, Schwarzkopf R. Total joint arthroplasty in human immunodeficiency virus positive patients. Orthop Surg. 2012;4:211–215. doi: 10.1111/os.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tornero E, Garcia S, Larrousse M, Gallart X, Bori G, Riba J, Rios J, Gatell J, Martinez E. Total hip arthroplasty in HIV-infected patients: a retrospective, controlled study. HIV Med. 2012;13:623–629. doi: 10.1111/j.1468-1293.2012.01017.x. [DOI] [PubMed] [Google Scholar]
- 22.Yombi JC, Vandercam B, Wilmes D, Dubuc JE, Vincent A, Docquier PL. Osteonecrosis of the femoral head in patients with type 1 human immunodeficiency virus infection: clinical analysis and review. Clin Rheumatol. 2009;28:815–823. doi: 10.1007/s10067-009-1156-5. [DOI] [PubMed] [Google Scholar]