Abstract
Large-scale surveys that assess cancer prevention and control behaviors are a readily-available, rich resource for public health researchers. Although these data are used by a subset of researchers who are familiar with them, their potential is not fully realized by the research community for reasons including lack of awareness of the data, and limited understanding of their content, methodology, and utility. Until now, no comprehensive resource existed to describe and facilitate use of these data. To address this gap and maximize use of these data, we catalogued the characteristics and content of four surveys that assessed cancer screening behaviors in 2005, the most recent year with concurrent periods of data collection: the National Health Interview Survey, Health Information National Trends Survey, Behavioral Risk Factor Surveillance System, and California Health Interview Survey. We documented each survey's characteristics, measures of cancer screening, and relevant correlates; examined how published studies (n=78) have used the surveys’ cancer screening data; and reviewed new cancer screening constructs measured in recent years. This information can guide researchers in deciding how to capitalize on the opportunities presented by these data resources.
Keywords: Surveillance systems, cancer screening, health behaviors, secondary data analysis, surveys
Introduction
The National Cancer Institute (NCI) supports large-scale surveys that collect health behavior data from populations residing in distinct geographic regions and at varying time intervals. Data from these surveys are used to produce national- and state-level estimates of behaviors, helping public health researchers to address issues related to behavioral patterns, trends, and geographic variation. Federal public health agencies may use items from these surveys to monitor progress toward reaching health objectives, determine the effectiveness of interventions, and identify disparities and other health indicators that suggest the need for focused attention. These surveys are often used by a subset of researchers who are familiar with them, yet the broader research community might benefit from increased awareness of the data and improved understanding of their content, methodology, and utility. Until now, no comprehensive resource existed to describe and facilitate use of these data. Thus, we at the NCI undertook a project to catalogue the characteristics, content, and strengths of these surveys as they relate to cancer screening, with the goal of maximizing their use.
We focus on cancer screening because it represents a well-defined set of health behaviors for which clinical guidelines exist. Population-based breast, cervical, and colorectal cancer screening of average-risk individuals is critical because it leads to reductions in morbidity and mortality (1–3). It is vital to know whether the U.S. population is receiving appropriate screening, and population-based surveys with self-reported information are the only mechanism available to obtain national and state-level estimates of screening utilization. Scientific agreement exists regarding a limited number of key cancer screening behaviors that need to be measured. Furthermore, past research has led to an understanding of correlates, such as individual socio-demographic characteristics, personal attitudes, beliefs, and healthcare access or system factors, which are commonly associated with cancer screening behaviors (4–9), and are typically incorporated into these surveys. Together, these features make cancer screening a good example for exploring the opportunities and challenges of using surveillance systems in public health research.
Cancer screening data from large-scale surveys can be used to measure test use (including underuse and overuse), assess screening patterns and trends, explore geographic variation, identify populations at the highest risk, examine disparities in screening uptake, and discern which factors may impede or facilitate screening so as to establish priorities for intervention research and practice (10,11). Especially in an era of fiscal retrenchment, these publicly-available surveys offer researchers a range of possible uses: preliminary data, alternatives to primary data collection, and comparison samples for data collected from other studies. Survey data can also be pooled across years (12) or combined to provide more precise estimates (13) for innovative analyses and knowledge synthesis. There are, however, several aspects of the surveillance of cancer screening behaviors that may impede the use of these data by researchers. One challenge is that although multiple surveillance systems collect data on key cancer screening behaviors, the specific ways in which these behaviors are measured may vary slightly from survey to survey. Moreover, surveys typically differ in the breadth, depth, and correlates of cancer screening behaviors measured. Surveys are designed to achieve specific yet varying goals, thus they use different sampling approaches and modes of administration, and they differ in their degree of geographic granularity. Though elucidating such differences can be challenging, this variability ultimately results in unique strengths for each survey which researchers can capitalize upon to address a specific research question.
Cataloguing large-scale survey characteristics to maximize use
This paper is designed to help researchers decide where to look, in terms of cancer screening surveys, to address different kinds of questions. Our goal is not to offer an evaluation of which survey is best in terms of content or methodology, but rather to present strengths of the various surveys and the differences among them to guide researchers toward the survey that is most appropriate for their specific research questions.
To this end, we examined four leading, federally-funded, publicly-available surveys that collect data on both: 1) cancer screening constructs, defined as concepts reflecting important aspects of cancer screening that can be assessed with multiple measures (e.g., screening test use, beliefs about screening, or provider recommendations about screening), and 2) a wide range of correlates of cancer screening, defined as variables reflecting general characteristics that past research has shown are frequently associated with cancer screening constructs (e.g., socio-demographics such as age, personal attitudes such as perceived risk of developing cancer, and healthcare access and system factors such as insurance coverage) (4–9). We selected two surveys that provide national-level data – the National Health Interview Survey (NHIS; www.cdc.gov/nchs/nhis.htm) and the Health Information National Trends Survey (HINTS; hints.cancer.gov). We also selected two surveys that provide state- and county-level data widely used outside of their local areas – the Behavioral Risk Factors Surveillance System (BRFSS; www.cdc.gov/brfss) and the California Health Interview Survey (CHIS; www.chis.ucla.edu).
Our analysis focused on each survey's characteristics and content in 2005 – the only year within the past decade when NHIS, HINTS, BRFSS, and CHIS concurrently collected cancer screening data. We were thus able to make direct comparisons across the different surveys at a single point in time, and to examine ways in which data from the surveys have been used in the published literature as examples of their utility. We documented survey content regarding those cancer screening behaviors for which guidelines existed from organizations such as the U.S. Preventive Services Task Force (USPSTF; 14–17) and American Cancer Society (ACS; 1); USPSTF recommendations are used in preventive services coverage determinations under the Patient Protection and Affordable Care Act (18), and ACS guidelines are a widely-used resource among primary care providers in the U.S. Thus, we focused on behaviors recommended at the time of data collection: breast (clinical breast examinations; mammography), cervical (Pap testing), colorectal (home and office fecal occult blood testing or FOBT; endoscopy including colonoscopy and sigmoidoscopy), and prostate (prostate-specific antigen or PSA testing; digital rectal examinations) cancer screening. To promote the use of these data resources by the research community, our analyses answered five primary questions related to the surveys’ characteristics, content, and use, described below. We will answer each of these questions in turn, based on our analyses of the surveys and related literature, and then provide conclusions and recommendations for public health researchers’ further use of these rich data resources.
What are the surveys’ characteristics?
To document the characteristics of each survey, we created a detailed data abstraction tool that was guided by a fixed set of indicators (e.g., sampling strategy, pretesting, and administration; extent of geocoding; data access procedures and costs) adapted from those of the National Collaborative on Childhood Obesity Research (NCCOR) Catalogue of Surveillance Systems (19). Members of the study team (JGH, NB, CNK, RPM, SCK) used each survey's online documentation to complete the data abstraction tool (findings presented in Table 1).
National Health Interview Survey (NHIS)
Begun in 1957, the purpose of NHIS is to collect cross-sectional data on the health status, behaviors, conditions and the use of health services in the U.S population. NHIS is conducted annually by the National Center for Health Statistics (NCHS) using face-to-face interviews of civilian, non-institutionalized individuals living in households or group quarters in all 50 states and Washington, DC.a Data for most of the cancer screening constructs are collected through periodic Cancer Control Supplements to the NHIS (most recently administered in 2000, 2005, and 2010); however, some basic measures of cancer screening were included in additional years (e.g., 2003, 2008, 2011, 2012, 2013, 2014). Survey content for NHIS is evaluated through cognitive testing, and through pilot and field testing (dependent on sponsor resources). Public-use NHIS datasets and sample weights (necessary for computing national-level estimates) are available free of charge from the NCHS website (20). Restricted-use data (e.g., geocoded data) can be accessed with permission through the NCHS Research Data Center.
Health Information National Trends Survey (HINTS)
The purpose of HINTS is to collect cross-sectional data regarding how people access, trust, and use health and cancer information, how they use information technology to manage health and health information, and the degree to which they engage in health behaviors, particularly those relevant to cancer prevention and control. HINTS is conducted periodically (i.e., 2003, 2005, 2007/2008, 2011/2012, 2012/2013, 2013), and samples from the population of U.S. civilian, non-institutionalized adults (ages 18 and older). HINTS has been administered by telephone as well as through the mail. Consistent with the survey's cancer focus, cancer screening constructs have appeared in all iterations of HINTS. Survey content is evaluated and refined through cognitive testing and extensive pilot testing. HINTS datasets and sample weighting information for both the final sample weight and the replicate weights (post-stratified on gender, race/ethnicity, age, and education) are publicly available free of charge from the HINTS website (21); users must consent to a “terms of use agreement.” Some geocoding variables are available; researchers must submit proposals to obtain these restricted-use data
Behavioral Risk Factor Surveillance System (BRFSS)
Begun in 1984, the purpose of BRFSS is to collect cross-sectional, state-specific data regarding health behaviors associated with premature morbidity and mortality from individuals in all 50 U.S. states, Washington, DC, American Samoa, Palau, Puerto Rico, the U.S. Virgin Islands, and Guam. BRFSS is conducted annually through telephone interviews with civilian, non-institutionalized adults (ages 18 and older). BRFSS consists of a core component of fixed questions that are asked by all states and territories, optional modules that individual states and territories can elect to include in their annual survey, and some state-added questions. Cancer screening constructs have been included in both core components and optional modules. In 2005, cancer screening-related content was included in optional modules that a subset of states and territories elected to administer. All core and optional survey content is evaluated through cognitive and field testing. Public-use BRFSS datasets and sample weighting information can be obtained free of charge from the BRFSS website (22). Some public-use geocoding variables exist, and the website's Selected Metropolitan/Micropolitan Area Risk Trends (SMART) data and documentation can be used to obtain information about metropolitan and micropolitan statistical areas with 500 or more respondents if states elect to pay for the required oversample.
California Health Interview Survey (CHIS)
The purpose of CHIS is to collect cross-sectional data regarding Californians’ health status, health behaviors, insurance coverage, and access to and use of healthcare. CHIS began in 2001 and is released biannually. CHIS collects data about civilian, non-institutionalized residents of California including one adult (ages 18 and older), one adolescent (ages 12-17), and one child (ages 0-11) from each household sampled through telephone interviews. Cancer screening constructs have appeared in all iterations of CHIS. CHIS has historically adopted many items from NHIS; survey content is further evaluated and refined through additional cognitive and pilot testing. Although not used in 2005, behavioral coding (23) has also been periodically employed to ensure the quality of CHIS survey content. After completing a one-time registration, researchers can access public-use CHIS datasets and sample weighting information free of charge from the survey website or use the on-line calculator AskCHIS to create their own tables using CHIS data (24). Proposals for analyses involving confidential and sensitive data (e.g., geographic identifiers) can be submitted to the CHIS Data Access Center; approvals must be obtained and fees may be charged to access such data.
How did the surveys measure cancer screening in 2005?
One coder (JGH) reviewed the surveys’ online documentation and 2005 questionnaires to document each item that assessed an aspect of cancer screening including the item wording, response options, and respondent eligibility. Next, two coders (JGH and SCK) reviewed the items and identified their discrete cancer screening constructs. Once a list of constructs and corresponding items was established, it was reviewed by the other study authors and refined until consensus was achieved. Table 2 shows the cancer screening constructs assessed by items within the four surveys (e.g., “Mam ever had”, or was a mammogram ever had by the respondent), and indicates which survey(s) included an item to measure the construct, as well as whether the wording, response options, and universe of eligible respondents for the item were the same across the surveys. Exact item wordings, response options, and eligibility requirements are provided in Supplemental Table 1.
These surveys included constructs related to clinical breast examinations (3 separate constructs), mammography (12), Pap testing (14), home or office FOBT (12), endoscopy (8), colorectal cancer screening in general (5), PSA testing (9), and digital rectal examinations (2). NHIS assessed the largest number of constructs (47), followed by CHIS (28), HINTS (24), and BRFSS (14).
The surveys differed in the specific types of screening constructs measured. BRFSS primarily assessed basic behavioral surveillance constructs related to ever using a screening test and the timing of the most recent test. NHIS and CHIS assessed additional details including abnormal screening test results and follow-up, reasons for using or not using tests, and receipt of provider recommendations for tests. HINTS assessed screening attitudes and beliefs including guideline knowledge, test intentions, and perceptions of tests (e.g., fear, benefits). Only the basic behavioral surveillance constructs of ever using a screening test and the timing of the most recent test appeared on all four surveys. Ever use of mammography was the only construct with item wording and response options that were identical across surveys, although the universe of respondents differed across surveys. For timing of mammography, and ever use and timing of Pap testing, home FOBT, endoscopy, and PSA testing, item wording, response options, and universe of eligible respondents differed across the four surveys. In no instance was the same cancer screening construct measured with identical item wording, response options, and universe of respondents in two or more of the surveys.
Which cancer screening correlates are available on the surveys?
Theoretical and empirical correlates of cancer screening constructs were identified through an examination of the 2005 NHIS, HINTS, BRFSS, and CHIS surveys’ online documentation and 2005 questionnaires (see Table 3).b Correlates within the broad categories of socio-demographics, healthcare access and utilization, cancer history, health behaviors, and health status appeared on multiple surveys. Certain constructs within these categories, including education, income, interview language, race/ethnicity, insurance status, personal cancer history, nutrition, physical activity, tobacco use, body mass index, self-reported health, and emotional health, appeared on all four surveys. Other specific correlates in these categories such as sexual orientation, time in the U.S., reason for lack of insurance coverage, family cancer history, health information seeking, and diagnosis of specific health conditions only appeared on one or two surveys. Similarly, correlates within the broad categories of physician communication, attitudes and beliefs, social integration and support, and objective disease risk calculators were only present on a few of the surveys.
Which research questions have been addressed with survey data?
In January 2014, we conducted for each survey a search of the Scopus and PubMed databases using the following broad search terms: [survey name] AND cancer screening AND 2005. Eligibility was limited to articles published in English since January 1, 2005. Publication lists available from the HINTS (25) and CHIS (26) websites were also reviewed. Each identified article was evaluated to determine whether it fit our objectives (e.g., used 2005 survey data relevant to a cancer site of interest). This search yielded a total of 147 publications; of these, 78 publications met the eligibility criteria, with 43 reporting on NHIS (7,27–68), 11 on HINTS (69–79), 7 on BRFSS (80–86), and 17 on CHIS (87–103). One coder (JGH) reviewed these publications in order to develop a broad categorization scheme for the types of research questions that had been addressed with the surveys’ data; publications were categorized as examining correlates of cancer screening behaviors, examining cancer screening trends over time, or enhancing understanding of cancer screening by linking different data sources (note that publications could, and often did, address more than one type of research question). Relevant information about each publication was abstracted (e.g., types of correlates examined; see Supplemental Table 2).
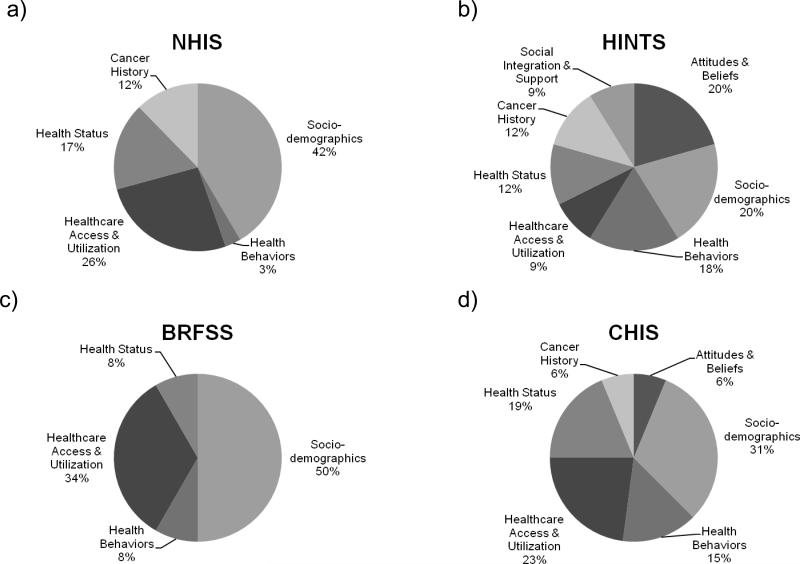
Across surveys, data were used most frequently to examine correlates of cancer screening behaviors (63% of identified research questions). Details about the different types of correlates examined are depicted in Figure 1. As shown, socio-demographic correlates and healthcare access and utilization correlates were frequently used in publications arising from each survey. Publications using HINTS data reported on the greatest variety of correlates; for example, Nelson and colleagues (73) examined the extent to which socio-demographics (age, race/ethnicity, education, marital status, English fluency), healthcare access and utilization (insurance status, frequency of provider utilization), health status (body mass index, self-reported health, emotional health), health behaviors (tobacco use, information seeking), cancer history (self and family history), and attitudes and beliefs (HPV knowledge) were associated with adherence to Pap testing recommendations. Conversely, publications using BRFSS data reported on fewer correlates, primarily involving socio-demographics and healthcare access and utilization. For example, BRFSS data were used to evaluate the association between insurance coverage and colorectal cancer screening in Virginia, while accounting for factors such as gender, age, and income (83). In general, BRFSS does not assess fewer correlates than the other surveys; thus, additional opportunities may exist for examining how correlates such as cancer history, health status, or health behaviors are associated with screening constructs with this data source.
Data from multiple survey iterations were also used to examine cancer screening trends over time (22% of identified research questions). For instance, NHIS data have been used to examine trends in mammography screening from 2000 to 2008 among women of varying ages (31), and CHIS data have been used to describe colorectal cancer screening rates among different ethnic groups in California over time (96).
Least frequently, data were used to enhance our understanding of cancer screening by linking different data sources (e.g., using data as control cohorts; 15% of identified research questions). In these instances, complex, multifaceted questions about cancer screening were addressed that could not be answered by a single data source. For example, the breast cancer screening practices of women whose mothers had breast cancer were examined in an investigator-designed survey, and contrasted with local breast cancer screening rates measured through BRFSS, in an effort to assess the unique effects of the familial cancer experience (86). In other cases, NHIS, HINTS, BRFSS, or CHIS data were compared or integrated with data from various sources such as U.S. Census (e.g., 34,39), international cancer screening (e.g., 88), or local insurance claims data (e.g., 49). In only three cases were data from the surveys under examination, namely NHIS and BRFSS, used together (51,52,63).
How have the surveys’ measurements of cancer screening evolved since 2005?
To describe where cancer screening surveillance has been focused in more recent years and where it may be heading in the future, we examined questionnaires fielded by each survey since 2005. Cancer screening constructs appeared on all of the surveys in the years following 2005, with related content appearing on NHIS in 2008, 2010, 2011, 2012, 2013, and 2014; HINTS in 2007/2008, 2011/2012, 2012/2013, and 2013; BRFSS in 2007, 2008, 2009, 2010, 2011, 2012, and 2013; and CHIS in 2007, 2009, and 2011/2012. We identified 80 new (i.e., content that was not collected by any survey in 2005) constructs (see Supplemental Table 3). Similar to 2005, few constructs were shared across multiple surveys, and in no instance was the exact same item wording, response options, and eligibility requirements found.
These 80 new constructs reflect advances and emerging issues in the field of cancer screening surveillance. Multiple constructs relevant to changes in screening guidelines, uncertainty due to conflicting expert recommendations, and a shift toward shared and informed decision-making have recently appeared. For instance, NHIS, HINTS, and CHIS all included measures to assess women's awareness of and discussions with their healthcare providers regarding recent changes to the USPSTF mammography recommendations and the resulting controversy surrounding these changes (17,104). Similarly, all four surveys have included measures assessing some aspect of men's awareness of conflicting risks and benefits of PSA testing, decision-making about this test, and discussions with providers regarding these issues.
Constructs relevant to the delivery and follow-up of screening tests have also begun to appear on these surveys, with measures of the cost of mammography (CHIS); adherence to recommendations for mammography follow-up tests (NHIS); receipt of Pap test results, adherence to recommendations for Pap follow-up tests, perceived utility of reminders for Pap tests (NHIS); experience of having a discussion about colorectal cancer screening tests with different types of healthcare providers, and perceived efficacy of different colorectal cancer screening tests (HINTS). Furthermore, there has been a greater emphasis on distinguishing between different types of endoscopic colorectal cancer screening tests.
Finally, a few of the surveys have begun to collect data regarding emerging cancer screening tests. NHIS has included measures of the use of breast magnetic resonance imaging (MRI) to screen for breast cancer, and CT colonography to screen for colorectal cancer. Both NHIS and HINTS have incorporated measures of respondents’ awareness and use of lung cancer screening tests – and given the recent positive findings of the National Lung Screening Trial (105), such measures will likely be adopted by the other surveys in future years. Measures regarding predictive cancer genetic testing have also been incorporated into recent iterations of NHIS and HINTS,c including measures of respondents’ awareness, use, beliefs, and discussions with providers regarding genetic tests that can identify an elevated risk for developing hereditary forms of cancer (e.g., hereditary breast and ovarian cancer).
Conclusions
The NHIS, HINTS, BRFSS, and CHIS surveys all provide data regarding the prevalence of important cancer screening behaviors, as each includes items designed to evaluate the use and timing of recommended cancer screening tests (1,14–17). Yet, our review identified differences in the specific wording, response options, and respondent eligibility requirements for these items. It may be worthwhile for survey sponsors to strive for greater consistency in the construction of such items – not only because item wording and response option variations (in terms of both the range and order of options presented) may impact respondents’ answers in unintended ways (106), but also because establishing a “gold standard” of measurement for public health researchers could ultimately allow for innovative data analyses that combine data. For example, common items would facilitate the integration of datasets and analyses that could not be done with data from any one of the surveys. Such integration could generate larger sample sizes (which are especially useful when trying to obtain sufficient samples for hard-to-reach populations), or allow for novel comparisons to be made across datasets (e.g., comparing sub-populations of respondents unique to each dataset, or testing for effects of methodologic differences or potential sampling biases on outcome estimates). Although several studies have integrated survey data to answer larger questions about previously unexplained statistical associations (51,52) and to evaluate large-scale public health programs (63), many additional opportunities for innovative analyses exist. Our efforts to document the characteristics and content of these surveys will help to promote this goal, but must be supplemented by training opportunities and analytic guidance for working with complex, integrated datasets.
Some differences observed among the surveys reflect the unique contributions of NHIS, HINTS, BRFSS, and CHIS. These surveys vary in their focus, and consequently in the depth and breadth of their measurement of cancer screening constructs such as abnormal test follow-up, provider test recommendations, and respondent attitudes and perceptions of screening. Furthermore, they differ in their measurement of theoretical and empirical correlates of cancer screening. For example, both NHIS and CHIS include extensive measures of socio-demographic characteristics, healthcare access and utilization, and health status – consistent with their roles as leading sources of population estimates for the health status and health services use of people in the U.S. and California, respectively. BRFSS, which is designed to assess behaviors associated with premature morbidity and mortality, collects detailed information regarding health behaviors and some common health conditions. HINTS predominantly focuses on people's understanding and use of cancer information; thus, HINTS collects extensive information regarding people's cancer-related attitudes, beliefs, social relationships, and discussions with healthcare providers. Each survey provides novel information that is distinct, yet complementary, to that provided by the others, while minimizing respondent burden. Each surveillance system makes an important contribution that allows researchers and other stakeholders to investigate the complexities of cancer screening at a population level.
Our review identified three primary categories of research questions that have been addressed with data collected by NHIS, HINTS, BRFSS, and CHIS in 2005: examining correlates of cancer screening behaviors, exploring cancer screening trends over time, and enhancing our understanding of cancer screening by linking data sources. Although a number of important questions have already been addressed with these data, researchers could continue to use these resources to answer novel questions (e.g., investigations of the prevalence, drivers, and consequences of guideline-concordant and guideline-discordant screening, characterization of screening experiences in subgroups such as the growing cancer survivor population or older adults with various comorbidities). Furthermore, researchers can continue to build on these approaches, both by undertaking innovative analyses and by capitalizing on the variety of new cancer screening constructs that have appeared in recent years. For instance, these data could be used to examine correlates of and trends in the uptake of novel screening tests and changes in screening behaviors in response to shifting policies (e.g., healthcare reform, tailoring of screening recommendations to specific subgroups based on factors such as age), and linked with other data sources for modeling to determine the cost effectiveness or resource demands of different screening-related scenarios. Although the nation's uptake of novel and emerging tests to detect breast, colorectal, and lung cancer as well as genetic predisposition to cancer is likely low at the present time, large-scale surveys are providing valuable baseline data that will allow researchers to better understand how these tests can be integrated into preventive health services in the future. It is noteworthy that, in the few instances where constructs were shared across surveys, there was variation in item wording, response options, or respondent eligibility requirements. Although some item differences arise as a consequence of the surveys’ different modes of administration, we at the NCI will evaluate future items with an eye toward consistency. Achieving greater consistency in the basic items of greatest interest to multiple stakeholders would pave the way for future innovative and integrative analyses while still maintaining the unique strengths of the individual surveys. In the meantime, we hope that this paper will provide researchers with more in-depth knowledge of these data resources, enabling them to capitalize on these surveys to a greater extent by addressing new questions in novel ways, and thus further our understanding of cancer screening in the U.S.
Supplementary Material
Figure 1.
Types of correlates of cancer screening behaviors examined in published studies using surveillance data from 2005. Data are presented for the: a) National Health Interview Study (NHIS; analysis of 43 studies); b) Health Information National Trends Surveys (HINTS; analysis of 11 studies); c) Behavioral Risk Factor Surveillance System (BRFSS; analysis of 7 studies); and d) California Health Interview Study (CHIS; analysis of 17 studies).
Table 1.
Summary of characteristics of the National Health Interview Survey (NHIS), Health Information National Trends Survey (HINTS), Behavioral Risk Factor Surveillance System (BRFSS), and California Health Interview Survey (CHIS)
| Characteristic | NHIS | HINTS | BRFSS | CHIS |
|---|---|---|---|---|
| Mode of administration (2005) | In-person interviews | RDD landline telephone interviewsa | RDD landline telephone interviewsb | RDD landline telephone interviewsc |
| Sample size (adults; 2005) | 31,428 | 5,586 | 328,485 | 43,020 |
| Overall response rate (adults; 2005) | 69% | 21% | 37% | 27% |
| Language of administration | English, Spanish | English, Spanish | English, Spanish | English, Spanish, Chinese, Korean, Vietnamese |
| Representativeness | Nationally-representative; oversamples African-American, Asian, and Hispanic respondents | Nationally-representative; oversamples African-American and Hispanic respondents | Representative of states and territories; oversampling varies by state | Representative of California; oversamples Korean and Vietnamese respondents |
| Geocoding (use restricted in some cases) | Census region, census division, state, county, zip code, census block/tract, municipality | Census region, census division, designated market area, rural urban continuum code | State, county, some metropolitan and micropolitan statistical areas (MMSA) | Stratum, county, zip code, census tract, latitude, longitude, address and cross streets |
| Existing data linkages | NCHS-linked mortality data, Medicare enrollment and claims, Social Security benefit history; MEPS | None | None | None |
| Estimates available for researchers | Data briefs and Health E-stats | HINTS briefs and item-specific estimates | Surveillance summaries | Interactive AskCHIS |
| Website | www.cdc.gov/nchs/nhis.htm | hints.cancer.gov | http://www.cdc.gov/brfss | www.chis.ucla.edu |
Notes: RDD = random-digit dial; NCHS = National Center for Health Statistics; MEPS= Medical Expenditure Panel Survey
In 2007/2008, HINTS was administered through random-digit dial interviews and mailing surveys to a national address-based sample; in 2011/2012, 2012/2013, and 2013 the survey was administered through mailing to a national address-based sample.
In 2011, BRFSS added cell phones as part of their regular sample.
In 2007, CHIS added cell phones as part of their regular sample.
Table 2.
Similarities in cancer screening constructs across the National Health Interview Survey (NHIS), Health Information National Trends Survey (HINTS), Behavioral Risk Factor Surveillance System (BRFSS), and California Health Interview Survey (CHIS), 2005
| Construct | NHIS | HINTS | BRFSS | CHIS | Wording same? | Response options same? | Universe same? |
|---|---|---|---|---|---|---|---|
| Clinical Breast Exam (CBE) | |||||||
| CBE ever had | ✓ | ✓ a | No | Yes | No | ||
| CBE most recent | ✓ | ✓ a | No | No | No | ||
| CBE past 12 months | ✓ | ||||||
| Mammography (Mam) | |||||||
| Mam ever had | ✓ | ✓ | ✓ a | ✓ | Yes | Yes | No. NHIS and CHIS the same |
| Mam most recent | ✓ | ✓ | ✓ a | ✓ | No. All similar; may be possible to merge | No. BRFSS and CHIS similar; NHIS could be coded to be similar to others | No. NHIS and CHIS the same |
| Mam frequency | ✓ | ✓ | Same question, CHIS has extra language | Yes. | Yes. | ||
| Mam age at first | ✓ | ||||||
| Mam main reason did not have | ✓ | ✓ | Yes. | Yes | No. | ||
| Mam main reason did have | ✓ | ✓ | No | No | No | ||
| Mam abnormal | ✓ | ✓ | No | Yes | Yes | ||
| Mam abnormal: cancer | ✓ | ||||||
| Mam abnormal: follow-up test or surgery | ✓ | ||||||
| Mam abnormal: follow-up type | ✓ | ||||||
| Mam provider recommendation (if screening off schedule) | ✓ | ✓ | No | No | Yes | ||
| Mam provider recommendation (if screening on schedule) | ✓ | ✓ | No | No | Yes | ||
| Pap Test (Pap) | |||||||
| Pap ever had | ✓ | ✓ | ✓ a | ✓ | No | Yes | Yes, if NHIS and CHIS skip cervical cancer survivors |
| Pap most recent | ✓ | ✓ | ✓ a | ✓ | No. NHIS and HINTS very similar | No. CHIS and BRFSS similar; NHIS could be coded to be similar to others | No |
| Pap frequency | ✓ | ✓ | No, but similar | Yes | Yes | ||
| Pap penultimate | ✓ | ||||||
| Pap main reason did not have | ✓ | ✓ | No, but similar | Yes | No | ||
| Pap main reason did have | ✓ | ✓ | No, but similar | No, but similar | Yes, if NHIS skips cervical cancer survivors | ||
| Pap abnormal | ✓ | ||||||
| Pap abnormal: follow-up test | ✓ | ||||||
| Pap abnormal: follow-up surgery | ✓ | ||||||
| Pap provider recommendation (if screening off schedule) | ✓ | ✓ | No, but similar | Yes | No | ||
| Pap provider recommendation (if screening on schedule) | ✓ | ||||||
| Pap intention | ✓ | ||||||
| Pap guideline knowledge | ✓ (2 items) | ||||||
| Pap willingness to extend interval | ✓ | ||||||
| Fecal Occult Blood Test (FOBT) | |||||||
| Home FOBT ever had | ✓ | ✓ | ✓ b | ✓ | No | Yes | No; CHIS and NHIS the same |
| Home FOBT most recent | ✓ | ✓ | ✓ b | ✓ | No | No. CHIS and BRFSS similar; NHIS could be coded to be similar to others | No; CHIS and NHIS the same |
| Home FOBT frequency | ✓ | ||||||
| Home FOBT main reason did not have | ✓ | ✓ | Yes | Yes | No | ||
| Home FOBT main reason did have | ✓ | ||||||
| Home FOBT abnormal | ✓ | ||||||
| Home FOBT abnormal: timing | ✓ | ||||||
| Home FOBT abnormal: follow-up type | ✓ | ||||||
| Home FOBT provider recommendation (if screening off schedule) | ✓ | ✓ | No | Yes | Yes | ||
| Home FOBT provider recommendation (if screening on schedule) | ✓ | ||||||
| Office FOBT ever had | ✓ | ||||||
| Office FOBT most recent | ✓ | ||||||
| Endoscopy (Endo) | |||||||
| Endo ever had | ✓ | ✓ | ✓ b | ✓ | No | Yes | No. CHIS and NHIS the same |
| Endo most recent | ✓ | ✓ | ✓ b | ✓ | No | No, may be possible to merge | No. CHIS and NHIS the same |
| Endo frequency | ✓ | ||||||
| Endo type | ✓ | ✓ | No | No | No | ||
| Endo main reason did not have | ✓ | ✓ | No | Yes | No | ||
| Endo main reason did have | ✓ | ||||||
| Endo provider recommendation (if screening off schedule) | ✓ | ✓ | No | Yes | No | ||
| Endo provider recommendation (if screening on schedule) | ✓ | ||||||
| Colorectal Cancer (CRC) Screening | |||||||
| CRC screening fear | ✓ | ||||||
| CRC screening benefit | ✓ | ||||||
| CRC risk reduction | ✓ | ||||||
| CRC screening test knowledge | ✓ | ||||||
| CRC screening provider recommendation | ✓ | ||||||
| Prostate-Specific Antigen Test (PSA) | |||||||
| PSA ever heard | ✓ | ✓ | Same question, CHIS has extra language | Yes | No | ||
| PSA ever had | ✓ | ✓ | ✓ c | ✓ | Yes | No; CHIS, BRFSS, and NHIS the same | No; CHIS, BRFSS, and NHIS the same |
| PSA most recent | ✓ | ✓ | ✓ c | ✓ | No; NHIS and HINTS the same | No. CHIS and BRFSS similar; NHIS could be coded to be similar to others | No; CHIS, BRFSS, and NHIS the same |
| PSA frequency | ✓ | ||||||
| PSA age at first | ✓ | ||||||
| PSA main reason did have | ✓ | ||||||
| PSA provider discussion | ✓ | ||||||
| PSA provider recommendation (for or against) | ✓ | ||||||
| PSA provider encourage questions | ✓ | ||||||
| Digital Rectal Exam (DRE) | |||||||
| DRE ever had | ✓ c | ||||||
| DRE most recent | ✓ c | ||||||
Notes: Gray columns indicate constructs that were only assessed by a single survey. For complete information about the items used to assess each construct, organized by survey, please refer to Supplemental Table 1.
Construct only assessed in 11 states (Arkansas, Georgia, Iowa, Maine, Mississippi, Nevada, New Jersey, Tennessee, Vermont, Virginia, Wyoming)
Construct only assessed in 7 states (Arizona, Florida, Georgia, Indiana, Iowa, Maine, Virginia)
Construct assessed by 0 states (i.e., item proposed but not adopted for use in any state surveys)
Table 3.
Correlates available on National Health Interview Survey (NHIS), Health Information National Trends Survey (HINTS), Behavioral Risk Factor Surveillance System (BRFSS), and California Health Interview Survey (CHIS), 2005
| Correlates available on the survey | NHIS | HINTS | BRFSS | CHIS |
|---|---|---|---|---|
| Socio-demographics | ||||
| Age | ✓ | ✓ | ✓ | ✓ |
| Citizenship/Immigration Status | ✓ | ✓ | ||
| Country of birth | ✓ | |||
| County of residence | ✓ | ✓ | ||
| Education | ✓ | ✓ | ✓ | ✓ |
| English fluency | ✓ | ✓ | ||
| Income | ✓ | ✓ | ✓ | ✓ |
| Interview language | ✓ | ✓ | ✓ | ✓ |
| Marital status | ✓ | ✓ | ✓ | ✓ |
| Occupational status | ✓ | ✓ | ✓ | ✓ |
| Poverty level | ✓ | |||
| Public program participation | ✓ | |||
| Race/ethnicity | ✓ | ✓ | ✓ | ✓ |
| Sexual orientation | ✓ | |||
| Time in the United States | ✓ | |||
| Healthcare access and utilization | ||||
| Communication with healthcare provider(s) | ✓ | ✓ | ||
| Frequency of utilization | ✓ | ✓ | ✓ | |
| Insurance status | ✓ | ✓ | ✓ | ✓ |
| Insurance status, detailed | ✓ | ✓ | ✓ | |
| Mental health services | ✓ | ✓ | ||
| Reason for lack of insurance coverage | ✓ | ✓ | ||
| Usual source of care | ✓ | ✓ | ✓ | |
| Physician Communication | ||||
| Discussion about cancer risk factors | ✓ | |||
| Discussion about online health information | ✓ | |||
| Attitudes and Beliefs | ||||
| Ambiguity | ✓ | |||
| Control perceptions | ✓ | |||
| Fatalism, cancer | ✓ | |||
| Risk perceptions | ✓ | |||
| Worry, cancer | ✓ | |||
| Disease-related knowledge | ✓ | ✓ | ||
| Social Integration and Support | ||||
| Social support | ✓ | ✓ | ||
| Community organization participation | ✓ | |||
| Cancer History | ||||
| Self | ✓ | ✓ | ✓ | ✓ |
| Family, generic | ✓ | ✓ | ||
| Family, detailed | ✓ | ✓ | ||
| Health Behaviors | ||||
| Alcohol use | ✓ | ✓ | ||
| Health information seeking | ✓ | |||
| Nutrition | ✓ | ✓ | ✓ | ✓ |
| Physical activity | ✓ | ✓ | ✓ | ✓ |
| Second-hand smoke exposure | ✓ | ✓ | ||
| Sexual health | ✓ | ✓ | ✓ | |
| Sleep | ✓ | ✓ | ||
| Sun protection | ✓ | ✓ | ||
| Tobacco use | ✓ | ✓ | ✓ | ✓ |
| Tobacco use, detailed | ✓ | ✓ | ✓ | |
| Vaccinations | ✓ | ✓ | ✓ | |
| Health Status | ||||
| AIDS | ✓ | ✓ | ||
| Arthritis | ✓ | ✓ | ✓ | |
| Asthma | ✓ | ✓ | ✓ | |
| Body mass index (e.g., height and weight) | ✓ | ✓ | ✓ | ✓ |
| Bronchitis | ✓ | |||
| Cardiovascular disease | ✓ | ✓ | ✓ | |
| Diabetes | ✓ | ✓ | ✓ | |
| Emphysema | ✓ | |||
| Epilepsy | ✓ | |||
| Hay fever | ✓ | |||
| Headache/migraine | ✓ | |||
| Hearing | ✓ | |||
| Kidney (weak or failing) | ✓ | |||
| Sinusitis | ✓ | |||
| Tuberculosis | ✓ | |||
| Ulcer | ✓ | |||
| Vision | ✓ | ✓ | ||
| Self-reported health | ✓ | ✓ | ✓ | ✓ |
| Emotional health (e.g., psychological distress) | ✓ | ✓ | ✓ | ✓ |
| Objective Disease Risk Calculators | ||||
| Gail model | ✓ |
Acknowledgments
We would like to thank Drs. Erik Augustson, William Klein, April Oh, and Heather Patrick for their valuable feedback during the development of this project.
Funding: JGH was supported in part by the National Cancer Institute Cancer Prevention Fellowship Program. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Conflicts of interest: The authors have no conflicts or financial interests to disclose.
NHIS also collects data on children. Children are not discussed here because they are not included in population-level screening guidelines for the examined cancers.
Although the correlates listed in Table 3 are specific to the 2005 iterations of NHIS, HINTS, BRFSS, and CHIS, many of these correlates are also found on other iterations of the surveys.
Constructs relevant to genetic testing did appear on the 2000 and 2005 iterations of NHIS. However, since predictive genetic testing is not generally categorized as a traditional cancer screening test, we have elected to discuss such measures in the context of important future directions.
References
- 1.Smith R, Brooks D. Cancer screening in the United States, 2013. CA Cancer J Clin. 2013;63:87–105. doi: 10.3322/caac.21174. [DOI] [PubMed] [Google Scholar]
- 2.Brawley OW, Kramer BS. Cancer prevention in the United States. In: Miller AB, editor. Epidemiologic studies in cancer prevention and screening. Springer; New York: 2013. pp. 109–20. [Google Scholar]
- 3.Meissner HI, Smith RA, Rimer BK, Wilson KM, Rakowski W, Vernon SW, et al. Promoting cancer screening: Learning from experience. Cancer. 2004;101:1107–17. doi: 10.1002/cncr.20507. [DOI] [PubMed] [Google Scholar]
- 4.Hiatt RA, Klabunde C, Breen N, Swan J, Ballard-Barbash R. Cancer screening practices from National Health Interview Surveys: Past, present, and future. J Natl Cancer Inst. 2002;94:1837–46. doi: 10.1093/jnci/94.24.1837. [DOI] [PubMed] [Google Scholar]
- 5.Subramanian S, Klosterman M, Amonkar MM, Hunt TL. Adherence with colorectal cancer screening guidelines: A review. Prev Med (Baltim) 2004;38:536–50. doi: 10.1016/j.ypmed.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Consedine NS, Magai C, Krivoshekova YS, Ryzewicz L, Neugut AI. Fear, anxiety, worry, and breast cancer screening behavior: A critical review. Cancer Epidemiol Biomarkers Prev. 2004;13:501–10. [PubMed] [Google Scholar]
- 7.Swan J, Breen N, Graubard BI, McNeel TS, Blackman D, Tangka FK, et al. Data and trends in cancer screening in the United States: Results from the 2005 National Health Interview Survey. Cancer. 2010;116:4872–81. doi: 10.1002/cncr.25215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vernon SW, Meissner H, Klabunde C, Rimer BK, Ahnen DJ, Bastani R, et al. Measures for ascertaining use of colorectal cancer screening in behavioral, health services, and epidemiologic research. Cancer Epidemiol Biomarkers Prev. 2004;13:898–905. [PubMed] [Google Scholar]
- 9.Rakowski W, Meissner H, Vernon SW, Breen N, Rimer B, Clark MA. Correlates of repeat and recent mammography for women ages 45 to 75 in the 2002 to 2003 Health Information National Trends Survey (HINTS 2003). Cancer Epidemiol Biomarkers Prev. 2006;15:2093–101. doi: 10.1158/1055-9965.EPI-06-0301. [DOI] [PubMed] [Google Scholar]
- 10.Breen N, Meissner HI. Toward a system of cancer screening in the United States: Trends and opportunities. Annu Rev Public Health. 2005;26:561–82. doi: 10.1146/annurev.publhealth.26.021304.144703. [DOI] [PubMed] [Google Scholar]
- 11.Rakowski W, Breslau ES. Perspectives on behavioral and social science research on cancer screening. Cancer. 2004;101:1118–30. doi: 10.1002/cncr.20503. [DOI] [PubMed] [Google Scholar]
- 12.Kagawa-Singer M, Pourat N. Asian American and Pacific Islander breast and cervical carcinoma screening rates and Healthy People 2000 objectives. Cancer. 2000;89:696–705. doi: 10.1002/1097-0142(20000801)89:3<696::aid-cncr27>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 13.Davis WW, Parsons VL, Xie D, Schenker N, Town M, Raghunathan TE, et al. State-based estimates of mammography screening rates based on information from two health surveys. Public Health Rep. 2010;125:567–78. doi: 10.1177/003335491012500412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moyer V. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156:880–91. doi: 10.7326/0003-4819-156-12-201206190-00424. [DOI] [PubMed] [Google Scholar]
- 15.Moyer V. Screening for prostate cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:120–34. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 16.Calonge N, Petitti D. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–38. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 17.Calonge N, Petitti D, DeWitt T. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716–26. doi: 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
- 18.The Patient Protection and Affordable Care Act of 2010. Pub. L. No. 114-48 (March 23, 2010), as ammended through May 1, 2010 [Internet]. United States; 2010 [cited 2013 Nov 6]. Available from: http://housedocs.house.gov/energycommerce/ppacacon.pdf
- 19.National Collaborative on Childhood Obesity Research [2011 Mar 16];Catalogue of surveillance systems [Internet] 2011 Available from: http://nccor.org/projects/catalogue/index.php.
- 20.Centers for Disease Control and Prevention [2014 Feb 28];National Health Interview Survey: Questionnaires, Datasets, and Related Documentation 1997 to the Present [Internet] Available from: http://www.cdc.gov/nchs/nhis/quest_data_related_1997_forward.htm.
- 21.National Cancer Institute [2014 Feb 28];Health Information National Trends Survey [Internet] Available from: http://hints.cancer.gov/
- 22.Centers for Disease Control and Prevention [2014 Feb 28];Behavioral Risk Factor Surveillance System: Survey Data and Documentation [Internet] Available from: http://www.cdc.gov/brfss/data_documentation/index.htm.
- 23.Visser PS, Krosnick JA, Lawrakas PJ. Survey research. In: Reis HT, Judd CM, editors. Handbook of Research Methods in Social and Personality Psychology. Cambridge University Press; Cambridge: 2000. pp. 223–52. [Google Scholar]
- 24.UCLA Center for Health Policy Research [2014 Feb 28];California Health Interview Survey: Get CHIS Data [Internet] Available from: http://healthpolicy.ucla.edu/chis/data/Pages/overview.aspx.
- 25.National Cancer Institute [2013 May 10];Published articles using HINTS data [Internet] Available from: http://hints.cancer.gov/publications.aspx.
- 26.UCLA Center for Health Policy Research [2013 May 10];Browse publications [Internet] Available from: http://healthpolicy.ucla.edu/publications/browsepubs/Pages/default.aspx.
- 27.Afable-Munsuz A, Liang S-Y, Ponce NA, Walsh JME. Acculturation and colorectal cancer screening among older Latino adults: Differential associations by national origin. J Gen Intern Med. 2009;24:963–70. doi: 10.1007/s11606-009-1022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bandi P, Cokkinides V, Smith RA, Jemal A. Trends in colorectal cancer screening with home-based fecal occult blood tests in adults ages 50 to 64 years, 2000-2008. Cancer. 2012;118:5092–9. doi: 10.1002/cncr.27529. [DOI] [PubMed] [Google Scholar]
- 29.Breen N, Cronin KA, Meissner HI, Taplin SH, Tangka FK, Tiro JA, et al. Reported drop in mammography: Is this cause for concern? Cancer. 2007;109:2405–9. doi: 10.1002/cncr.22723. [DOI] [PubMed] [Google Scholar]
- 30.Breen N, Cronin KA, Tiro JA, Meissner HI, McNeel TS, Sabatino SA, et al. Was the drop in mammography rates in 2005 associated with the drop in hormone therapy use? Cancer. 2011;117:5450–60. doi: 10.1002/cncr.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breen N, Gentleman JF, Schiller JS. Update on mammography trends: Comparisons of rates in 2000, 2005, and 2008. Cancer. 2011;117:2209–18. doi: 10.1002/cncr.25679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chagpar AB, McMasters KM. Trends in mammography and clinical breast examination: A population-based study. J Surg Res. 2007;140:214–9. doi: 10.1016/j.jss.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 33.Chen X, White MC, Peipins LA, Seeff LC. Increase in screening for colorectal cancer in older Americans: Results from a national survey. J Am Geriatr Soc. 2008;56:1511–6. doi: 10.1111/j.1532-5415.2008.01796.x. [DOI] [PubMed] [Google Scholar]
- 34.Dailey AB, Brumback BA, Livingston MD, Jones BA, Curbow BA, Xu X. Area-level socioeconomic position and repeat mammography screening use: Results from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2011;20:2331–44. doi: 10.1158/1055-9965.EPI-11-0528. [DOI] [PubMed] [Google Scholar]
- 35.Deshpande AD, McQueen A, Coups EJ. Different effects of multiple health status indicators on breast and colorectal cancer screening in a nationally representative US sample. Cancer Epidemiol. 2012;36:270–5. doi: 10.1016/j.canep.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drake BF, Lathan CS, Okechukwu CA, Bennett GG. Racial differences in prostate cancer screening by family history. Ann Epidemiol. 2008;18:579–83. doi: 10.1016/j.annepidem.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drazer MW, Huo D, Schonberg MA, Razmaria A, Eggener SE. Population-based patterns and predictors of prostate-specific antigen screening among older men in the United States. J Clin Oncol. 2011;29:1736–43. doi: 10.1200/JCO.2010.31.9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drew JAR, Short SE. Disability and Pap smear receipt among U.S. Women, 2000 and 2005. Perspect Sex Reprod Health. 2010;42:258–66. doi: 10.1363/4225810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eltoum IA, Roberson J. Impact of HPV testing, HPV vaccine development, and changing screening frequency on national Pap test volume: Projections from the National Health Interview Survey (NHIS). Cancer. 2007;111:34–40. doi: 10.1002/cncr.22487. [DOI] [PubMed] [Google Scholar]
- 40.Fagan HB, Myers RE, Daskalakis C, Sifri R, Mainous AG, Wender R. Race/Ethnicity, gender, weight status, and colorectal cancer screening. J Obes. 2011:2011. doi: 10.1155/2011/314619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffman KE, Nguyen PL, Ng AK, D'Amico A V. Prostate cancer screening in men 75 years old or older: An assessment of self-reported health status and life expectancy. J Urol. 2010;183:1798–802. doi: 10.1016/j.juro.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Kapp JM, Ryerson AB, Coughlin SS, Thompson TD. Racial and ethnic differences in mammography use among U.S. women younger than age 40. Breast Cancer Res Treat. 2009;113:327–37. doi: 10.1007/s10549-008-9919-2. [DOI] [PubMed] [Google Scholar]
- 43.Kapp JM, Yankaskas BC, LeFevre ML. Are mammography recommendations in women younger than 40 related to increased risk? Breast Cancer Res Treat. 2010;119:485–90. doi: 10.1007/s10549-008-0305-x. [DOI] [PubMed] [Google Scholar]
- 44.Klabunde CN, Cronin KA, Breen N, Waldron WR, Ambs AH, Nadel MR. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20:1611–21. doi: 10.1158/1055-9965.EPI-11-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kunitake H, Zheng P, Yothers G, Land SR, Fehrenbacher L, Giguere JK, et al. Routine preventive care and cancer surveillance in long-term survivors of colorectal cancer: Results from National Surgical Adjuvant Breast and Bowel Project Protocol LTS-01. J Clin Oncol. 2010;28:5274–9. doi: 10.1200/JCO.2010.30.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leone LA, Campbell MK, Satia JA, Bowling JM, Pignone MP. Race moderates the relationship between obesity and colorectal cancer screening in women. Cancer Causes Control. 2010;21:373–85. doi: 10.1007/s10552-009-9469-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li J, Berkowitz Z, Richards TB, Richardson LC. Shared decision making in prostate-specific antigen testing with men older than 70 years. J Am Board Fam Med. 2013;26:401–8. doi: 10.3122/jabfm.2013.04.120267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meissner HI, Tiro JA, Haggstrom D, Lu-Yao G, Breen N. Does patient health and hysterectomy status influence cervical cancer screening in older women? J Gen Intern Med. 2008;23:1822–8. doi: 10.1007/s11606-008-0775-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miglioretti DL, Rutter CM, Bradford SC, Zauber AG, Kessler LG, Feuer EJ, et al. Improvement in the diagnostic evaluation of a positive fecal occult blood test in an integrated health care organization. Med Care. 2008;46:S91–6. doi: 10.1097/MLR.0b013e31817946c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pace LE, He Y, Keating NL. Trends in mammography screening rates after publication of the 2009 US Preventive Services Task Force recommendations. Cancer. 2013;119:2518–23. doi: 10.1002/cncr.28105. [DOI] [PubMed] [Google Scholar]
- 51.Rakowski W, Clark M, Rogers M, Weitzen S. Investigating reversals of association for utilization of recent mammography among Hispanic and non-Hispanic black women. Cancer Causes Control. 2009;20:1483–95. doi: 10.1007/s10552-009-9345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rakowski W, Clark MA, Rogers ML, Weitzen SH. Reversals of association for Pap, colorectal, and prostate cancer testing among Hispanic and non-Hispanic black women and men. Cancer Epidemiol Biomarkers Prev. 2011;20:876–89. doi: 10.1158/1055-9965.EPI-10-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ross LE, Berkowitz Z, Ekwueme DU. Use of the prostate-specific antigen test among U.S. men: Findings from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2008;17:636–44. doi: 10.1158/1055-9965.EPI-07-2709. [DOI] [PubMed] [Google Scholar]
- 54.Ross LE, Taylor YJ, Howard DL. Trends in prostate-specific antigen test use, 2000-2005. Public Health Rep. 2011;126:228–39. doi: 10.1177/003335491112600214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sabatino S, Coates R, Uhler R. Disparities in mammography use among US women aged 40-64 years, by race, ethnicity, income, and health insurance status, 1993 and 2005. Med Care. 2008;46:692–700. doi: 10.1097/MLR.0b013e31817893b1. [DOI] [PubMed] [Google Scholar]
- 56.Sabatino S, Thompson T. Health insurance and other factors associated with mammography surveillance among breast cancer survivors: Results from a national survey. Med Care. 2012;50:270–6. doi: 10.1097/MLR.0b013e318244d294. [DOI] [PubMed] [Google Scholar]
- 57.Schonberg MA, Leveille SG, Marcantonio ER. Preventive health care among older women: Missed opportunities and poor targeting. Am J Med. 2008;121:974–81. doi: 10.1016/j.amjmed.2008.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shapiro JA, Seeff LC, Thompson TD, Nadel MR, Klabunde CN, Vernon SW. Colorectal cancer test use from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2008;17:1623–30. doi: 10.1158/1055-9965.EPI-07-2838. [DOI] [PubMed] [Google Scholar]
- 59.Shavers VL, Jackson MC, Sheppard VB. Racial/ethnic patterns of uptake of colorectal screening, National Health Interview Survey 2000-2008. J Natl Med Assoc. 2010;102:621–35. doi: 10.1016/s0027-9684(15)30640-4. [DOI] [PubMed] [Google Scholar]
- 60.Slomiany BA, McMasters KM, Chagpar AB. The recent decline in mammography rates is limited to low-to average-risk women. Am J Surg. 2008;196:821–6. doi: 10.1016/j.amjsurg.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 61.Stock C, Knudsen AB, Lansdorp-Vogelaar I, Haug U, Brenner H. Colorectal cancer mortality prevented by use and attributable to nonuse of colonoscopy. Gastrointest Endosc. 2011;73:435–443.e5. doi: 10.1016/j.gie.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 62.Subramanian S, Bobashev G, Morris RJ. Modeling the cost-effectiveness of colorectal cancer screening: Policy guidance based on patient preferences and compliance. Cancer Epidemiol Biomarkers Prev. 2009;18:1971–8. doi: 10.1158/1055-9965.EPI-09-0083. [DOI] [PubMed] [Google Scholar]
- 63.Tangka FKL, O'Hara B, Gardner JG, Turner J, Royalty J, Shaw K, et al. Meeting the cervical cancer screening needs of underserved women: The National Breast and Cervical Cancer Early Detection Program, 2004-2006. Cancer Causes Control. 2010;21:1081–90. doi: 10.1007/s10552-010-9536-3. [DOI] [PubMed] [Google Scholar]
- 64.Trivers KF, Shaw KM, Sabatino SA, Shapiro JA, Coates RJ. Trends in colorectal cancer screening disparities in people aged 50-64 years, 2000-2005. Am J Prev Med. 2008;35:185–93. doi: 10.1016/j.amepre.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 65.Vidal L, LeBlanc WG, McCollister KE, Arheart KL, Chung-Bridges K, Christ S, et al. Cancer screening in US workers. Am J Public Health. 2009;99:59–65. doi: 10.2105/AJPH.2008.135699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu T-C, Chou C-F, Johnson PJ, Ward A. Persistent disparities in pap test use: Assessments and predictions for Asian women in the U.S., 1982-2010. J Immigr Minor Heal. 2010;12:445–53. doi: 10.1007/s10903-009-9255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou J, Enewold L. Trends in cancer screening among Hispanic and white non-Hispanic women, 2000–2005. J Women's Heal. 2010;19:2167–74. doi: 10.1089/jwh.2009.1909. [DOI] [PubMed] [Google Scholar]
- 68.Zhou J, Enewold L. Colorectal, prostate, and skin cancer screening among Hispanic and White non-Hispanic men, 2000-2005. J Natl Med Assoc. 2011;103:343–50. doi: 10.1016/s0027-9684(15)30315-1. [DOI] [PubMed] [Google Scholar]
- 69.Han PKJ, Moser RP, Klein WMP. Perceived ambiguity about cancer prevention recommendations: Associations with cancer-related perceptions and behaviours in a US population survey. Heal Expect. 2007;10:321–36. doi: 10.1111/j.1369-7625.2007.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jun J, Oh K. Asian and Hispanic Americans’ cancer fatalism and colon cancer screening. Am J Health Behav. 2013;37:145–54. doi: 10.5993/AJHB.37.2.1. [DOI] [PubMed] [Google Scholar]
- 71.Koshiol J, Rutten LF, Moser RP, Hesse N. Knowledge of human papillomavirus: Differences by self-reported treatment for genital warts and sociodemographic characteristics. J Health Commun. 2009;14:331–45. doi: 10.1080/10810730902873067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meissner HI, Tiro JA, Yabroff KR, Haggstrom DA, Coughlin SS. Too much of a good thing? Physician practices and patient willingness for less frequent pap test screening intervals. Med Care. 2010;48:249–59. doi: 10.1097/MLR.0b013e3181ca4015. [DOI] [PubMed] [Google Scholar]
- 73.Nelson W, Moser RP, Gaffey A, Waldron W. Adherence to cervical cancer screening guidelines for U.S. women aged 25-64: Data from the 2005 Health Information National Trends Survey (HINTS). J Women's Heal. 2009;18:1759–68. doi: 10.1089/jwh.2009.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Redmond N, Baer HJ, Clark CR, Lipsitz S, Hicks LS. Sources of health information related to preventive health behaviors in a national study. Am J Prev Med. 2010;38:620–627.e2. doi: 10.1016/j.amepre.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sullivan HW, Rutten LJF, Hesse BW, Moser RP, Rothman AJ, McCaul KD. Lay representations of cancer prevention and early detection: Associations with prevention behaviors. Prev Chronic Dis. 2010;7:A14. [PMC free article] [PubMed] [Google Scholar]
- 76.Thomas KB, Simpson SL, Tarver WL, Gwede CK. Is social support from family associated with PSA testing? An exploratory analysis using the Health Information National Trends Survey (HINTS) 2005. Am J Mens Health. 2010;4:50–9. doi: 10.1177/1557988308328541. [DOI] [PubMed] [Google Scholar]
- 77.Tian Y, Robinson JD. Incidental health information use on the Internet. Health Commun. 2009;24:41–9. doi: 10.1080/10410230802606984. [DOI] [PubMed] [Google Scholar]
- 78.Tiro JA, Meissner HI, Kobrin S, Chollette V. What do women in the U.S. know about human papillomavirus and cervical cancer? Cancer Epidemiol Biomarkers Prev. 2007;16:288–94. doi: 10.1158/1055-9965.EPI-06-0756. [DOI] [PubMed] [Google Scholar]
- 79.Ye J, Williams SD, Xu Z. The association between social networks and colorectal cancer screening in American males and females: Data from the 2005 Health Information National Trends Survey. Cancer Causes Control. 2009;20:1227–33. doi: 10.1007/s10552-009-9335-x. [DOI] [PubMed] [Google Scholar]
- 80.Bennett KJ. Rural population estimates: An analysis of a large secondary data set. J Rural Heal. 2013;29:233–8. doi: 10.1111/j.1748-0361.2012.00446.x. [DOI] [PubMed] [Google Scholar]
- 81.Centers for Disease Control and Prevention Use of mammograms among women aged greater than or equal to 40 years - United States, 2000-2005. MMWR. 2007;56:49–51. [PubMed] [Google Scholar]
- 82.Cole AM, Jackson JE, Doescher M. Urban-rural disparities in colorectal cancer screening: Cross-sectional analysis of 1998-2005 data from the Centers for Disease Control's Behavioral Risk Factor Surveillance Study. Cancer Med. 2012;1:350–6. doi: 10.1002/cam4.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Bosset V, Atashili J, Miller W, Pignone M. Health insurance-related disparities in colorectal cancer screening in Virginia. Cancer Epidemiol biomarkers Prev. 2008;17:834–7. doi: 10.1158/1055-9965.EPI-07-2760. [DOI] [PubMed] [Google Scholar]
- 84.Kim J, Jang SN. Socioeconomic disparities in breast cancer screening among US women: Trends from 2000 to 2005. J Prev Med Public Health. 2008;41:186–94. doi: 10.3961/jpmph.2008.41.3.186. [DOI] [PubMed] [Google Scholar]
- 85.Welch C, Miller C, James N. Sociodemographic and health-related determinants of breast and cervical cancer screening behavior, 2005. J Obstet Gynecol Neonatal Nurs. 2008;37:51–7. doi: 10.1111/j.1552-6909.2007.00190.x. [DOI] [PubMed] [Google Scholar]
- 86.Wilson DB, Quillin J, Bodurtha JN, McClish D. Comparing screening and preventive health behaviors in two study populations: Daughters of mothers with breast cancer and women responding to the Behavioral Risk Factor Surveillance System survey. J Women's Heal. 2011;20:1201–6. doi: 10.1089/jwh.2010.2256. [DOI] [PubMed] [Google Scholar]
- 87.Boehmer U, Miao X, Linkletter C, Clark MA. Adult health behaviors over the life course by sexual orientation. Am J Public Health. 2012;102:292–300. doi: 10.2105/AJPH.2011.300334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choi K, Lee S, Park E. Comparison of breast cancer screening rates between Korean women in America versus Korea. J Women's Heal. 2010;19:1089–96. doi: 10.1089/jwh.2009.1584. [DOI] [PubMed] [Google Scholar]
- 89.Crawley LM, Ahn DK, Winkleby MA. Perceived medical discrimination and cancer screening behaviors of racial and ethnic minority adults. Cancer Epidemiol Biomarkers Prev. 2008;17:1937–44. doi: 10.1158/1055-9965.EPI-08-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Heslin KC, Gore JL, King WD, Fox SA. Sexual orientation and testing for prostate and colorectal cancers among men in California. Med Care. 2008;46:1240–8. doi: 10.1097/MLR.0b013e31817d697f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jackson MC, Davis WW, Waldron W, McNeel TS, Pfeiffer R, Breen N. Impact of geography on mammography use in California. Cancer Causes Control. 2009;20:1339–53. doi: 10.1007/s10552-009-9355-6. [DOI] [PubMed] [Google Scholar]
- 92.Johnson-Kozlow M. Colorectal cancer screening of Californian adults of Mexican origin as a function of acculturation. J Immigr Minor Heal. 2010;12:454–61. doi: 10.1007/s10903-009-9236-9. [DOI] [PubMed] [Google Scholar]
- 93.Johnson-Kozlow M, Roussos S. Colorectal cancer test use among Californians of Mexican origin: Influence of language barriers. Ethn Dis. 2009;19:315–22. [PMC free article] [PubMed] [Google Scholar]
- 94.Lee HY, Ju E, Der Vang P, Lundquist M. Breast and cervical cancer screening disparity among Asian American women: Does race/ethnicty matter? J Women's Heal. 2010;19:1877–84. doi: 10.1089/jwh.2009.1783. [DOI] [PubMed] [Google Scholar]
- 95.Lee HY, Lundquist M, Ju E, Luo X, Townsend A. Colorectal cancer screening disparities in Asian Americans and Pacific Islanders: Which groups are most vulnerable? Ethn Health. 2011;16:501–18. doi: 10.1080/13557858.2011.575219. [DOI] [PubMed] [Google Scholar]
- 96.Maxwell AE, Crespi CM. Trends in colorectal cancer screening utilization among ethnic groups in California: Are we closing the gap? Cancer Epidemiol biomarkers Prev. 2009;18:752–9. doi: 10.1158/1055-9965.EPI-08-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maxwell AE, Crespi CM, Antonio CM, Lu P. Explaining disparities in colorectal cancer screening among five Asian ethnic groups: A population-based study in California. BMC Cancer. 2010;10:214. doi: 10.1186/1471-2407-10-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ponce NA, Tsui J, Knight SJ, Afable-Munsuz A, Ladabaum U, Hiatt RA, et al. Disparities in cancer screening in individuals with a family history of breast or colorectal cancer. Cancer. 2012;118:1656–63. doi: 10.1002/cncr.26480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rakowski W, Wyn R, Breen N, Meissner H, Clark MA. Prevalence and correlates of recent and repeat mammography among California women ages 55-79. Cancer Epidemiol. 2010;34:168–77. doi: 10.1016/j.canep.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rodriguez HP, Herrera AP, Wang Y, Jacobson DM. Local health department assurance of services and the health of California's seniors. J Public Heal Manag Pract. 2013;19:550–61. doi: 10.1097/PHH.0b013e31828e25e5. [DOI] [PubMed] [Google Scholar]
- 101.Shariff-Marco S, Breen N, Stinchcomb DG, Klabunde CN. Multilevel predictors of colorectal cancer screening use in California. Am J Manag Care. 2013;19:205–16. [PMC free article] [PubMed] [Google Scholar]
- 102.Simonds V, Colditz G, Rudd R, Sequist T. Cancer screening among Native Americans in California. Ethn Dis. 2011;21:202–9. [PubMed] [Google Scholar]
- 103.Townsend JS, Steele CB, Richardson LC, Stewart SL. Health behaviors and cancer screening among Californians with a family history of cancer. Genet Med. 2013;15:212–21. doi: 10.1038/gim.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Squiers LB, Holden DJ, Dolina SE, Kim AE, Bann CM, Renaud JM. The public's response to the U.S. Preventive Services Task Force's 2009 recommendations on mammography screening. Am J Prev Med. 2011;40:497–504. doi: 10.1016/j.amepre.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 105.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dillman DA, Smyth JD, Christian LM. Internet, mail, and mixed-mode surveys: The Tailored Design Method. 3rd ed. John Wiley & Sons; New Jersey: 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.