Abstract
To date, all population-based epidemiologic data on gastrointestinal stromal tumor (GIST) in the United States predate the 2001 implementation of GIST-specific histology coding. As such, results from previous studies were limited due to inclusion of non-GIST abdominal or gastrointestinal sarcomas. We utilized a national cancer registry with modern day histological codes to gain greater insight into the true epidemiology of GIST in the United States. We identified 6,142 patients diagnosed with GIST between 2001 and 2011 in the Surveillance, Epidemiology, and End Results database. Incidence, survival, demographic risk factors, and prognostic factors were analyzed. Annual age-adjusted incidence rose from 0.55/100,000 in 2001 to 0.78/100,000 in 2011 and increased with age, peaking among 70-79 year olds (3.06/100,000). GIST was also more common in males than females [rate ratio (RR)=1.35], non-Hispanics than Hispanics (RR=1.23), and blacks (RR=2.07) or Asians/Pacific Islanders (RR=1.50) than whites. The study period had 5-year overall and GIST-specific survival rates of 65% and 79%, respectively. The 5-year overall survival rates for those with localized, regional, and metastatic disease at diagnosis were 77%, 64%, and 41%, respectively. Multivariate analyses demonstrated that older age at diagnosis, male sex, black race, and advanced stage at diagnosis were independent risk factors of worse overall survival. Multivariate analysis also showed the four aforementioned characteristics, along with earlier year of diagnosis, to be independent risk factors of worse GIST-specific survival. As the first population-based, epidemiological study of histologically confirmed disease, our findings provide a robust representation of GIST in the era of immunohistochemical diagnoses.
Introduction
Gastrointestinal stromal tumor (GIST) represents the most common sarcoma of the gastrointestinal (GI) tract. However, until the late 1990s, it was often confused with submucosal and abdominal tumors (1), including leiomyoma and leiomyosarcoma, as well as S100-positive spindle cell tumor, myofibroblastic tumor, desmoid, and KIT-positive metastatic melanoma (2, 3). The lack of uniform nomenclature and histologic distinction resulted in frequent misdiagnoses by pathologists, as well as miscoding by cancer registrars (3). Therefore, before the implementation of a GIST-specific histology code in 2001, population-based studies were frequently limited due to inclusion of non-GIST abdominal and GI sarcomas (1, 4–7). In turn, this reduced the accuracy of older GIST epidemiology research. This began to change in 1998, when Hirota and colleagues reported that GIST was initiated by gain-of-function mutations in KIT (c-KIT, CD117) with associated KIT-positive immunostaining (8), thereby providing a more objective basis upon which to identify and diagnose GIST. The purpose of this study is to define the epidemiology of GIST in the modern era of precise pathologic diagnoses.
Materials and Methods
Using the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database (9), we identified 6,142 patients diagnosed with histologically confirmed GIST between 2001 and 2011 using GI tumor site codes (C150-C189, C199, C209-C212, C218, C220-C221, C239-C260, C268-C269, C480-C482, C488) and the GIST-specific histology code (ICD-O-3 code 8936). Follow-up extended through December 2011, at which point 4,170 patients were alive. Age adjusted incidence rates per 100,000 subjects were calculated based on 5-year age categories as calculated by SEER-Stat software. The 2000 United States standard population was used as the denominator in generating these age-adjusted rates (10). Ninety-five percent confidence intervals for incidence rates were determined with the Tiwari method (11). Overall survival was determined using Kaplan-Meier curves, and GIST-specific survival was determined using cumulative incidence analyses. Risk factors for overall survival were assessed with a multivariate Cox regression, and risk factors for GIST-specific survival were assessed with a Fine-Gray regression. Multivariate analysis included age as a continuous variable, sex, race, ethnicity, tumorsite and size, disease stage at diagnosis (SEER historic stage), and year of diagnosis. Sixteen percent of patients had missing tumor size, and these were categorized into a “missing” group for analysis. Associations were considered statistically significant if the 95% confidence interval excluded the null value. Incidence rates were calculated with SEER-Stat (version 8.1.5, Calverton, MD), and survival analyses were conducted with SAS (version 9.4, Cary, NC.
Results
Table 1 shows incidence rates stratified by demographic and GIST-specific factors. The annual incidence rate for the entire study period was 0.68 per 100,000 (95% CI 0.66-0.69 per 100,000) and increased steadily over the study period, from 0.55 per 100,000 (95% CI 0.50-0.61 per 100,000) in 2001 to 0.78 per 100,000 (95% CI 0.72-0.84 per 100,000) in 2011. Annual incidence also increased with age, from 0.01 per 100,000 (95% CI 0.01-0.02/100,000) for those under age 20 years old to 2.72 per 100,000 (95% CI 2.53-2.91 per 100,000) for those above age 80 years old, with a peak of 3.06 per 100,000 (95% CI 2.91-3.23/100,000) for those between 70 and 79 years of age. The median age at diagnosis was 64 years old, and the range extended between 8 and 101 years of age. GIST was 36% more common in men than women [rate ratio (RR) 1.35], and 23% more common in non-Hispanics than Hispanics (RR 1.23). Additionally, blacks and Asians/Pacific Islanders were 2.07 and 1.50 times more likely to develop GIST than whites, respectively. The most common tumor sites were the stomach (55%) and small intestine (29%); the remaining 16% was comprised of all other sites.
Table 1.
Age-Adjusted Incidence of GIST Stratified by Demographic Characteristics and Tumor Site in the Surveillance, Epidemiology, and End Results Program, 2001-2011.
| Characteristic | Number (%) | Rate per 100,000 (95% CI)* |
|---|---|---|
| All | 6,142 | 0.678 (0.661-0.695) |
| Age at diagnosis (years) | ||
| 8-20 | 30 (0.5) | 0.011 (0.008-0.016) |
| 20-29 | 76 (1.2) | 0.059 (0.046-0.074) |
| 30-39 | 249 (4.1) | 0.194 (0.170-0.219) |
| 40-49 | 739 (12.0) | 0.535 (0.497-0.575) |
| 50-59 | 1289 (21.0) | 1.118 (1.058-1.181) |
| 60-69 | 1553 (25.3) | 2.189 (2.081-2.301) |
| 70-79 | 1398 (22.8) | 3.064 (2.906-3.229) |
| 80-101 | 808 (13.2) | 2.718 (2.534-2.912) |
| Race | ||
| White | 4317 (70.3) | 0.592 (0.575-0.610) |
| Black | 1083 (17.6) | 1.224 (1.150-1.301) |
| American Indian/Alaska Native | 18 (0.3) | 0.233 (0.133-0.374) |
| Asian or Pacific Islander | 692 (11.3) | 0.889 (0.823-0.959) |
| Ethnicity | ||
| Non-Hispanic white | 5535 (90.1) | 0.695 (0.676-0.713) |
| Hispanic/Latino | 607 (9.9) | 0.565 (0.518-0.615) |
| Sex | ||
| Male | 3263 (53.1) | 0.790 (0.763-0.818) |
| Female | 2879 (46.9) | 0.585 (0.564-0.607) |
| Tumor Site | ||
| Stomach | 3394 (55.3) | 0.376 (0.363-0.389) |
| Small Intestine | 1765 (28.7) | 0.193 (0.184-0.203) |
| Colon | 179 (2.9) | 0.020 (0.017-0.023) |
| Rectum | 164 (2.7) | 0.018 (0.015-0.021) |
| Peritoneum, Omentum and Mesentery | 126 (2.1) | 0.014 (0.011-0.016) |
| Retroperitoneum | 58 (0.9) | 0.006 (0.005-0.008) |
| Esophagus | 33 (0.5) | 0.004 (0.003-0.005) |
| Hepatopancreaticobiliary | 26 (0.4) | 0.003 (0.002-0.004) |
| Anus, Anal Canal and Anorectum | 11 (0.2) | 0.001 (0.001-0.002) |
| Gastrointestinal (Not Otherwise Specified) | 384 (6.3) | 0.042 (0.038-0.047) |
| Tumor Stage | ||
| Localized | 3343 (54.4) | 0.369 (0.356-0.382) |
| Regional | 895 (14.6) | 0.099 (0.092-0.105) |
| Distant | 1348 (22.0) | 0.149 (0.141-0.157) |
| Unknown | 556 (9.1) | 0.062 (0.057-0.067) |
| Year of diagnosis | ||
| 2001 | 412 (6.7) | 0.552 (0.500-0.608) |
| 2002 | 528 (8.6) | 0.687 (0.630-0.748) |
| 2003 | 507 (8.3) | 0.655 (0.599-0.714) |
| 2004 | 525 (8.5) | 0.663 (0.607-0.723) |
| 2005 | 534 (8.7) | 0.657 (0.602-0.715) |
| 2006 | 496 (8.1) | 0.607 (0.554-0.663) |
| 2007 | 518 (8.4) | 0.622 (0.569-0.678) |
| 2008 | 578 (9.4) | 0.688 (0.633-0.747) |
| 2009 | 604 (9.8) | 0.682 (0.628-0.739) |
| 2010 | 733 (11.9) | 0.818 (0.759-0.880) |
| 2011 | 707 (11.5) | 0.781 (0.724-0.842) |
Rates are age-adjusted to the 2000 U.S. Standard Population; CI represents 95% confidence intervals using the Tiwari modification.
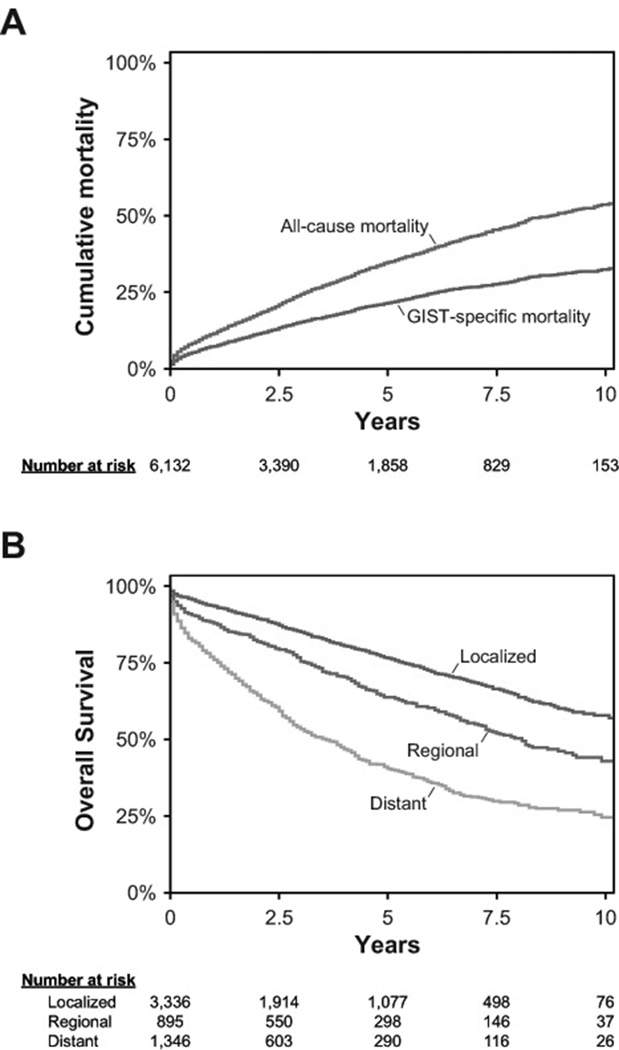
Kaplan-Meier curves are shown in Figure 1, both for the entire study period and for patients stratified by disease stage. For all GIST patients, the 5-year cancer-specific survival rate was 79% (95% CI 77-80%), and the 5-year overall survival rate was 65%(95% CI 64-67%) (Figure 1A). Of the 3,336 patients with localized disease at diagnosis, the 5-year overall survival rate was 77% (95% CI 75-78%), and of the 895 patients with regional disease at diagnosis, the 5-year overall survival rate was 64% (95% CI 60-67%). These were superior to the 5-year overall survival rate of 41% (95% CI 38-44%) among the 1,346 patients with metastatic disease at diagnosis (Figure 1B).
Figure 1. Kaplan-Meier Survival Curves.
A. Kaplan-Meier curves for overall and cancer-specific survival after GIST diagnosis. The 5-year overall survival (OS) was 65% (95% CI 64-67%), and 5-year GIST-specific survival was 79% (95% CI 77-80%).
B. Kaplan-Meier curves for overall survival stratified by disease stage at GIST diagnosis. In the era of tyrosine kinase inhibitor therapy, 3,336 patients with localized GIST had a 5-year overall survival (OS) of 77% (95% CI 75-78%), 895 patients with regional disease had a 5-year OS of 64% (95% CI 60-67%), and 1,346 patients with distant metastases had a 5-year OS of 41% (95% CI 38-44%).
Risk factors for overall and GIST-specific survival are shown in Table 2. After controlling for sex, age at diagnosis, race and ethnicity, tumor site and size, disease stage, and year of diagnosis, we found the following factors to be independently associated with worse overall survival: increased age at diagnosis [hazard ratio (HR) 1.58], male sex (HR 1.41), black race (HR 1.26), and regional (HR 1.59) or metastatic (HR 2.81) disease at diagnosis. Hispanic versus non-Hispanic ethnicity, tumors in the stomach versus the small intestine, primary tumors greater than 5 cm versus smaller than 2 cm, and earlier versus later diagnostic years did not seem to be independently associated with worse overall survival.
Table 2.
Characteristics Associated with Overall Survival (OS) and Disease-Specific Survival in GIST Patients, Surveillance, Epidemiology, and End Results Program, 2001-2011.
| Multivariate analysis for OS |
Multivariate analysis for GSS | |||
|---|---|---|---|---|
| Characteristic | 5-year OS (95% CI) |
Hazard ratio (95% CI) |
5-year GSS (95% CI) |
Sub-distribution hazard ratio (95% CI) |
| Age at Diagnosis (Continuous) | - | 1.58 (1.52-1.63) | - | 1.27 (1.22-1.33) |
| Race | ||||
| White | 66% (64-67%) | 1 | 79% (81-78%) | 1 |
| Asian or Pacific Islander | 68% (63-72%) | 0.93 (0.80-1.08) | 78% (82-74%) | 1.12 (0.92-1.35) |
| Black | 61% (57-65%) | 1.26 (1.12-1.42) | 75% (78-72%) | 1.24 (1.06-1.47) |
| American Indian/Alaska Native | 63% (31-83%) | 0.97 (0.40-2.35) | 70% (92-43%) | 1.56 (0.63-3.82) |
| Ethnicity | ||||
| Non-Hispanic/unknown | 66% (61-71%) | 1 | 79% (80-77%) | 1 |
| Hispanic | 65% (64-67%) | 1.05 (0.89-1.24) | 77% (81-73%) | 1.12 (0.90-1.40) |
| Sex | ||||
| Female | 62% (60-64%) | 1 | 81% (83-79%) | 1 |
| Male | 69% (67-71%) | 1.41 (1.28-1.54) | 76% (78-74%) | 1.36 (1.20-1.55) |
| Tumor site | ||||
| Stomach | 66% (64-68%) | 1 | 80% (82-78%) | 1 |
| Esophagus | 63% (41-79%) | 1.10 (0.60-1.99) | 72% (88-54%) | 1.43 (0.66-3.12) |
| Small Intestine | 70% (68-73%) | 0.93 (0.83-1.04) | 82% (84-80%) | 0.95 (0.82-1.11) |
| Colon and Rectum | 63% (56-69%) | 1.06 (0.88-1.29) | 74% (80-69%) | 1.17 (0.90-1.53) |
| Anus | 66% (27-88%) | 1.94 (0.72-5.21) | 75% (97-42%) | 1.79 (0.44-7.19) |
| Hepatopancreaticobiliary | 53% (28-72%) | 1.16 (0.66-2.06) | 56% (80-35%) | 1.63 (0.89-2.96) |
| Gastrointestinal (Not Otherwise Specified) | 45% (41-50%) | 1.25 (1.08-1.43) | 64% (68-59%) | 1.13 (0.93-1.38) |
| Year of diagnosis | ||||
| 2001 | 57% (52-62%) | 1 | 73% (77-68%) | 1 |
| 2002 | 62% (58-66%) | 1.12 (0.94-1.33) | 77% (80-73%) | 0.92 (0.74-1.16) |
| 2003 | 62% (58-66%) | 0.95 (0.80-1.14) | 77% (80-73%) | 0.86 (0.68-1.08) |
| 2004 | 67% (62-70%) | 0.84 (0.70-1.01) | 78% (82-75%) | 0.75 (0.59-0.95) |
| 2005 | 65% (61-69%) | 0.84 (0.69-1.01) | 80% (83-76%) | 0.71 (0.55-0.90) |
| 2006 | 67% (62-71%) | 0.76 (0.62-0.93) | 78% (82-75%) | 0.69 (0.53-0.89) |
| 2007 | - | 0.87 (0.70-1.07) | - | 0.66 (0.50-0.87) |
| 2008 | - | 0.71 (0.57-0.88) | - | 0.69 (0.53-0.91) |
| 2009 | - | 0.85 (0.67-1.07) | - | 0.60 (0.44-0.82) |
| 2010 | - | 0.84 (0.66-1.08) | - | 0.54 (0.38-0.75) |
| 2011 | - | 0.86 (0.64-1.17) | - | 0.55 (0.35-0.87) |
| Disease Stage at Diagnosis | ||||
| Localized | 77% (75-78%) | 1 | 87% (89-86%) | 1 |
| Regional | 64% (60-67%) | 1.59 (1.39-1.83) | 78% (81-74%) | 1.66 (1.37-2.01) |
| Distant | 41% (38-44%) | 2.81 (2.50-3.15) | 60% (63-57%) | 3.11 (2.65-3.64) |
| Unknown | 63% (59-68%) | 1.49 (1.26-1.75) | 75% (79-71%) | 1.92 (1.54-2.38) |
| GIST size | ||||
| ≤2 cm | 71% (65-76%) | 1 | 82% (86-77%) | 1 |
| >2, ≤5 cm | 76% (73-79%) | 0.74 (0.59-0.93) | 88% (90-85%) | 0.69 (0.50-0.95) |
| >5, ≤10 cm | 71% (68-73%) | 0.89 (0.72-1.11) | 83% (85-81%) | 0.81 (0.60-1.09) |
| >10 cm | 59% (56-62%) | 1.07 (0.86-1.33) | 74% (76-71%) | 1.09 (0.81-1.46) |
| Unknown | 49% (46-53%) | 1.29 (1.03-1.61) | 66% (69-63%) | 1.16 (0.86-1.58) |
The 5-year overall survival represents the results of Kaplan-Meier analysis, and the multivariate analysis for overall survival represents the results of a multivariate Cox regression. The 5-year GIST-specific survival represents the results of a cumulative incidence analysis treating death from other causes as a competing event, and the multivariate analysis for GIST-specific survival represents the results of a Fine-Gray regression.
Like overall survival, increased age at diagnosis (HR 1.27), male sex (HR 1.36), black race (HR 1.24), and regional (HR 1.66) or metastatic (HR3.11) disease at diagnosis were statistically significantly associated with worse GIST-specific survival on multivariate analysis. However, Hispanic versus non-Hispanic ethnicity, tumors in the stomach versus the small intestine, and primary tumors greater than 5 cm versus smaller than 2 cm than 2 cm were not statistically significantly associated wit hGIST-specific mortality.
Discussion
Our study represents the first population-based assessment of GIST epidemiology in the United States using ICD-O-3 coding. While some of our results supported previous studies, others differed from earlier findings, providing a comprehensive description and statistical examination of GIST in the modern era of immunohistochemical diagnoses. We examined incidence trends by demographic and tumor-specific characteristics, determined survival trends, and found several risk factors to be independent predictors of mortality.
Among the patients in our study period, age-adjusted incidence rose 42% over a decade, from 0.55 per 100,000 in 2001 to 0.78 per 100,000 in 2011. A previous study with older SEER data found a 25-fold increase in disease incidence from 1992 to 2002 (e.g., 0.03 per 100,000 to 0.69 per 100,000) (1), though the considerable increase was likely attributable to the prevalent re-classification of misdiagnosed smooth muscle tumors as GIST during the latter years. Two additional studies reported average GIST incidences of 0.32 per 100,000 between 1993 and 2002 (7) and 0.68 per 100,000 between 1992 and 2000 (4), but these results were likely limited by aforementioned pathologic and coding issues. Therefore, though the overall incidence in our study (0.68 per 100,000) was similar to the one reported in a previous SEER-based report (4), our findings more accurately reflect he present-day incidence of GIST in the United States.
We found GIST to be more common in males, non-Hispanics, blacks, and Asians/Pacific Islanders. As such, our analyses confirmed results of several previous population-based studies that found increased incidence among males (4, 6, 12), as well as one study reporting that GIST incidence in blacks was approximately twice the incidence in whites (4). We also found increased incidence among Asians and Pacific Islanders in the United States, which would be consistent with the much higher age-adjusted incidence (1.97 per 100,000) reported in an earlier study from Taiwan (12). In addition, the anatomic distribution of tumor sites [e.g., stomach (55%), small intestines (29%), colon (2.9%), and rectum (2.7%)] was similar to the distribution reported in several earlier studies (2, 4, 12, 13). Finally, we contributed new evidence to the literature by showing that incidence peaked among patients between 70 and 79 years of age.
The 5-year overall survival rate of 65% was higher than the figure reported by Tran et al. in a SEER study between 1992 and 2000, which found a rate of 45% (4). The 5-year GIST-specific survival rate of 79% was also higher than the figure previously reported by DeMatteo et al. in a study at Memorial Sloan Kettering between 1982 and 1998, which found a rate of 35% (14), and a single-institution study by Hassan et al. between 1978 and 2004, which reported a rate of 68% (15). While treatment data is not available in the SEER database, the improved survival is likely attributable to the introduction of Imatinib in 2000 (1), as 80-90% of patients with metastatic disease responded to imatinib, which was highly effective in shrinking or stabilizing tumors with continuous therapy (16).
Like the report by Tran et al.,(4), we also found black race, older age, and advanced stage to be associated with worse prognoses, which conflicted with prior claims of a lack of survival disparity between black and white patients (6). In addition, our results supported a Taiwanese study showing male sex to be associated with worse overall survival, but contradicts its claim that tumor location in the small intestine rather than stomachis a significant independent factor correlated with higher mortality (12). After controlling for other variables such as tumor site and disease stage, we also found that primary tumors larger than 5 cm were not indicative of worse overall survival when compared with primary tumors less than 2 cm.
Results from the multivariate Cox analysis also contradicted previous findings showing tumor size as a significant predictor of GIST-specific survival (14), as we did not find tumors larger than 5 cm to be associated with worse GIST-specific survival than tumors smaller than 2 cm. Unlike Cox analyses conducted previously (15), we also did not find tumors located in the small intestines to be associated with worse GIST-specific survival than tumors located in the stomach. Finally, our results indicated an improvement in GIST-specific survival during the latter years of our study (2004-2011), though this failed to translate into an improvement in overall survival. This finding suggests that the improvement in GIST-specific survival in later years was offset by a concurrent increased risk in non-GIST mortality.
As the first epidemiological study to incorporate histology coding, we were able to more precisely identify GIST patients, and, by extension, GIST epidemiology in the United States. However, since cancers were identified using histology and site-specific codes, misdiagnosis and miscoding were still potential sources of error. Also, since the current ICD-O-3GIST histology code was instated in 2001, we likely underestimated the actual number of patients with GIST in SEER, particularly at the beginning of our study period. This might explain, in part, the 42% increase in incidence between 2001 and 2011, which was likely due to the increased application of GIST histology codes over time.
In summary, older population-based reports on GIST epidemiology suffer from misdiagnoses and miscoding, which could bias results and skew analyses. The advent of a GIST-specific histology code in the early 2000s allowed us to more accurately identify GIST and describe its epidemiology, which may carry important diagnostic, prognostic and therapeutic implications.
Acknowledgments
Funding Sources: The authors acknowledge the support of NIH KL2 RR031978 (J.D. Murphy) and the GIST Research Fund (J.K. Sicklick).
Footnotes
Conflict of Interest: In 2013, J.K.S. received honoraria from Novartis Pharmaceuticals Corporation for advisory board consultancy and speaking, as well as reimbursement for travel, lodging, and meals. J.K.S. received honoraria from Genentech Inc. for speaking. No additional authors have conflicts of interest to declare.
References
- 1.Perez EA, Livingstone AS, Franceschi D, Rocha-Lima C, Lee DJ, Hodgson N, et al. Current incidence and outcomes of gastrointestinal mesenchymal tumors including gastrointestinal stromal tumors. Journal of the American College of Surgeons. 2006;202:623–629. doi: 10.1016/j.jamcollsurg.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Archiv : an international journal of pathology. 2001;438:1–12. doi: 10.1007/s004280000338. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Human pathology. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 4.Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. The American journal of gastroenterology. 2005;100:162–168. doi: 10.1111/j.1572-0241.2005.40709.x. [DOI] [PubMed] [Google Scholar]
- 5.Blanke C, Eisenberg BL, Heinrich M. Epidemiology of GIST. The American journal of gastroenterology. 2005;100:2366. doi: 10.1111/j.1572-0241.2005.50650_6.x. [DOI] [PubMed] [Google Scholar]
- 6.Cheung MC, Zhuge Y, Yang R, Koniaris LG. Disappearance of racial disparities in gastrointestinal stromal tumor outcomes. Journal of the American College of Surgeons. 2009;209:7–16. doi: 10.1016/j.jamcollsurg.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Rubin JL, Sanon M, Taylor DC, Coombs J, Bollu V, Sirulnik L. Epidemiology, survival, and costs of localized gastrointestinal stromal tumors. International journal of general medicine. 2011;4:121–130. doi: 10.2147/IJGM.S16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 9.Surveillance, Epidemiology, and End Results (SEER) Program (http://www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2013 Sub (2000-2011) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969-2012 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014 (updated 5/7/2014), based on the November 2013 submission.;
- 10.Day JC. US Bureau of the Census, Current Population Reports, P25-1130. US Government Printing Office; Washington, DC: 1996. Population Projections of the United States by Age, Sex, Race, and Hispanic Origin: 1995 to 2050. [Google Scholar]
- 11.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Statistical methods in medical research. 2006;15:547–569. doi: 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]
- 12.Chiang NJ, Chen LT, Tsai CR, Chang JS. The epidemiology of gastrointestinal stromal tumors in Taiwan, 1998–2008: a nation-wide cancer registry-based study. BMC cancer. 2014;14:102. doi: 10.1186/1471-2407-14-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of surgical oncology. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 14.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Annals of surgery. 2000;231:51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassan I, You YN, Shyyan R, Dozois EJ, Smyrk TC, Okuno SH, et al. Surgically managed gastrointestinal stromal tumors: a comparative and prognostic analysis. Annals of surgical oncology. 2008;15:52–59. doi: 10.1245/s10434-007-9633-z. [DOI] [PubMed] [Google Scholar]
- 16.Eisenberg BL, Judson I. Surgery and imatinib in the management of GIST: emerging approaches to adjuvant and neoadjuvant therapy. Annals of surgical oncology. 2004;11:465–475. doi: 10.1245/ASO.2004.09.011. [DOI] [PubMed] [Google Scholar]