Abstract
Costello syndrome is a rare, autosomal dominant syndrome caused by activating missense mutations in the Harvey rat sarcoma viral oncogene homolog (HRAS), most often p.G12S. Several rare mutations have consistently been associated with a more severe phenotype which is often lethal in infancy. Cause of death is most often respiratory failure with hypertrophic cardiomyopathy playing a significant role in morbidity. Impaired fibroblast elastogenesis is thought to contribute to the Costello phenotype, but reports of histologic evidence of disordered elastogenesis at autopsy are limited. We report a patient with Costello syndrome due to a rare tandem base substitution (c.35_36GC>AA) resulting in the p.G12E missense change. The proband died at age 3 months from respiratory failure, with minimal evidence for cardiomyopathy. Autopsy disclosed pulmonary vascular dysplasia affecting small arteries and veins associated with abnormal elastin distribution in tortuous dilated arteries and veins, with non-uniform wall thickness and semi-obstructive lesions at artery branch points typical of early pulmonary hypertensive vascular disease. Elastic fibers in the dermis were abnormally short and fragmented. This case suggests that disordered elastogenesis in the pulmonary vasculature and undiagnosed (or underdiagnosed) pulmonary hypertension may contribute to morbidity in patients with Costello syndrome.
Keywords: Costello syndrome, elastin, HRAS, pulmonary, pulmonary hypertension, vasculopathy
Introduction
Costello syndrome is a rare autosomal dominant disorder caused by activating mutations in the Harvey rat sarcoma viral oncogene homolog (HRAS) [1, 2]. Characteristics of Costello syndrome include course craniofacial features, short stature, poor feeding and growth, cardiovascular anomalies, developmental delay/intellectual disability, loose/extensible skin and joins, laryngomalacia, and increased risk for solid tumors. The most commonly reported HRAS mutations in Costello syndrome are p.G12S followed by p.G12A [3]. Several rare mutations have consistently been associated with a more severe phenotype which is often lethal in infancy, for example p.G12D, p.G12C, and p.G12E [2, 4, 5](see Table 1 for summary). Cause of death in neonatal lethal cases is most often respiratory failure with hypertrophic cardiomyopathy playing a significant factor in morbidity. Persistent pulmonary hypertension is described in one infant [6]. Fibroblasts in Costello syndrome have an increased rate of proliferation and impaired elastogenesis which are thought to contribute to the phenotype [7]. Reports of disordered elastogenesis at autopsy of patients with Costello syndrome provide limited histologic support. We describe a patient, with Costello syndrome caused by the rare p.G12E mutation, who died at age 3 months from respiratory failure without significant cardiomyopathy. Post-mortem evaluation was significant for the novel finding of pulmonary vascular dysplasia and widespread early lesions of pulmonary hypertension, which we speculate are related to disordered elastogenesis and likely contributed to death.
Table 1.
Early Lethal Costello Syndrome
| Reference | Mutation | Age at death |
Cause of death | Reported histologic findings and relevant clinical features |
|---|---|---|---|---|
| Current report | G12E | 3 m | Respiratory failure | Atrial tachyarrythmia Mild cardiomegaly Pulmonary vascular dysplasia Pharyngomalacia Obstructive sleep apnea |
| Lorenz et al 2012[1] | G12C | 3.5 m | Heart failure | 29 wga HCM Tachyarrhythmias IVH, hydrocephalus (shunted) Seizures |
| G12D | 2 w | Cardiocirculatory and respiratory failure | HCM (prenatal onset) Dysplastic pulmonary valve Supraventricular tachycardia |
|
| Burkitt-Wright et al 2012[2] | G12V | 6 w | Bronchopneumonia | Biventricular and septal hypertrophy Bronchopulmonary dysplasia (37 wga) Ventilator dependent |
| G12V | 1 m | Respiratory failure | PFO, mild asymmetric septal hypertrophy Ventilator dependent Autopsy: Congenital alveolar dysplasia |
|
| G12V | 6 w | D/C of life support | Multifocal atrial tachycardia Biventricular concentric hypertrophy Ventilator dependence |
|
| G12V | 2 w | Respiratory failure | HCM Ventilator dependence |
|
| Lin et al 2009[3] | G12S | 4 m | Treatment-resistant, hemodynamically significant atrial tachyarryhthmia | Pulmonic valve stenosis Atrial tachyarrhythmia Tracheostomy for soft tissue airway obstruction |
| G12C | 40 d | Multi-organ failure | 32 weeks gestation Wide QRS tachycardia Prenatal onset, recurrent pleural effusion Chylothorax Autopsy: lung hypoplasia, nephromegaly, liver hemangioma |
|
| G12S | 13 m | Cardiovascular collapse (ventricular tachycardia and fibrillation) | Tracheostomy Gastrostomy tube Wide QRS tachycardia Autopsy: Left ventricle hypertrophy, mild subaortic stenosis. Disorganized myofibers. Normal coronary arteries, intra-myocardial vessels, and pulmonary artery. |
|
| Kuniba et al 2009[4] | G12D | <4 w | Multiple organ failure | 31 weeks gestation Cardiac Hypertrophy Respiratory failure Renal failure Hypoglycemia |
| Lo et al 2007[5] | G12D | 3 m | Respiratory failure | ASD, septal hypertrophy Multifocal atrial tachycardia Tracheobronchomalacia Autopsy: Lymphangiectasia (lung, liver, spleen); Alveolar-capillary dysplasia |
| G12D | 3 m | Sepsis, renal failure | HCM Dysplastic pulmonary valve Atrial fibrillation Cardiac failure Ventilator dependent |
|
| G12C | 3 m | Respiratory insufficiency | Atrial tachyarrhythmia | |
| Kerr et al 2006[6] | G12C | 7 m | Metastatic rhabdomyosarcoma; Respiratory failure | Cardiomyopathy Rhabdomyosarcoma (prostate) |
| G12E | 6 m | Respiratory failure | Cardiomyopathy Bronchomalacia Tracheostomy Autopsy: Fibromuscular dysplasia of coronary arteries |
|
| Mori 1996[7] | Not reported | 6 m | Rhabdomyolysis related to viral infection | Multifocal ventricular tachycardia Autopsy: Fine, disrupted elastic fibers in skin, tongue, pharynx, larynx, upper esophagus. Narrow pulmonary arteries with intact elastic fibers. |
Lorenz S, Petersen C, Kordass U, Seidel H, Zenker M, Kutsche K. Two cases with severe lethal course of Costello syndrome associated with HRAS p.G12C and p.G12D. European journal of medical genetics 2012, 55:615–9.
Burkitt-Wright EM, Bradley L, Shorto J, et al. Neonatal lethal Costello syndrome and unusual dinucleotide deletion/insertion mutations in HRAS predicting p.Gly12Val. Am J Med Genet A 2012, 158A:1102–10.
Lin AE, O'Brien B, Demmer LA, et al. Prenatal features of Costello syndrome: ultrasonographic findings and atrial tachycardia. Prenat Diagn 2009, 29:682–90.
Kuniba H, Pooh RK, Sasaki K, et al. Prenatal diagnosis of Costello syndrome using 3D ultrasonography amniocentesis confirmation of the rare HRAS mutation G12D. Am J Med Genet A 2009, 149A:785–7.
Lo IF, Brewer C, Shannon N, et al. Severe neonatal manifestations of Costello syndrome. J Med Genet 2008, 45:167–71.
Kerr B, Delrue MA, Sigaudy S, et al. Genotype-phenotype correlation in Costello syndrome: HRAS mutation analysis in 43 cases. J Med Genet 2006, 43:401–5.
Mori M, Yamagata T, Mori Y, et al. Elastic fiber degeneration in Costello syndrome. American journal of medical genetics 1996, 61:304–9.
Methods
We report detailed clinical, genetic, and pathologic findings in an infant with Costello syndrome caused by a rare mutation. Written permission from the infant’s parents was obtained prior to publication.
Results
Clinical and Phenotypic Report
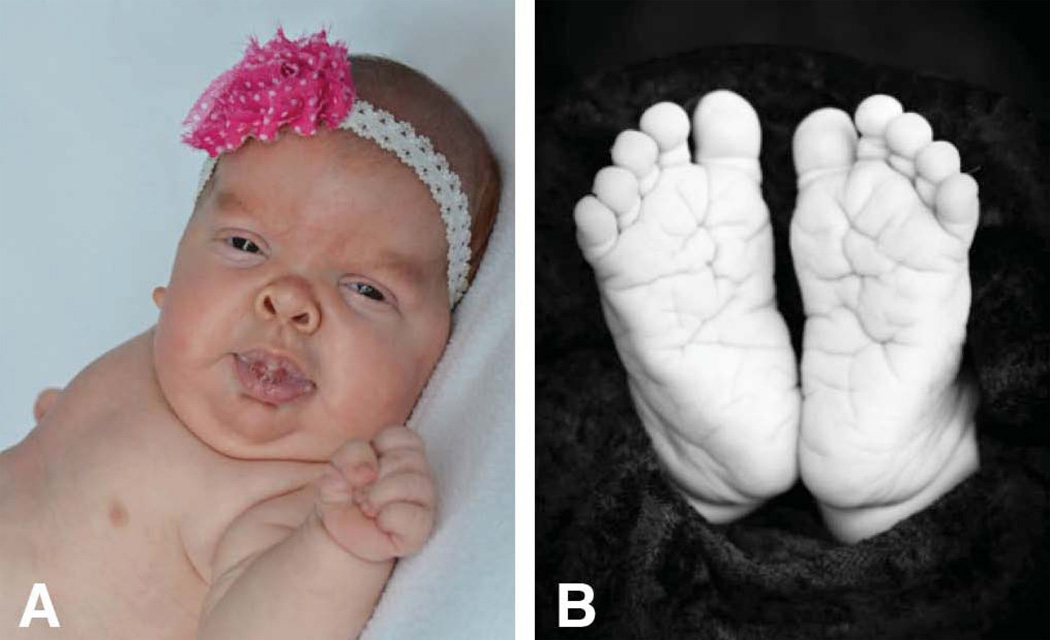
The female patient was the second child born to healthy non-consanguineous parents. Maternal age was 35 and paternal age was 34 years. The pregnancy was complicated by gestational diabetes diagnosed at 32 weeks, polyhydramnios, and concern for multiple fetal anomalies, including macrocephaly, ventriculomegaly, short long bones, and possible coarctation of the aorta. The patient was delivered by C-section at 37 weeks due to fetal heart rate decelerations. She required positive pressure ventilation (PPV) and continuous positive airway pressure (CPAP) in the delivery room for low heart rate and poor color. Apgar scores were 6 and 8. Birth weight was 3350 grams (45th centile), length 47 cm (16th centile), and head circumference 36 cm (75th centile). She was hyperteloric with down-slanting palpebral fissures, flat nasal bridge, and upturned nasal tip (Figure 1A). She had a short neck with excess nuchal skin. Her ears were low-set and posteriorly rotated, with over-folded helices. She had a wide space between her first and second toes bilaterally, and excess skin on her hands and feet with deep palmar and plantar creases (Figure 1B). Limbs appeared to have mild rhizomelic shortening.
Figure 1.
A. Craniofacial features typical of Costello syndrome (CS) include coarse face (full cheeks, thick lips, full nasal tip) short neck, epicanthal folds, and depressed nasal bridge. B. Deep plantar creases are typical for CS.
Because of hypotonia and retractions, she required intubation, which was difficult due to poor glottis exposure. The initial echocardiogram demonstrated mild biventricular hypertrophy, moderate PDA with bidirectional shunting, and small patent foramen ovale with left to right shunting.
The patient was hospitalized for 5 consecutive weeks after birth. She briefly required treatment for hypoglycemia. She failed extubation on day-of-life (DOL) 3, demonstrating excessive secretions with increased work of breathing. Upper GI series and water soluble enema were performed on DOL 5 due to emesis and feeding intolerance. Imaging findings suggested atypical duodenal malrotation but no obstruction. Microlaryngoscopy and bronchoscopy performed on DOL 6 demonstrated pharyngomalacia, subglottic edema and ulceration. Echocardiogram on DOL 9 showed septal flattening and RV dysfunction, and she was started on inhaled nitric oxide (NO) and milrinone to treat pulmonary hypertension. She was successfully extubated on DOL 10 to CPAP, gradually weaned off support to room air by DOL 50 and transitioned to Sildenafil by DOL 16. Evidence for pulmonary hypertension gradually declined. Atrial premature beats on DOL 9 initially improved but on DOL 34 she developed sustained atrial tachycardia for several hours. This was controlled with digoxin and flecainide. On DOL 34 there was also concern for clinical seizures, but prolonged EEG was inconclusive. Brain MRI on DOL 34 demonstrated mild prominence and asymmetry of the lateral ventricles with left larger than right. Ophthalmologic exam demonstrated a very small polar cataract in each eye. Poor feeding persisted and a gastrostomy tube was placed on DOL 38. A Ladd’s procedure corrected malrotation. Endoscopic evaluation of swallowing on DOL 34 showed moderate laryngomalacia, increased secretions with decreased swallowing, and laryngeal penetration without frank aspiration. She was discharged on DOL 53 feeding entirely by G-tube and with no respiratory support. Medications included flecainide, digoxin, and sildenafil.
A polysomnogram (PSG) was performed at 87 days of age due to snoring and respiratory pauses during sleep. During 310 minutes of sleep there were 133 obstructive apneas and 54 obstructive hypopneas, for an obstructive index of 36.8. The minimum oxygen saturation associated with the obstructive events was 84.7%. The overall impression was severe obstructive sleep apnea with mild-moderate degree of oxygen desaturation. Oxygen therapy at ¼ L per minute decreased the frequency of respiratory events and improved oxygenation.
The patient was readmitted to the hospital on DOL 99 due to diaphoresis, prolonged episodes of atrial tachycardia, and respiratory distress. Echocardiogram performed on DOL 100 demonstrated mild left ventricular hypertrophy, no outflow tract obstruction, and qualitatively normal systolic function (EF 55–60%). Atrial tachycardia was controlled with IV amiodarone and she was transitioned to flecainide and propranolol. She did not tolerate weaning of respiratory support and required either CPAP or high-flow nasal cannula. Bronchoscopy demonstrated multiple levels of airway obstruction, including pharyngomalacia, glossoptosis, and laryngomalacia. After supraglottoplasty respiratory support was weaned to 1L/min by nasal cannula. However, episodic worsening of respiratory status continued and she died on DOL 123 from respiratory failure.
Genetic Studies
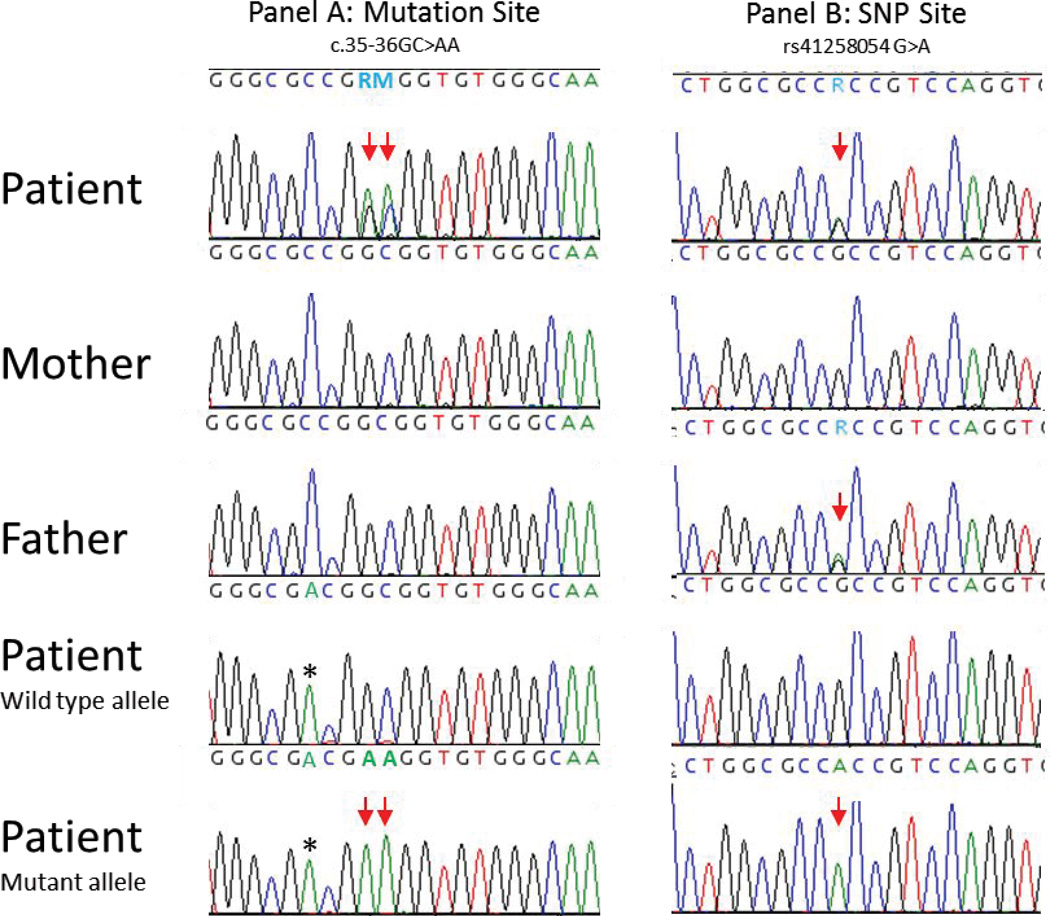
Routine chromosomes and SNP microarray had normal results. Noonan-spectrum testing sent to a CLIA certified lab reported a heterozygous c.35_36delinsAA mutation in exon 2 of HRAS which is predicted to result in p.Gly12Glu (G12E). Using an allele specific amplification protocol that took advantage of informative SNP rs41258054 (c.111+15G>A), follow up laboratory studies demonstrated the de novo HRAS mutation results from a tandem nucleotide base substitutions of paternal origin (Figure 8). Additionally, a heterozygous c.368G>A (p.Arg123Lys) variant of uncertain significance was identified in NRAS. Both parents were subsequently tested and the NRAS variant was found to be maternally inherited and therefore likely benign given the normal phenotype of the patient’s mother.
Figure 8.
Family HRAS sequencing report. Panel A identifies the nucleotide sequence around the c.35–36GC>AA mutation site (double arrow), while panel B represents the region surrounding rs41258054 (single arrow). In each panel, the top 3 chromatograms display PCR sequencing of the family members as indicated. The bottom 2 chromatograms represent allele specific (wild type or mutant) sequencing of the patient. Panel B shows that the patient and his father are G/A, while the mother is homozygous G, making the “A” allele a marker for the paternal germline allele. Allele specific amplification (ASA) used modified primers* targeting the G>A change at c.35. The asterisk denotes a mis-match purposely created in the ASA primer to increase allelic discrimination. The wild type allele carries the maternally inherited “G” SNP allele, while the “A” allele derived from the father is present in the mutant ASA product. This ASA also confirms that both substitutions (c.35 and c.36) are adjacent on the same allele and thus result in the Gly>Glu amino acid change.
Autopsy Findings
Postmortem examination demonstrated borderline low body length (55cm, expected 59cm; 50th centile for individuals with Costello syndrome,[8]]). Craniofacial and other body characteristics were as described above. The heart weight of 34.8 gm was mildly enlarged for body length (normal 25 gm). The brain weight of 587 gm was slightly heavier than expected (normal 489 gm), which may be explained by edema. There were no structural abnormalities identified in the brain. Overall, there was mild growth delay compared with expected for a normal 4 month old infant girl using autopsy charts [9].
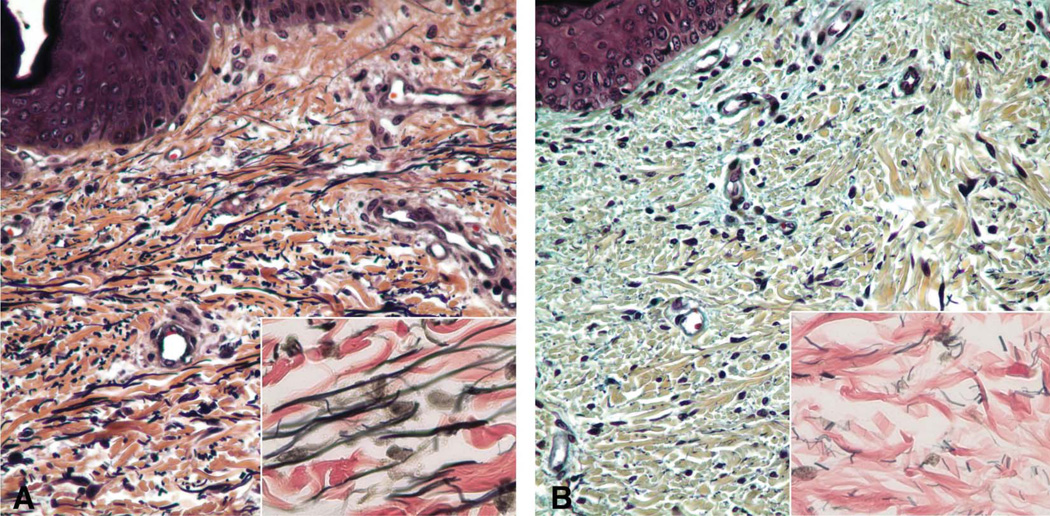
Although clinical imaging and mildly increased cardiac weight suggested ventricular overload, there was no myocyte hypertrophy or myofiber disarray. No systemic vasculopathy was identified in a conventional organ sample at autopsy. Elastic staining of skin demonstrated markedly decreased elastic fibers that were thin and fragmented compared to age-matched control (Figure 2). Pentachrome stain, performed to assess presence of glycosaminoglycans, demonstrated increased bluish amorphous deposition of glycosaminoglycans in the dermis compared to normal.
Figure 2.
Skin in CS. A. Age-matched normal abdominal skin dermis has expected amount and caliber of elastic fibers. Pentachrome ×400; Inset: VVG elastic stain ×1000. B. Abdominal skin dermis in CS shows markedly reduced elastic fibers that are thin, short and fragmented. Pentachrome stain × 400. Inset: VVG elastic stain × 1000.
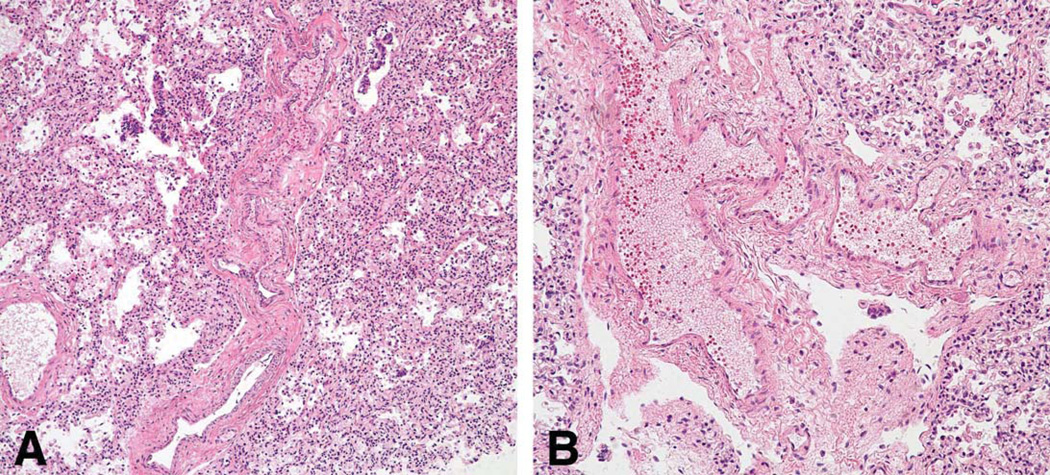
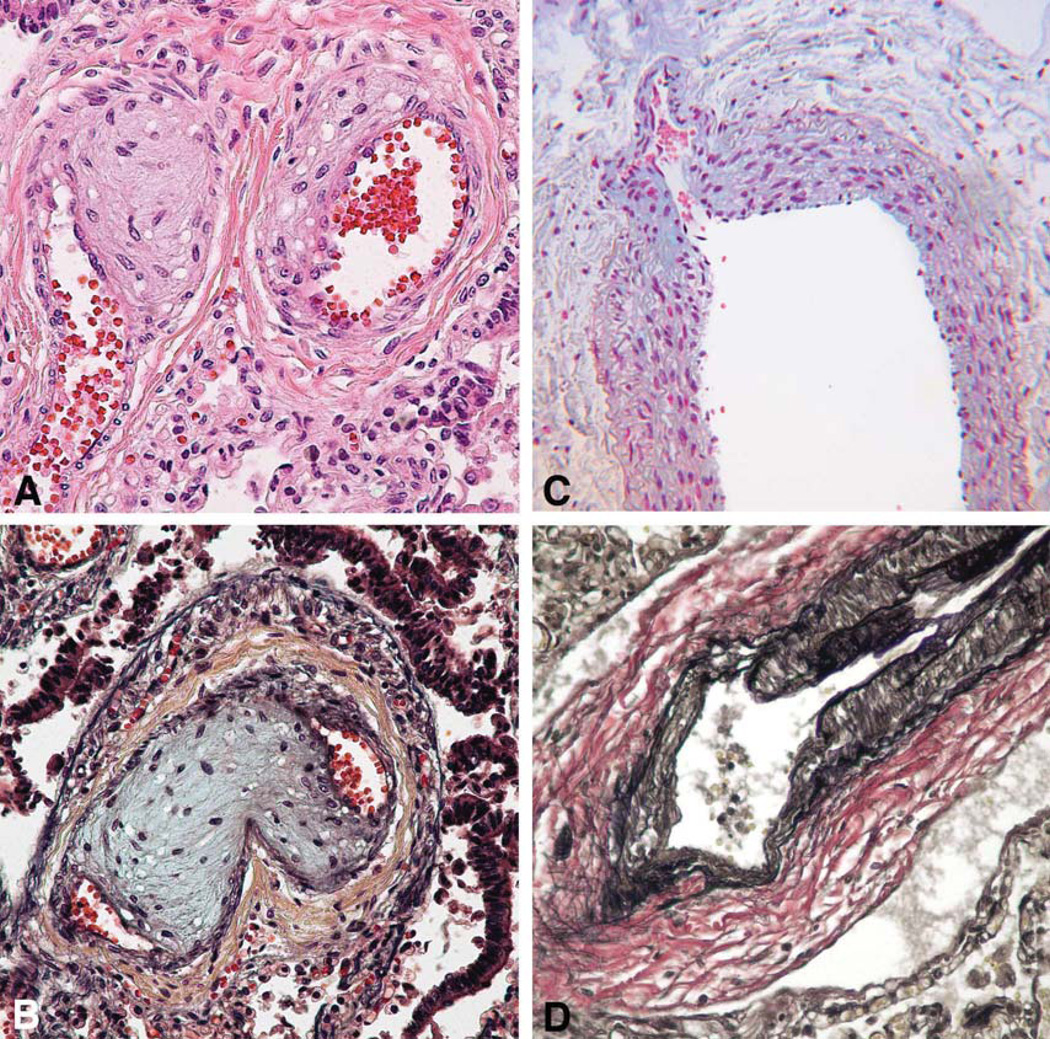
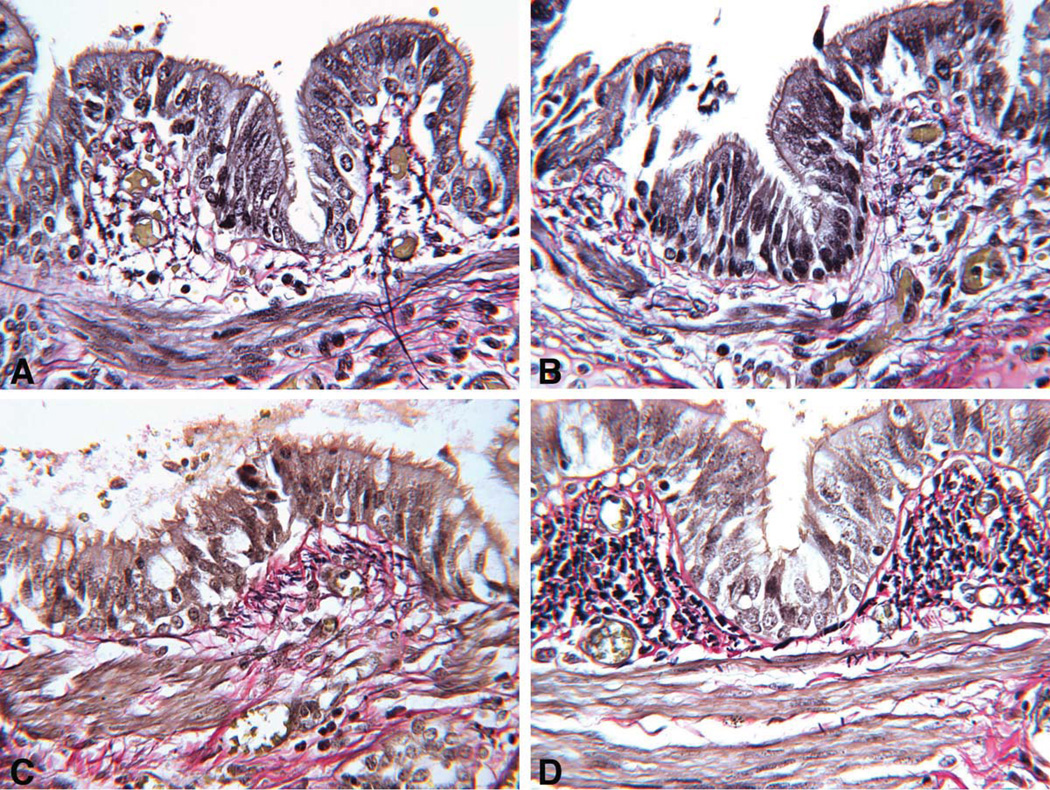
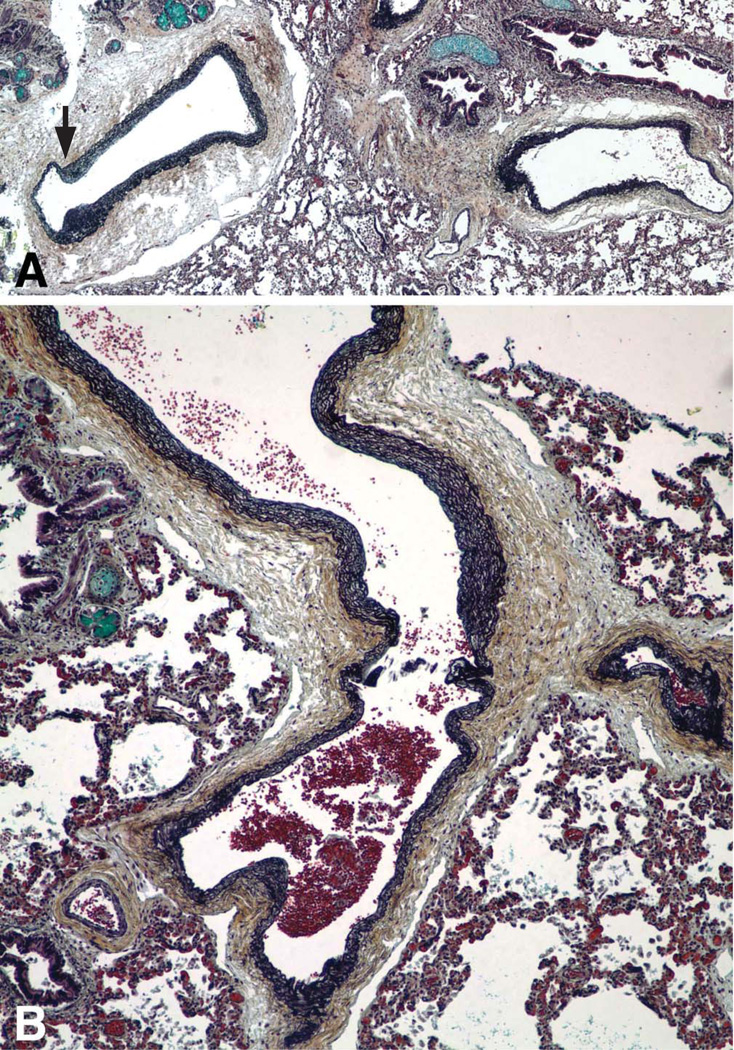
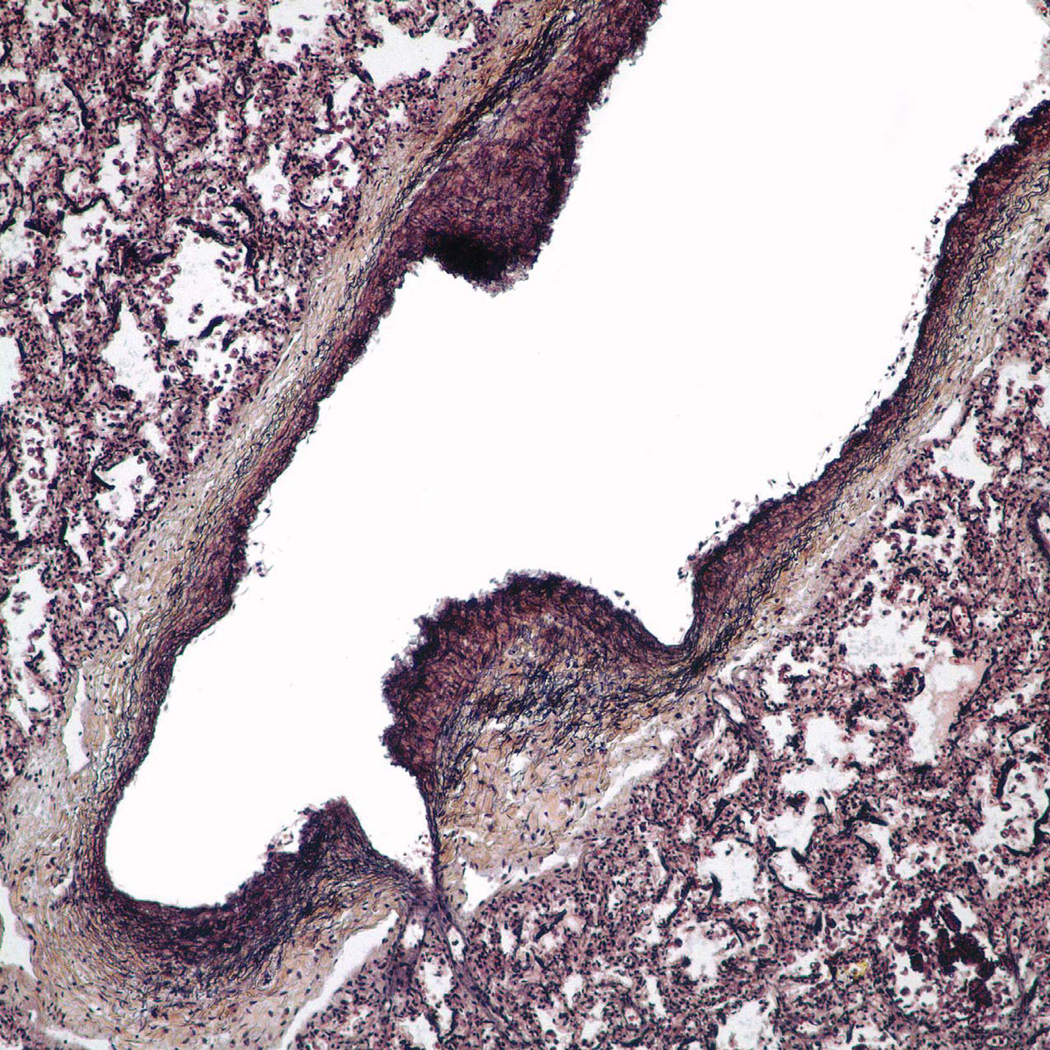
Multifocal pulmonary vascular abnormalities involved the intra-pulmonary pulmonary veins and arteries (Figure 3–5). Elastic staining of pulmonary arteries and veins demonstrated disorganized non-uniform elastic fiber composition as well as foci of both deficiency and excess of medial smooth muscle, particularly in medium-sized arteries. Many tortuous intrapulmonary veins had asymmetric musculoelastic wall thickening. The alignment of the pulmonary veins was normal. The walls of pulmonary hilar arteries and veins were not systematically sampled but seemed normal. Arteries that accompanied cartilage-bearing bronchi were tortuous and irregularly dilated or hypertrophied, often abruptly transitioning from thick to thin-walled at or near branch points (Figure 6). Depending on the orientation of the section, branch point stenosis and post-stenotic local dilation were often seen. Rare bulky deposits of myxoid acid glycosaminoglycans obstructed small pulmonary arteries. Thrombosis was extremely rare; the only example in six large samples of paraffin-embedded lung was located in an exaggerated branch point cushion. There was no vasculitis. The pulmonaryvascular changes at arterial branch points resemble those reported i n early primary and secondary pulmonary hypertension but typical features of hypertensive vasculopathy such as uniform muscularization of small pulmonary arteries, intimal fibrosis and muscularization of pulmonary arterioles were absent. [10, 11]. Elastic fibers in the bronchi were fine, short, and disorganized compared to the bronchus of an age and size-matched control bronchus (Figure 7).
Figure 3.
Small pulmonary artery and vein are tortuous with marked thinning of the media without intimal fibrosis or focal muscular hypertrophy. A, artery; B, vein; H&E, ×200.
Figure 6.
The small pulmonary arteries show focal wall thickening resulting in exaggerated cushions and mild stenosis at branch points with locally increased glycosaminoglycan matrix. A, H&E: ×400; B, Pentachrome stain: ×400; C, Alcian blue stain, ×400; D, VVG elastic stain: ×400.
Figure 7.
A and B, The elastic fibers in a CS bronchus (1.5mm diameter) are fine and short and somewhat disorganized, pentachrome stain ×250. C and D, The elastic fibers in an age and size-matched normal control bronchus are larger and are focally arrayed in compact submucosal bundles not seen in CS bronchi of similar diameter, pentachrome stain ×250.
Discussion
We report a patient with early lethal Costello syndrome caused by a rare HRAS mutation predicting G12E. This mutation has been reported previously only once, in an infant who died at 6 months of age [2]. That infant also had a particularly severe phenotype, including a severe cardiomyopathy, persistent hypoglycemia attributed to hyperinsulinemia and hyperplasia of Langerhans’ islets. The patient we report had mild cardiomegaly and transient hypoglycemia but no lesion of the islets at autopsy.
Two infants with early lethal Costello syndrome caused by G12D and G12C mutations both had severe hypertrophic cardiomyopathy [5]. Moreover, the mutation G12V has been established as a consistent cause of early lethal Costello syndrome with cardiomyopathy [12]. Our patient is unique in that severe cardiomyopathy was not detected by echocardiogram. Although the heart was slightly heavy for body length, the myocardium was normal by microscopy. Her death was due to respiratory failure, which was likely a combination of multi-level airway obstruction in addition to pulmonary hypertension to which vascular dysplasia contributed.
Pulmonary vascular dysplasia associated with abnormal elastin morphology has not previously been described in Costello syndrome. Previous studies with qualitative and quantitative histological analyses showed impairment in elastin deposition in the skin, tongue, pharynx, larynx, alveoli, aorta, or coronary arteries of patients with Costello syndrome[13, 14]. In these tissues elastic fibers appeared fine, disrupted, and loosely constructed. Although multiple previous reports have established that fibroblasts from patients with Costello syndrome display disordered elastogenesis, abnormal elastin architecture has not been previously identified in the pulmonary vasculature. We suspect that the pulmonary vascular dysplasia contributed to the initial echocardiographic findings of pulmonary hypertension. Furthermore, distinctive semi-obstructivearterial branch point lesions support the clinical impression of pulmonary hypertension. Cardiopulmonary reserve may have been further reduced by hypoxia due to obstructive apnea.
Primary disorders of elastogenesis, such as the genetically heterogeneous cutis laxa, provide insight into the complex process of elastin biogenesis. There are at least 10 distinct types of cutis laxa, including dominant, recessive, and X-linked forms, of which 9 have known genetic defects. The causative genes include those for elastin (ELN), fibulin 4 and 5 (FBLN4 and FBLN5) and latent TGFβ binding protein 4 (LTBP4). As a group, there is significant overlap with the Costello syndrome phenotype, including lax skin and joints, postnatal growth delay, developmental delay and intellectual disability, and hypotonia [15]. Arterial tortuosity has been reported as a feature of four cutis laxa types. Abnormal elastin in the extracellular matrix leads to increased TGF-β signaling in an attempt to upregulate the genes needed for production of elastic fibers [16]. Upregulation of TGF-β signaling is associated with wide-spread vasculopathy in Loeys-Dietz syndrome [17]. A child with an 11p duplication of including HRAS was reported and the HRAS duplication was speculated to cause the phenotype of severe cutis laxa and skin histology demonstrating almost complete absence of elastin in the papillary dermis[18]. We hypothesize that the disordered elastogenesis in Costello syndrome is the result of activating HRAS mutations, and may lead to upregulation of TGF-β signaling and resulting vasculopathy. This mechanism may be independent of or in addition to the increased deposition of glycosaminoglycan causing decreased EBP and resultant abnormal elastin deposition.
Three previous reports have noted disordered lung development in infants with neonatal lethal Costello syndrome. Specifically, Burkitt-Wright et al [2012] noted congenital alveolar dysplasia in a patient with p.G12V who died around 1 month of age. Lo et al [2008] noted pulmonary lymphangiectasia and possible alveolar-capillary dysplasia in an infant with p.G12D who died at age 3 months due to respiratory failure[4]. O’Shea [2008] reported persistent pulmonary hypertension in a newborn with Costello syndrome. In addition, severe lethal primary pulmonary hypertension is reported in Noonan syndrome, a related disorder [19]. Although our patient did not have evidence of pulmonary alveolar-capillary dysplasia, she had multifocal lymphangiectasia in pleura and septa. This could be secondary to lymphatic obstruction by tortuous, asymmetrically thickened and dilated pulmonary veins, rather than a primary abnormality. Disordered pulmonary vascular development in neonatal lethal Costello syndrome lacking evidence for severe cardiomyopathy, suggests a significant role for pulmonary vascular abnormalities in the early mortality. Table 1 provides a summary of reported histopathologic findings in neonatal lethal Costello syndrome
A small series suggested that airway obstruction is prevalent and may be of significant morbidity [20].The authors recommended that Costello patients should be routinely assessed with polysomnography (PSG). The PSG findings in the patient reported here of severe obstructive sleep apnea support this recommendation, especially since disordered breathing, coupled with upper airway malacia, were significant factors in her death.
This is the first reported patient with Costello syndrome with congenital cataracts. Ocular manifestations are associated with Noonan syndrome, with cataracts rarely reported [21]. Hashida et al [2013] reported local activation of MAPK signaling pathway in the lens of a patient with Noonan syndrome who had a mature cataract diagnosed in adulthood[22]. A child with Noonan syndrome (severe pulmonary stenosis) with a moderately dense asymmetric mixed nuclear and cortical cataract has been reported [21]. Taken together, these findings suggest that cataract is a rare manifestation of RAS/MAPK disorders.
Interestingly, the G12E somatic mutation is unrelated to cancer, whereas the G12V mutation is the most common somatic HRAS mutation associated with cancer (COSMIC database). The severe phenotype associated with heterozygous germline G12V mutations as well as the increased incidence in cancers is thought to be related to the strongly activating mutation. However, the presence of G12E germline mutation in two cases of lethal Costello syndrome and absence in human cancers suggests an alternative mechanism might be at play or that the young age of those affected provides insufficient time for neoplastic transformation.
In this patient, we demonstrated using allele specific amplification that both base changes are on the same paternally inherited allele and result in a pG12E mutation of the protein. The identification of the G12E mutation as paternally-derived is in keeping with previous reports that de novo Costello syndrome is more often caused by paternally-derived mutations [23, 24]. It is thought that activating HRAS mutations in sperm are enriched through a process termed selfish spermatogonial selection and that this explains the preponderance of paternally derived mutations [25]. This patient’s pG12E mutation is likely caused by simultaneous substitution of the dinucleotide at c.35–36GC>AA, in the paternal germline. Such tandem base substitutions are overrepresented in the germline compared to blood or malignancies, and their occurrence in sperm correlates strongly with the donor’s age [25]. While this particular tandem substitution was seen in only one patient previously [2], based on its occurrence in sperm it may be more common in neonates than is currently appreciated.
Conclusion
In summary, we report a patient with neonatal lethal Costello syndrome caused by a rare HRAS tandem base substitution (c.35–36GC>AA), which results in a p.G12E change. This case is unique due to the minimal evidence for cardiomyopathy and the novel finding of generalized pulmonary vascular dysplasia with stigmata of pulmonary hypertensive vascular disease. Also, this is the first reported Costello syndrome patient with congenital cataracts. This case supports that amino acid substitutions other than serine at the 12th position are associated with a particularly severe phenotype, but challenges the notion that severe hypertrophic cardiomyopathy is the sole major contributor to morbidity. The identification of a third case of Costello syndrome with disordered lung and respiratory tract function supports the inclusion of this finding as part of the phenotype. Finally, the finding of congenital cataracts in conjunction with previous reports of cataracts in Noonan syndrome supports the role of RAS/MAPK in cataract pathophysiology.
Figure 4.
The walls of these pulmonary arteries are asymmetric with foci of both thick and thin media. Note mild stenosis at the arterial branch point (arrow). A. Pentachrome stain, ×40; B, Pentachrome stain, ×100.
Figure 5.
The elastic fibers in a medium sized pulmonary vein are disorganized with highly variable deposition of elastic. VVG elastic stain, × 400.
Acknowledgements
We thank the Center for Pediatric Research COBRE-funded Biomolecular Core for sequencing support. This work was supported in part by the Nemours Foundation, and by the NIH-NIGMS grants P20GM103464 and grant P20GM103446.
This work was supported in part by the Nemours Foundation, and by the NIH-NIGMS grants P20GM103464 and grant P20GM103446.
References
- 1.Aoki Y, Niihori T, Kawame H, et al. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat Genet. 2005;37:1038–1040. doi: 10.1038/ng1641. [DOI] [PubMed] [Google Scholar]
- 2.Kerr B, Delrue MA, Sigaudy S, et al. Genotype-phenotype correlation in Costello syndrome: HRAS mutation analysis in 43 cases. J Med Genet. 2006;43:401–405. doi: 10.1136/jmg.2005.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gripp KW, Lin AE. Costello syndrome: a Ras/mitogen activated protein kinase pathway syndrome (rasopathy) resulting from HRAS germline mutations. Genet Med. 2012;14:285–292. doi: 10.1038/gim.0b013e31822dd91f. [DOI] [PubMed] [Google Scholar]
- 4.Lo IF, Brewer C, Shannon N, et al. Severe neonatal manifestations of Costello syndrome. J Med Genet. 2008;45:167–171. doi: 10.1136/jmg.2007.054411. [DOI] [PubMed] [Google Scholar]
- 5.Lorenz S, Petersen C, Kordass U, Seidel H, Zenker M, Kutsche K. Two cases with severe lethal course of Costello syndrome associated with HRAS p.G12C and p.G12D. European journal of medical genetics. 2012;55:615–619. doi: 10.1016/j.ejmg.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 6.O'Shea J, Lynch SA, Macken S. A case of persistent pulmonary hypertension in a newborn with Costello syndrome. Clinical dysmorphology. 2008;17:287–288. doi: 10.1097/MCD.0b013e3283079e68. [DOI] [PubMed] [Google Scholar]
- 7.Hinek A, Smith AC, Cutiongco EM, Callahan JW, Gripp KW, Weksberg R. Decreased elastin deposition and high proliferation of fibroblasts from Costello syndrome are related to functional deficiency in the 67-kD elastin-binding protein. Am J Hum Genet. 2000;66:859–872. doi: 10.1086/302829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sammon MR, Doyle D, Hopkins E, et al. Normative growth charts for individuals with Costello syndrome. Am J Med Genet A. 2012;158A:2692–2699. doi: 10.1002/ajmg.a.35534. [DOI] [PubMed] [Google Scholar]
- 9.Schulz DM, Giordano DA, Schulz DH. Weights of organs of fetuses and infants. Archives of pathology. 1962;74:244–250. [PubMed] [Google Scholar]
- 10.Wagenvoort CA. Lung biopsy specimens in the evaluation of pulmonary vascular disease. Chest. 1980;77:614–625. doi: 10.1378/chest.77.5.614. [DOI] [PubMed] [Google Scholar]
- 11.Rabinovitch M, Haworth SG, Vance Z, et al. Early pulmonary vascular changes in congenital heart disease studied in biopsy tissue. Human pathology. 1980;11:499–509. [PubMed] [Google Scholar]
- 12.Burkitt-Wright EM, Bradley L, Shorto J, et al. Neonatal lethal Costello syndrome and unusual dinucleotide deletion/insertion mutations in HRAS predicting p.Gly12Val. Am J Med Genet A. 2012;158A:1102–1110. doi: 10.1002/ajmg.a.35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mori M, Yamagata T, Mori Y, et al. Elastic fiber degeneration in Costello syndrome. American journal of medical genetics. 1996;61:304–309. doi: 10.1002/(SICI)1096-8628(19960202)61:4<304::AID-AJMG2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 14.Vila Torres J, Pineda Marfa M, Gonzalez Ensenat MA, Lloreta Trull J. Pathology of the elastic tissue of the skin in Costello syndrome. An image analysis study using mathematical morphology. Analytical and quantitative cytology and histology / the International Academy of Cytology [and] American Society of Cytology. 1994;16:421–429. [PubMed] [Google Scholar]
- 15.Berk DR, Bentley DD, Bayliss SJ, Lind A, Urban Z. Cutis laxa: a review. Journal of the American Academy of Dermatology. 2012;66:842 e1–842 e17. doi: 10.1016/j.jaad.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Urban Z, Davis EC. Cutis laxa: Intersection of elastic fiber biogenesis, TGFbeta signaling, the secretory pathway and metabolism. Matrix biology : journal of the International Society for Matrix Biology. 2013 doi: 10.1016/j.matbio.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallo EM, Loch DC, Habashi JP, et al. Angiotensin II-dependent TGF-beta signaling contributes to Loeys-Dietz syndrome vascular pathogenesis. J Clin Invest. 2014;124:448–460. doi: 10.1172/JCI69666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardeitchik T, de Leeuw N, Nijtmans L, et al. Infant with MCA and severe cutis laxa due to a de novo duplication 11p of paternal origin. Am J Med Genet A. 2012;158A:469–472. doi: 10.1002/ajmg.a.34410. [DOI] [PubMed] [Google Scholar]
- 19.Tinker A, Uren N, Schofield J. Severe pulmonary hypertension in Ullrich-Noonan syndrome. British heart journal. 1989;62:74–77. doi: 10.1136/hrt.62.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Della Marca G, Vasta I, Scarano E, et al. Obstructive sleep apnea in Costello syndrome. Am J Med Genet A. 2006;140:257–262. doi: 10.1002/ajmg.a.31076. [DOI] [PubMed] [Google Scholar]
- 21.Lee NB, Kelly L, Sharland M. Ocular manifestations of Noonan syndrome. Eye (Lond) 1992;6(Pt 3):328–334. doi: 10.1038/eye.1992.66. [DOI] [PubMed] [Google Scholar]
- 22.Hashida N, Ping X, Nishida K. MAPK activation in mature cataract associated with Noonan syndrome. BMC ophthalmology. 2013;13:70. doi: 10.1186/1471-2415-13-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sol-Church K, Stabley DL, Nicholson L, Gonzalez IL, Gripp KW. Paternal bias in parental origin of HRAS mutations in Costello syndrome. Hum Mutat. 2006;27:736–741. doi: 10.1002/humu.20381. [DOI] [PubMed] [Google Scholar]
- 24.Zampino G, Pantaleoni F, Carta C, et al. Diversity, parental germline origin, and phenotypic spectrum of de novo HRAS missense changes in Costello syndrome. Hum Mutat. 2007;28:265–272. doi: 10.1002/humu.20431. [DOI] [PubMed] [Google Scholar]
- 25.Giannoulatou E, McVean G, Taylor IB, et al. Contributions of intrinsic mutation rate and selfish selection to levels of de novo HRAS mutations in the paternal germline. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:20152–20157. doi: 10.1073/pnas.1311381110. [DOI] [PMC free article] [PubMed] [Google Scholar]