Abstract
Non-melanoma skin cancer, derived from epidermal keratinocytes, is the most common malignancy in organ transplant recipients, causes serious morbidity and mortality, and is strongly associated with solar ultraviolet (UV) exposure. Preventing and treating skin cancer in these individuals has been extraordinarily challenging. Following organ transplantation, the immunosuppressants are used to prevent graft rejection. Until now, immunosuppression has been assumed to be the major factor leading to skin cancer in this setting. However, the mechanism of skin carcinogenesis in organ transplant recipients has not been understood to date; specifically, it remains unknown whether these cancers are immunosuppression-dependent or -independent. In particular, it remains poorly understood what is the mechanistic carcinogenic action of the newer generation of immunosuppressants including tacrolimus and mycophenolate mofetil (MMF). Here we show that tacrolimus and MMF impairs UVB-induced DNA damage repair and apoptosis in human epidermal keratinocytes. In addition, tacrolimus inhibits UVB-induced checkpoint signaling. However, MMF had no effect. Our findings have demonstrated that tacrolimus and MMF compromises proper UVB response in keratinocytes, suggesting an immunosuppression-independent mechanism in the tumor-promoting action of these immunosuppressants.
INTRODUCTION
Skin cancer is the most common cancer in the United States. Its major environmental risk factor is UVB radiation in sunlight, which causes DNA damage (1). Incomplete DNA repair, impaired checkpoint function, and deregulated cell survival and growth can lead to tumor initiation and promotion, which ultimately will accelerate the development of skin cancer (1, 2).
Organ transplant recipients are a population at particularly high risk for cancer, and skin cancers are their most common malignant conditions. Among skin cancers, squamous cell carcinoma (SCC) is the most common type among transplant recipients, occurring 65–250 times as frequently as in the general population (3). Many organ transplant recipients are given the immunosuppressants to suppress their immune system. However, a lifetime course of such treatment causes a dramatic increase in risk of skin cancer as a major adverse effect (3–5). The first immunosuppressant is cyclosporin A, a transforming medicine that substantially increases graft survival and lifespan of OTRs.
Due to the nephrotoxicity, newer immunosuppressants have been developed to replace cyclosporin A. These include tacrolimus (FK506, FK) and mycophenolate mofetil (MMF). Similar to cyclosporin A, Tacrolimus is an inhibitor for calcineurin, a serine/threonine phosphatase dephosphorylating family of transcription factor NFATs, which mediate an immune response (6). Tacrolimus is the current mainstream immunosuppressant widely used for preventing or treating graft rejection in organ transplantation patients, and for treating autoimmune diseases including myasthenia gravis, arthritis, and atopic dermatitis (7). Mycophenolate mofetil (MMF) has been used widely as a part of various combination regimens of immunosuppressive agents. It is an ester prodrug of the active immunosuppressant mycophenolic acid (MPA). MMF and/or MPA inhibit the proliferation of lymphocytes and the production of antibodies induced by a variety of mitogens and antigens (8). Large clinical trials have shown that MMF is effective in preventing acute rejection and improving graft and patient survival in combination with calcineurin inhibitors (cyclosporin A and tacrolimus) (9).
UVB radiation in sunlight is the major human skin carcinogen and is a profound factor in increased skin cancer risk in OTRs (5). The main mechanism in UVB-induced skin cancer is causing DNA damage. The predominant UVB-induced DNA photoproducts caused are cyclobutane pyrimidine dimers (CPD) and pyrimidine(6-4)pyrimidone dimers (6-4PP) (10, 11). In response to DNA damage, the cells activate a specific DNA repair mechanism, i.e., global genome nucleotide excision repair (GG-NER), which involves well-coordinated action of multiple proteins (12–15). In addition, the damaged cells activate the DNA damage response (DDR) signal-transduction pathway to coordinate cell-cycle transitions, DNA replication, DNA repair, and apoptosis. The checkpoint activation involves the phosphorylation of the Ser/Thr kinase checkpoint kinase-1 (Chk1) (16, 17) and 2 (Chk2) (18–21). Defects in the Chk1 or Chk2 pathways are known to increase cancer risk (22–27).
We have recently shown that, independent of immunosuppression, cyclosporin A suppresses the expression of the tumor suppressor PTEN and UVB-induced DNA damage repair and checkpoint activation in human keratinocytes, and promotes UVB-dependent skin tumorigenesis (28, 29). However, it remains unknown about the immunosuppression-independent action of tacrolimus and MMF on human epidermal keratinocytes. In this study we seek to determine the influence of tacrolimus and MMF on human epidermal keratinocytes on UVB-induced molecular and cellular responses critical for suppressing tumorigenesis.
MATERIALS AND METHODS
Cell culture and UVB treatment
HaCaT cells (kindly provided by Dr. Fusenig) were cultured in 60 mm dishes. For UVB treatment, the Stratalinker 2400 equipped with 312-nm UVB bulbs (Stratagene, La Jolla, CA) (UVC 0%, UVB 51%, and UVA 49%) was used. The UV exposure was performed in PBS after washing the cells with PBS twice to avoid the photosensitization effect of components in culture medium on the cells. In all experiments, cells were preincubated with vehicle (Veh), Tacrolimus (FK506, FK, 0.1, 1 or 10 μg/ml, or MMF (0.1 μg/ml) for 1 week prior to UVB irradiation. These concentrations were selected based on the literature (30, 31) and our data on the toxicity of FK506 or MMF in human keratinocytes (data not shown). Cells were collected at predetermined time points for analysis of DNA repair, apoptosis, and checkpoint activation based on our recent work (32–34).
Western blotting
Protein concentrations were determined using the BCA assay (Pierce, Rockford, IL, USA). Equal amounts of protein were subjected to electrophoresis. Western blotting was performed as described previously (35, 36). Antibodies used included Chk1, Chk2, PARP, GAPDH (Santa Cruz), p-Chk1, and p-Chk2 (Cell Signaling Technology).
Determination of UVB-induced CPD/6-4PP level in genomic DNA by slot blot assay
Slot blot assays of CPD/6-4PP were performed as described previously (37). Briefly, cells were collected at different time points post-UVB, and DNA was isolated using a QIAamp DNA Mini Kit (Qiagen, Valencia, CA, USA). The DNA concentration was calculated from the absorbance at 260 nm using NanoDrop 1000 (NanoDrop products, Wilmington, DE, USA). The CPD in DNA were quantified by slot-blot (Bio-Rad, Hercules, CA, USA) with a monoantibody (TDM-2 for CPD and 6-4PP, COSMO BIO Co., Tokyo, Japan) as described previously (38). The chemiluminescence was detected with a Carestream Imaging Station (Carestream, Rochester, NY, USA). For examining repair kinetics, the percentage of repair was calculated by comparing the optical density at the indicated time with that of the corresponding absorbance at time zero, when there was no opportunity for repair, and 100% of CPDs were present post-UVB.
Sub-G1 flow cytometric analysis
Sub-G1 flow cytometric analysis was performed to determine apoptosis as described previously (39). Briefly, cells were fixed and stained with propidium iodide, and the percentage of cells at the sub-G1 phase was quantified by flow cytometry.
Statistical analyses
Statistical analyses were performed using Prism 5 (GraphPad software, San Diego, CA). Data were expressed as the mean of three independent experiments and analyzed by Student’s t-test. A P value of less than 0.05 was considered statistically significant.
Results
FK506 or MMF inhibit UVB-induced DNA damage repair in HaCaT cells
To determine the effect of FK506 and MMF on UVB-induced DNA damage repair, we measured the percentage of CPDs and 64-PPs remaining at different time points post-UVB, and thereby determined the percentage of DNA repair, in vehicle, FK, or MMF-pretreated HaCaT cells for 1 week. FK506 significantly inhibited repair of CPDs (Fig. 1A–D) and this inhibitory effect was dose-dependent, but it had no effect on repair of 64-PPs (Fig. 1A–D). Similarly, MMF significantly suppressed repair of CPDs (Fig. 1E–F) but not 64-PPs (Fig. 1E–F). Our findings demonstrated that in human epidermal non-tumorigenic HaCaT cells FK506 and MMF inhibit UVB-induced DNA repair of CPD, but not 64-PP at the time points examined.
Figure 1. Chronic treatment with FK506 and MMF in HacaT cells inhibits global genome nucleotide excision repair.
(A) Slot-blot analysis of percent repair of CPD and 64-PP at different time points post-UVB (20 mJ/cm2) in HaCaT cells treated with vehicle (Veh) or FK506 (0.1 μg/ml or 1μg/ml) for 1 week. (B) Quantification of percentage (%) of CPD and 64-PP repair in A. (C) Slot-blot analysis of percent repair of CPD and 64-PP at different time points post-UVB (20 mJ/cm2) in HaCaT cells treated with vehicle or FK506 (10 μg/ml) for 1 week. (D) Quantification of repair percentage (%) of CPD and 64-PP repair in C. (E) Slot-blot analysis of percent of CPD and 64-PP repair at different time points post-UVB (20 mJ/cm2) in HaCaT cells treated with vehicle (Veh) or MMF (0.1 μg/ml) for 1 week. (F) Quantification of percentage (%) of CPD and 64-PP repair in E.
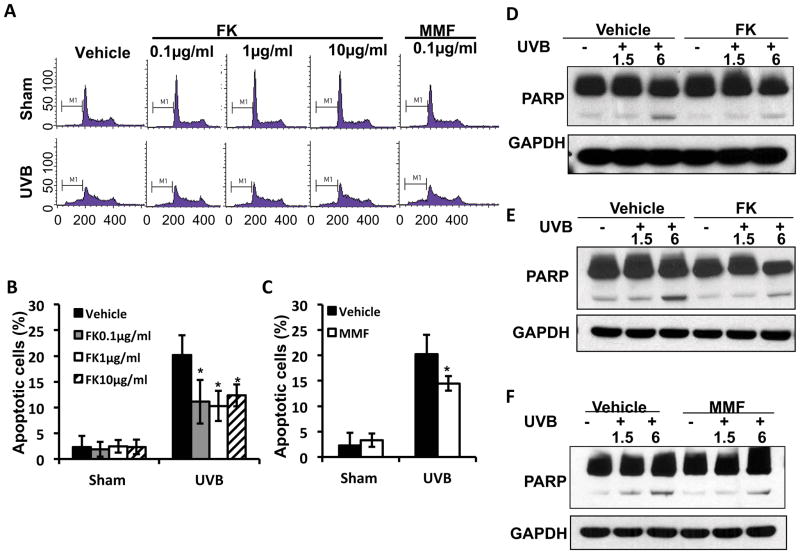
FK506 or MMF suppresses UVB-induced apoptosis in HaCaT cells
It has been reported that FK506 inhibited proliferation and induced apoptosis of fibroblasts in vitro (40). Here we explored the effect of FK506 and MMF in HaCaT cells on apoptosis by flow cytometry. FK506 or MMF suppressed UVB-induced apoptosis dose-dependently (Fig. 2A–C). Cleaved PARP, a hallmark for apoptosis, was reduced by FK506 or MMF post-UVB irradiation (Fig. 2D–F). These findings suggested that chronic FK506 or MMF suppresses UVB-induced apoptosis.
Figure 2. FK506 and MMF suppresses UVB-induced apoptosis in HaCaT cells.
(A) Flow cytometric analysis of UVB (30mJ/cm2)-induced apoptosis in HaCaT cells treated with vehicle, FK506 (0.1, 1 or 10 μg/ml) or MMF (0.1 μg/ml) for 1 week. (B–C) Quantitation of apoptotic cells (%) in A for FK506 treatment (B) and MMF treatment (C). *, P<0.05, t-test, significant differences between FK506 (0.1, 1 or 10 μg/ml), MMF (0.1 μg/ml) and vehicle control. (D–F) Immunoblot analysis of PARP and GAPDH in HaCaT cells at 1.5 or 6 h post-UVB (20mJ/cm2) after treatment with vehicle, FK (1 μg/ml) (D), FK (10 μg/ml) (E), or MMF (0.1 μg/ml) (F) for 1 week.
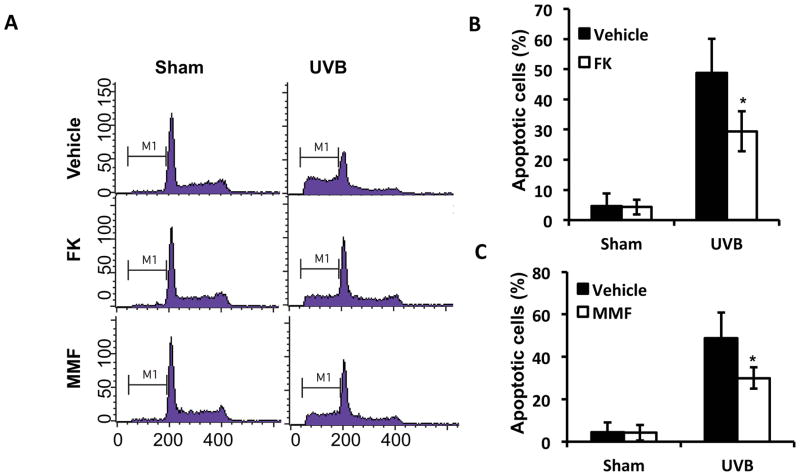
FK506 or MMF inhibits UVB-induced apoptosis in A431 cells
In addition to non-tumorigenic HaCaT cells, we determined the effect of FK506 or MMF on UVB-induced apoptosis in human A431 skin cancer cells by flow cytometric analysis. Both FK (1 μg/ml) and MMF (0.1 μg/ml) significantly reduced UVB-induced apoptosis, as compared with vehicle control (Fig. 3A–C). These data further indicate that either FK506 or MMF suppressed apoptosis in both non-tumorigenic keratinocytes and skin cancer cells.
Figure 3. FK506 and MMF inhibits UVB-induced apoptosis in A431 cells.
(A) Flow cytometric analysis of UVB (30 mJ/cm2)-induced apoptosis in A431 cells pretreated with vehicle, FK506 (1 μg/ml), or MMF (0.1 μg/ml) for 1 week. (B–C) Quantitation of apoptotic cells (%) in A for FK506 (1 μg/ml) (B) and MMF treatment (0.1 μg/ml) (C). *, P<0.05, t-test, significant differences between FK506 (1 μg/ml), MMF (0.1 μg/ml) and Vehicle control.
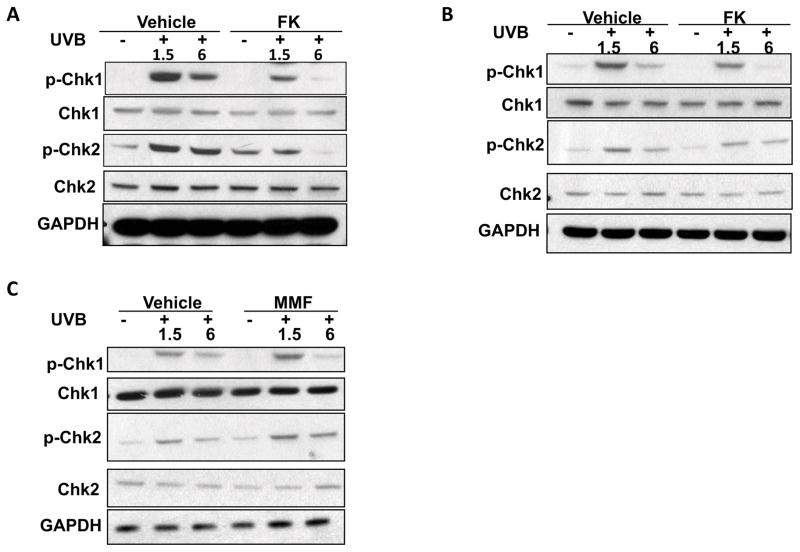
FK506 but not MMF inhibits UVB-induced checkpoint signaling in HaCaT cells
To determine the effect of FK506 or MMF on UVB-induced checkpoint signaling, we assessed the phosphorylation of Chk1 and Chk2 in vehicle, FK506 (1 μg/ml, 10μg/ml), or MMF (0.1μg/ml)-treated HacaT cells. UVB irradiation (20 mJ/cm2) increased Chk1 phosphorylation at serine 345 (p-Chk1) and Chk2 phosphorylation at threonine 68 (p-Chk2) at 1.5 and 6 hours (Fig. 4A–C). However, in HaCaT cells at 1.5 and 6 hours post-UVB irradiation (20mJ/cm2), FK506 significantly reduced UVB-induced phosphorylation of both Chk1 and Chk2 (Fig. 4A–B), whereas MMF had no effect (Fig. 4C). These data indicated that, following UVB irradiation, FK506 inhibited UVB-induced checkpoint signaling.
Figure 4. FK506 but not MMF inhibits UVB-induced checkpoint signaling in HaCaT cells.
(A–C) Immunoblot analysis of p-Chk1, Chk1, p-Chk2, Chk2, and GAPDH in HaCaT cells at 1.5 and 6 h post-UVB (20 mJ/cm2) prior to treatment with vehicle, FK506 (1 μg/ml) (A), FK506 (10 μg/ml) (B), or MMF (0.1μg/ml) (C) for 1 week.
DISCUSSION
FK506 and MMF have been widely used to prevent graft rejection in organ transplantation. It is generally believed that the use of drugs that suppress cellular immune function is associated with an increased risk of cancer development. Here we have shown in epidermal keratinocytes in vitro, we found that FK506 and MMF impaired UVB-induced DNA damage repair and apoptosis. FK506 but not MMF reduced UVB-induced checkpoint signaling. These findings suggest that FK506 and MMF promote tumor initiation.
Recent evidence in mouse models have shown that topical FK506 on the skin with a mouse model revealed a marked tumor-promoting effect of topical FK506, associated with evidence that a significant decrease in CD4/CD8 ratio (41). In contrast in another report, it has been shown that topical application of FK506 inhibited TPA-induced tumor promotion, epidermal ornithine decarboxylase induction and skin inflammation caused by TPA in a dose-related manner (42). It has been reported that immunosuppressant FK506 acts as a growth factor for VSMCs (43).
Here we have shown that in human epidermal HaCaT keratinocytes, FK506 and MMF inhibited UVB-induced CPD repair apoptosis, which supports their oncogenic effect in tumor initiation by increasing survival of unrepaired cells. Our findings suggest a cocarcinogenic effect of FK506 and MMF on skin carcinogenesis (41), which may be different from those studies by Jiang and colleagues (42), who reported that topical FK506 suppressed tumorigenesis in CD-1 mice treated with DMBA and TPA. FK506 was reported to up-regulate C/EBP family members, especially C/EBPbeta and CHOP, and overexpression of either C/EBPbeta or CHOP significantly attenuated TNF-alpha-triggered NF-kappaB activation (30). Recent studies have shown that JNK and ERK are involved in FK506-induced fibroblast apoptosis (40). However, the role of these signaling pathways in the carcinogenic effect of FK506 or MMF requires future investigation.
Inhibition of UVB-induced DNA repair by FK506 and MMF may be mediated through altering the key factors in this DNA repair machinery, including xeroderma pigmentosum proteins (12–15). It is possible that FK506 or MMF inhibits the expression or the activity of these factors (16, 17). Inhibition of checkpoint signaling by FK506 may be resulted from the impact of FK506 on the upstream pathways in the checkpoint activation such as the kinases ATR or ATM (18–21). Future investigation will elucidate the molecular mechanism for the influence of FK506 and MMF in keratinocytes.
In summary, our findings have demonstrated that tacrolimus and MMF inhibits UVB-induced DNA damage repair and apoptosis. Furthermore, tacrolimus reduces UVB-induced checkpoint signaling, whereas MMF has no effect. Our studies suggest an immunosuppression-independent carcinogenic role of the two newer immunosuppressants in skin cancer and may provide valuable knowledge for future investigation of the tumorigenecity of tacrolimus and MMF in animal models and human patients.
Acknowledgments
This work was supported by the NIH/NIEHS grant ES016936 (YYH), the American Cancer Society (ACS) grant RSG-13-078-01 (YYH), the University of Chicago Cancer Research Center (P30 CA014599), the CTSA (NIH UL1 TR000430), and the University of Chicago Friends of Dermatology Endowment Fund.
Footnotes
This paper is part of the Special Issue commemorating the 65th birthday of Dr. Craig A. Elmets
References
- 1.Bowden GT. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signalling. Nat Rev Cancer. 2004;4:23–35. doi: 10.1038/nrc1253. [DOI] [PubMed] [Google Scholar]
- 2.Kraemer KH. Sunlight and skin cancer: another link revealed. Proc Natl Acad Sci U S A. 1997;94:11–14. doi: 10.1073/pnas.94.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jensen P, Hansen S, Moller B, Leivestad T, Pfeffer P, Geiran O, Fauchald P, Simonsen S. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol. 1999;40:177–186. doi: 10.1016/s0190-9622(99)70185-4. [DOI] [PubMed] [Google Scholar]
- 4.Berg D, Otley CC. Skin cancer in organ transplant recipients: Epidemiology, pathogenesis, and management. J Am Acad Dermatol. 2002;47:1–17. doi: 10.1067/mjd.2002.125579. quiz 18–20. [DOI] [PubMed] [Google Scholar]
- 5.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348:1681–1691. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 6.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 7.Iwasaki K. Metabolism of tacrolimus (FK506) and recent topics in clinical pharmacokinetics. Drug Metab Pharmacokinet. 2007;22:328–335. doi: 10.2133/dmpk.22.328. [DOI] [PubMed] [Google Scholar]
- 8.Fulton B, Markham A. Mycophenolate mofetil. A review of its pharmacodynamic and pharmacokinetic properties and clinical efficacy in renal transplantation. Drugs. 1996;51:278–298. doi: 10.2165/00003495-199651020-00007. [DOI] [PubMed] [Google Scholar]
- 9.Jeong H, Kaplan B. Therapeutic monitoring of mycophenolate mofetil. Clin J Am Soc Nephrol. 2007;2:184–191. doi: 10.2215/CJN.02860806. [DOI] [PubMed] [Google Scholar]
- 10.Niggli HJ, Rothlisberger R. Cyclobutane-type pyrimidine photodimer formation and induction of ornithine decarboxylase in human skin fibroblasts after UV irradiation. J Invest Dermatol. 1988;91:579–584. doi: 10.1111/1523-1747.ep12477095. [DOI] [PubMed] [Google Scholar]
- 11.Vink AA, Berg RJ, de Gruijl FR, Roza L, Baan RA. Induction, repair and accumulation of thymine dimers in the skin of UV-B-irradiated hairless mice. Carcinogenesis. 1991;12:861–864. doi: 10.1093/carcin/12.5.861. [DOI] [PubMed] [Google Scholar]
- 12.Kraemer KH, Lee MM, Scotto J. DNA repair protects against cutaneous and internal neoplasia: evidence from xeroderma pigmentosum. Carcinogenesis. 1984;5:511–514. doi: 10.1093/carcin/5.4.511. [DOI] [PubMed] [Google Scholar]
- 13.Kraemer KH, Lee MM, Andrews AD, Lambert WC. The role of sunlight and DNA repair in melanoma and nonmelanoma skin cancer. The xeroderma pigmentosum paradigm. Arch Dermatol. 1994;130:1018–1021. [PubMed] [Google Scholar]
- 14.Sugasawa K, Ng JM, Masutani C, Iwai S, van der Spek PJ, Eker AP, Hanaoka F, Bootsma D, Hoeijmakers JH. Xeroderma pigmentosum group C protein complex is the initiator of global genome nucleotide excision repair. Mol Cell. 1998;2:223–232. doi: 10.1016/s1097-2765(00)80132-x. [DOI] [PubMed] [Google Scholar]
- 15.Sugasawa K. UV-induced ubiquitylation of XPC complex, the UV-DDB-ubiquitin ligase complex, and DNA repair. J Mol Histol. 2006;37:189–202. doi: 10.1007/s10735-006-9044-7. [DOI] [PubMed] [Google Scholar]
- 16.Brown EJ, Baltimore D. Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev. 2003;17:615–628. doi: 10.1101/gad.1067403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, Donehower LA, Elledge SJ. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- 19.Chaturvedi P, Eng WK, Zhu Y, Mattern MR, Mishra R, Hurle MR, Zhang X, Annan RS, Lu Q, Faucette LF, Scott GF, Li X, Carr SA, Johnson RK, Winkler JD, Zhou BB. Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene. 1999;18:4047–4054. doi: 10.1038/sj.onc.1202925. [DOI] [PubMed] [Google Scholar]
- 20.Matsuoka S, Rotman G, Ogawa A, Shiloh Y, Tamai K, Elledge SJ. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc Natl Acad Sci U S A. 2000;97:10389–10394. doi: 10.1073/pnas.190030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahn JY, Schwarz JK, Piwnica-Worms H, Canman CE. Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res. 2000;60:5934–5936. [PubMed] [Google Scholar]
- 22.Brown EJ, Baltimore D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 2000;14:397–402. [PMC free article] [PubMed] [Google Scholar]
- 23.Menoyo A, Alazzouzi H, Espin E, Armengol M, Yamamoto H, Schwartz S., Jr Somatic mutations in the DNA damage-response genes ATR and CHK1 in sporadic stomach tumors with microsatellite instability. Cancer Res. 2001;61:7727–7730. [PubMed] [Google Scholar]
- 24.Swift M, Reitnauer PJ, Morrell D, Chase CL. Breast and other cancers in families with ataxia-telangiectasia. N Engl J Med. 1987;316:1289–1294. doi: 10.1056/NEJM198705213162101. [DOI] [PubMed] [Google Scholar]
- 25.Renwick A, Thompson D, Seal S, Kelly P, Chagtai T, Ahmed M, North B, Jayatilake H, Barfoot R, Spanova K, McGuffog L, Evans DG, Eccles D, Easton DF, Stratton MR, Rahman N. ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat Genet. 2006;38:873–875. doi: 10.1038/ng1837. [DOI] [PubMed] [Google Scholar]
- 26.Hannan MA, Hellani A, Al-Khodairy FM, Kunhi M, Siddiqui Y, Al-Yussef N, Pangue-Cruz N, Siewertsen M, Al-Ahdal MN, Aboussekhra A. Deficiency in the repair of UV-induced DNA damage in human skin fibroblasts compromised for the ATM gene. Carcinogenesis. 2002;23:1617–1624. doi: 10.1093/carcin/23.10.1617. [DOI] [PubMed] [Google Scholar]
- 27.Hirao A, Cheung A, Duncan G, Girard PM, Elia AJ, Wakeham A, Okada H, Sarkissian T, Wong JA, Sakai T, De Stanchina E, Bristow RG, Suda T, Lowe SW, Jeggo PA, Elledge SJ, Mak TW. Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol Cell Biol. 2002;22:6521–6532. doi: 10.1128/MCB.22.18.6521-6532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han W, Ming M, He TC, He YY. Immunosuppressive cyclosporin A activates AKT in keratinocytes through PTEN suppression: implications in skin carcinogenesis. J Biol Chem. 2010;285:11369–11377. doi: 10.1074/jbc.M109.028142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han W, Soltani K, Ming M, He YY. Deregulation of XPC and CypA by cyclosporin A: an immunosuppression-independent mechanism of skin carcinogenesis. Cancer Prev Res (Phila) 2012;5:1155–1162. doi: 10.1158/1940-6207.CAPR-12-0185-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du S, Hiramatsu N, Hayakawa K, Kasai A, Okamura M, Huang T, Yao J, Takeda M, Araki I, Sawada N, Paton AW, Paton JC, Kitamura M. Suppression of NF-kappaB by cyclosporin a and tacrolimus (FK506) via induction of the C/EBP family: implication for unfolded protein response. J Immunol. 2009;182:7201–7211. doi: 10.4049/jimmunol.0801772. [DOI] [PubMed] [Google Scholar]
- 31.Voisard R, Viola S, Kaspar V, Weber CM, von Muller L, Baur R, Gastrock-Balitsch I, Hombach V. Effects of mycophenolate mofetil on key pattern of coronary restenosis: a cascade of in vitro and ex vivo models. BMC Cardiovasc Disord. 2005;5:9. doi: 10.1186/1471-2261-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ming M, Shea CR, Guo X, Li X, Soltani K, Han W, He YY. Regulation of global genome nucleotide excision repair by SIRT1 through xeroderma pigmentosum C. Proc Natl Acad Sci U S A. 2010;107:22623–22628. doi: 10.1073/pnas.1010377108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ming M, Feng L, Shea CR, Soltani K, Zhao B, Han W, Smart RC, Trempus CS, He YY. PTEN positively regulates UVB-induced DNA damage repair. Cancer Res. 2011;71:5287–5295. doi: 10.1158/0008-5472.CAN-10-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han W, Ming M, He YY. Caffeine promotes ultraviolet B-induced apoptosis in human keratinocytes without complete DNA repair. J Biol Chem. 2011;286:22825–22832. doi: 10.1074/jbc.M111.222349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He YY, Pi J, Huang JL, Diwan BA, Waalkes MP, Chignell CF. Chronic UVA irradiation of human HaCaT keratinocytes induces malignant transformation associated with acquired apoptotic resistance. Oncogene. 2006;25:3680–3688. doi: 10.1038/sj.onc.1209384. [DOI] [PubMed] [Google Scholar]
- 36.Ming M, Han W, Maddox J, Soltani K, Shea CR, Freeman DM, He YY. UVB-induced ERK/AKT-dependent PTEN suppression promotes survival of epidermal keratinocytes. Oncogene. 29:492–502. doi: 10.1038/onc.2009.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han W, Ming M, Zhao R, Pi J, Wu C, He YY. Nrf1 CNC-bZIP protein promotes cell survival and nucleotide excision repair through maintaining glutathione homeostasis. J Biol Chem. 287:18788–18795. doi: 10.1074/jbc.M112.363614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maeda T, Chua PP, Chong MT, Sim AB, Nikaido O, Tron VA. Nucleotide excision repair genes are upregulated by low-dose artificial ultraviolet B: evidence of a photoprotective SOS response? J Invest Dermatol. 2001;117:1490–1497. doi: 10.1046/j.0022-202x.2001.01562.x. [DOI] [PubMed] [Google Scholar]
- 39.Ni YG, Wang N, Cao DJ, Sachan N, Morris DJ, Gerard RD, Kuro OM, Rothermel BA, Hill JA. FoxO transcription factors activate Akt and attenuate insulin signaling in heart by inhibiting protein phosphatases. Proc Natl Acad Sci U S A. 2007;104:20517–20522. doi: 10.1073/pnas.0610290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Que J, Cao Q, Sui T, Du S, Kong D, Cao X. Effect of FK506 in reducing scar formation by inducing fibroblast apoptosis after sciatic nerve injury in rats. Cell Death Dis. 4:e526. doi: 10.1038/cddis.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niwa Y, Terashima T, Sumi H. Topical application of the immunosuppressant tacrolimus accelerates carcinogenesis in mouse skin. Br J Dermatol. 2003;149:960–967. doi: 10.1111/j.1365-2133.2003.05735.x. [DOI] [PubMed] [Google Scholar]
- 42.Jiang H, Yamamoto S, Nishikawa K, Kato R. Anti-tumor-promoting action of FK506, a potent immunosuppressive agent. Carcinogenesis. 1993;14:67–71. doi: 10.1093/carcin/14.1.67. [DOI] [PubMed] [Google Scholar]
- 43.Giordano A, Romano S, Mallardo M, D’Angelillo A, Cali G, Corcione N, Ferraro P, Romano MF. FK506 can activate transforming growth factor-beta signalling in vascular smooth muscle cells and promote proliferation. Cardiovasc Res. 2008;79:519–526. doi: 10.1093/cvr/cvn079. [DOI] [PubMed] [Google Scholar]