Summary
Background
Sickle cell trait may increase risk of venous thromboembolism, but this is not fully established.
Objectives
We sought to determine the association of sickle cell trait with deep vein thrombosis and pulmonary embolism.
Methods
Middle-aged African Americans participating in a prospective, population-based cohort investigation, the Atherosclerosis Risk in Communities Study, were followed from 1987 through 2011 for incident hospitalized pulmonary embolism (n = 111) or isolated deep vein thrombosis (n = 138), verified by physician review of medical records. Sickle cell trait (heterozygosity for hemoglobin S, n = 268) was compared with no sickle cell trait (n = 3,748).
Results
Over a median of 22 years of follow-up, 249 participants had an incident venous thromboembolism. The hazard ratio of venous thromboembolism was 1.50 (95% CI 0.96, 2.36) for participants with versus without sickle cell trait, after adjustment for age, sex, ancestry, hormone replacement therapy (women), body mass index, diabetes, and estimated glomerular filtration rate. This hazard ratio was 2.05 (95% CI 1.12, 3.76) for pulmonary embolism and 1.15 (95% CI 0.58, 2.27) for deep vein thrombosis without pulmonary embolism.
Conclusions
Sickle cell trait in African Americans carries a 2-fold increased risk of pulmonary embolism, but does not elevate deep vein thrombosis risk. Because neonatal screening for sickle hemoglobin is being conducted in the United States currently, consideration should be paid to the increased pulmonary embolism risk of individuals with sickle cell trait.
Keywords: epidemiology, prospective study, risk factors, sickle cell trait, venous thromboembolism
Introduction
Sickle cell disease (homozygosity for hemoglobin S (HbSS)) increases the risk of venous thromboembolism (VTE), particularly pulmonary embolism (PE) [1-3], and is associated with coagulation abnormalities, such as enhanced thrombin generation [4]. The enhanced thrombin generation in sickle cell disease is thought to be a result of multiple component mechanisms involving all the cellular elements of the blood and vascular endothelial cells [5].
In contrast, sickle cell trait (heterozygosity for HbS (HbAS)) has not been widely recognized as a risk factor for VTE, despite evidence that chronic hemostatic abnormalities such as elevated D-dimer are present in affected individuals [6]. To date, only three large-scale studies have evaluated the risk of VTE related to sickle cell trait. A case-control study of African Americans reported a 1.8 (95% CI 1.2-2.9) fold increased risk of VTE and 3.9 (95% CI 2.2-6.9) fold increased risk for isolated PE in individuals with sickle cell trait compared with controls without sickle cell trait [7]. Two hospital-based record linkage studies reported an approximately 40% increased risk of PE for sickle cell trait [8, 9].
To expand existing evidence on this topic, we conducted a prospective study of sickle cell trait with incidence of venous thromboembolism among African American participants in the Atherosclerosis Risk in Communities (ARIC) Study cohort. In addition, we determined the association of HbAS with hemostatic factors measured in the ARIC cohort.
Materials and methods
Study population
The ARIC Study [10] and its methods for identification and classification of VTE have been described in detail elsewhere [11, 12]. In brief, 15,792 men and women aged 45 to 64 years enrolled in the ARIC study in 1987-1989, and had subsequent examinations in 1990-92, 1993-95, and 1996-98 and annual telephone contact. The 4,266 African-Americans were mostly enrolled from Jackson, MS (n = 3,728) and Forsyth County, NC (n = 483), but a few enrolled from suburban Minneapolis, MN (n = 22) and Washington County, MD (n = 33). The institutional review committees at each study center approved the methods, and staff obtained informed participant consent.
Measurement of sickle trait and VTE risk factors
At ARIC visits, staff drew and processed blood samples, and DNA was isolated. Carriers of HbS were identified from biallelic variation [missense change (Glu7Val) – labeled Glu6Val in older literature] in the single nucleotide polymorphism, rs334, and were grouped into HbSS, HbAS, and no HbS (wild type). In addition, carriers of HbC were identified from rs33930165 [missense change (Glu7Lys) – labeled Glu6Lys in older literature], and were grouped into HbCC, HbAC, and no HbC (wild type). Individuals who were compound heterozygous for both HbS and HbC (HbSC) were grouped separately. Genotyping was performed using functionally tested TaqMan® SNP Genotyping Assays in accordance with manufacturer protocols (Life Technologies, Grand Island, NY; www.lifetechnologies.com). The following custom primer and probe sequences were used to capture biallelic variation: rs334 (A/T) Forward-TCAAACAGACACCATGGTGCAT, Reverse-CCCCACAGGGCAGTAACG, VIC-CTGACTCCTGAGGAGAA-MGB, 6FAM-CTGACTCCTGTGGAGAA-MGB; and rs33930165 (A/G) Forward-AAACAGACACCATGGTGCATCT, Reverse-CCCCACAGGGCAGTAACG, VIC-CAGACTTCTCCTTAGGAGTC-MGB, 6FAM-ACTTCTCCTCAGGAGTC-MGB (designed on complement strand). PCR product in a 5.5 μL reaction volume was amplified utilizing 0.9 μM of each forward and reverse primer, 0.2 μM of each FAM and VIC sequence-specific probe, 3 ng DNA, and 1X TaqMan® Universal PCR Master Mix containing AmpliTaq Gold DNA Polymerase and no AmpErase UNG. After an initial step of 10 min at 95°C, the products were amplified using 50 cycles of 15 s at 92°C and 1 min at 60°C. Allele detection and genotype calling were performed using the ABI 7900HT and the Sequence Detection System software (Life Technologies, formerly Applied Biosystems). DNA sequencing had also been performed on 2,768 overlapping individuals using Illumina HiSeqs after exome capture with NimbleGen’s VChrome2.1. Genotypes were derived using Mercury [13], and results for HbS and HbC were compared with TaqMan® results. Genotyping of 43 individuals with discordant genotypes (HbS n = 36 and HbC n = 10; 3 individuals were discrepant at both sites) was repeated and 288 samples that had previously been genotyped were also included to establish clustering patterns. Discrepancies were adjudicated by review of quality control data and re-genotyping to yield the final HbS and HbC classifications.
Hemoglobin, hematocrit, white blood cell count and differential were measured by standard methods on fresh samples at local laboratories. Monocyte count was estimated from the white cell count times the monocyte proportion. Other blood assays were performed in central research laboratories. Diabetes was defined as a fasting blood glucose of 126 mg/dl or higher, non-fasting blood glucose of 200 mg/dl or higher, a reported physician diagnosis of diabetes, or reported use of antidiabetic medication in the past 2 weeks. Glomerular filtration rate (eGFR) was estimated from creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) algorithm [14]. Body mass index was calculated as weight (kg)/height (m)2. Activated partial thromboplastin time (aPTT), factor VIII, von Willebrand factor, factor VII, fibrinogen, protein C, and antithrombin III were measured as previously described [15, 16]. In order to control for population stratification in African Americans, we used exome chip data [13] to derive ten principal components of ancestry using EIGENSTRAT [17].
VTE occurrence
ARIC participants were contacted annually by phone and asked about all hospitalizations in the previous year. Hospital records with discharge diagnoses for possible VTE events were obtained from baseline through 2011. To validate the VTE events, two physicians reviewed the records using standardized criteria [11]. A diagnosis of deep vein thrombosis (DVT) or PE required positive imaging tests. We restricted DVTs for this analysis to those in the lower extremity or vena cava, because upper extremity DVTs were relatively few and almost always the result of venous catheters. Cases were classified by the reviewers as provoked (associated with cancer, major trauma, surgery, marked immobility) or unprovoked.
Statistical analysis
Of the 4,266 self-reported African American ARIC participants at baseline, we excluded those who declined DNA use for research and those for whom genotyping of either HbS or HbC was unsuccessful (n = 146), reported a VTE prior to baseline (n = 78), or were taking anticoagulants at baseline (n = 14). This left a maximum of 4,028 participants for the present analyses (n = 4,019 for HbS and n = 4,027 for HbC).
Analyses were done separately for sickle cell trait versus no sickle cell trait (ignoring HbC) and for HbAC versus no HbC (ignoring HbS). Thus, the reference group was analysis-specific. We compared means or prevalences of various characteristics for sickle cell trait (yes, no) or HbC (yes, no) using t-tests or chi-squared tests. Our main hypothesis was that sickle trait would be associated with increased VTE incidence. Time at risk was computed from the baseline to the earliest of the following: date of hospital discharge with incident VTE, date of death, date of last follow-up contact, or end of follow-up. Cox proportional hazards models were used to calculate hazard ratios (HR) and 95% confidence intervals of incident VTE. We verified the proportional hazards assumption of the Cox models by inspection of ln(-ln) survival curves for sickle trait (yes, no). We selected possible confounding variables for regression models based on previous prospective findings from the Longitudinal Investigation of Thromboembolism Etiology (LITE) [12, 15, 16]. Model 1 adjusted for age (continuous), sex, and ten principal components of ancestry; Model 2 for age, ancestry, sex/hormone replacement therapy (male, female on HRT, female not on HRT), diabetes status (yes or no), body mass index (continuous), and eGFR (categorized ≥ 90, 60-90, <60 ml/min/1.73m2); Model 3 additionally for factor VIII and aPTT (both continuous); and Model 4 added other hemostatic factors. Adjustment for hemoglobin, hematocrit, or monocyte count was also explored but dropped as noncontributory.
Results
Descriptive data
Among the 4,019 African Americans with HbS genotyping, 93.3% had no HbS, 6.7% had sickle cell trait (HbAS), and 0.07% (n = 3) had HbSS. For HbC, 97.5% had no HbC, 2.5% were HbAC, and 0.02% (n = 1) were HbCC; 0.12% (n = 5) were HbSC.
As shown in Table 1, demographic, hemostatic factors and other baseline characteristics were similar between participants with versus without sickle cell trait, with the exceptions that participants with sickle cell trait had somewhat lower eGFR values and higher mean factor VII, von Willebrand factor, and protein C values. Participants with HbAC were similar to those without HbC on all baseline characteristics, except estimated monocyte count (Table 1).
Table 1.
Baseline characteristics [mean (SD) or %] of participants according to hemoglobin (Hb) S or C genotype, ARIC African Americans, 1987-1989
| HbS Genotype |
HbC Genotype |
|||||
|---|---|---|---|---|---|---|
| No S | AS (sickle cell trait) |
P-value | No C | AC | P-value | |
| N* | 3,748 | 268 | 3,926 | 100 | ||
| Age, years | 53.5 (5.8) |
53.8 (5.9) | 0.32 | 53.5 (5.8) |
53.2 (5.8) | 0.60 |
| Men, % | 38.3 | 42.0 | 0.37 | 38.6 | 35.0 | 0.47 |
| Hormone replacement, women, % | 13.5 | 10.1 | 0.23 | 13.4 | 9.2 | 0.33 |
| Body mass index, kg/m2 | 29.5 (6.1) |
29.9 (6.3) | 0.39 | 29.5 (6.1) |
29.7 (5.7) | 0.81 |
| Diabetes, % | 19.4 | 20.2 | 0.77 | 19.6 | 14.1 | 0.18 |
| eGFR, ml/min/1.73m2 | ||||||
| ≥90, % | 86.6 | 80.2 | 86.1 | 88.0 | ||
| 60-90, % | 11.4 | 16.7 | 0.01 | 11.8 | 11.0 | 0.72 |
| <60, % | 2.0 | 3.2 | 2.1 | 1.0 | ||
| Hematocrit, % | 40.3 (4.4) |
40.1 (4.5) | 0.59 | 40.3 (4.4) |
39.5 (5.4) | 0.16 |
| Hemoglobin, g/dL | 13.2 (1.5) |
13.3 (1.5) | 0.53 | 13.2 (1.5) |
13.2 (1.8) | 0.94 |
| Monocyte count, cells/mm3 | 346 (197) |
357 (190) | 0.38 | 345 (196) |
399 (217) | 0.007 |
| Activated partial thromboplastin time, sec |
29.1 (3.2) |
29.0 (3.1) | 0.44 | 29.1 (3.2) |
29.0 (3.1) | 0.75 |
| Factor VII, % | 118 (31) | 125 (35) | 0.001 | 118 (31) | 115 (35) | 0.35 |
| Factor VIII, % | 147 (47) | 153 (53) | 0.06 | 147 (47) | 151 (47) | 0.40 |
| von Willebrand factor, % | 133 (55) | 142 (61) | 0.02 | 134 (56) | 134 (55) | 0.99 |
| Fibrinogen, mg/dL | 320 (72) | 323 (78) | 0.60 | 320 (73) | 323 (64) | 0.70 |
| Protein C, μg/mL | 3.1 (0.6) | 3.2 (0.7) | 0.02 | 3.1 (0.6) | 3.2 (0.7) | 0.78 |
| Antithrombin III, % | 115 (23) | 114 (23) | 0.33 | 115 (23) | 115 (19) | 0.89 |
N's vary between 3,789 and 4,016 because of characteristics missing on some participants. Participants with HbSS (n = 3) or HbCC (n = 1) were omitted from the table due to small numbers.
Associations with total VTE
Over a median of 22 years of follow-up, 249 participants had an incident VTE. As shown in Table 2 and Figure 1, panel (a), the crude incidence rate of VTE per 1,000 person-years among individuals with sickle cell trait was 4.83 (95% CI 3.24, 7.20), 1.58 fold greater (95% CI 1.04, 2.40) than those with no HbS. Although those with HbSS had about a 28.7-fold greater hazard, this was based on only one VTE occurrence among three HbSS participants and thus was a very imprecise estimate. HbAC did not appreciably increase VTE risk and is not discussed further.
Table 2.
Incidence rate of venous thromboembolism (VTE) according to hemoglobin (Hb) S or C genotype, ARIC African Americans, 1987-2011
| HbS Genotype |
HbC Genotype |
|||||
|---|---|---|---|---|---|---|
| No S | AS (sickle cell trait) |
SS | No C | AC | CC | |
| N at risk | 3,748 | 268 | 3 | 3,926 | 100 | 1 |
| VTE Cases* | 223 | 24 | 1 | 242 | 7 | 0 |
| Person-years | 71,109 | 4,972 | 28 | 74,261 | 1,928 | 23 |
| VTE rate (95% CI) †‡ | 3.14 (2.75-3.58) | 4.83 (3.24-7.20) | 35.8 (5.04-254.0) | 3.26 (2.87-3.70) | 3.63 (1.73-7.62) | 0 (0, 0) |
| Hazard ratio (95% CI) | 1 (Ref.) | 1.58 (1.04-2.40) | 28.7 (3.99-206.9) | 1 (Ref.) | 1.08 (0.51-2.30) | --- |
One VTE case had missing information on HbS genotype.
Crude VTE rate per 1,000 person years.
For HbS genotypes, the VTE rate per 1,000 person-years for <70 year olds was 2.10 (1.74-2.51) among No HbS and 2.65 (1.39-4.69) among HbAS participants, and for >70 year olds was 6.40 (5.29-7.69) among No HbS and 11.7 (6.73-19.2) among HbAS participants.
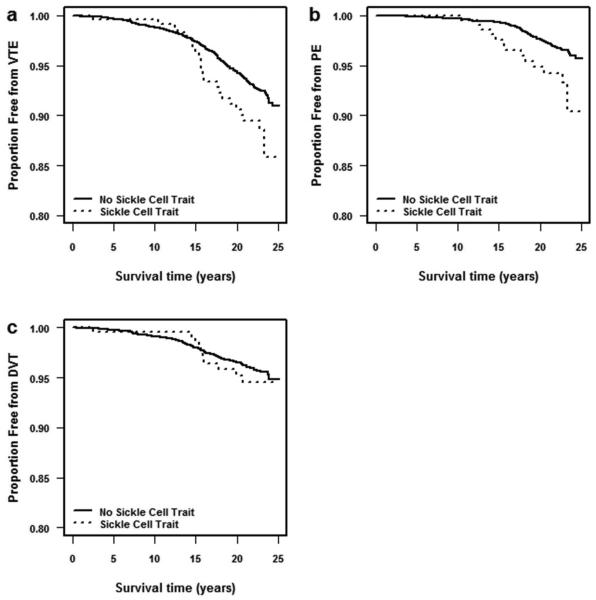
Figure 1.
Kaplan-Meier survival probabilities for (a) venous thromboembolism (VTE), (b) pulmonary embolism (PE) with or without deep vein thrombosis (DVT), and (c) isolated DVT, comparing participants with sickle cell trait versus no sickle cell trait, ARIC, 1987-2011.
Table 3 shows that adjustment for age, sex, and ancestry raised the hazard ratio for sickle cell trait to 1.60 (95% CI 1.05, 2.45). This hazard ratio was 1.63 (95% CI 0.82, 3.25) for unprovoked VTE (n = 88 VTEs) and 1.64 (95% CI 0.96, 2.79) for provoked VTE (n = 147 VTEs). Adjustment for other VTE risk factors (Models 2 and 3) attenuated the hazard ratio of sickle cell trait on VTE to a hazard ratio near 1.5. Additional adjustment for other hemostatic factors (Model 4) had no further impact.
Table 3.
Hazard ratios (HR) and 95% confidence intervals (CI) of total venous thromboembolism (VTE), pulmonary embolus, and deep vein thrombosis, in relation to hemoglobin S (HbS) genotype, ARIC African Americans, 1987-2011
| Model | Successive Adjustment | Total VTE |
Pulmonary Embolus* |
Deep Vein Thrombosis† |
||||
|---|---|---|---|---|---|---|---|---|
| No S | Sickle Cell Trait |
No S | Sickle Cell Trait |
No S | Sickle Cell Trait |
|||
| 1 | Age, sex, ancestry | N events‡
Person-years HR (95% CI) |
211 67, 233 1 (Ref.) |
24 4,807 1.60 (1.05-2.45) |
92 65,503 1 (Ref.) |
14 4,654 2.24 (1.28-3.95) |
119 65,661 1 (Ref.) |
10 4,566 1.18 (0.62-2.26) |
| 2 | Added HRT, BMI, diabetes, eGFR |
N events Person-years HR (95% CI) |
205 65,621 1 (Ref.) |
21 4,469 1.50 (0.96-2.36) |
90 63,940 1 (Ref.) |
12 4,337 2.05 (1.12-3.76) |
115 64,082 1 (Ref.) |
9 4,254 1.15 (0.58-2.27) |
| 3 | Add aPTT, factor VIII | N events Person-years HR (95% CI) |
201 64,101 1 (Ref.) |
21 4,367 1.44 (0.92-2.27) |
89 62,481 1 (Ref.) |
12 4,235 2.04 (1.11-3.75) |
112 62,578 1 (Ref.) |
9 4,152 1.08 (0.54-2.13) |
| 4 | Full§ | N events Person-years HR (95% CI) |
199 62,582 1 (Ref.) |
21 4,271 1.44 (0.91-2.27) |
88 60,968 1 (Ref.) |
12 4,139 2.13 (1.15-3.94) |
111 61,082 1 (Ref.) |
9 4,056 1.05 (0.53-2.09) |
Pulmonary embolus with or without deep vein thrombosis.
Deep vein thrombosis without pulmonary embolus.
Numbers of events and person-years differ from Table 2 and diminish across models due to missing data on ancestry or other adjusting variables.
Full: Model 3 plus factor VII, von Willebrand factor, fibrinogen, protein C, and antithrombin III.
Associations with PE versus DVT
Strikingly, those with versus without sickle cell trait had a 2-fold greater risk of PE, with or without DVT (Figure 1), and in all adjustment models (Table 3). In contrast, HbAS showed no appreciable association with isolated DVT.
Discussion
This prospective population-based study of African Americans found that sickle cell trait was associated with an approximately 1.5-fold increased risk of VTE, compared with those without any HbS allele, primarily due to a 2-fold increased risk of PE. HbAC was not associated with VTE risk, as previously reported [7-9]. Our results are consistent with the only previous three investigations on sickle cell trait and VTE. One, a case-control study [7], reported that sickle cell trait carried a 1.8-fold increased risk of VTE (95% CI 1.2, 2.9), principally for PE (odds ratios = 3.9 for PE only and 2.5 for PE with DVT). Likewise, record linkage studies reported PE odds ratios of 1.46 (95% CI 1.14, 1.89) in Veterans Administration hospitals and 1.37 (95% CI 1.07, 1.75) in a large California HMO, with no significantly increased risk for DVT [8, 9]. Sickle cell disease (HbSS) also has been associated with a 3-fold greater co-occurrence of PE hospitalization, but no excess risk of DVT [2], compared with other African Americans.
Our study demonstrated that the increased VTE risk associated with sickle cell trait is not due to confounding by several other risk factors, nor is it appreciably mediated by altered levels of several hemostasis variables. Among the hemostasis variables available in all ARIC participants, those most strongly associated with VTE in ARIC were factor VIII (or highly correlated von Willebrand factor) and aPTT [15, 16]. We found participants with sickle cell trait had modestly higher factor VIII and von Willebrand factor levels and slightly shorter aPTT, compared with HbAA participants free of sickle cell trait (Table 1), but this explained little of the excess PE risk for sickle cell trait (Table 3). A small clinical study reported that sickle cell trait was associated with other coagulation abnormalities [6]. Specifically, compared with controls, patients with sickle cell trait (n = 23) had higher basal mean values for D-dimer, thrombin-antithrombin complexes, prothrombin fragment 1.2, and monocyte count, although levels were significantly lower than for HbSC or HbSS patients [6]. Even though this suggests a hypercoagulable state with sickle cell trait, the controls in that study were “random laboratory and non-laboratory personnel” and may not have been comparable to the sickle cell trait patients on confounding factors. We did not confirm a higher estimated monocyte count in sickle cell trait. Co-existent α-thalassemia heterozygosity, present in up to 30% of African Americans, has been reported to reduce the severity of the complications of sickle cell disease [18] and sickle cell trait [19] due to a reduced intra-erythrocytic concentration of hemoglobin S that may undergo polymerization. Unfortunately, we were unable to evaluate the effect of α-thalassemia on VTE risk in this study.
Why sickle cell trait is a risk factor for PE, and not DVT, is unclear. Other genetic thrombophilias such as prothrombin G20210A are also associated with a higher risk of PE than DVT [20] and this difference in risk remains unexplained. If real, an association for PE and not DVT would imply sickle cell trait may influence either (a) the composition, size, venous adhesion, or fibrinolytic degradation of the DVT, and therefore risk to embolize; (b) the size or symptoms of emboli in the pulmonary arterial system; or (c) the occurrence of in situ pulmonary artery thrombi [21, 22]. With respect to possibility (a), the extreme hypoxia present in the venous valve sinuses of the lower extremities [23], where deep vein thrombi generally originate, may promote sickling of susceptible red cells. These sickle trait red cells may be unusually pro-thrombotic due to abnormal membrane phosphatidylserine exposure and may be incorporated in a venous ‘red clot’, altering clot structure [24]. Further mechanistic studies are required to explain this “paradox”. Likewise regarding possibility (a), elevated D-dimer in sickle cell trait [6] could be a marker of not just thrombin generation but also hyperfibrinolysis and thus the risk to embolize. With respect to possibility (c), in sickle cell disease, hypoxia-induced sickling in the pulmonary vasculature is thought to lead to in situ pulmonary thrombi, contributing to the pathophysiology of acute chest syndrome and pulmonary hypertension [25-27]. Although the degree of hypoxia in the pulmonary arterial vasculature alone is unlikely to induce sickling in sickle cell trait, future studies are needed to determine if a similar mechanism may occur in the setting of additional triggers or stress.
Several studies have suggested that not only is the VTE rate higher in African Americans than whites, but that the proportion of VTEs that are PE also is higher in African Americans than whites [28, 29]. One might therefore speculate that sickle cell trait, which is present in approximately 7% of African Americans, could partly account for a higher PE proportion in African Americans. In ARIC, however, while the rate of VTE was approximately 50% higher in African Americans than whites [11], the proportion of VTEs that involved PE was actually slightly lower, not higher, in African Americans than whites (45% versus 49%).
Methodological aspects of this ARIC analysis warrant consideration. Previous studies were not population-based or prospective [7-9] or were not able to directly validate VTE diagnoses [8, 9]. Although our study was population-based, it was not a sample of African Americans across the entire U.S. We had a moderate number of VTEs, but the sample size was still small enough that the confidence limits of the hazard ratio estimates were wide. Nevertheless, the consistency of our finding with the previous reports on sickle cell trait and VTE [7-9], and analogous findings for sickle cell disease [1-3], offers reassurance that the association is not due to chance and may be causal. Unmeasured confounding variables seem unlikely to explain the observed association, because sickle cell trait is genetically determined and the association is specific for PE and not present for isolated DVT. Selection bias at intake is unlikely in a prospective study, but could occur with differential losses to follow-up. However, follow-up rates in ARIC were quite high. Diagnostic suspicion bias is also unlikely, because clinicians generally would not know that a patient has sickle cell trait, nor should they suspect PE more often in sickle cell trait carriers than other patients. Compared to PEs, more DVTs may be subclinical and missed, or tests for DVTs may be omitted for clear PEs; however, again, it seems unlikely that these diagnostic issues would vary by HbS status and bias our results. ARIC missed VTEs diagnosed and treated in the outpatient setting, but small validation studies in ARIC have found relatively few outpatient VTEs over the course of the study.
Austin et al estimated that if sickle cell trait is present in 7% of the African American population and doubles VTE risk, then the population attributable risk (i.e., the percentage of VTEs in the population “caused” by HbAS) would be approximately 7%. Based on the approximate relative risks we observed of 1.5 for total VTE and 2.0 for PE, the population attributable risks would be 3% for VTE and 7% for PE. These are higher than the estimated population attributable risk for the prothrombin G20210A mutation in whites [30].
Conclusions
Because testing for sickle hemoglobin is currently being performed in the United States in several contexts, including the universal newborn screening program and college athletics, characterization of complications of sickle cell trait is imperative to guide policy and to inform counseling of identified sickle cell trait individuals. As clinical complications of sickle cell trait are elucidated, the ongoing process of informing parents about their child’s sickle trait status must involve directed genetic counseling to provide an accurate assessment of risks, dispel myths, and offer reproductive recommendations. Genetic counseling must also incorporate the uncertainties of current research in sickle cell trait. Although the population attributable risk of sickle cell trait for VTE is likely too small to impact treatment recommendations, future research into the pathophysiology and modifying factors for VTE among sickle cell trait carriers could be used to identify high risk individuals and improve health outcomes.
STROBE Statement–checklist of items that should be included in reports of observational studies
| Item No |
Recommendation | Reported on page |
|
|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study's design with a commonly used term in the title or the abstract |
1 |
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found |
2 | ||
| Introduction | |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported |
3 |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | 3 |
| Methods | |||
| Study design | 4 | Present key elements of study design early in the paper | 3-4 |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection |
3,4,6 |
| Participants | 6 | (a) Cohort study—Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up Case-control study—Give the eligibility criteria, and the sources and methods of case ascertainment and control selection. Give the rationale for the choice of cases and controls Cross-sectional study—Give the eligibility criteria, and the sources and methods of selection of participants |
3,4,6 |
| (b) Cohort study—For matched studies, give matching criteria and number of exposed and unexposed Case-control study—For matched studies, give matching criteria and the number of controls per case |
NA | ||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable |
4-6 |
| Data sources/ measurement |
8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group |
4-6 |
| Bias | 9 | Describe any efforts to address potential sources of bias | |
| Study size | 10 | Explain how the study size was arrived at | |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why |
6,7 |
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding |
6,7 |
| (b) Describe any methods used to examine subgroups and interactions | 6,7 | ||
| (c) Explain how missing data were addressed | 6 | ||
| (d) Cohort study—If applicable, explain how loss to follow-up was addressed Case-control study—If applicable, explain how matching of cases and controls was addressed Cross-sectional study—If applicable, describe analytical methods taking account of sampling strategy |
6 | ||
| (e) Describe any sensitivity analyses | NA | ||
| Results | |||
| Participants | 13* | (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed |
6 |
| (b) Give reasons for non-participation at each stage | 6 | ||
| (c) Consider use of a flow diagram | --- | ||
| Descriptive data |
14* | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders |
Table 1 |
| (b) Indicate number of participants with missing data for each variable of interest |
Table 1 | ||
| (c) Cohort study—Summarise follow-up time (eg, average and total amount) | 7 | ||
| Outcome data | 15* |
Cohort study—Report numbers of outcome events or summary measures over time |
7 |
|
Case-control study—Report numbers in each exposure category, or summary measures of exposure |
NA | ||
|
Cross-sectional study—Report numbers of outcome events or summary measures |
NA | ||
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included |
Tables 2 and 3 |
| (b) Report category boundaries when continuous variables were categorized | 7 | ||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period |
Table 2 | ||
| Other analyses | 17 | Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses |
8 |
| Discussion | |||
| Key results | 18 | Summarise key results with reference to study objectives | 8 |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias |
11 |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence |
8-12 |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | 11-12 |
| Other information | |||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based |
12-13 |
Give information separately for cases and controls in case-control studies and, if applicable, for exposed and unexposed groups in cohort and crosssectional studies.
Note: An Explanation and Elaboration article discusses each checklist item and gives methodological background and published examples of transparent reporting. The STROBE checklist is best used in conjunction with this article (freely available on the Web sites of PLoS Medicine at http://www.plosmedicine.org/, Annals of Internal Medicine at http://www.annals.org/, and Epidemiology at http://www.epidem.com/). Information on the STROBE Initiative is available at www.strobe-statement.org.
Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370:1453-7
Acknowledgments
The authors thank the staff and participants of the ARIC Study for their important contributions.
This research was funded by National Heart, Lung, and Blood Institute (NHLBI) R01 HL59367, R01 HL117659, R01 HL087097, U01 HL117659, K12 HL087097, and ARIC contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C. Support for ARIC whole exome sequencing and exome chip genotyping was provided by Building on GWAS for NHLBI-diseases: the U.S. CHARGE consortium through the National Institutes of Health (NIH) American Recovery and Reinvestment Act of 2009 (ARRA) (5RC2 HL102419).
Footnotes
Addendum
A. R. Folsom, A. V. Kshirsagar, V. K. Derebail, N. S. Key, and M. Cushman designed the research. A. R. Folsom, M. L. Grove, and M. Cushman conducted the research. N. S. Roetker and S. Basu analyzed data or performed statistical analysis. A. R. Folsom wrote the paper and had primary responsibility for final content. W. Tang, N. S. Roetker, A. V. Kshirsagar, V. K. Derebail, P. L. Lutsey, R. Naik, J. S. Pankow, M. L. Grove, S. Basu, N. S. Key, and M. Cushman made critical comments on the paper. All authors read and approved the final manuscript.
Disclosure of Conflict of Interests
R. Naik reports grants from NHLBI, outside the submitted work. All other authors state that they have no conflicts of interests.
References
- 1.Naik RP, Streiff MB, Haywood C, Jr, Nelson JA, Lanzkron S. Venous thromboembolism in adults with sickle cell disease: A serious and under-recognized complication. Am J Med. 2013;126:443–9. doi: 10.1016/j.amjmed.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stein PD, Beemath A, Meyers FA, Skaf E, Olson RE. Deep venous thrombosis and pulmonary embolism in hospitalized patients with sickle cell disease. Am J Med. 2006;119:897.e7–11. doi: 10.1016/j.amjmed.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Porter B, Key NS, Jauk VC, Adam S, Biggio J, Tita A. Impact of sickle hemoglobinopathies on pregnancy-related venous thromboembolism. Am J Perinatol. 2014;31:805–10. doi: 10.1055/s-0033-1361931. [DOI] [PubMed] [Google Scholar]
- 4.Lim MY, Ataga KI, Key NS. Hemostatic abnormalities in sickle cell disease. Curr Opin Hemato. 2013;20:472–7. doi: 10.1097/MOH.0b013e328363442f. [DOI] [PubMed] [Google Scholar]
- 5.Ataga KI, Key NS. Hypercoagulability in sickle cell disease: new approaches to an old problem. Hematology Am Soc Hematol Educ Program. 2007;91:6. doi: 10.1182/asheducation-2007.1.91. [DOI] [PubMed] [Google Scholar]
- 6.Westerman MP, Green D, Gilman-Sachs A, Beaman K, Freels S, Boggio L, Allen S, Schlegel R, Williamson P. Coagulation changes in individuals with sickle cell trait. Am J Hematol. 2002;69:89–94. doi: 10.1002/ajh.10021. [DOI] [PubMed] [Google Scholar]
- 7.Austin H, Key NS, Benson JM, Lally C, Dowling NF, Whitsett C, Hooper WC. Sickle cell trait and the risk of venous thromboembolism among blacks. Blood. 2007;110:908–12. doi: 10.1182/blood-2006-11-057604. [DOI] [PubMed] [Google Scholar]
- 8.Bucknor MD, Goo JS, Coppolino ML. The risk of potential thromboembolic, renal and cardiac complications of sickle cell trait. Hemoglobin. 2014;38:28–32. doi: 10.3109/03630269.2013.832689. [DOI] [PubMed] [Google Scholar]
- 9.Heller P, Best WR, Nelson RB, Becktel J. Clinical implications of sickle-cell trait and glucose-6-phosphate dehydrogenase deficiency in hospitalized black male patients. N Engl J Med. 1979;300:1001–5. doi: 10.1056/NEJM197905033001801. [DOI] [PubMed] [Google Scholar]
- 10.The ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 11.Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, Enright P, Folsom AR. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR. Cardiovascular risk factors and venous thromboembolism incidence: the Longitudinal Investigation of Thromboembolism Etiology. Arch Intern Med. 2002;162:1182–9. doi: 10.1001/archinte.162.10.1182. [DOI] [PubMed] [Google Scholar]
- 13.Reid JG, Carroll A, Veeraraghavan N, Dahdouli M, Sundquist A, English A, Bainbridge M, White S, Salerno W, Buhay C, Yu F, Muzny D, Daly R, Duyk G, Gibbs RA, Boerwinkle E. Launching genomics into the cloud: deployment of Mercury, a next generation sequence analysis. BMC Bioinformatics. 2014;15:30. doi: 10.1186/1471-2105-15-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS, CKD-EPI Investigators Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–9. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Tracy RP, Aleksic N, Folsom AR. Coagulation factors, inflammation markers, and venous thromboembolism: The Longitudinal Investigation of Thromboembolism Etiology (LITE) Am J Med. 2002;113:636–42. doi: 10.1016/s0002-9343(02)01345-1. [DOI] [PubMed] [Google Scholar]
- 16.Zakai NA, Ohira T, White R, Folsom AR, Cushman M. Activated partial thromboplastin time and risk of future venous thromboembolism. Am J Med. 2008;121:231–8. doi: 10.1016/j.amjmed.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–9. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 18.Embury SH, Clark MR, Monroy G, Mohandas N. Concurrent sickle cell anemia and alpha-thalassemia. Effect on pathological properties of sickle erythrocytes. J Clin Invest. 1984;73:116–23. doi: 10.1172/JCI111181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta AK, Kirchner KA, Nicholson R, adams JG, 3rd, Schechter AN, Noguchi CT, Steinberg MH. Effects of alpha-thalassemia and sickle polymerization tendency on the urine-concentrating defect of individuals with sickle cell trait. J Clin Invest. 1991;88:1963–8. doi: 10.1172/JCI115521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinelli I, Battaglioli T, Razzari C, Mannucci PM. Type and location of venous thromboembolism in patients with factor V Leiden or prothrombin G20210A and in those with no thrombophilia. J Thromb Haemost. 2007;5:98–101. doi: 10.1111/j.1538-7836.2006.02291.x. [DOI] [PubMed] [Google Scholar]
- 21.van Langevelde K, Srámek A, Vincken PW, van Rooden JK, Rosendaal FR, Cannegieter SC. Finding the origin of pulmonary emboli with a total-body magnetic resonance direct thrombus imaging technique. Haematologica. 2013;98:309–15. doi: 10.3324/haematol.2012.069195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Langevelde K, Flinterman LE, van Hylckama Vileg A, Rosendaal FR, Cannegieter SC. Broadening the factor V Leiden paradox: pulmonary embolism and deep-vein thrombosis as 2 sides of the spectrum. Blood. 2012;120:933–46. doi: 10.1182/blood-2012-02-407551. [DOI] [PubMed] [Google Scholar]
- 23.Hamer JD, Malone PC, Silver IA. The PO2 in venous valve pockets: its possible bearing on thrombogenesis. Br J Surg. 1981;68:166–70. doi: 10.1002/bjs.1800680308. [DOI] [PubMed] [Google Scholar]
- 24.Whelihan MF, Mooberry MJ, Zachary V, Bradford RL, Ataga KI, Mann KG, Key NS. The contribution of red blood cells to thrombin generation in sickle cell disease: meizothrombin generation on sickled red blood cells. J Thromb Haemost. 2013;11:2187–9. doi: 10.1111/jth.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mekontso Dessap A, Deux JF, Abidi N, Lavenu-Bombled C, Melica G, Renaud B, Godeau B, Adnot S, Brochard L, Brun-Buisson C, Galacteros F, Rahmouni A, Habbi A, Maitre B. Pulmonary artery thrombosis during acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med. 2011;184:1022–9. doi: 10.1164/rccm.201105-0783OC. [DOI] [PubMed] [Google Scholar]
- 26.Adedeji MO, Cespedes J, Allen K, Subramony C, Hughson MD. Pulmonary thrombotic arteriopathy in patients with sickle cell disease. Arch Pathol Lab Med. 2001;125:1436–41. doi: 10.5858/2001-125-1436-PTAIPW. [DOI] [PubMed] [Google Scholar]
- 27.Naik RP, Streiff MB, Lanzkron S. Sickle cell disease and venous thromboembolism: what the anticoagulation expert needs to know. J Thromb Thrombolysis. 2013;35:352–8. doi: 10.1007/s11239-013-0895-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White RH, Dager WE, Zhou H, Murin S. Racial and gender differences in the incidence of recurrent venous thromboembolism. Thromb Haemost. 2006;96:267–73. doi: 10.1160/TH06-07-0365. [DOI] [PubMed] [Google Scholar]
- 29.Stein PD, Kayali F, Olson RE, Milford CE. Pulmonary thromboembolism in American Indians and Alaskan natives. Arch Intern Med. 2004;164:1804–6. doi: 10.1001/archinte.164.16.1804. [DOI] [PubMed] [Google Scholar]
- 30.Sode BF, Allin KH, Dahl M, Gyntelberg F, Nordestgaard BG. Risk of venous thromboembolism and myocardial infarction associated with factor V Leiden and prothrombin mutations and blood type. CMAJ. 2013;185:E229–37. doi: 10.1503/cmaj.121636. [DOI] [PMC free article] [PubMed] [Google Scholar]