Abstract
Autoimmunity, gastrointestinal (GI) disorders and schizophrenia have been associated with one another for a long time. This paper reviews these connections and provides a context by which multiple risk factors for schizophrenia may be related. Epidemiological studies strongly link schizophrenia with autoimmune disorders including enteropathic celiac disease. Exposure to wheat gluten and bovine milk casein also contribute to non-celiac food sensitivities in susceptible individuals. Co-morbid GI inflammation accompanies humoral immunity to food antigens, occurs early during the course of schizophrenia and appears to be independent from antipsychotic-generated motility effects. This inflammation impacts endothelial barrier permeability and can precipitate translocation of gut bacteria into systemic circulation. Infection by the neurotropic gut pathogen, Toxoplasma gondii, will elicit an inflammatory GI environment. Such processes trigger innate immunity, including activation of complement C1q, which also functions at synapses in the brain. The emerging field of microbiome research lies at the center of these interactions with evidence that the abundance and diversity of resident gut microbiota contribute to digestion, inflammation, gut permeability and behavior. Dietary modifications of core bacterial compositions may explain inefficient gluten digestion and how immigrant status in certain situations is a risk factor for schizophrenia. Gut microbiome research in schizophrenia is in its infancy, but data in related fields suggest disease-associated altered phylogenetic compositions. In summary, this review surveys associative and experimental data linking autoimmunity, GI activity and schizophrenia, and proposes that understanding of disrupted biological pathways outside of the brain can lend valuable information regarding pathogeneses of complex, polygenic brain disorders.
Keywords: Autoimmunity, Psychosis, Microbiota, Immune system, Psychiatry, Intestinal
1. Introduction
Schizophrenia is a complex brain disorder with lifetime prevalence rates estimated to range from 1.6–12.1/1000 persons, with some variability according to age and sex (Eaton et al., 2011; Pedersen et al., 2014). The disorder is characterized by behavioral abnormalities and is diagnosed by a set of criteria defined in the Diagnostic and Statistic Manual of Mental Disorders, 5th edition (DSM-5) (APA, 2013). Among the criteria used to diagnose schizophrenia are the presence of psychotic symptoms, such as delusions and hallucinations, as well as cognitive disorganization, apathy and withdrawal (APA, 2013). The causes of schizophrenia have not been fully defined, but prevailing evidence supports an interaction of genetic and environmental variables as central to its etiology (Demjaha et al., 2012; Modinos et al., 2013; Tsuang, 2000).
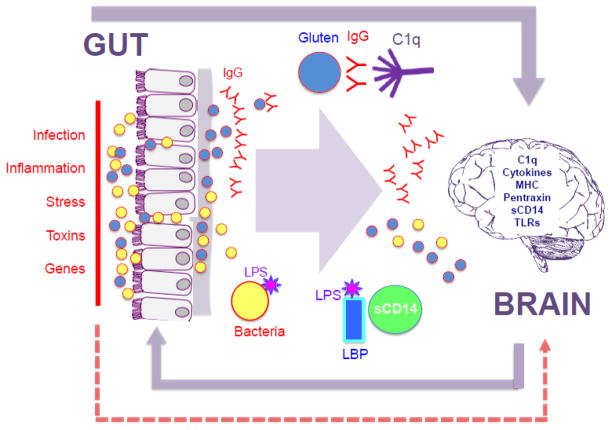
The relationship between autoimmune diseases and schizophrenia has been studied for more than half a century. In the past decade, the focus of this autoimmune research has been narrowed to a certain extent to gastrointestinal (GI) disorders, while at the same time has broadened somewhat to include dysfunctions of the immune system. As the largest immune organ in the body, the GI tract is a plausible junction to reconcile hypotheses regarding how the autoimmune response and GI-related products can become neuropathogenic. This paper traces some of these developments and, following the logic of translational research, links the epidemiologic observations to molecular studies of the gut-brain axis and the gut’s main bio-processor, its resident microbiota. An overview of the interactions described in this review is depicted in Figure 1.
Figure 1. Overview of autoimmunity, GI and brain connections in schizophrenia.
Aspects of the gut to brain axis described in this review are depicted. Multiple environmental and genetic factors can contribute to inflammation in the GI tract and a compromised GI epithelial and endothelial barrier (left side of diagram, text in red). The same series of factors may also cause permeability of the blood brain barrier (dashed red arrow). Loss of barrier integrity can lead to translocation of food-derived peptides (blue circle) and resident gut microbiota (yellow circle). In individuals with celiac disease, an autoimmune response following ingestion of wheat gluten (blue circle) causes the formation of antibodies (red Y structure) that attack the self-protein tTg (not shown). In individuals who do not have a biomarker profile of classically-defined celiac disease, an IgG sensitivity to wheat gluten and to bovine milk casein can occur, and the humoral immune response (also red Y structure) leads to activation of innate immunity. C1q forms immune complexes with invading antigens including gluten, casein and bacterial peptides and corresponding antibodies. Soluble CD14 (sCD14) is activated in the presence of serological lipopolysaccharide (LPS) and its binding protein, LBP. C1q and sCD14 are also both active in the brain, as are other innate immune molecules such as the major histocompatibility complex (MHC), cytokines, pentraxin and toll-like receptors (TLRs). Alternatively, gut-derived food and bacterial peptides, and corresponding antibodies, might exert direct effects on the brain. The gut-brain axis is bi-directional, but this review focuses on GI factors impacting the brain in schizophrenia. Details are described in the main text.
2. Autoimmunity and schizophrenia
2.1 Origins
The earliest interest connecting autoimmunity and schizophrenia stems from a repeated finding of a low prevalence of rheumatoid arthritis in individuals with schizophrenia, beginning with studies in the 1950’s and including analyses up to the present (Eaton et al., 1992; Oken and Schulzer, 1999; Pilkington, 1955; Sellgren et al., 2014; Trevathan and Tatum, 1953; Vinogradov et al., 1991). The finding was replicated more than a dozen times, and various explanations were offered, such as that neuroleptic medications had a protective effect; or that persons with schizophrenia were less likely to report pain; or that life as an inpatient was less physically active and thereby less prone to raising risk for rheumatoid arthritis. Each of these hypotheses was evaluated in studies with research designs that effectively discounted those explanations (Mellsop et al., 1974; Mohamed et al., 1982; Pilkington, 1955; Trevathan and Tatum, 1953). There have been several studies of genes, immune-related factors, and common infections which might explain the inverse association (de la Fontaine et al., 2006; Genevay et al., 2002; Hopkins et al., 1988; Taylor, 1978; Torrey and Yolken, 2001; Wright et al., 1998), including a compelling meta-analysis showing that the antagonist to the receptor of the inflammatory cytokine IL-1 was more prevalent in individuals with schizophrenia, thus protecting them from rheumatoid arthritis (Potvin et al., 2008).
2.2 Autoimmunity link to the GI tract
Autoimmune disorders can be triggered by dietary components and antigens derived from the GI tract. One such autoimmune condition characterized by altered GI structure and functioning is celiac disease, a disorder that arises from the interaction of gene and environmental factors. Upon the ingestion of wheat gluten by people who are genetically susceptible, an immune reaction is launched that damages the epithelial lining of the small intestine (Green et al., 2005; Guandalini and Assiri, 2014). During digestion, the gluten protein in wheat is broken down into toxic peptides that are modified through deamidation and/or transamidation by tissue Transglutaminase (tTG), so that the modulated peptide might stimulate the immune system more effectively and thus be more easily cleared. Through the process of molecular mimicry, tTG becomes the target of a T-cell-mediated immune attack, and resulting inflammation causes extensive histopathological changes in the small intestine (Alaedini and Green, 2008; Di Sabatino et al., 2012; van Heel and West, 2006). Clinically-defined celiac disease will be the focus of this portion of this discussion, and this condition differs in diagnosis from non-celiac disease gluten sensitivity, which will be discussed later.
The GI system connection with autoimmune issues in schizophrenia came about with clinical observations of co-associations of celiac disease and schizophrenia, starting with Bender in 1953 (Bender, 1953), and later in 1961, when two residents in psychiatry reported a higher number than expected of people with celiac disease among their psychiatric patients (Graff and Handford, 1961). This finding interested F. Curtis Dohan who spent the remainder of his career focusing on a link between wheat consumption and schizophrenia (Dohan, 1973, 1980; Dohan, 1970). His first epidemiologic study of wartime admissions for schizophrenia showed that populations in countries whose consumption of wheat had decreased during the war saw a decrease in hospital admissions for schizophrenia, whereas those populations in countries with an increase in consumption of wheat during the war had an increase in hospital admissions for schizophrenia (Dohan, 1966a, b). Dohan analyzed other epidemiologic data consistent with the hypothesis that eating wheat was linked to higher rates of schizophrenia, as evident by areas of the south Pacific region where consumption of wheat was low having lower rates of schizophrenia than areas where wheat consumption was high (Dohan et al., 1984). He also conducted several controlled trials removing gluten from the diet (Dohan and Grasberger, 1973; Dohan et al., 1969), the results of which supported the hypothesis that celiac disease was related to schizophrenia. Clinical trials conducted by others were sometimes supportive of the idea (Rice et al., 1978; Singh and Kay, 1976; Vlissides et al., 1986), and sometimes failed to replicate it (Potkin et al., 1981; Storms et al., 1982). One explanation for the diverse results is the effect of random sampling in a population of persons with a heterogeneously complex disease such as schizophrenia, of whom only a small proportion might actually have celiac disease (King, 1985). Thus, some studies might have had enough persons with schizophrenia to detect an effect, but other samples would have few or none with celiac disease, with no possibility of a positive effect of removal of gluten from the diet. There are several case studies of gluten withdrawal showing dramatic improvement or even elimination of symptoms (De Santis et al., 1997; Jackson et al., 2012; Jansson et al., 1984; Kraft and Westman, 2009).
2.3 Shared HLA predisposition in celiac disease and schizophrenia
The association between celiac disease and schizophrenia was hypothesized by Dohan, and others, to be the result of a gene by environment interaction (Dohan, 1988; Wei and Hemmings, 2005). A surge in interest in the 6p21 region as a susceptibility locus for schizophrenia was generated following publication of three genome-wide association studies (Purcell et al., 2009; Shi et al., 2009; Stefansson et al., 2009). This chromosomal region houses the major histocompatibility complex (MHC) locus and its resident human leukocyte antigen (HLA) genes which comprise an immune gene network designed to respond to a variety of antigens, with some gene products that function in the brain during synapse development (Boulanger, 2009; Corvin and Morris, 2014). Individuals with celiac disease almost exclusively have the HLA-DQ2 heterodimer or DQ8 haplotype (van Heel and West, 2006). A linkage study of schizophrenia in western Ireland highlighted the short arm of chromosome 6, telomeric from the HLA region (Straub et al., 1995). This region, which includes the dysbindin locus, has attracted attention of several replications (Benson et al., 2004; Camargo et al., 2007; Fanous et al., 2005; Guo et al., 2009; Papaleo and Weinberger, 2011; Riley et al., 2009; Schwab et al., 1995; Schwab et al., 1998; Schwab et al., 2003; Shi et al., 2009; Van Den Bogaert et al., 2003). An independent study of celiac disease in western Ireland pointed to the same general area of chromosome 6 (Zhong et al., 1996), but there has yet to be further conclusive evidence about the dysbindin locus and celiac disease. Another gene with a history of association to celiac disease is the MY09B locus in the short arm of chromosome 19, and this has been associated with schizophrenia in a study by Jungerius (Jungerius et al., 2007).
2.4 Epidemiology links schizophrenia with celiac disease
The most convincing epidemiologic data connecting celiac disease and schizophrenia came from studies using health registers. In 1980, data from the Oxford Health Register revealed a cross sectional association of schizophrenia with celiac disease (relative odds of about 3)(Baldwin, 1980). In a prospective study based in the national register system of Denmark (Eaton et al., 2004), the diagnosis of celiac disease in the individual or the family raised the risk for later diagnosis of schizophrenia by 2.7. In contrast, the risk for Crohn’s disease or irritable bowel syndrome, two other autoimmune diseases with similar symptom presentations as celiac disease, was not significantly different from 1.0. Of note, however, a Finnish birth cohort study had previously found that inflammatory diseases of the bowel including ulcerative colitis and Crohn’s disease were significantly overrepresented in schizophrenia compared to non-psychiatric controls, 3.4% vs 0.3% (Makikyro et al., 1998). The association between non-celiac disease wheat sensitivity, intestinal inflammation and schizophrenia will be discussed further in subsequent sections.
2.5 Current focus on autoimmunity and schizophrenia
The linkage of two distinct autoimmune diseases to schizophrenia (albeit in opposite directions) contributed to interest in the role of autoimmune diseases in general to schizophrenia. The epidemiologic literature soon yielded results broader than the initial focus on rheumatoid arthritis and celiac disease, including several small family studies (Gilvarry et al., 1996; Wright et al., 1996). A further study in Denmark showed a 45% increased relative risk for schizophrenia (adjusted for other major risk factors such as urbanicity of birth, family history of schizophrenia, and wealth) for persons who had one or more of 30 autoimmune diseases in their own history or in their first degree relatives, prior to diagnosis of schizophrenia (Eaton et al., 2006). This same positive association turned up in a cross-sectional analysis in Taiwan (Chen et al., 2012), and in a later prospective study from Denmark, with the additional detail that the results were not similar for bipolar disorder (Eaton et al., 2010). Another study in Denmark showed that a history of serious infection was predictive of onset of schizophrenia, and that the history of infection interacted with a history of autoimmune diseases in raising risk (Benros et al., 2011). An analysis of this Danish register which reversed the temporal direction indicated that people with schizophrenia were at a significantly increased risk for the development of autoimmune diseases, with that risk further elevated in those with a history of an infection requiring hospitalization or a family member with schizophrenia (Benros et al., 2014).
Another emerging area of interest in the context of pathogenic autoantibody production and schizophrenia are the human endogenous retroviruses (HERVs), which are fossil infectious retroviruses that have integrated into the human genome. HERVs have been associated with schizophrenia and other psychiatric diseases in a number of studies (Dickerson et al., 2012; Huang et al., 2011; Karlsson et al., 2001; Lillehoj et al., 2000), and it is thought that HERV expression may induce an autoimmune state through molecular mimicry especially when coupled with viral or other infections (Brodziak et al., 2012; Leboyer et al., 2013; Ogasawara et al., 2010; Perron et al., 2012). A recent report of HERV-related immunoreactivity in plasmacytoid dendritic cells of the duodenum from patients with myalgic encephalomyelitis further links the gut to neuroinflammatory autoimmune processes by means of this retroelement mechanism (De Meirleir et al., 2013).
3. Extra-autoimmune GI connection to schizophrenia
3.1 The dietary exorphin hypothesis
As thus far described, numerous studies indicate that schizophrenia may have a strong autoimmune component. However, the broader nature of the risk structure, beyond celiac disease to other autoimmune diseases and infections, suggested a wider range of possible etiologic pathways. An alternative or additional explanation for the wheat and schizophrenia connection is suggested by Dohan who called these peptides “exorphins” because he felt they resembled the brain-reactive chemicals endorphins, suggestive of a capacity to bind to opioid receptors found throughout the body, but particularly in the brain (Dohan, 1988). Early studies document increased levels of neuroactive food antigen peptides in the bloodstream, CSF and urine in individuals with schizophrenia (Cade et al., 1990; Drysdale et al., 1982; Lindstrom et al., 1986; Reichelt et al., 1981; Reichelt et al., 1996; Reichelt and Stensrud, 1998). The presence of these exorphins has also been found in some analyses of urine samples from children with autism, and in one study, peptide levels correlated with symptom severity scores (Reichelt et al., 2012; Sokolov et al., 2014). A third study failed to replicate these findings (Hunter et al., 2003), but overall these studies have low sample sizes, and this area of research would benefit from larger, well-designed, case-control analyses. The pathological potential of these exorphins might also occur in a non- or extra-autoimmune capacity that is not due to the classically-defined celiac disease. For example, these exorphins may be related to the more general condition known as gluten enteropathy (Kabbani et al., 2014) or alternatively, to the potency potential of the peptide ligands at opioid receptors (Fukudome and Yoshikawa, 1992; Zioudrou et al., 1979). Furthermore, the neuroactive peptides hypothesis is not exclusive to gluten, and Dohan and Reichelt theorized that the digestion of the principal component of bovine milk, casein, also resulted in the production of these exorphins (Reichelt et al., 1996).
For the exorphin hypothesis to operate, a means of penetrating the GI epithelial and endothelial barriers would be necessary. The production of IgG antibodies against these proteins thus might suggest barrier penetrance, but additional simultaneous measures are needed for proof of concept. Direct activation of the GI immune mucosa could also result in secretory IgA antibody production, provided the peptides can penetrate the mucosal layer and interact with the gut’s lymphoid tissue. Peyer’s patches are located in the lamina propria layer of the mucosa in the ileum and are where T-, M- and B-cells are activated in the presence of an antigen (Mantis et al., 2011). The presence of these peptides in multiple clinical and experimental biospecimen types support that these exorphins have the ability to extend their range beyond gut walls.
3.2 Diet-generated humoral immune activation
Activation of an IgG antibody response against a number of morphologically related food exorphins suggests the presence of an IgG sensitivity or intolerance that is different from the biomarker profiles used to diagnose celiac disease. For wheat, this non-celiac IgG sensitivity is accompanied by a diversity of GI and extra-intestinal symptoms that appear immediately following the ingestion of gluten and disappear upon removal of gluten from the diet. GI inflammation, barrier permeability changes and intestinal dysbioses are all thought to contribute to the pathogenesis of this non-celiac disease gluten intolerance (Catassi et al., 2013; Kabbani et al., 2014; Sapone et al., 2011). As early as 1979, Ashkenazi showed that there was a general immune reaction to wheat in persons with psychosis (Ashkenazi et al., 1979), and in 1998, Reichelt described, with a small sample, that antibodies to the gliadins (the soluble components of gluten) were more common in persons with schizophrenia than in the general population (Reichelt and Landmark, 1995). This gliadin-antibody link was replicated in the CATIE sample (Cascella et al., 2011), and later in three further replications in Taiwan (Jin et al., 2010), Tunisia (Sidhom et al., 2012), and Germany (Okusaga et al., 2013) with a similar association, reported in slightly different form in Baltimore as well (Dickerson et al., 2010). A meta-analysis of gluten sensitivity studies suggests that the nature of this immune response to wheat by individuals with schizophrenia differs from the classic celiac disease immune response (Lachance and McKenzie, 2014). Persons with schizophrenia tend to have three or four times the proportion with antibodies to tTG as the general population (i.e., about 5% versus 1.5 percent), and three or four times the proportion with anti-gliadin antibodies (i.e., about 20% versus about 5%). This pattern expands the immunologic picture beyond the adaptive immune system, implicated in the tTG antibodies, to the innate immune system, implicated by the anti-gliadin antibodies.
There is also precedence for the formation of a non-celiac disease autoimmune response against other gluten-associated cellular entities (Vojdani et al., 2004a; Vojdani et al., 2004b; Vojdani et al., 2003). In children with autism, a strong association between anti-gliadin and anti-peptidase antibodies suggested that during processing, gliadin might bind to aminopeptidases to induce autoantibodies, perhaps in people with predisposing HLA molecules (Vojdani et al., 2004a). Similarly, anti-gliadin antibodies were associated with autoantibodies to the immune molecules, CD26 and CD69, indicating that these peptides bind to these immune receptors and an autoimmune reaction is generated (Vojdani et al., 2003). Ultimately, the concern with respect to autoimmune pathogenicity in brain diseases is whether there is cross-reactivity of anti-gliadin antibodies with brain proteins, and several studies provide support for this hypothesis (Alaedini et al., 2007; Briani et al., 2008; Hadjivassiliou et al., 2002).
An increased seroprevalence of antibodies to another type of food-derived exorphin, bovine milk casein, has also been reported in schizophrenia compared to controls (Niebuhr et al., 2011; Severance et al., 2010). Studies on a prospective cohort indicated that increases in antibodies to casein can be detected as early as two years prior to the onset of psychiatric symptoms or the initiation of therapy, suggesting the possibility that the pathology may precede the onset of diagnosis (Niebuhr et al., 2011). Bovine milk casein itself has not been associated with the same celiac disease-related pathology as gluten, yet levels of antibodies directed against these food antigens were often extremely well-correlated in both control and schizophrenia groups (Severance et al., 2012a). Furthermore, anti-food antigen antibodies were significantly correlated with a surrogate marker of intestinal inflammation and microbial translocation, anti-Saccharomyces cerevisiae antibodies (ASCA), in the schizophrenia but not control groups (Severance et al., 2012a). These findings suggest a role of inflammation in enabling gut-located antigens to activate the immune system or penetrate the GI endothelial barrier. Although ASCA is predominately a marker that can help discriminate GI-related inflammatory diseases, this marker is also elevated in patients with a variety of other autoimmune conditions such as systemic lupus erythematosus, Graves’ disease, cryoglobulinemia and vasculitis (Ben-Ami Shor et al., 2012).
3.3 GI inflammation and schizophrenia
3.3.1 Early evidence of co-morbid GI inflammation
GI co-morbidities in mental illness have been described for as long as such maladies have been documented, with purgatives and emetics offered as predominant treatment strategies in the older literature (Prichard, 1837). Among recent and historical accounts are reports of extensive inflammatory changes throughout the GI tract of patients with psychiatric symptoms (Alander et al., 2005; Buscaino, 1953; Hemmings, 2004; Reiter, 1926; Schneck, 1946). In one autopsy study of 82 patients with schizophrenia, as many as 50% had gastritis, 88% enteritis and 92% colitis (Buscaino, 1953; Hemmings, 2004). In hindsight, this extensive inflammation could have reflected any number of states including the aforementioned and described celiac disease, but the prevalence seems too high to be accounted for by a single enteropathic disease. Studies of inflammatory indices in schizophrenia support the possibility that there exists a non-celiac disease GI pathology inherent to schizophrenia. Gluten sensitivity, for example, in the absence of celiac disease may also produce intestinal pathologies (Catassi et al., 2013; Kabbani et al., 2014; Sapone et al., 2011). Furthermore, as mentioned previously, there are other reports of increased rates of inflammatory bowel disease, including ulcerative colitis and Crohn’s disease, and of irritable bowel syndrome in schizophrenia (Gupta et al., 1997; Makikyro et al., 1998).
3.3.2 The effects of antipsychotics on GI inflammation
It is difficult to distinguish GI conditions generated by lifestyle factors or antipsychotic effects from GI symptoms that are part of the disease pathology of schizophrenia. Both first and second generation antipsychotics are suspected to have strong intestinal motility consequences resulting in numerous GI conditions such as constipation and bowel obstruction (Dean, 2010; Dome et al., 2007; McNamara et al., 2011; Watanabe et al., 2010). Of note, however, a number of reports that document GI-related inflammation preceded the development of antipsychotics that were first discovered in the 1950s (Preskorn, 2010). As mentioned earlier, measures of serological ASCA are used diagnostically for inflammatory bowel diseases including ulcerative colitis and Crohn’s disease (Ashorn et al., 2009; Desplat-Jego et al., 2007; Kotze et al., 2010; Mallant-Hent et al., 2006; Oshitani et al., 2000). In a recent study of gut inflammation in schizophrenia, the highest levels of ASCA were found in individuals who were in the early stages of disease and/or who were medication-naïve (Severance et al., 2012a). Thus while antipsychotic agents may affect the type or degree of inflammation (Beumer et al., 2012a; Drexhage et al., 2010; Drexhage et al., 2011; Leonard et al., 2012; Miller et al., 2012; Steiner et al., 2012), some part of disease-associated inflammation is likely present before the start of pharmacological treatment.
3.3.3 GI permeability
Inherent to the rationale of a GI role in psychiatric disorders is the idea of disease-associated GI permeability that impacts barriers both in the gut and in the central nervous system (CNS). Inflammation and stress are potent perpetrators of endothelial barrier permeability (Collins and Bercik, 2009; Lambert, 2009; Soderholm and Perdue, 2001). GI-derived antigenic peptides presumably enter the general circulation because of compromised GI epithelial and/or endothelial barriers, but they also may selectively breach intra-epithelial tight junction proteins. Tight junctions (zonula occludens) are present between the epithelial cells that line the lumen of the GI tract; similar tight junctions comprise the blood brain barrier (Deli, 2009; Jong and Huang, 2005). The cerebrospinal fluid (CSF)- brain and CSF-blood barrier at the choroid plexus and arachnoid membrane also represent a junction by which faulty architecture might allow passage of bioactive or infectious peptides (Laterra et al., 1999). There is evidence that gluten peptides directly induce zonulin release from intestinal epithelial cells thereby modulating barrier permeability (Clemente et al., 2003; Fasano, 2012; Lammers et al., 2008; Thomas et al., 2006). In addition to gluten, exposure to bacteria is another powerful trigger of zonulin release in the gut (Fasano, 2012). Zonulin, a precursor for haptoglobin-2, is able to reversibly regulate intestinal permeability and is thus thought to represent a point of pathway convergence in the gut for allergic, inflammatory and autoimmune diseases (Tripathi et al., 2009). Haptoglobin is an acute phase protein that has been implicated both genetically and physiologically in studies of schizophrenia (Maes et al., 2001; Wan et al., 2007; Yang et al., 2006).
The integrity of epithelial and endothelial barriers could also be compromised by an infection of the gut by a virus or parasite. Exposure to the parasite Toxoplasma gondii, a gut pathogen, is a well-known risk factor for the development of schizophrenia (Mortensen et al., 2007; Torrey et al., 2007; Xiao et al., 2009; Yolken et al., 2009). This parasite also provides a tool for modeling inflammatory bowel disorders in rodents (Bereswill et al., 2010; Craven et al., 2012; Erridge et al., 2010; Hand et al., 2012; Munoz et al., 2009; Schreiner and Liesenfeld, 2009). If the inflammatory state in schizophrenia were the result of a generalized, polyspecific activated immune state, it would be expected that antibody levels to food antigens would correlate with those of infectious disease antigens. An analysis of this type of relationship, however, showed that antibodies to food-based antigens, while significantly and expectedly inter-correlated, were correlated with only one of the studied infectious agents, T. gondii (Severance et al., 2012a). This association was especially evident in individuals with schizophrenia who had a recent onset of disease. These findings were supported by a mouse model of peroral T. gondii infection where infected animals had elevated antibody levels to dietary gluten compared to those that were uninfected (Severance et al., 2012c). Links between measures of T. gondii exposure and celiac disease have also been reported, although it was not possible to determine if infection or the enteropathic state came first (Lidar et al., 2009; Rostami Nejad et al., 2011). Additional studies have documented that T. gondii infection impacts the resident microbiota communities and brings about a state of inflammation conducive to the process of bacterial translocation, a surrogate measure of GI permeability (Craven et al., 2012; Grainger et al., 2013; Hand et al., 2012; Heimesaat et al., 2006).
When the GI tract is compromised, resident gut microbiota can translocate into systemic circulation and create a state of low-level inflammation, as the immune system is activated to clear invading antigenic microbes from circulation (Brenchley et al., 2006; Lambert, 2009; Sandler and Douek, 2012). This process of bacterial translocation and its occurrence in major depressive disorder is a current topic of extensive research and appears to be a demonstrable source of inflammation associated with this disease (Maes et al., 2008; Maes et al., 2012a; Maes et al., 2012b). In a study of bacterial translocation in schizophrenia, two surrogate biomarkers, soluble CD14 (sCD14) and lipopolysaccharide (LPS) binding protein (LBP), were found to be inter-correlated in both case and control groups, as expected based on their similar physiological roles in alerting the immune system to the bloodstream presence of a bacterial endotoxin (Severance et al., 2013). The two markers were also both significantly correlated with a general marker of inflammation, C-reactive protein, and gluten IgG in individuals with schizophrenia, suggesting a common pathway of associated inflammation. Marker levels, however, were only significantly elevated in schizophrenia compared to controls for sCD14 (multivariate OR=3.09). LBP, but not sCD14, was found to correlate with BMI scores in schizophrenia. Obesity and its accompanying metabolic state are increasingly implicated in gut permeability, bacterial translocation and resulting inflammatory processes, all as mediated by gut microbiota (Shen et al., 2013; Turnbaugh et al., 2009). The discordant patterns of sCD14 and LBP implicate additional pathogenic mechanisms related to bacterial translocation and dysregulated monocyte activation inherent to schizophrenia. Other investigators have reported abnormal monocyte or macrophage activation in schizophrenia and other psychoses, and it is thought that this might reflect a state of innate immune hyper-reactivity (Beumer et al., 2012a; Beumer et al., 2012b; Drexhage et al., 2010; Drexhage et al., 2011; Miller et al., 2012; Muller et al., 2012).
3.3.4 GI activation of innate immunity and relevance to the brain
There is increasing evidence that the activation of the innate immune pathways by gut processes may impact the brain. Immune proteins, such as complement C1q and MHC I, have been identified as critically important to neuronal development in the brain with effects on synapses by mechanisms that are not part of historically described immune pathways (Boulanger, 2009; Goddard et al., 2007; Lackner et al., 2008; Perry and O’Connor, 2008; Rupprecht et al., 2007; Stevens et al., 2007; Yuzaki, 2010). C1q and MHC I may selectively tag cells or excessive synapses in the CNS for clearance by microglia (Boulanger, 2009; Chu et al., 2010; Fourgeaud and Boulanger, 2007; Huh et al., 2000; Ma et al., 2013; Stevens et al., 2007).
Complement activity can be genetically and functionally linked to schizophrenia. Genetic studies of the complement pathway in schizophrenia reveal disease-associations of the C1QB gene, complement control-related genes, and complement surface receptor gene CD46 (Havik et al., 2011; Zakharyan et al., 2011). In multiple reports, elevated levels of complement-containing circulating immune complexes were found in individuals with schizophrenia compared to controls (Arakelyan et al., 2011; Boyajyan et al., 2008; Mailian et al., 2005; Mayilyan et al., 2008; Severance et al., 2012b; Vetlugina et al., 1984). This complement activity can be triggered by exposure to gluten and casein. In many individuals with schizophrenia, C1q forms immune complexes with these food antigens at increased rates compared to controls (Severance et al., 2012b). Interestingly, gut C1q was linked to psychopathology in a study of intestinal biopsies where IgG deposition and C1q infiltration of the duodenum was present in autism, a disorder as etiologically complex as schizophrenia, but not in celiac disease, cerebral palsy, mental retardation and normal controls (Torrente et al., 2004; Torrente et al., 2002). In rodent models, serological C1q elevations accompanied increased gluten IgG levels in animals infected with T. gondii compared to those that were not infected (Severance et al., 2012c). In a separate rodent study, T. gondii infection induced inflammatory cascades including heightened brain C1q expression (Hermes et al., 2008). It has also been found that in mice, microglial activation and brain C1q synthesis precedes experimentally-induced blood brain barrier dysfunction (Lynch et al., 2004). Thus, peripheral activation of C1q by antigenic peptides of gut origin may have the propensity to activate inflammatory pathways in the brain, given the correct set of factors are in place, including food sensitivity, pathogen exposure, gut and systemic inflammation, and barrier permeability defects.
The role of innate immune activation in schizophrenia can be additionally presented in the context of the prenatal exposure hypotheses; yet for the purposes of this section, the proposition that immune molecules might function peripherally as well as centrally is the focus rather than the timing of the immune activation. MHC I and C1q are perhaps the best known of these types of multi-venue functioning molecules; however, as described above, sCD14 may also contribute to brain processes in this context. Plasma and CSF levels of sCD14 were associated with cognitive function in patients with HIV dementia presumably as a result of CNS invasion by peripheral blood-derived monocytes and precipitated by microbial translocation (Ancuta et al., 2008; Fischer-Smith et al., 2001; Ryan et al., 2001). Cytokines, pentraxin, and the toll-like receptors (TLRs) may all be active in neurogenesis and other brain functions, with the TLRs especially implicated in blood brain barrier integrity and neurodegenerative disorders (Frodl and Amico, 2014; Garate et al., 2013; Nagyoszi et al., 2010; Pribiag and Stellwagen, 2014; Trotta et al., 2014). Research described in previous sections supports an association of gluten immunoreactivity with sCD14 (Severance et al., 2013), and opioid ligands may be potent modulators of TLR in the gut (Meng et al., 2013). By the same rationale, bacteria residing in the gut mucosa might produce peptides or themselves might directly interact with the intestinal lumina and resident recognition receptors including TLRs and NOD2 (Hong and Rhee, 2014).
4. The microbiome and implications for schizophrenia
The human GI tract is a reservoir of a large diversity of bacteria, and we are only just beginning to understand the multiplicity of functions that these microbial agents confer to the human body in terms of nutrition, metabolism, and immune system development (Douglas-Escobar et al., 2013; Hornig, 2013). An estimated 1014 cells make up the human microbiome, and the five bacterial phyla include Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria and Fusobacteria (Caminero et al., 2014; Cryan and Dinan, 2012). These phyla are found throughout the gut in varying degrees with the colon characterized predominantly by Firmicutes, Bacteroidetes and Actinobacteria (Caminero et al., 2014; Hong and Rhee, 2014)). A current working hypothesis is that dysbioses of GI microbial communities contribute to inflammation in the GI tract; thus, changes in bacterial communities in patients with inflammatory bowel disorders compared to controls are expected, with the requisite qualifications that differences in biospecimen type (fecal vs biopsy, luminal vs mucosal), intestinal location sampled, diet, antibiotic use, probiotic use, age of patient and other factors will impact the results (Daulatzai, 2014; Gevers et al., 2014; Hong and Rhee, 2014; Kostic et al., 2014). In patients with irritable bowel syndrome, most recent studies support disease-associated Firmicutes abundances and decreased Bacteroides, although there is a variability in relative species dynamics among the studies reviewed (Hong and Rhee, 2014). As such, enteropathologies in schizophrenia, as evident by autoimmune conditions, food sensitivities, and inflammation might be the result of altered gut microbial compositions.
4.1 The gut microbiome and the brain
Mental health research currently is hampered by a dearth of human data regarding resident biota and consequent effects on the CNS. Thus, the mechanisms by which microorganisms located at anatomical sites apart from the brain might impact neuronal connections are not known, but are surmised to involve metabolic, endocrine (cortisol), immunological or neuronal (vagus, enteric) routes (Clarke et al., 2013; Collins et al., 2012; Cryan and Dinan, 2012; Diaz Heijtz et al., 2011; Gareau et al., 2011). An analogous association between gut microbiota and behavior in people with schizophrenia may be gleaned from studies of autism spectrum disorders, where there are numerous reports of autism-related altered communities of the intestinal microbiome (Adams et al., 2011; Finegold et al., 2010; Finegold et al., 2012; Kang et al., 2013; Parracho et al., 2005; Williams et al., 2011; Williams et al., 2012). Further information regarding how the microbiome might impact the brain can be extrapolated from rodent studies, where research thus far shows that manipulations of gut microbiota result in behavioral, biochemical and molecular changes (Collins et al., 2012; Foster and McVey Neufeld, 2013; Hsiao et al., 2013; Stilling et al., 2014). Diaz-Heijtz, for example, documented that these behavioral effects were accompanied by modulations of the synaptic markers, synaptophysin and PSD95, in the striatum (Diaz Heijtz et al., 2011). In these animal studies, the use of gnotobiotic (germ-free) animals, vagectomy, probiotics and/or antibiotics each has been used to effectively recover animal phenotypes.
Preliminary studies in humans indicate that the pharyngeal and intestinal microbiome are altered in individuals with schizophrenia as compared to controls. These studies investigating the biological and pathogenic consequences of this altered microbiome are ongoing (Yolken and Dickerson, 2014).
4.2 Dietary considerations, immigrant status and the microbiome
The microbiome and its diversity are greatly affected by diet (David et al., 2014; Hong and Rhee, 2014; Kau et al., 2011). Dietary options are impacted by a multitude of cultural and lifestyle factors and may in turn play a role in contributing to certain risk factors for schizophrenia such as immigrant status. With some variability, being an immigrant from sub-Saharan African or Caribbean nations to the United Kingdom is a well-replicated risk factor for the development of schizophrenia (Cantor-Graae and Selten, 2005; Morgan et al., 2010; Selten et al., 2007; Sharpley et al., 2001). Some have attributed this risk to social explanations involving stress, discrimination, and exclusion (Eaton and Harrison, 2000, 2001; Selten and Cantor-Graae, 2005); others to vitamin D levels (McGrath, 1999); and yet others to cephalopelvic disproportion in the fetus (Warner, 1995); but see (Hutchinson et al., 1997). The high prevalence among Afro-Caribbeans is likely not due to genetic differences, because the prevalence in the countries of origin is not elevated when compared to rates in the UK’s general population (Bhugra et al., 1996; Hickling, 1991).
It could be that dietary influences related to wheat may contribute as one hit in the two-hit hypothesis scenarios in schizophrenia. Consumption and preparation of grains in Africa are usually different than in Europe and the U.S, both of which are heavy consumers of wheat- and corn-based products. In Africa, the primary type of grain produced is maize (corn) followed by millet, rice and sorghum foods, with wheat production at a very distant 5th (Haard et al., 1999). African grains are prepared via fermentation to produce an acid porridge that lacks the difficult-to-digest outer hull component (Gaffa et al., 2002; Steinkraus, 1996; Tou et al., 2006; Vieira-Dalode et al., 2007). Thus, when an immigrant raised on a grain fermentation-based diet first encounters western wheat-based foods, the individual is being exposed to a brand new, potentially antigenic, protein structure. The consequences of a suddenly altered diet on the microbiome, in turn, are likely to be significant.
In a study of the gut microbiome in children from urban centers in Europe compared to rural Africa, African children had significant abundances of Bacteroidetes, minimal Firmicutes and a number of unique species from the genuses Prevotella and Xylanobacter. These latter two species contain a set of genes needed for cellulose and xylan hydrolysis not typically required by people with an urban diet (De Filippo et al., 2010). The ability to digest certain foods is thus likely dictated by the resident microbiota repertoire, and the differential processing of food proteins by inefficient, dysfunctional or absent intestinal peptidases may be the result of altered resident gut microbiota. The diet-gut microbiome functional connection is further evident in an experimental comparison of a short-term animal-based diet with one predominantly composed of plant products fed to U.S. volunteers. The animal-based diet was associated with increased abundances of bile-tolerant microbes and decreases in those necessary to metabolize plant polysaccharides (David et al., 2014). The gut microbiome of rodent consumers of a Western diet has been found to reflect a generally low diversity of bacteria, accompanied by a higher circulating LPS and blood brain barrier permeability (Bested et al., 2013; Cani et al., 2007; Davidson et al., 2012; Freeman and Granholm, 2012).
4.3 Digestion of caseins and glutens by bacteria
Inefficiencies or disruption of the digestion powerhouses of the gut commensal bacteria might impact how certain food peptides become antigenic. Casein and gluten-derived peptides might be incompletely or aberrantly digested in schizophrenia, thus forming antigens associated with the foods that are novel. These novel antigens might prime the immune system differently upon first exposure to the subject food. Indeed, individuals with psychiatric disorders recognize epitopes within the food-derived antigenic proteins, which are not recognized by control individuals (Samaroo et al., 2010; Severance et al., 2010). Gluten is notoriously difficult to completely digest by resident digestive proteases (Caminero et al., 2014). In experimental analyses of human gut bacteria isolated from fecal samples, it was predominantly the Firmicutes and Actinobacteria that were deemed effective in metabolizing gluten and especially the Bifidobacterium and Lactobacillus genera (Caminero et al., 2014). Of interest, the bioactive peptides that are believed to trigger the inflammatory response in celiac disease were susceptible to digestion by several bacterial species including Lactobacillus mucosae, L. rhamnosus and Clostridium botulinum/sporogenes (Caminero et al., 2014). Another study identified in the oral microbiome, a series of bacteria with gluten-degrading enzymes including species of Rothia, Actinomyces, Streptococcus, Neisseria and Capnocytophaga (Fernandez-Feo et al., 2013). Given this preliminary evidence that the gut bacterial profile likely impacts an indivdual’s ability to digest gluten, it is not surprising that a gluten-free diet might aid in limiting the abundance and diversity of the Bifidobacteria (De Palma et al., 2009; Nistal et al., 2012). Interestingly, autistic patients on casein-free and gluten-free diets compared to those who received unrestricted diets generally show less immune-associated infiltration and intestinal permeability (Ashwood et al., 2003; Ashwood et al., 2004; de Magistris et al., 2010).
5. Conclusions and future directions
Our understanding of the gut and microbial effects on the brain in individuals with schizophrenia would be greatly advanced with carefully designed, case-control, longitudinal studies that incorporate biomarkers of physiological processes and behavioral indices. Biomarkers might include measurement of those molecules depicted in Figure 1 in conjunction with microbiome sampling from multiple biological sites and behavioral measures such as symptom and cognitive scores from Positive and Negative Syndrome Scale (PANSS) and the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (Kay et al., 1987; Randolph et al., 1998). Informative markers that associate with schizophrenia could be developed into screening tools to help define physiologically-based inclusion criteria for use in clinical trials. Toward this end, those individuals with schizophrenia who would most benefit from specific GI- and immune-based treatments could be identified. In autism, for example, a variety of outcomes of gluten-free and casein-free diets on symptoms have been reported, yet success of these diets were only apparent in those trials that specifically targeted individuals with GI pathologies who might respond to treatment (Elder, 2008; Hyman et al., 2010; Marcason, 2009; Reichelt and Knivsberg, 2009; Sponheim, 1991; Whiteley et al., 2010).
Other treatment modalities in need of careful clinical evaluation for safety and efficacy in schizophrenia include anti-inflammatory agents, dietary interventions, digestive enzyme-based aids, prebiotics, and probiotic supplementation. Additional compounds in development to treat celiac disease and gluten sensitivity might also be candidates for clinical trials in individuals with schizophrenia (Freeman, 2013). For example, recent reports in experimental in vitro and mouse models of celiac disease suggest that intestinal treatment with elafin, an endogenous serine protease inhibitor, normalized inflammation and intestinal barrier function (Galipeau et al., 2014). Another compound, sevelamer, that is used to treat symptoms of chronic kidney disease may be applicable to GI pathologies, as evident by a recent finding that this drug can bind bacterial proteins, prevent these proteins from translocating from the gut into circulation, and effectively reversing the “leaky gut” syndrome (Kristoff et al., 2014).
The dysfunction of gut and immune processes in schizophrenia hints at dysregulated pathways that might serve as promising targets for gene susceptibility studies. Genes in pathways that impact endothelial barriers, peptidases, antigen recognition or detection and monocyte activation are all possible candidates. Schizophrenia susceptibility loci identified by techniques such as GWAS should be re-evaluated for activity in these related biological pathways. Another intriguing hypothesis relates to the role of epigenetic machinery on host-microbe, gut-brain interactions as extensively reviewed by Stilling et al (Stilling et al., 2014).
In this paper, we have proposed a number of means by which the immune system, the GI tract, the microbiome and the brain might be connected in schizophrenia. While some of these links are hypothetical, they are consistent with data derived from numerous epidemiological, biological and experimental studies and consistent with a focus away from the traditional view that schizophrenia is a disease originating solely from the brain. Although some of the concepts presented here have been around for a long time, progress in this field is only beginning to accelerate.
Acknowledgments
Role of funding source
This work was supported by a NIMH P50 Silvio O. Conte Center at Johns Hopkins (grant# MH-94268) and by the Stanley Medical Research Institute. Dr. Eaton was supported by NIMH grant# 1R34MH100776-01. These funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
We thank Zita Erbowor-Becksen for a critical review of the manuscript and Ann Cusic for administrative support. This work was supported by a NIMH P50 Silvio O. Conte Center at Johns Hopkins (grant# MH-94268) and by the Stanley Medical Research Institute. Dr. Eaton was supported by NIMH grant# 1R34MH100776-01.
Footnotes
Contributors
Drs. Severance, Yolken and Eaton all wrote and approved the final manuscript.
Conflict of Interest
Robert Yolken is a member of the Stanley Medical Research Institute Board of Directors and Scientific Advisory Board. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict of interest policies. None of the other authors report any potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism--comparisons to typical children and correlation with autism severity. BMC Gastroenterology. 2011;11:22. doi: 10.1186/1471-230X-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaedini A, Green PH. Autoantibodies in celiac disease. Autoimmunity. 2008;41(1):19–26. doi: 10.1080/08916930701619219. [DOI] [PubMed] [Google Scholar]
- Alaedini A, Okamoto H, Briani C, Wollenberg K, Shill HA, Bushara KO, Sander HW, Green PH, Hallett M, Latov N. Immune cross-reactivity in celiac disease: anti-gliadin antibodies bind to neuronal synapsin I. Journal of Immunology. 2007;178(10):6590–6595. doi: 10.4049/jimmunol.178.10.6590. [DOI] [PubMed] [Google Scholar]
- Alander T, Svardsudd K, Johansson SE, Agreus L. Psychological illness is commonly associated with functional gastrointestinal disorders and is important to consider during patient consultation: a population-based study. BMC Med. 2005;3:8. doi: 10.1186/1741-7015-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancuta P, Kamat A, Kunstman KJ, Kim EY, Autissier P, Wurcel A, Zaman T, Stone D, Mefford M, Morgello S, Singer EJ, Wolinsky SM, Gabuzda D. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PloS One. 2008;3(6):e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition: DSM-5. 5. American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- Arakelyan A, Zakharyan R, Khoyetsyan A, Poghosyan D, Aroutiounian R, Mrazek F, Petrek M, Boyajyan A. Functional characterization of the complement receptor type 1 and its circulating ligands in patients with schizophrenia. BMC Clin Pathol. 2011;11:10. doi: 10.1186/1472-6890-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi A, Krasilowsky D, Levin S, Idar D, Kalian M, Or A, Ginat Y, Halperin B. Immunologic reaction of psychotic patients to fractions of gluten. American Journal of Psychiatry. 1979;136(10):1306–1309. doi: 10.1176/ajp.136.10.1306. [DOI] [PubMed] [Google Scholar]
- Ashorn S, Valineva T, Kaukinen K, Ashorn M, Braun J, Raukola H, Rantala I, Collin P, Maki M, Luukkaala T, Iltanen S. Serological responses to microbial antigens in celiac disease patients during a gluten-free diet. Journal of Clinical Immunology. 2009;29(2):190–195. doi: 10.1007/s10875-008-9255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Anthony A, Pellicer AA, Torrente F, Walker-Smith JA, Wakefield AJ. Intestinal lymphocyte populations in children with regressive autism: evidence for extensive mucosal immunopathology. Journal of Clinical Immunology. 2003;23(6):504–517. doi: 10.1023/b:joci.0000010427.05143.bb. [DOI] [PubMed] [Google Scholar]
- Ashwood P, Anthony A, Torrente F, Wakefield AJ. Spontaneous mucosal lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms: mucosal immune activation and reduced counter regulatory interleukin-10. Journal of Clinical Immunology. 2004;24(6):664–673. doi: 10.1007/s10875-004-6241-6. [DOI] [PubMed] [Google Scholar]
- Baldwin J. Schizophrenia and physical disease: a preliminary analysis of the data from the Oxford Record Linkage Study. In: Hemmings G, editor. Biochemistry of Schizophrenia and Addiction. MTP Press; Lancaster, England: 1980. [Google Scholar]
- Ben-Ami Shor D, Orbach H, Boaz M, Altman A, Anaya J, Bizzaro N, Tincani A, Cervera R, Espinosa G, Stojanovich L, Rozman B, Bombardier S, De Vita S, Damoiseaux J, Villalta D, Tonutti E, Tozzoli R, Barzilai O, Ram M, Blank M, Agmon-Levin N, Shoenfeld Y. Gastrointestinal-associated autoantibodies in different autoimmune diseases. American Journal of Clinical and Experimental Immunology. 2012;1(1):49–55. [PMC free article] [PubMed] [Google Scholar]
- Bender L. Childhood schizophrenia. Psychiatric Quarterly. 1953;27(4):663–681. doi: 10.1007/BF01562517. [DOI] [PubMed] [Google Scholar]
- Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. The American Journal of Psychiatry. 2011;168(12):1303–1310. doi: 10.1176/appi.ajp.2011.11030516. [DOI] [PubMed] [Google Scholar]
- Benros ME, Pedersen MG, Rasmussen H, Eaton WW, Nordentoft M, Mortensen PB. A nationwide study on the risk of autoimmune diseases in individuals with a personal or a family history of schizophrenia and related psychosis. American Journal of Psychiatry. 2014;171(2):218–226. doi: 10.1176/appi.ajp.2013.13010086. [DOI] [PubMed] [Google Scholar]
- Benson MA, Sillitoe RV, Blake DJ. Schizophrenia genetics: dysbindin under the microscope. Trends Neurosci. 2004;27(9):516–519. doi: 10.1016/j.tins.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Bereswill S, Munoz M, Fischer A, Plickert R, Haag LM, Otto B, Kuhl AA, Loddenkemper C, Gobel UB, Heimesaat MM. Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS One. 2010;5(12):e15099. doi: 10.1371/journal.pone.0015099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bested AC, Logan AC, Selhub EM. Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances: Part II - contemporary contextual research. Gut Pathog. 2013;5(1):3. doi: 10.1186/1757-4749-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beumer W, Drexhage RC, De Wit H, Versnel MA, Drexhage HA, Cohen D. Increased level of serum cytokines, chemokines and adipokines in patients with schizophrenia is associated with disease and metabolic syndrome. Psychoneuroendocrinology. 2012a;37(12):1901–1911. doi: 10.1016/j.psyneuen.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Beumer W, Gibney SM, Drexhage RC, Pont-Lezica L, Doorduin J, Klein HC, Steiner J, Connor TJ, Harkin A, Versnel MA, Drexhage HA. The immune theory of psychiatric diseases: a key role for activated microglia and circulating monocytes. J Leukoc Biol. 2012b;92(5):959–975. doi: 10.1189/jlb.0212100. [DOI] [PubMed] [Google Scholar]
- Bhugra D, Hilwig M, Hossein B, Marceau H, Neehall J, Leff J, Mallett R, Der G. First-contact incidence rates of schizophrenia in Trinidad and one-year follow-up. British Journal of Psychiatry. 1996;169(5):587–592. doi: 10.1192/bjp.169.5.587. [DOI] [PubMed] [Google Scholar]
- Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64(1):93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Boyajyan A, Khoyetsyan A, Tsakanova G, Sim RB. Cryoglobulins as indicators of upregulated immune response in schizophrenia. Clin Biochem. 2008;41(6):355–360. doi: 10.1016/j.clinbiochem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Briani C, Zara G, Alaedini A, Grassivaro F, Ruggero S, Toffanin E, Albergoni MP, Luca M, Giometto B, Ermani M, De Lazzari F, D’Odorico A, Battistin L. Neurological complications of celiac disease and autoimmune mechanisms: a prospective study. J Neuroimmunol. 2008;195(1–2):171–175. doi: 10.1016/j.jneuroim.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Brodziak A, Ziolko E, Muc-Wierzgon M, Nowakowska-Zajdel E, Kokot T, Klakla K. The role of human endogenous retroviruses in the pathogenesis of autoimmune diseases. Med Sci Monit. 2012;18(6):RA80–88. doi: 10.12659/MSM.882892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscaino V. Patologia extraneurale della schizofrenia. Fegato, tubo digerente, sistema reticolo-endoteliale. Acta neurologica. 1953;VIII:1–60. [Google Scholar]
- Cade R, Wagemaker H, Privette RM, Fregly MJ, Rogers J, Orlando J. The Effect of Dialysis and Diet on Schizophrenia. Elsevier; 1990. [Google Scholar]
- Camargo LM, Collura V, Rain JC, Mizuguchi K, Hermjakob H, Kerrien S, Bonnert TP, Whiting PJ, Brandon NJ. Disrupted in Schizophrenia 1 Interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Molecular Psychiatry. 2007;12(1):74–86. doi: 10.1038/sj.mp.4001880. [DOI] [PubMed] [Google Scholar]
- Caminero A, Herran AR, Nistal E, Perez-Andres J, Vaquero L, Vivas S, Ruiz de Morales JM, Albillos SM, Casqueiro J. Diversity of the cultivable human gut microbiome involved in gluten metabolism: isolation of microorganisms with potential interest for coeliac disease. FEMS Microbiol Ecol. 2014;88(2):309–319. doi: 10.1111/1574-6941.12295. [DOI] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Cantor-Graae E, Selten JP. Schizophrenia and migration: a meta-analysis and review. American Journal of Psychiatry. 2005;162(1):12–24. doi: 10.1176/appi.ajp.162.1.12. [DOI] [PubMed] [Google Scholar]
- Cascella NG, Kryszak D, Bhatti B, Gregory P, Kelly DL, Mc Evoy JP, Fasano A, Eaton WW. Prevalence of celiac disease and gluten sensitivity in the United States clinical antipsychotic trials of intervention effectiveness study population. Schizophrenia Bulletin. 2011;37(1):94–100. doi: 10.1093/schbul/sbp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catassi C, Bai JC, Bonaz B, Bouma G, Calabro A, Carroccio A, Castillejo G, Ciacci C, Cristofori F, Dolinsek J, Francavilla R, Elli L, Green P, Holtmeier W, Koehler P, Koletzko S, Meinhold C, Sanders D, Schumann M, Schuppan D, Ullrich R, Vecsei A, Volta U, Zevallos V, Sapone A, Fasano A. Non-celiac gluten sensitivity: the new frontier of gluten related disorders. Nutrients. 2013;5(10):3839–3853. doi: 10.3390/nu5103839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S-J, Chao Y-L, Chen C-Y, Chang C-M, Wu EC-H, Wu C-S, Yeh H-H, Chen C-H, Tsai H-J. Prevalence of autoimmune diseases in in-patients with schizophrenia: nationwide population-based study. British Journal of Psychiatry. 2012;200(5):374–380. doi: 10.1192/bjp.bp.111.092098. [DOI] [PubMed] [Google Scholar]
- Chu Y, Jin X, Parada I, Pesic A, Stevens B, Barres B, Prince DA. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc Natl Acad Sci U S A. 2010;107(17):7975–7980. doi: 10.1073/pnas.0913449107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Molecular Psychiatry. 2013;18(6):666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- Clemente MG, De Virgiliis S, Kang JS, Macatagney R, Musu MP, Di Pierro MR, Drago S, Congia M, Fasano A. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut. 2003;52(2):218–223. doi: 10.1136/gut.52.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology. 2009;136(6):2003–2014. doi: 10.1053/j.gastro.2009.01.075. [DOI] [PubMed] [Google Scholar]
- Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10(11):735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- Corvin A, Morris DW. Genome-wide association studies: findings at the major histocompatibility complex locus in psychosis. Biological Psychiatry. 2014;75(4):276–283. doi: 10.1016/j.biopsych.2013.09.018. [DOI] [PubMed] [Google Scholar]
- Craven M, Egan CE, Dowd SE, McDonough SP, Dogan B, Denkers EY, Bowman D, Scherl EJ, Simpson KW. Inflammation drives dysbiosis and bacterial invasion in murine models of ileal Crohn’s disease. PloS One. 2012;7(7):e41594. doi: 10.1371/journal.pone.0041594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nature reviews. Neuroscience. 2012;13(10):701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- Daulatzai MA. Chronic functional bowel syndrome enhances gut-brain axis dysfunction, neuroinflammation, cognitive impairment, and vulnerability to dementia. Neurochem Res. 2014;39(4):624–644. doi: 10.1007/s11064-014-1266-6. [DOI] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Monnot A, Neal AU, Martin AA, Horton JJ, Zheng W. The effects of a high-energy diet on hippocampal-dependent discrimination performance and blood-brain barrier integrity differ for diet-induced obese and diet-resistant rats. Physiol Behav. 2012;107(1):26–33. doi: 10.1016/j.physbeh.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107(33):14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fontaine L, Schwarz MJ, Riedel M, Dehning S, Douhet A, Spellmann I, Kleindienst N, Zill P, Plischke H, Gruber R, Muller N. Investigating disease susceptibility and the negative correlation of schizophrenia and rheumatoid arthritis focusing on MIF and CD14 gene polymorphisms. Psychiatry Res. 2006;144(1):39–47. doi: 10.1016/j.psychres.2006.01.006. [DOI] [PubMed] [Google Scholar]
- de Magistris L, Familiari V, Pascotto A, Sapone A, Frolli A, Iardino P, Carteni M, De Rosa M, Francavilla R, Riegler G, Militerni R, Bravaccio C. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr. 2010;51(4):418–424. doi: 10.1097/MPG.0b013e3181dcc4a5. [DOI] [PubMed] [Google Scholar]
- De Meirleir KL, Khaiboullina SF, Fremont M, Hulstaert J, Rizvanov AA, Palotas A, Lombardi VC. Plasmacytoid dendritic cells in the duodenum of individuals diagnosed with myalgic encephalomyelitis are uniquely immunoreactive to antibodies to human endogenous retroviral proteins. In Vivo. 2013;27(2):177–187. [PMC free article] [PubMed] [Google Scholar]
- De Palma G, Nadal I, Collado MC, Sanz Y. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult human subjects. Br J Nutr. 2009;102(8):1154–1160. doi: 10.1017/S0007114509371767. [DOI] [PubMed] [Google Scholar]
- De Santis A, Addolorato G, Romito A, Caputo S, Giordano A, Gambassi G, Taranto C, Manna R, Gasbarrini G. Schizophrenia symptoms and SPECT abnormalities in a coeliac patient: regression after gluten-free diet. Journal of Internal Medicine. 1997;242(5):421–423. doi: 10.1046/j.1365-2796.1997.00200.x. [DOI] [PubMed] [Google Scholar]
- Dean B. Understanding the role of inflammatory-related pathways in the pathophysiology and treatment of psychiatric disorders: evidence from human peripheral studies and CNS studies. Int J Neuropsychopharmacol. 2010;14(7):997–1012. doi: 10.1017/S1461145710001410. [DOI] [PubMed] [Google Scholar]
- Deli MA. Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. Biochimica Et Biophysica Acta-Biomembranes. 2009;1788(4):892–910. doi: 10.1016/j.bbamem.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Demjaha A, MacCabe JH, Murray RM. How genes and environmental factors determine the different neurodevelopmental trajectories of schizophrenia and bipolar disorder. Schizophrenia Bulletin. 2012;38(2):209–214. doi: 10.1093/schbul/sbr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplat-Jego S, Johanet C, Escande A, Goetz J, Fabien N, Olsson N, Ballot E, Sarles J, Baudon JJ, Grimaud JC, Veyrac M, Chamouard P, Humbel RL. Update on anti-Saccharomyces cerevisiae antibodies, anti-nuclear associated anti-neutrophil antibodies and antibodies to exocrine pancreas detected by indirect immunofluorescence as biomarkers in chronic inflammatory bowel diseases: results of a multicenter study. World Journal of Gastroenterology: WJG. 2007;13(16):2312–2318. doi: 10.3748/wjg.v13.i16.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sabatino A, Vanoli A, Giuffrida P, Luinetti O, Solcia E, Corazza GR. The function of tissue transglutaminase in celiac disease. Autoimmun Rev. 2012;11(10):746–753. doi: 10.1016/j.autrev.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108(7):3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Lillehoj E, Stallings C, Wiley M, Origoni A, Vaughan C, Khushalani S, Sabunciyan S, Yolken R. Antibodies to retroviruses in recent onset psychosis and multi-episode schizophrenia. Schizophrenia Research. 2012;138(2–3):198–205. doi: 10.1016/j.schres.2012.03.037. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Leister F, Yang S, Krivogorsky B, Alaedini A, Yolken R. Markers of gluten sensitivity and celiac disease in recent-onset psychosis and multi-episode schizophrenia. Biological Psychiatry. 2010;68(1):100–104. doi: 10.1016/j.biopsych.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Dohan F. Coeliac disease and schizophrenia. British Medical Journal. 1973;3(5870):51–52. doi: 10.1136/bmj.3.5870.51-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohan F. Celiac disease and schizophrenia. The New England Journal of Medicine. 1980;302(22):1262. doi: 10.1056/NEJM198005293022216. [DOI] [PubMed] [Google Scholar]
- Dohan F. Genetic hypothesis of idiopathic schizophrenia: its exorphin connection. Schizophrenia Bulletin. 1988;14(4):489–494. doi: 10.1093/schbul/14.4.489. [DOI] [PubMed] [Google Scholar]
- Dohan FC. Wartime changes in hospital admissions for schizophrenia. A comparison of admission for schizophrenia and other psychoses in six countries during World War II. Acta Psychiatr Scand. 1966a;42(1):1–23. doi: 10.1111/j.1600-0447.1966.tb01912.x. [DOI] [PubMed] [Google Scholar]
- Dohan FC. Wheat “consumption” and hospital admissions for schizophrenia during World War II. A preliminary report. Am J Clin Nutr. 1966b;18(1):7–10. doi: 10.1093/ajcn/18.1.7. [DOI] [PubMed] [Google Scholar]
- Dohan FC. Coeliac disease and schizophrenia. Lancet. 1970;1(7652):897–898. doi: 10.1016/s0140-6736(70)91729-0. [DOI] [PubMed] [Google Scholar]
- Dohan FC, Grasberger JC. Relapsed schizophrenics: earlier discharge from the hospital after cereal-free, milk-free diet. American Journal of Psychiatry. 1973;130(6):685–688. doi: 10.1176/ajp.130.6.685. [DOI] [PubMed] [Google Scholar]
- Dohan FC, Grasberger JC, Lowell FM, Johnston HT, Jr, Arbegast AW. Relapsed schizophrenics: more rapid improvement on a milk- and cereal-free diet. Br J Psychiatry. 1969;115(522):595–596. doi: 10.1192/bjp.115.522.595. [DOI] [PubMed] [Google Scholar]
- Dohan FC, Harper EH, Clark MH, Rodrigue RB, Zigas V. Is schizophrenia rare if grain is rare? Biol Psychiatry. 1984;19(3):385–399. [PubMed] [Google Scholar]
- Dome P, Teleki Z, Kotanyi R. Paralytic ileus associated with combined atypical antipsychotic therapy. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):557–560. doi: 10.1016/j.pnpbp.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Douglas-Escobar M, Elliott E, Neu J. Effect of intestinal microbial ecology on the developing brain. JAMA Pediatr. 2013;167(4):374–379. doi: 10.1001/jamapediatrics.2013.497. [DOI] [PubMed] [Google Scholar]
- Drexhage RC, Knijff EM, Padmos RC, Heul-Nieuwenhuijzen L, Beumer W, Versnel MA, Drexhage HA. The mononuclear phagocyte system and its cytokine inflammatory networks in schizophrenia and bipolar disorder. Expert Rev Neurother. 2010;10(1):59–76. doi: 10.1586/ern.09.144. [DOI] [PubMed] [Google Scholar]
- Drexhage RC, Weigelt K, van Beveren N, Cohen D, Versnel MA, Nolen WA, Drexhage HA. Immune and neuroimmune alterations in mood disorders and schizophrenia. Int Rev Neurobiol. 2011;101:169–201. doi: 10.1016/B978-0-12-387718-5.00007-9. [DOI] [PubMed] [Google Scholar]
- Drysdale A, Deacon R, Lewis P, Olley J, Electricwala A, Sherwood R. A peptide-containing fraction of plasma from schizophrenic-patients which binds to opiate receptors and induces hyperreactivity in rats. Neuroscience. 1982;7(6):1567–1573. doi: 10.1016/0306-4522(82)90265-2. [DOI] [PubMed] [Google Scholar]
- Eaton W, Chen C-Y, Bromet EJ. Epidemiology of Schizophrenia. In: Tsuang M, Tohen M, Jones PB, editors. Textbook of Psychiatric Epidemiology. 3. Wiley-Blackwell; West Sussex, U.K: 2011. [Google Scholar]
- Eaton W, Hayward C, Ram R. Schizophrenia and rheumatoid arthritis: a review. Schizophrenia Research. 1992;6:181–192. doi: 10.1016/0920-9964(92)90001-l. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Byrne M, Ewald H, Mors O, Chen C-Y, Agerbo E, Mortensen PB. The association of schizophrenia and autoimmune diseases; linkage of Danish national registers. American Journal of Psychiatry. 2006;163:521–528. doi: 10.1176/appi.ajp.163.3.521. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Harrison G. Ethnic disadvantage and schizophrenia. Acta Psychiatric Scandinavica, supplementum. 2000;102(407):38–43. doi: 10.1034/j.1600-0447.2000.00007.x. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Harrison G. Life chances, life planning, and schizophrenia: a review and interpretation of research on social deprivation. International Journal of Mental Health. 2001;30(1):58–81. [Google Scholar]
- Eaton WW, Mortensen PB, Agerbo E, Byrne M, Mors O, Ewald H. Coeliac disease and schizophrenia: population based case control study with linkage of Danish national registers. BMJ. 2004;328(7437):438–439. doi: 10.1136/bmj.328.7437.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton WW, Pedersen MG, Nielsen PR, Mortensen PB. Autoimmune diseases, bipolar disorder, and non-affective psychosis. Bipolar Disorders. 2010;12(6):638–646. doi: 10.1111/j.1399-5618.2010.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder JH. The gluten-free, casein-free diet in autism: an overview with clinical implications. Nutr Clin Pract. 2008;23(6):583–588. doi: 10.1177/0884533608326061. [DOI] [PubMed] [Google Scholar]
- Erridge C, Duncan SH, Bereswill S, Heimesaat MM. The induction of colitis and ileitis in mice is associated with marked increases in intestinal concentrations of stimulants of TLRs 2, 4, and 5. PloS One. 2010;5(2):e9125. doi: 10.1371/journal.pone.0009125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanous AH, van den Oord EJ, Riley BP, Aggen SH, Neale MC, O’Neill FA, Walsh D, Kendler KS. Relationship between a high-risk haplotype in the DTNBP1 (dysbindin) gene and clinical features of schizophrenia. American Journal of Psychiatry. 2005;162(10):1824–1832. doi: 10.1176/appi.ajp.162.10.1824. [DOI] [PubMed] [Google Scholar]
- Fasano A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann N Y Acad Sci. 2012;1258:25–33. doi: 10.1111/j.1749-6632.2012.06538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Feo M, Wei G, Blumenkranz G, Dewhirst FE, Schuppan D, Oppenheim FG, Helmerhorst EJ. The cultivable human oral gluten-degrading microbiome and its potential implications in coeliac disease and gluten sensitivity. Clin Microbiol Infect. 2013;19(9):E386–394. doi: 10.1111/1469-0691.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, Youn E, Summanen PH, Granpeesheh D, Dixon D, Liu M, Molitoris DR, Green JA., 3rd Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16(4):444–453. doi: 10.1016/j.anaerobe.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Finegold SM, Downes J, Summanen PH. Microbiology of regressive autism. Anaerobe. 2012;18(2):260–262. doi: 10.1016/j.anaerobe.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L’Heureux D, Regulier EG, Richardson MW, Amini S, Morgello S, Khalili K, Rappaport J. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7(6):528–541. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Fourgeaud L, Boulanger LM. Synapse remodeling, compliments of the complement system. Cell. 2007;131(6):1034–1036. doi: 10.1016/j.cell.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Freeman HJ. Non-dietary forms of treatment for adult celiac disease. World J Gastrointest Pharmacol Ther. 2013;4(4):108–112. doi: 10.4292/wjgpt.v4.i4.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman LR, Granholm AC. Vascular changes in rat hippocampus following a high saturated fat and cholesterol diet. J Cereb Blood Flow Metab. 2012;32(4):643–653. doi: 10.1038/jcbfm.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Amico F. Is there an association between peripheral immune markers and structural/functional neuroimaging findings? Progress in Neuropsychopharmacology & Biological Psychiatry. 2014;48:295–303. doi: 10.1016/j.pnpbp.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Fukudome S, Yoshikawa M. Opioid peptides derived from wheat gluten: their isolation and characterization. FEBS Lett. 1992;296(1):107–111. doi: 10.1016/0014-5793(92)80414-c. [DOI] [PubMed] [Google Scholar]
- Gaffa T, Jideani IA, Nkama I. Traditional production, consumption and storage of Kunu--a non alcoholic cereal beverage. Plant Foods Hum Nutr. 2002;57(1):73–81. doi: 10.1023/a:1013129307086. [DOI] [PubMed] [Google Scholar]
- Galipeau HJ, Wiepjes M, Motta JP, Schulz JD, Jury J, Natividad JM, Pinto-Sanchez I, Sinclair D, Rousset P, Martin-Rosique R, Bermudez-Humaran L, Leroux JC, Murray JA, Smecuol E, Bai JC, Vergnolle N, Langella P, Verdu EF. Novel role of the serine protease inhibitor elafin in gluten-related disorders. Am J Gastroenterol. 2014;109(5):748–756. doi: 10.1038/ajg.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garate I, Garcia-Bueno B, Madrigal JL, Caso JR, Alou L, Gomez-Lus ML, Mico JA, Leza JC. Stress-induced neuroinflammation: role of the Toll-like receptor-4 pathway. Biological Psychiatry. 2013;73(1):32–43. doi: 10.1016/j.biopsych.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, Macqueen G, Sherman PM. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307–317. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- Genevay S, Di Giovine FS, Perneger TV, Silvestri T, Stingelin S, Duff G, Guerne PA. Association of interleukin-4 and interleukin-1B gene variants with Larsen score progression in rheumatoid arthritis. Arthritis Rheum. 2002;47(3):303–309. doi: 10.1002/art.10394. [DOI] [PubMed] [Google Scholar]
- Gevers D, Kugathasan S, Denson LA, Vazquez-Baeza Y, Van Treuren W, Ren B, Schwager E, Knights D, Song SJ, Yassour M, Morgan XC, Kostic AD, Luo C, Gonzalez A, McDonald D, Haberman Y, Walters T, Baker S, Rosh J, Stephens M, Heyman M, Markowitz J, Baldassano R, Griffiths A, Sylvester F, Mack D, Kim S, Crandall W, Hyams J, Huttenhower C, Knight R, Xavier RJ. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe. 2014;15(3):382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilvarry CM, Sham PC, Jones PB, Cannon M, Wright P, Lewis SW, Bebbington P, Toone BK, Murray RM. Family history of autoimmune diseases in psychosis. Schizophrenia Research. 1996;19(1):33–40. doi: 10.1016/0920-9964(95)00045-3. [DOI] [PubMed] [Google Scholar]
- Goddard CA, Butts DA, Shatz CJ. Regulation of CNS synapses by neuronal MHC class I. Proc Natl Acad Sci U S A. 2007;104(16):6828–6833. doi: 10.1073/pnas.0702023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff H, Handford A. Celiac Syndrome in the case histories of five schizophrenics. Psychiatric Quarterly. 1961;35:306–313. doi: 10.1007/BF01566581. [DOI] [PubMed] [Google Scholar]
- Grainger JR, Wohlfert EA, Fuss IJ, Bouladoux N, Askenase MH, Legrand F, Koo LY, Brenchley JM, Fraser ID, Belkaid Y. Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection. Nat Med. 2013;19(6):713–721. doi: 10.1038/nm.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PH, Alaedini A, Sander HW, Brannagan TH, 3rd, Latov N, Chin RL. Mechanisms underlying celiac disease and its neurologic manifestations. Cell Mol Life Sci. 2005;62(7–8):791–799. doi: 10.1007/s00018-004-4109-9. [DOI] [PubMed] [Google Scholar]
- Guandalini S, Assiri A. Celiac disease: a review. JAMA Pediatr. 2014;168(3):272–278. doi: 10.1001/jamapediatrics.2013.3858. [DOI] [PubMed] [Google Scholar]
- Guo AY, Sun J, Riley BP, Thiselton DL, Kendler KS, Zhao Z. The dystrobrevin-binding protein 1 gene: features and networks. Molecular Psychiatry. 2009;14(1):18–29. doi: 10.1038/mp.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Masand PS, Kaplan D, Bhandary A, Hendricks S. The relationship between schizophrenia and irritable bowel syndrome (IBS) Schizophrenia Research. 1997;23(3):265–268. doi: 10.1016/s0920-9964(96)00099-0. [DOI] [PubMed] [Google Scholar]
- Haard N, Odunfa S, Lee C, Qunitero-Ramirez R, Lorence-Quinones A, Wacher-Radarte C. Food and Agriculture Organization of the United Nations. 1999. Fermented cereals. A global perspective. [Google Scholar]
- Hadjivassiliou M, Boscolo S, Davies-Jones GA, Grunewald RA, Not T, Sanders DS, Simpson JE, Tongiorgi E, Williamson CA, Woodroofe NM. The humoral response in the pathogenesis of gluten ataxia. Neurology. 2002;58(8):1221–1226. doi: 10.1212/wnl.58.8.1221. [DOI] [PubMed] [Google Scholar]
- Hand TW, Dos Santos LM, Bouladoux N, Molloy MJ, Pagan AJ, Pepper M, Maynard CL, Elson CO, 3rd, Belkaid Y. Acute gastrointestinal infection induces long-lived microbiota-specific T cell responses. Science. 2012;337(6101):1553–1556. doi: 10.1126/science.1220961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havik B, Le Hellard S, Rietschel M, Lybaek H, Djurovic S, Mattheisen M, Muhleisen TW, Degenhardt F, Priebe L, Maier W, Breuer R, Schulze TG, Agartz I, Melle I, Hansen T, Bramham CR, Nothen MM, Stevens B, Werge T, Andreassen OA, Cichon S, Steen VM. The complement control-related genes CSMD1 and CSMD2 associate to schizophrenia. Biological Psychiatry. 2011;70(1):35–42. doi: 10.1016/j.biopsych.2011.01.030. [DOI] [PubMed] [Google Scholar]
- Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, Jahn HK, Dunay IR, Moter A, Gescher DM, Schumann RR, Gobel UB, Liesenfeld O. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. Journal of Immunology. 2006;177(12):8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- Hemmings G. Schizophrenia. Lancet. 2004;364(9442):1312–1313. doi: 10.1016/S0140-6736(04)17181-X. [DOI] [PubMed] [Google Scholar]
- Hermes G, Ajioka JW, Kelly KA, Mui E, Roberts F, Kasza K, Mayr T, Kirisits MJ, Wollmann R, Ferguson DJ, Roberts CW, Hwang JH, Trendler T, Kennan RP, Suzuki Y, Reardon C, Hickey WF, Chen L, McLeod R. Neurological and behavioral abnormalities, ventricular dilatation, altered cellular functions, inflammation, and neuronal injury in brains of mice due to common, persistent, parasitic infection. J Neuroinflammation. 2008;5:48. doi: 10.1186/1742-2094-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickling FW. Psychiatric hospital admission rates in Jamaica, 1971 and 1988. British Journal of Psychiatry. 1991;159:817–821. doi: 10.1192/bjp.159.6.817. [DOI] [PubMed] [Google Scholar]
- Hong SN, Rhee PL. Unraveling the ties between irritable bowel syndrome and intestinal microbiota. World Journal of Gastroenterology: WJG. 2014;20(10):2470–2481. doi: 10.3748/wjg.v20.i10.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins SJ, Humphreys M, Jayson MI. Cytokines in synovial fluid. I. The presence of biologically active and immunoreactive IL-1. Clinical and experimental immunology. 1988;72(3):422–427. [PMC free article] [PubMed] [Google Scholar]
- Hornig M. The role of microbes and autoimmunity in the pathogenesis of neuropsychiatric illness. Curr Opin Rheumatol. 2013;25(4):488–795. doi: 10.1097/BOR.0b013e32836208de. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Li S, Hu Y, Yu H, Luo F, Zhang Q, Zhu F. Implication of the env gene of the human endogenous retrovirus W family in the expression of BDNF and DRD3 and development of recent-onset schizophrenia. Schizophrenia Bulletin. 2011;37(5):988–1000. doi: 10.1093/schbul/sbp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290(5499):2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter LC, O’Hare A, Herron WJ, Fisher LA, Jones GE. Opioid peptides and dipeptidyl peptidase in autism. Dev Med Child Neurol. 2003;45(2):121–128. [PubMed] [Google Scholar]
- Hutchinson G, Takei N, Bhugra D, Fahy TA, Gilvarry C, Mallett R, Moran P, Leff J, Murray RM. Increased rate of psychosis among African-Caribbeans in Britain is not due to an excess of pregnancy and birth complications. British Journal of Psychiatry. 1997;171:145–147. doi: 10.1192/bjp.171.2.145. [DOI] [PubMed] [Google Scholar]
- Hyman S, Stewart PA, Smith T, Foley J, Cain U, Peck R, Morris DD, Wang H. The gluten free and casein free (GFCF) diet: a double blind, placebo controlled challenge study. International Meeting for Autism Research; Philadelphia, PA: 2010. [Google Scholar]
- Jackson J, Eaton W, Cascella N, Fasano A, Warfel D, Feldman S, Richardson C, Vyas G, Linthicum J, Santora D, Warren KR, Carpenter WT, Jr, Kelly DL. A gluten-free diet in people with schizophrenia and anti-tissue transglutaminase or anti-gliadin antibodies. Schizophrenia Research. 2012;140(1–3):262–263. doi: 10.1016/j.schres.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson N, Kristjansson E, Nilsson L. Schizophrenic psychosis disappearing after patient is given gluten-free diet. Lakartidningen. 1984;81(6):448–449. [PubMed] [Google Scholar]
- Jin SZ, Wu N, Xu Q, Zhang X, Ju GZ, Law MH, Wei J. A study of circulating gliadin antibodies in schizophrenia among a Chinese population. Schizophrenia Bulletin. 2010;38(3):514–518. doi: 10.1093/schbul/sbq111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong A, Huang SH. Blood-brain barrier drug discovery for central nervous system infections. Curr Drug Targets Infect Disord. 2005;5(1):65–72. doi: 10.2174/1568005053174672. [DOI] [PubMed] [Google Scholar]
- Jungerius BJ, Bakker SC, Monsuur AJ, Sinke RJ, Kahn RS, Wijmenga C. Is MYO9B the missing link between schizophrenia and celiac disease? Am J Med Genet B Neuropsychiatr Genet. 2007;147(3):351–355. doi: 10.1002/ajmg.b.30605. [DOI] [PubMed] [Google Scholar]
- Kabbani TA, Vanga RR, Leffler DA, Villafuerte-Galvez J, Pallav K, Hansen J, Mukherjee R, Dennis M, Kelly CP. Celiac disease or non-celiac gluten sensitivity? An approach to clinical differential diagnosis. Am J Gastroenterol. 2014;109(5):741–746. doi: 10.1038/ajg.2014.41. [DOI] [PubMed] [Google Scholar]
- Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, Krajmalnik-Brown R. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PloS One. 2013;8(7):e68322. doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson H, Bachmann S, Schroder J, McArthur J, Torrey EF, Yolken RH. Retroviral RNA identified in the cerebrospinal fluids and brains of individuals with schizophrenia. Proc Natl Acad Sci U S A. 2001;98(8):4634–4639. doi: 10.1073/pnas.061021998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474(7351):327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia bulletin. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- King DS. Statistical power of the controlled research on wheat gluten and schizophrenia. Biological Psychiatry. 1985;20(7):785–787. doi: 10.1016/0006-3223(85)90157-x. [DOI] [PubMed] [Google Scholar]
- Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel diseases: current status and the future ahead. Gastroenterology. 2014;146(6):1489–1499. doi: 10.1053/j.gastro.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotze LM, Nisihara RM, Utiyama SR, Kotze PG, Theiss PM, Olandoski M. Antibodies anti-Saccharomyces cerevisiae (ASCA) do not differentiate Crohn’s disease from celiac disease. Arq Gastroenterol. 2010;47(3):242–245. doi: 10.1590/s0004-28032010000300006. [DOI] [PubMed] [Google Scholar]
- Kraft BD, Westman EC. Schizophrenia, gluten, and low-carbohydrate, ketogenic diets: a case report and review of the literature. Nutr Metab (Lond) 2009;6:10. doi: 10.1186/1743-7075-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristoff J, Haret-Richter G, Ma D, Ribeiro RM, Xu C, Cornell E, Stock JL, He T, Mobley AD, Ross S, Trichel A, Wilson C, Tracy R, Landay A, Apetrei C, Pandrea I. Early microbial translocation blockade reduces SIV-mediated inflammation and viral replication. J Clin Invest. 2014;124(6):2802–2806. doi: 10.1172/JCI75090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachance LR, McKenzie K. Biomarkers of gluten sensitivity in patients with non-affective psychosis: a meta-analysis. Schizophrenia Research. 2014;152(2–3):521–527. doi: 10.1016/j.schres.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Lackner P, Hametner C, Beer R, Burger C, Broessner G, Helbok R, Speth C, Schmutzhard E. Complement factors C1q, C3 and C5 in brain and serum of mice with cerebral malaria. Malar J. 2008;7:207. doi: 10.1186/1475-2875-7-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert GP. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J Anim Sci. 2009;87(14 Suppl):E101–108. doi: 10.2527/jas.2008-1339. [DOI] [PubMed] [Google Scholar]
- Lammers KM, Lu R, Brownley J, Lu B, Gerard C, Thomas K, Rallabhandi P, Shea-Donohue T, Tamiz A, Alkan S, Netzel-Arnett S, Antalis T, Vogel SN, Fasano A. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology. 2008;135(1):194–204. e193. doi: 10.1053/j.gastro.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laterra J, Keep R, Betz LA, Goldstein G. Blood-Cerebrospinal Fluid Barrier. Lippincott-Raven; Philadelphia: 1999. [Google Scholar]
- Leboyer M, Tamouza R, Charron D, Faucard R, Perron H. Human endogenous retrovirus type W (HERV-W) in schizophrenia: a new avenue of research at the gene-environment interface. The World Journal of Biological Psychiatry. 2013;14(2):80–90. doi: 10.3109/15622975.2010.601760. [DOI] [PubMed] [Google Scholar]
- Leonard BE, Schwarz M, Myint AM. The metabolic syndrome in schizophrenia: is inflammation a contributing cause? J Psychopharmacol. 2012;26(5 Suppl):33–41. doi: 10.1177/0269881111431622. [DOI] [PubMed] [Google Scholar]
- Lidar M, Lipschitz N, Langevitz P, Barzilai O, Ram M, Porat-Katz BS, Pagnoux C, Guilpain P, Sinico RA, Radice A, Bizzaro N, Damoiseaux J, Tervaert JW, Martin J, Guillevin L, Bombardieri S, Shoenfeld Y. Infectious serologies and autoantibodies in Wegener’s granulomatosis and other vasculitides: novel associations disclosed using the Rad BioPlex 2200. Ann N Y Acad Sci. 2009;1173:649–657. doi: 10.1111/j.1749-6632.2009.04641.x. [DOI] [PubMed] [Google Scholar]
- Lillehoj EP, Ford GM, Bachmann S, Schroder J, Torrey EF, Yolken RH. Serum antibodies reactive with non-human primate retroviruses identified in acute onset schizophrenia. J Neurovirol. 2000;6(6):492–497. doi: 10.3109/13550280009091949. [DOI] [PubMed] [Google Scholar]
- Lindstrom LH, Besev G, Gunne LM, Terenius L. CSF levels of receptor-active endorphins in schizophrenic patients: correlations with symptomatology and monoamine metabolites. Psychiatry Research. 1986;19(2):93–100. doi: 10.1016/0165-1781(86)90001-6. [DOI] [PubMed] [Google Scholar]
- Lynch NJ, Willis CL, Nolan CC, Roscher S, Fowler MJ, Weihe E, Ray DE, Schwaeble WJ. Microglial activation and increased synthesis of complement component C1q precedes blood-brain barrier dysfunction in rats. Mol Immunol. 2004;40(10):709–716. doi: 10.1016/j.molimm.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Ma Y, Ramachandran A, Ford N, Parada I, Prince DA. Remodeling of dendrites and spines in the C1q knockout model of genetic epilepsy. Epilepsia. 2013;54(7):1232–1239. doi: 10.1111/epi.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Delanghe J, Bocchio Chiavetto L, Bignotti S, Tura GB, Pioli R, Zanardini R, Altamura CA. Haptoglobin polymorphism and schizophrenia: genetic variation on chromosome 16. Psychiatry Research. 2001;104(1):1–9. doi: 10.1016/s0165-1781(01)00298-0. [DOI] [PubMed] [Google Scholar]
- Maes M, Kubera M, Leunis JC. The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett. 2008;29(1):117–124. [PubMed] [Google Scholar]
- Maes M, Kubera M, Leunis JC, Berk M. Increased IgA and IgM responses against gut commensals in chronic depression: Further evidence for increased bacterial translocation or leaky gut. J Affect Disord. 2012a;141(1):55–62. doi: 10.1016/j.jad.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Maes M, Kubera M, Leunis JC, Berk M, Geffard M, Bosmans E. In depression, bacterial translocation may drive inflammatory responses, oxidative and nitrosative stress (O&NS), and autoimmune responses directed against O&NS-damaged neoepitopes. Acta Psychiatr Scand. 2012b;127(5):344–354. doi: 10.1111/j.1600-0447.2012.01908.x. [DOI] [PubMed] [Google Scholar]
- Mailian KR, Boiadzhian AS, Sogoian AF, Sim RB, Manukian LA. Concentration and protein composition of circulating immune complexes in the blood of patients with schizophrenia and subjects with positive familial history of disease. Zh Nevrol Psikhiatr Im S S Korsakova. 2005;105(4):55–60. [PubMed] [Google Scholar]
- Makikyro T, Karvonen JT, Hakko H, Nieminen P, Joukamaa M, Isohanni M, Jones P, Jarvelin MR. Comorbidity of hospital-treated psychiatric and physical disorders with special reference to schizophrenia: a 28 year follow-up of the 1966 northern Finland general population birth cohort. Public Health. 1998;112(4):221–228. doi: 10.1038/sj.ph.1900455. [DOI] [PubMed] [Google Scholar]
- Mallant-Hent R, Mary B, von Blomberg E, Yuksel Z, Wahab PJ, Gundy C, Meyer GA, Mulder CJ. Disappearance of anti-Saccharomyces cerevisiae antibodies in coeliac disease during a gluten-free diet. Eur J Gastroenterol Hepatol. 2006;18(1):75–78. doi: 10.1097/00042737-200601000-00013. [DOI] [PubMed] [Google Scholar]
- Mantis NJ, Rol N, Corthesy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4(6):603–611. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcason W. What is the current status of research concerning use of a gluten-free, casein-free diet for children diagnosed with autism? J Am Diet Assoc. 2009;109(3):572. doi: 10.1016/j.jada.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Mayilyan KR, Weinberger DR, Sim RB. The complement system in schizophrenia. Drug News Perspect. 2008;21(4):200–210. doi: 10.1358/dnp.2008.21.4.1213349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. Hypothesis: is low prenatal vitamin D a risk-modifying factor for schizophrenia? Schizophrenia Research. 1999;40(3):173–177. doi: 10.1016/s0920-9964(99)00052-3. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P. Chronic risperidone normalizes elevated pro-inflammatory cytokine and C-reactive protein production in omega-3 fatty acid deficient rats. Eur J Pharmacol. 2011;652(1–3):152–156. doi: 10.1016/j.ejphar.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellsop G, Koadlow L, Syme J, Whittingham S. Absence of rheumatoid arthritis on schizophrenia. Aust N Z J Med. 1974;4(3):247–252. doi: 10.1111/j.1445-5994.1974.tb03183.x. [DOI] [PubMed] [Google Scholar]
- Meng J, Yu H, Ma J, Wang J, Banerjee S, Charboneau R, Barke RA, Roy S. Morphine induces bacterial translocation in mice by compromising intestinal barrier function in a TLR-dependent manner. PloS One. 2013;8(1):e54040. doi: 10.1371/journal.pone.0054040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Mellor A, Buckley P. Total and differential white blood cell counts, high-sensitivity C-reactive protein, and the metabolic syndrome in non-affective psychoses. Brain, Behavior, and Immunity. 2012;31:82–89. doi: 10.1016/j.bbi.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modinos G, Iyegbe C, Prata D, Rivera M, Kempton MJ, Valmaggia LR, Sham PC, van Os J, McGuire P. Molecular genetic gene-environment studies using candidate genes in schizophrenia: a systematic review. Schizophrenia Research. 2013;150(2–3):356–365. doi: 10.1016/j.schres.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Mohamed S, Merskey H, Kazarian S, Disney T. An investigation of the possible inverse relationships between the occurrence of rheumatoid arthritis, osteoarthritis and schizophrenia. Can J Psychiatry. 1982;27(5):381–383. doi: 10.1177/070674378202700505. [DOI] [PubMed] [Google Scholar]
- Morgan C, Charalambides M, Hutchinson G, Murray RM. Migration, ethnicity, and psychosis: toward a sociodevelopmental model. Schizophrenia Bulletin. 2010;36(4):655–664. doi: 10.1093/schbul/sbq051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen PB, Norgaard-Pedersen B, Waltoft BL, Sorensen TL, Hougaard D, Yolken RH. Early infections of Toxoplasma gondii and the later development of schizophrenia. Schizophrenia Bulletin. 2007;33(3):741–744. doi: 10.1093/schbul/sbm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N, Wagner JK, Krause D, Weidinger E, Wildenauer A, Obermeier M, Dehning S, Gruber R, Schwarz MJ. Impaired monocyte activation in schizophrenia. Psychiatry Research. 2012;198(3):341–346. doi: 10.1016/j.psychres.2011.12.049. [DOI] [PubMed] [Google Scholar]
- Munoz M, Heimesaat MM, Danker K, Struck D, Lohmann U, Plickert R, Bereswill S, Fischer A, Dunay IR, Wolk K, Loddenkemper C, Krell HW, Libert C, Lund LR, Frey O, Holscher C, Iwakura Y, Ghilardi N, Ouyang W, Kamradt T, Sabat R, Liesenfeld O. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrixmetalloproteinase-2 and IL-22 but independent of IL-17. The Journal of Experimental Medicine. 2009;206(13):3047–3059. doi: 10.1084/jem.20090900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagyoszi P, Wilhelm I, Farkas AE, Fazakas C, Dung NT, Hasko J, Krizbai IA. Expression and regulation of toll-like receptors in cerebral endothelial cells. Neurochem Int. 2010;57(5):556–564. doi: 10.1016/j.neuint.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Niebuhr DW, Li Y, Cowan DN, Weber NS, Fisher JA, Ford GM, Yolken R. Association between bovine casein antibody and new onset schizophrenia among US military personnel. Schizophrenia Research. 2011;128(1–3):51–55. doi: 10.1016/j.schres.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Nistal E, Caminero A, Vivas S, Ruiz de Morales JM, Saenz de Miera LE, Rodriguez-Aparicio LB, Casqueiro J. Differences in faecal bacteria populations and faecal bacteria metabolism in healthy adults and celiac disease patients. Biochimie. 2012;94(8):1724–1729. doi: 10.1016/j.biochi.2012.03.025. [DOI] [PubMed] [Google Scholar]
- Ogasawara H, Kageyama M, Yamaji K, Takasaki Y. The possibility that autoimmune disease can be induced by a molecular mimicry mechanism between autoantigen and human endogenous retrovirus. Lupus. 2010;19(1):111–113. doi: 10.1177/0961203309106767. [DOI] [PubMed] [Google Scholar]
- Oken R, Schulzer M. At issue: Schizophrenia and rheumatoid arthritis: the negative association revisited. Schizophrenia Bulletin. 1999;25(4):625–638. doi: 10.1093/oxfordjournals.schbul.a033407. [DOI] [PubMed] [Google Scholar]
- Okusaga O, Yolken RH, Langenberg P, Sleemi A, Kelly DL, Vaswani D, Giegling I, Hartmann AM, Konte B, Friedl M, Mohyuddin F, Groer MW, Rujescu D, Postolache TT. Elevated gliadin antibody levels in individuals with schizophrenia. The World Journal of Biological Psychiatry. 2013;14(7):509–515. doi: 10.3109/15622975.2012.747699. [DOI] [PubMed] [Google Scholar]
- Oshitani N, Hato F, Matsumoto T, Jinno Y, Sawa Y, Hara J, Nakamura S, Seki S, Arakawa T, Kitano A, Kitagawa S, Kuroki T. Decreased anti-Saccharomyces cerevisiae antibody titer by mesalazine in patients with Crohn’s disease. J Gastroenterol Hepatol. 2000;15(12):1400–1403. doi: 10.1046/j.1440-1746.2000.02357.x. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Weinberger DR. Dysbindin and schizophrenia: it’s dopamine and glutamate all over again. Biol Psychiatry. 2011;69(1):2–4. doi: 10.1016/j.biopsych.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parracho HM, Bingham MO, Gibson GR, McCartney AL. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol. 2005;54(Pt 10):987–991. doi: 10.1099/jmm.0.46101-0. [DOI] [PubMed] [Google Scholar]
- Pedersen CB, Mors O, Bertelsen A, Waltoft BL, Agerbo E, McGrath JJ, Mortensen PB, Eaton WW. A comprehensive nationwide study of the incidence rate and lifetime risk for treated mental disorders. JAMA Psychiatry. 2014;71(5):573–581. doi: 10.1001/jamapsychiatry.2014.16. [DOI] [PubMed] [Google Scholar]
- Perron H, Hamdani N, Faucard R, Lajnef M, Jamain S, Daban-Huard C, Sarrazin S, LeGuen E, Houenou J, Delavest M, Moins-Teisserenc H, Bengoufa D, Yolken R, Madeira A, Garcia-Montojo M, Gehin N, Burgelin I, Ollagnier G, Bernard C, Dumaine A, Henrion A, Gombert A, Le Dudal K, Charron D, Krishnamoorthy R, Tamouza R, Leboyer M. Molecular characteristics of Human Endogenous Retrovirus type-W in schizophrenia and bipolar disorder. Transl Psychiatry. 2012;2:e201. doi: 10.1038/tp.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, O’Connor V. C1q: the perfect complement for a synaptic feast? Nat Rev Neurosci. 2008;9(11):807–811. doi: 10.1038/nrn2394. [DOI] [PubMed] [Google Scholar]
- Pilkington T. The coincidence of rheumatoid arthritis and schizophrenia. Journal of Nervous and Mental Disease. 1955;124(6):604–606. doi: 10.1097/00005053-195612000-00007. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Weinberger D, Kleinman J, Nasrallah H, Luchins D, Bigelow L, Linnoila M, Fischer SH, Bjornsson TD, Carman J, Gillin JC, Wyatt RJ. Wheat gluten challenge in schizophrenic patients. American Journal of Psychiatry. 1981;138(9):1208–1211. doi: 10.1176/ajp.138.9.1208. [DOI] [PubMed] [Google Scholar]
- Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63(8):801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Preskorn SH. CNS drug development. Part I: The early period of CNS drugs. J Psychiatr Pract. 2010;16(5):334–9. doi: 10.1097/01.pra.0000388628.44405.c0. [DOI] [PubMed] [Google Scholar]
- Pribiag H, Stellwagen D. Neuroimmune regulation of homeostatic synaptic plasticity. Neuropharmacology. 2014;78:13–22. doi: 10.1016/j.neuropharm.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Prichard JC. A treatise on insanity and other disorders affecting the mind. E.L. Carey & A. Hart; Philadelphia: 1837. [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN. The repeatable battery for the assessment of neuropsychological status (RBANS): Preliminary clinical validity. Journal of Clinical and Experimental Neuropsychology. 1998;20(3):310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- Reichelt KL, Hole K, Hamberger A, Saelid G, Edminson PD, Braestrup CB, Lingjaerde O, Ledaal P, Orbeck H. Biologically active peptide-containing fractions in schizophrenia and childhood autism. Adv Biochem Psychopharmacol. 1981;28:627–643. [PubMed] [Google Scholar]
- Reichelt KL, Knivsberg AM. The possibility and probability of a gut-to-brain connection in autism. Ann Clin Psychiatry. 2009;21(4):205–211. [PubMed] [Google Scholar]
- Reichelt KL, Landmark J. Specific IgA antibody increases in schizophrenia. Biological Psychiatry. 1995;37(6):410–413. doi: 10.1016/0006-3223(94)00176-4. [DOI] [PubMed] [Google Scholar]
- Reichelt KL, Seim AR, Reichelt WH. Could schizophrenia be reasonably explained by Dohan’s hypothesis on genetic interaction with a dietary peptide overload? Progress in Neuro-Psychopharmacology & Biological Psychiatry. 1996;20(7):1083–1114. doi: 10.1016/s0278-5846(96)00099-1. [DOI] [PubMed] [Google Scholar]
- Reichelt KL, Stensrud M. Increase in urinary peptides prior to the diagnosis of schizophrenia. Schizophrenia Research. 1998;34(3):211–213. doi: 10.1016/s0920-9964(98)00104-2. [DOI] [PubMed] [Google Scholar]
- Reichelt KL, Tveiten D, Knivsberg AM, Bronstad G. Peptides’ role in autism with emphasis on exorphins. Microb Ecol Health Dis. 2012:23. doi: 10.3402/mehd.v23i0.18958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter P. Extrapyramidal motor disturbances in dementia praecox. Acta Psychiatrica et Neurologica (KjøBenhavn) 1926;1:287–304. [Google Scholar]
- Rice JR, Ham CH, Gore WE. Another look at gluten in schizophrenia. American Journal of Psychiatry. 1978;135(11):1417–1418. doi: 10.1176/ajp.135.11.1417. [DOI] [PubMed] [Google Scholar]
- Riley B, Kuo PH, Maher BS, Fanous AH, Sun J, Wormley B, O’Neill FA, Walsh D, Zhao Z, Kendler KS. The dystrobrevin binding protein 1 (DTNBP1) gene is associated with schizophrenia in the Irish Case Control Study of Schizophrenia (ICCSS) sample. Schizophrenia Research. 2009;115(2–3):245–253. doi: 10.1016/j.schres.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami Nejad M, Rostami K, Cheraghipour K, Nazemalhosseini Mojarad E, Volta U, Al Dulaimi D, Zali MR. Celiac disease increases the risk of Toxoplasma gondii infection in a large cohort of pregnant women. Am J Gastroenterol. 2011;106(3):548–549. doi: 10.1038/ajg.2010.425. [DOI] [PubMed] [Google Scholar]
- Rupprecht TA, Angele B, Klein M, Heesemann J, Pfister HW, Botto M, Koedel U. Complement C1q and C3 are critical for the innate immune response to Streptococcus pneumoniae in the central nervous system. J Immunol. 2007;178(3):1861–1869. doi: 10.4049/jimmunol.178.3.1861. [DOI] [PubMed] [Google Scholar]
- Ryan LA, Zheng J, Brester M, Bohac D, Hahn F, Anderson J, Ratanasuwan W, Gendelman HE, Swindells S. Plasma levels of soluble CD14 and tumor necrosis factor-alpha type II receptor correlate with cognitive dysfunction during human immunodeficiency virus type 1 infection. The Journal of Infectious Diseases. 2001;184(6):699–706. doi: 10.1086/323036. [DOI] [PubMed] [Google Scholar]
- Samaroo D, Dickerson F, Kasarda DD, Green PH, Briani C, Yolken RH, Alaedini A. Novel immune response to gluten in individuals with schizophrenia. Schizophrenia Research. 2010;118(1–3):248–255. doi: 10.1016/j.schres.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol. 2012;10(9):655–666. doi: 10.1038/nrmicro2848. [DOI] [PubMed] [Google Scholar]
- Sapone A, Lammers KM, Casolaro V, Cammarota M, Giuliano MT, De Rosa M, Stefanile R, Mazzarella G, Tolone C, Russo MI, Esposito P, Ferraraccio F, Carteni M, Riegler G, de Magistris L, Fasano A. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC Med. 2011;9:23. doi: 10.1186/1741-7015-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneck JM. Gastro-intestinal symptomatology in schizophrenia. Am J Dig Dis. 1946;13:257–260. doi: 10.1007/BF03002853. [DOI] [PubMed] [Google Scholar]
- Schreiner M, Liesenfeld O. Small intestinal inflammation following oral infection with Toxoplasma gondii does not occur exclusively in C57BL/6 mice: review of 70 reports from the literature. Mem Inst Oswaldo Cruz. 2009;104(2):221–233. doi: 10.1590/s0074-02762009000200015. [DOI] [PubMed] [Google Scholar]
- Schwab SG, Albus M, Hallmayer J, Honig S, Borrmann M, Lichtermann D, Ebstein RP, Ackenheil M, Lerer B, Risch N. Evaluation of a susceptibility gene for schizophrenia on chromosome 6p by multipoint affected sib-pair linkage analysis. Nature Genetics. 1995;11(3):325–327. doi: 10.1038/ng1195-325. [DOI] [PubMed] [Google Scholar]
- Schwab SG, Knapp M, Freymann J, Albus M, Lerer B, Hallmayer J, Maier W, Wildenauer DB. The HLA DRB1 gene locus in schizophrenia: an association study in 55 families with linkage to chromosome 6p. Ninth Biennial Winter Workshop on Schizophrenia Research; Davos, Switzerland: Schizophrenia Research; 1998. pp. 1–216. [Google Scholar]
- Schwab SG, Knapp M, Mondabon S, Hallmayer J, Borrmann-Hassenbach M, Albus M, Lerer B, Rietschel M, Trixler M, Maier W, Wildenauer DB. Support for association of schizophrenia with genetic variation in the 6p22.3 gene, dysbindin, in sib-pair families with linkage and in an additional sample of triad families. Am J Hum Genet. 2003;72(1):185–190. doi: 10.1086/345463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellgren C, Frisell T, Lichtenstein P, Landen M, Askling J. Schizophrenia Bulletin. 2014. The association between schizophrenia and rheumatoid arthritis: a nationwide population-based Swedish study on intra-individual and familial risks. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selten JP, Cantor-Graae E. Social defeat: risk factor for schizophrenia? Br J Psychiatry. 2005;187:101–102. doi: 10.1192/bjp.187.2.101. [DOI] [PubMed] [Google Scholar]
- Selten JP, Cantor-Graae E, Kahn RS. Migration and schizophrenia. Current opinion in psychiatry. 2007;20(2):111–115. doi: 10.1097/YCO.0b013e328017f68e. [DOI] [PubMed] [Google Scholar]
- Severance EG, Alaedini A, Yang S, Halling M, Gressitt KL, Stallings CR, Origoni AE, Vaughan C, Khushalani S, Leweke FM, Dickerson FB, Yolken RH. Gastrointestinal inflammation and associated immune activation in schizophrenia. Schizophrenia Research. 2012a;138(1):48–53. doi: 10.1016/j.schres.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Dickerson FB, Halling M, Krivogorsky B, Haile L, Yang S, Stallings CR, Origoni AE, Bossis I, Xiao J, Dupont D, Haasnoot W, Yolken RH. Subunit and whole molecule specificity of the anti-bovine casein immune response in recent onset psychosis and schizophrenia. Schizophrenia Research. 2010;118(1–3):240–247. doi: 10.1016/j.schres.2009.12.030. [DOI] [PubMed] [Google Scholar]
- Severance EG, Gressitt KL, Halling M, Stallings CR, Origoni AE, Vaughan C, Khushalani S, Alaedini A, Dupont D, Dickerson FB, Yolken RH. Complement C1q formation of immune complexes with milk caseins and wheat glutens in schizophrenia. Neurobiol Dis. 2012b;48(3):447–453. doi: 10.1016/j.nbd.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Gressitt KL, Stallings CR, Origoni AE, Khushalani S, Leweke FM, Dickerson FB, Yolken RH. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophrenia Research. 2013;148(1–3):130–137. doi: 10.1016/j.schres.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Kannan G, Gressitt KL, Xiao J, Alaedini A, Pletnikov MV, Yolken RH. Anti-gluten immune response following Toxoplasma gondii infection in mice. PloS One. 2012c;7(11):e50991. doi: 10.1371/journal.pone.0050991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpley M, Hutchinson G, McKenzie K, Murray RM. Understanding the excess of psychosis among the African-Caribbean population in England. Review of current hypotheses. Br J Psychiatry Suppl. 2001;40:s60–68. doi: 10.1192/bjp.178.40.s60. [DOI] [PubMed] [Google Scholar]
- Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Mol Aspects Med. 2013;34(1):39–58. doi: 10.1016/j.mam.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe’er I, Dudbridge F, Holmans PA, Whittemore AS, Mowry BJ, Olincy A, Amin F, Cloninger CR, Silverman JM, Buccola NG, Byerley WF, Black DW, Crowe RR, Oksenberg JR, Mirel DB, Kendler KS, Freedman R, Gejman PV. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460(7256):753–757. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhom O, Laadhar L, Zitouni M, Ben AN, Rafrafi R, Kallel-Sellami M, Lahmar H, El HZ, Makni S. Spectrum of autoantibodies in tunisian psychiatric inpatients. Immunol Invest. 2012;41(5):538–549. doi: 10.3109/08820139.2012.685537. [DOI] [PubMed] [Google Scholar]
- Singh MM, Kay SR. Wheat gluten as a pathogenic factor in schizophrenia. Science. 1976;191(4225):401–402. doi: 10.1126/science.1246624. [DOI] [PubMed] [Google Scholar]
- Soderholm JD, Perdue MH. Stress and gastrointestinal tract. II. Stress and intestinal barrier function. Am J Physiol Gastrointest Liver Physiol. 2001;280(1):G7–G13. doi: 10.1152/ajpgi.2001.280.1.G7. [DOI] [PubMed] [Google Scholar]
- Sokolov O, Kost N, Andreeva O, Korneeva E, Meshavkin V, Tarakanova Y, Dadayan A, Zolotarev Y, Grachev S, Mikheeva I, Varlamov O, Zozulya A. Autistic children display elevated urine levels of bovine casomorphin-7 immunoreactivity. Peptides. 2014;56C:68–71. doi: 10.1016/j.peptides.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Sponheim E. Gluten-free diet in infantile autism. A therapeutic trial. Tidsskr Nor Laegeforen. 1991;111(6):704–707. [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, Werge T, Pietilainen OP, Mors O, Mortensen PB, Sigurdsson E, Gustafsson O, Nyegaard M, Tuulio-Henriksson A, Ingason A, Hansen T, Suvisaari J, Lonnqvist J, Paunio T, Borglum AD, Hartmann A, Fink-Jensen A, Nordentoft M, Hougaard D, Norgaard-Pedersen B, Bottcher Y, Olesen J, Breuer R, Moller HJ, Giegling I, Rasmussen HB, Timm S, Mattheisen M, Bitter I, Rethelyi JM, Magnusdottir BB, Sigmundsson T, Olason P, Masson G, Gulcher JR, Haraldsson M, Fossdal R, Thorgeirsson TE, Thorsteinsdottir U, Ruggeri M, Tosato S, Franke B, Strengman E, Kiemeney LA, Melle I, Djurovic S, Abramova L, Kaleda V, Sanjuan J, de Frutos R, Bramon E, Vassos E, Fraser G, Ettinger U, Picchioni M, Walker N, Toulopoulou T, Need AC, Ge D, Yoon JL, Shianna KV, Freimer NB, Cantor RM, Murray R, Kong A, Golimbet V, Carracedo A, Arango C, Costas J, Jonsson EG, Terenius L, Agartz I, Petursson H, Nothen MM, Rietschel M, Matthews PM, Muglia P, Peltonen L, St Clair D, Goldstein DB, Stefansson K, Collier DA. Common variants conferring risk of schizophrenia. Nature. 2009;460(7256):744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J, Bernstein HG, Schiltz K, Muller UJ, Westphal S, Drexhage HA, Bogerts B. Immune system and glucose metabolism interaction in schizophrenia: A chicken-egg dilemma. Progress in Neuropsychopharmacology & Biological Psychiatry. 2012;48:287–294. doi: 10.1016/j.pnpbp.2012.09.016. [DOI] [PubMed] [Google Scholar]
- Steinkraus K. Handbook of indigenous fermented foods. Marcel Dekker; New York: 1996. [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131(6):1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Stilling RM, Dinan TG, Cryan JF. Microbial genes, brain & behaviour - epigenetic regulation of the gut-brain axis. Genes Brain Behav. 2014;13(1):69–86. doi: 10.1111/gbb.12109. [DOI] [PubMed] [Google Scholar]
- Storms LH, Cloopton JM, Wright C. Effects of gluten on schizophrenics. Archives of General Psychiatry. 1982;39(3):323–327. doi: 10.1001/archpsyc.1982.04290030055010. [DOI] [PubMed] [Google Scholar]
- Straub RE, MacLean CJ, O’Neill FA, Burke J, Murphy B, Duke F, Shinkwin R, Web BT, Zhang J, Walsh D, Kendler KS. A potential vulnerability locus for schizophrenia on chromosome 6p24–22: evidence for genetic heterogeneity. Nature Genetics. 1995;11(3):287–293. doi: 10.1038/ng1195-287. [DOI] [PubMed] [Google Scholar]
- Taylor W. Schizophrenia, rheumatoid arthritis and trytophan metabolism. J Clin Psychiatry. 1978;39(6):499–503. [PubMed] [Google Scholar]
- Thomas KE, Sapone A, Fasano A, Vogel SN. Gliadin stimulation of murine macrophage inflammatory gene expression and intestinal permeability are MyD88-dependent: role of the innate immune response in celiac disease. Journal of immunology. 2006;176(4):2512–2521. doi: 10.4049/jimmunol.176.4.2512. [DOI] [PubMed] [Google Scholar]
- Torrente F, Anthony A, Heuschkel RB, Thomson MA, Ashwood P, Murch SH. Focal-enhanced gastritis in regressive autism with features distinct from Crohn’s and Helicobacter pylori gastritis. Am J Gastroenterol. 2004;99(4):598–605. doi: 10.1111/j.1572-0241.2004.04142.x. [DOI] [PubMed] [Google Scholar]
- Torrente F, Ashwood P, Day R, Machado N, Furlano RI, Anthony A, Davies SE, Wakefield AJ, Thomson MA, Walker-Smith JA, Murch SH. Small intestinal enteropathy with epithelial IgG and complement deposition in children with regressive autism. Molecular Psychiatry. 2002;7(4):375–382. 334. doi: 10.1038/sj.mp.4001077. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Bartko JJ, Lun ZR, Yolken RH. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophrenia Bulletin. 2007;33(3):729–736. doi: 10.1093/schbul/sbl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey EF, Yolken RH. The schizophrenia-rheumatoid arthritis connection: infectious, immune, or both? Brain Behav Immun. 2001;15(4):401–410. doi: 10.1006/brbi.2001.0649. [DOI] [PubMed] [Google Scholar]
- Tou EH, Guyot JP, Mouquet-Rivier C, Rochette I, Counil E, Traore AS, Treche S. Study through surveys and fermentation kinetics of the traditional processing of pearl millet (Pennisetum glaucum) into ben-saalga, a fermented gruel from Burkina Faso. Int J Food Microbiol. 2006;106(1):52–60. doi: 10.1016/j.ijfoodmicro.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Trevathan R, Tatum J. Rarity of concurrence of psychosis and rheumatoid arthritis in individual patients. Journal of Nervous and Mental Disease. 1953;120(1–2):83–84. [PubMed] [Google Scholar]
- Tripathi A, Lammers KM, Goldblum S, Shea-Donohue T, Netzel-Arnett S, Buzza MS, Antalis TM, Vogel SN, Zhao A, Yang S, Arrietta MC, Meddings JB, Fasano A. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci U S A. 2009;106(39):16799–16804. doi: 10.1073/pnas.0906773106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta T, Porro C, Calvello R, Panaro MA. Biological role of Toll-like receptor-4 in the brain. J Neuroimmunol. 2014;268(1–2):1–12. doi: 10.1016/j.jneuroim.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Tsuang M. Schizophrenia: genes and environment. Biological Psychiatry. 2000;47(3):210–220. doi: 10.1016/s0006-3223(99)00289-9. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Bogaert A, Schumacher J, Schulze TG, Otte AC, Ohlraum S, Kovalenko S, Becker T, Freudenberg J, Jonsson EG, Marja M-E, Sedvall GC, Czerski PM, Kapelski P, Hauser J, Maier W, Rietschel M, Propping P, Nothen MM, Cichon S. The DTNBP1 (Dysbindin) gene contributes to schizophrenia, depending on family history of the disease. American Journal of Human Genetics. 2003;73(6):1438–1443. doi: 10.1086/379928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heel DA, West J. Recent advances in coeliac disease. Gut. 2006;55(7):1037–1046. doi: 10.1136/gut.2005.075119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetlugina TP, Logvinovich GV, Maslennikova SN, Vasil’eva OA. Circulating immune complexes in the serum of mental patients and healthy subjects. Zh Nevropatol Psikhiatr Im S S Korsakova. 1984;84(3):422–426. [PubMed] [Google Scholar]
- Vieira-Dalode G, Jespersen L, Hounhouigan J, Moller PL, Nago CM, Jakobsen M. Lactic acid bacteria and yeasts associated with gowe production from sorghum in Benin. J Appl Microbiol. 2007;103(2):342–349. doi: 10.1111/j.1365-2672.2006.03252.x. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Gottesman II, Moises HW, Nicol S. Negative association between schizophrenia and rheumatoid arthritis. Schizophrenia Bulletin. 1991;17(4):669–678. doi: 10.1093/schbul/17.4.669. [DOI] [PubMed] [Google Scholar]
- Vlissides DM, Venulet A, Jenner FA. A double-blind gluten-free/gluten-load controlled trial in a secure ward population. Br J Psychiatry. 1986;148:447–452. doi: 10.1192/bjp.148.4.447. [DOI] [PubMed] [Google Scholar]
- Vojdani A, Bazargan M, Vojdani E, Samadi J, Nourian AA, Eghbalieh N, Cooper EL. Heat shock protein and gliadin peptide promote development of peptidase antibodies in children with autism and patients with autoimmune disease. Clin Diagn Lab Immunol. 2004a;11(3):515–524. doi: 10.1128/CDLI.11.3.515-524.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojdani A, O’Bryan T, Green JA, McCandless J, Woeller KN, Vojdani E, Nourian AA, Cooper EL. Immune response to dietary proteins, gliadin and cerebellar peptides in children with autism. Nutr Neurosci. 2004b;7(3):151–161. doi: 10.1080/10284150400004155. [DOI] [PubMed] [Google Scholar]
- Vojdani A, Pangborn JB, Vojdani E, Cooper EL. Infections, toxic chemicals and dietary peptides binding to lymphocyte receptors and tissue enzymes are major instigators of autoimmunity in autism. Int J Immunopathol Pharmacol. 2003;16(3):189–199. doi: 10.1177/039463200301600302. [DOI] [PubMed] [Google Scholar]
- Wan C, La Y, Zhu H, Yang Y, Jiang L, Chen Y, Feng G, Li H, Sang H, Hao X, Zhang G, He L. Abnormal changes of plasma acute phase proteins in schizophrenia and the relation between schizophrenia and haptoglobin (Hp) gene. Amino Acids. 2007;32(1):101–108. doi: 10.1007/s00726-005-0292-8. [DOI] [PubMed] [Google Scholar]
- Warner R. Time trends in schizophrenia: changes in obstetric risk factors with industrialization. Schizophrenia Bulletin. 1995;21(3):483–500. doi: 10.1093/schbul/21.3.483. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Someya T, Nawa H. Cytokine hypothesis of schizophrenia pathogenesis: evidence from human studies and animal models. Psychiatry and Clinical Neurosciences. 2010;64(3):217–230. doi: 10.1111/j.1440-1819.2010.02094.x. [DOI] [PubMed] [Google Scholar]
- Wei J, Hemmings GP. Gene, gut and schizophrenia: the meeting point for the gene-environment interaction in developing schizophrenia. Medical Hypotheses. 2005;64(3):547–552. doi: 10.1016/j.mehy.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Whiteley P, Haracopos D, Knivsberg AM, Reichelt KL, Parlar S, Jacobsen J, Seim A, Pedersen L, Schondel M, Shattock P. The ScanBrit randomised, controlled, single-blind study of a gluten- and casein-free dietary intervention for children with autism spectrum disorders. Nutr Neurosci. 2010;13(2):87–100. doi: 10.1179/147683010X12611460763922. [DOI] [PubMed] [Google Scholar]
- Williams BL, Hornig M, Buie T, Bauman ML, Cho Paik M, Wick I, Bennett A, Jabado O, Hirschberg DL, Lipkin WI. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PloS One. 2011;6(9):e24585. doi: 10.1371/journal.pone.0024585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BL, Hornig M, Parekh T, Lipkin WI. Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. MBio. 2012;3(1) doi: 10.1128/mBio.00261-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P, Dawson E, Donaldson PT, Underhill JA, Sham PC, Zhao J, Gill M, Nanko S, Owen MJ, McGuffin P, Murray RM. A transmission/disequilibrium study of the DRB1*04 gene locus on chromosome 6p21.3 with schizophrenia. Schizophrenia Research. 1998;32(2):75–80. doi: 10.1016/s0920-9964(98)00050-4. [DOI] [PubMed] [Google Scholar]
- Wright P, Sham PC, Gilvarry CM, Jones PB, Cannon M, Sharma T, Murray RM. Autoimmune diseases in the pedigrees of schizophrenic and control subjects. Schizophrenia Research. 1996;20(3):261–267. doi: 10.1016/0920-9964(96)82950-1. [DOI] [PubMed] [Google Scholar]
- Xiao J, Jones-Brando L, Talbot CC, Jr, Yolken RH. Differential effects of three canonical Toxoplasma strains on gene expression in human neuroepithelial cells. Infection and immunity. 2009;79(3):1363–1373. doi: 10.1128/IAI.00947-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wan C, Li H, Zhu H, La Y, Xi Z, Chen Y, Jiang L, Feng G, He L. Altered levels of acute phase proteins in the plasma of patients with schizophrenia. Anal Chem. 2006;78(11):3571–3576. doi: 10.1021/ac051916x. [DOI] [PubMed] [Google Scholar]
- Yolken R, Dickerson F. The microbiome - the missing link in the pathogenesis of schizophrenia. Schizophrenia Research. 2014;153(Supplement 1):S16. [Google Scholar]
- Yolken RH, Dickerson FB, Torrey EF. and schizophrenia. Parasite Immunol. 2009;31(11):706–715. doi: 10.1111/j.1365-3024.2009.01131.x. [DOI] [PubMed] [Google Scholar]
- Yuzaki M. Synapse formation and maintenance by C1q family proteins: a new class of secreted synapse organizers. Eur J Neurosci. 2010;32(2):191–197. doi: 10.1111/j.1460-9568.2010.07346.x. [DOI] [PubMed] [Google Scholar]
- Zakharyan R, Khoyetsyan A, Arakelyan A, Boyajyan A, Gevorgyan A, Stahelova A, Mrazek F, Petrek M. Association of C1QB gene polymorphism with schizophrenia in Armenian population. BMC Med Genet. 2011;12:126. doi: 10.1186/1471-2350-12-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong F, McCombs CC, Olson JM, Elston RC, Stevens FM, McCarthy CF, Michalski JP. An autosomal screen for genes that predispose to celiac disease in the western counties of Ireland. Nature Genetics. 1996;14(3):329–333. doi: 10.1038/ng1196-329. [DOI] [PubMed] [Google Scholar]
- Zioudrou C, Streaty RA, Klee WA. Opioid peptides derived from food proteins. The exorphins. J Biol Chem. 1979;254(7):2446–2449. [PubMed] [Google Scholar]