Abstract
Background and Purpose
Mild cognitive impairment (MCI) precedes both Alzheimer's disease (AD) dementia and with Lewy bodies (DLB). We investigated proton magnetic resonance spectroscopy (MRS) characteristics of MCI patients who progressed to DLB compared to those who progressed to AD dementia or remained stable.
Methods
Consecutive MCI patients who underwent single voxel MRS at baseline and progressed to DLB (n=10) were identified during a median follow-up period of 18 months. From the same cohort, we identified age- and sex-matched MCI patients who progressed to AD dementia (n=27) or remained stable (n=20) during a similar follow-up period. This study was approved by the Institutional Review Board and informed consent was from every subject.
Results
MCI patients who progressed to AD dementia were characterized by lower N-acetylaspartate (NAA)/Cr ratio in the posterior cingulate voxel compared to those who progressed to DLB (p=0.001). Decreased NAA/Cr in the posterior cingulate voxel differentiated MCI patients who progressed to DLB from those who progressed to AD with an area under the receiver operating characteristic curve of 0.85 (p<0.001) on logistic regression analysis.
Conclusions
MRS may be useful in differentiating MCI patients with prodromal AD dementia from those with prodromal DLB for early disease-specific interventions.
Keywords: Magnetic resonance spectroscopy (MRS), mild cognitive impairment (MCI), dementia with Lewy Bodies (DLB), Alzheimer's disease, MRI
Introduction
Alzheimer's disease (AD) is the most common pathology underlying mild cognitive impairment (MCI)[1-4], but dementia with Lewy bodies (DLB)[5, 6] may also contribute to MCI in older adults[7]. There is increasing interest in identifying MCI patients with a high risk of progression to DLB and differentiating them from those at a greater risk for AD dementia in order to potentially treat or delay disease progression at the prodromal stages[5, 8-11]. Because of the heterogeneous pathologies underlying MCI, markers of neuronal integrity on proton magnetic resonance spectroscopy (MRS) may help to distinguish patients with MCI who progress to DLB or AD dementia[12].
MRS is a non-invasive technique for detecting brain biochemical changes associated with neurodegenerative diseases[12]. Lower levels of neuronal integrity marker N-acetylaspartate (NAA) to creatine (Cr) ratio in the posterior cingulate gyrus on MRS is associated with a higher risk of progression to AD dementia in patients with MCI[13]. However, it is not known whether this pattern of biochemical alterations is also evident in those with MCI who progress to DLB. DLB is characterized by occipital hypometabolism on 18F-fluoro-deoxy glucose positron emission tomography (FDG PET), which is not present in those with AD dementia[14]. We hypothesized that posterior cingulate gyrus and medial occipital lobe MRS metabolites might be useful in distinguishing patients with MCI who progress to AD dementia or DLB or remain MCI during follow-up. Furthermore, we hypothesized that the superior frontal gyrus MRS metabolites would be similar across MCI patients who progress to AD dementia or DLB or remain MCI during follow-up because superior frontal gyrus is not typically involved with AD and DLB in the early stages of the disease process.
Our objective in this study was to compare regional single-voxel MRS metabolite ratios in the posterior cingulated gyrus, superior frontal and medial occipital lobes in patients with MCI who progressed to AD dementia, DLB or were stable during an average follow-up period of 18 months.
Material and Methods
Patients
Patients with MCI were from two cohorts: 1) Mayo Clinic Alzheimer Disease Research Center (ADRC) which is a prospectively followed sample of patients with MCI or dementia; 2) Mayo Clinic Study of Aging, which is a prospective, population-based cohort of older adults without dementia in Olmsted County, Minnesota. We identified consecutive patients with MCI who underwent single voxel MRS studies at baseline from August 2005 through December 2011 and were subsequently diagnosed with probable DLB (n=10) after an average follow-up of 18 (range=12 to 32) months. From the same cohort, we also identified patients with MCI who underwent MRS studies at baseline and were diagnosed with AD dementia (n=27) or remained as MCI (stable MCI; n=20) during a similar follow-up period, and frequency matched on age, sex and education to patients with MCI who progressed to DLB. This study was approved by the Mayo Clinic Institutional Review Board, and informed consent for participation was obtained from every subject.
Diagnosis at the time of the MRS exams and at follow-up was made according to the established clinical criteria during a consensus conference involving neurologists, neuropsychologists, and nurses who evaluated the patients. The operational definition of MCI was based on criteria by Petersen et al[15] for the broad definition of MCI: cognitive complaint, cognitive function not normal for age, decline in cognition, essentially normal functional activities, and no dementia. Patients with MCI who were diagnosed as probable DLB at follow-up met the third Report of the DLB Consortium criteria[16], and those who were diagnosed as probable AD at follow-up met the National Institute of Neurological and Communicative Disorders and Stroke and the AD and Related Disorders Association (NINCDS-ADRDA) criteria[17]. Severity of parkinsonism was rated with the Unified Parkinson's Disease Rating Scale (UPDRS)[18, 19]. Patients with probable rapid eye movement sleep behavior disorder (pRBD) met the International Classification of Sleep Disorders-II diagnostic criteria B for pRBD[20]. All subjects underwent clinical evaluation and MRS studies within a five month period.
MRS
Patients underwent single voxel MRS studies on a 1.5-T scanner (GE Healthcare). A 3D high resolution magnetization prepared rapid gradient echo (MPRAGE) acquisition with TR/TE/TI = 7/3/900 ms; flip angle, 8 degrees; in-plane resolution of 1.0 mm, and a slice thickness of 1.2 mm was performed for MRS voxel placements.
MRS studies were performed with an automated single-voxel MRS package (PROBE/SV). The prescan algorithm of PROBE automatically adjusted the transmitter and receiver gains and the center frequency. The local magnetic field homogeneity was optimized with the three-plane auto-shim procedure, and the flip angle of the third water suppression pulse was adjusted for chemical shift water suppression prior to MRS acquisition. Point-resolved spectroscopy (PRESS) pulse sequence (repetition time, 2000 milliseconds; echo time, 30 milliseconds; 2048 data points; 128 excitations) was used for the examinations. Three 8cm3(2×2×2cm3) voxels were localized on the mid-sagittal T1-weighted image: 1) Posterior cingulate voxel included right and left posterior cingulate gyri and inferior precunei; 2) Frontal lobe voxel included the right and left medial superior frontal gyri; 3) Occipital lobe voxel included right and left medial occipital lobes (Figure 1).
Figure 1. Voxel Locations for Proton MR Spectroscopy.
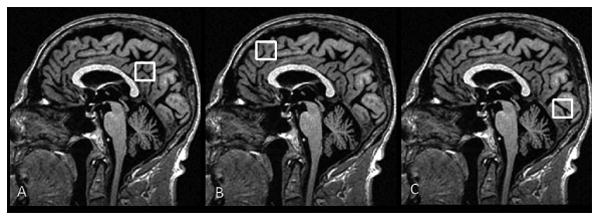
Three 8 cm3 (2×2×2cm3) voxels are localized on mid-sagittal T1-weighted images. A: Posterior cingulate voxel includes right and left posterior cingulate gyri and inferior precunei. Anterior inferior corner of the voxel is the anterior border of the splenium of the corpus callosum. B: Frontal lobe voxel includes the right and left medial superior frontal gyri. Posterior inferior corner of the voxel is the cingulate sulcus at the level of the anterior margin of the lateral ventricles. C: Occipital lobe voxel includes right and left medial occipital lobes. Anterior superior corner of the voxel is the parieto-occipital sulcus.
A radiologist (B.Z.) blinded to all clinical information performed quality control on the spectra and assessed the metabolite intensity ratios, which were automatically calculated at the end of each PROBE/SV acquisition using Cr as an internal reference metabolite. The metabolite ratios analyzed from posterior cingulate, occipital and frontal lobe voxels included NAA/Cr, Choline (Cho)/Cr and myoinositol (mI)/Cr ratios.
Statistical Analyses
Kruskal-Wallis and Chi-square tests were used to compare demographic variables, clinical characteristics such as the short test of mental status, Clinical Dementia Rating (CDR) sum of Boxes, Mini-Mental State Examination (MMSE), UPDRS, pRBD and follow-up time among the MCI patients who progressed to DLB, AD dementia, or were stable. Median (interquartile range) was reported for the MRS metabolite ratios and compared among MCI patients who progressed to DLB, AD dementia, or were stable using Wilcoxon rank-sum tests.
Receiver operator curves (ROC) were constructed using JMP9.0.0 (SAS Institute Inc., Cary, NC) for the significantly different MRS metabolite ratios in differentiating clinical groups. Accuracy of discrimination was based on the area under the ROC curve from logistic regression modeling.
Results
Patient Characteristics
Demographic and clinical characteristics of patients are presented in Table 1. Patients with MCI who progressed to DLB, AD dementia or were stable did not differ significantly on age, sex, and follow-up time. Education and cognitive function as indicated by Short Test of Mental Status, MMSE and CDR sum of boxes scores did not differ across the MCI groups at baseline. UPDRS differed significantly across MCI groups and the patients with MCI who progressed to DLB. (p<0.001).
Table 1. Patient characteristics at baseline MRI.
| MCI/DLB (n = 10) |
MCI/AD (n = 27) |
MCI Stable (n = 20) |
P-value | |
|---|---|---|---|---|
| Female (%) | 1(10.0%) | 11(40.7%) | 7(35.0%) | 0.21 |
| Age at scan, yr. | 68.3 (66.4, 82.9) | 79.9 (71.9, 84.2) | 79.5 (75.9, 83.3) | 0.16 |
| Time interval, day | 494 (366,867) | 494 (438,953) | 654(475,962) | 0.19 |
| Education, yr. | 14 (12, 19) | 13 (10, 17) | 14 (12, 16) | 0.33 |
| Short Test of Mental Status | 31 (30, 33) | 30 (28, 32) | 31 (29, 34) | 0.25 |
| CDR Sum of Boxes | 2 (0.88, 2.5) | 1 (0.5, 1.5) | 0.5 (0.5, 1.5) | 0.07 |
| MMSE | 26 (23, 27) | 25 (24, 27) | 26 (24, 28) | 0.61 |
| UPDRS | 8 (6, 11) | 0 (0, 3) | 0 (0, 5) | <0.001 |
Median (interquartile range) was reported for continuous variables. P-values for group-comparison are from Chi-square and Kruskal-Wallis Tests. MCI/DLB= Mild cognitive impairment (MCI) patients who progressed to dementia with Lewy bodies (DLB); MCI/AD =MCI patients who progressed to Alzheimer's disease (AD) dementia; MCI stable = MCI patients who remained MCI during follow up; MMSE: Mini Mental State Examination; CDR: Clinical Dementia Rating Sum of Boxes; UPDRS: Unified Parkinson's Disease Rating Scale;
MRS Findings
At baseline, patients with MCI who progressed to AD dementia were characterized by lower NAA/Cr ratio in the posterior cingulate voxel compared to MCI patients who progressed to DLB (p=0.001) (Figure 2). For an alpha of 0.05 and 80% power, NAA/Cr difference of at least 0.09 was was detectable when comparing MCI/AD and MCI/DLB. An NAA/Cr difference of 0.11 between these groups in the current study gave us 90% power at an alpha level of 0.05. There was a trend for lower NAA/Cr levels in patients with MCI who progressed to AD dementia compared to the stable MCI group (p=0.07) in the posterior cingulate voxel. No NAA/Cr differences were identified in occipital and frontal lobe voxels among the entire group of patients with MCI who progressed to DLB, AD dementia or were stable.
Figure 2. MRS findings from the Posterior Cingulate Voxel.
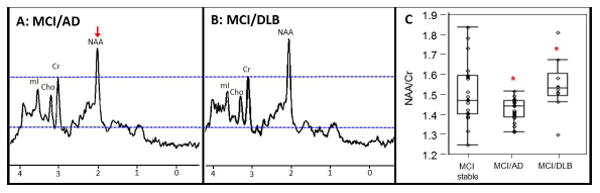
Spectra from the posterior cingulate voxel demonstrate decreased NAA in a patient with MCI who progressed to AD dementia (A) compared with a patient with MCI who progressed to DLB (B). All spectra are scaled to the height of the reference peak Cr, shown with a dotted line (A and B). Box plots show the differences in NAA/Cr among MCI patients who progressed to AD dementia (MCI/AD) (n=27), DLB (MCI/DLB) (n=10) and remained stable (MCI stable) (n=20), * p value =0.001 (C).
MRS metabolite ratios in clinical groups are summarized in Table 2. No differences in Cho/Cr levels of occipital and posterior cingulate voxel were identified among the clinical groups. There was a trend for higher Cho/Cr in the frontal lobe voxel in those with MCI who progressed to DLB compared to patients with MCI who progressed to AD dementia or were stable, but this did not reach statistical significance (p=0.08). We did not find differences in mI/Cr ratios across the clinical groups.
Table 2. MRS metabolite ratios at baseline in median (interquartile range) in MCI groups.
| Voxel and Metabolite ratio | MCI/DLB (n = 10) |
MCI/AD (n = 27) |
MCI Stable (n = 20) |
|---|---|---|---|
| NAA/Cr | |||
| Frontal voxel | 1.37 (1.33,1.43) | 1.34 (1.28,1.41) | 1.37 (1.25,1.49) |
| Occipital voxel | 1.58 (1.49,1.73) | 1.58 (1.49,1.66) | 1.57 (1.49,1.63) |
| Posterior cingulate voxel | 1.53 (1.49,1.61) | 1.45 (1.39,1.48) * | 1.48 (1.41,1.60) |
| Cho/Cr | |||
| Frontal voxel | 0.87 (0.84,0.96) | 0.81 (0.77,0.89) | 0.81 (0.74,0.95) |
| Occipital voxel | 0.48 (0.46,0.50) | 0.47 (0.43,0.52) | 0.49 (0.42,0.54) |
| Posterior cingulate voxel | 0.74 (0.63,0.78) | 0.72 (0.65,0.75) | 0.67 (0.63,0.76) |
| MI/Cr | |||
| Frontal voxel | 0.69 (0.65,0.73) | 0.72 (0.67,0.76) | 0.75 (0.66,0.81) |
| Occipital voxel | 0.65(0.58,0.76) | 0.61 (0.58,0.69) | 0.62 (0.60,0.70) |
| Posterior cingulate voxel | 0.71(0.66, 0.76) | 0.69 (0.66,0.75) | 0.73 (0.65,0.79) |
MCI/AD lower than MCI/DLB p=0.001 on Wilcoxon Rank Sum tests
MCI/DLB= Mild cognitive impairment (MCI) patients who progressed to dementia with Lewy bodies (DLB); MCI/AD =MCI patients who progressed to Alzheimer's disease (AD); MCI stable = MCI patients who remained MCI during follow up.
Receiver Operating Characteristic (ROC) Analysis
Among metabolite ratios from three regions, only NAA/Cr ratio in the posterior cingulate voxel distinguished patients with MCI who progressed to AD dementia from those who progressed to DLB. We investigated accuracy of the baseline NAA/Cr in distinguishing these patients with MCI using ROC analysis. Decreased NAA/Cr levels in the posterior cingulate voxel differentiated MCI patients who progressed to DLB from those who progressed to AD dementia with an area under the ROC curve of 0.85 (p<0.001) on logistic regression analysis as displayed in Table 3 and Figure 3.
Table 3. The ROC analysis between groups by NAA/Cr in PC.
| NAA/Cr in PC | |||
|---|---|---|---|
|
| |||
| MCI/DLB# vs. MCI/AD | MCI/DLB# vs. MCI stable | MCI/AD# vs. MCI stable | |
| AUROC | 0.85 | 0.61 | 0.66 |
| Specificity | 89% | 60% | 50% |
| Sensitivity | 80% | 80% | 80% |
| Cut-off value | 1.50 | 1.50 | 1.49 |
| P value | 0.00* | 0.61 | 0.01* |
positive level in ROC curve;
significant different (p < 0.05)
AUROC = area under ROC curve; PC= voxel in Posterior cingulate
Figure 3. ROC curves discriminating MCI patients with posterior cingulate voxel NAA/Cr.
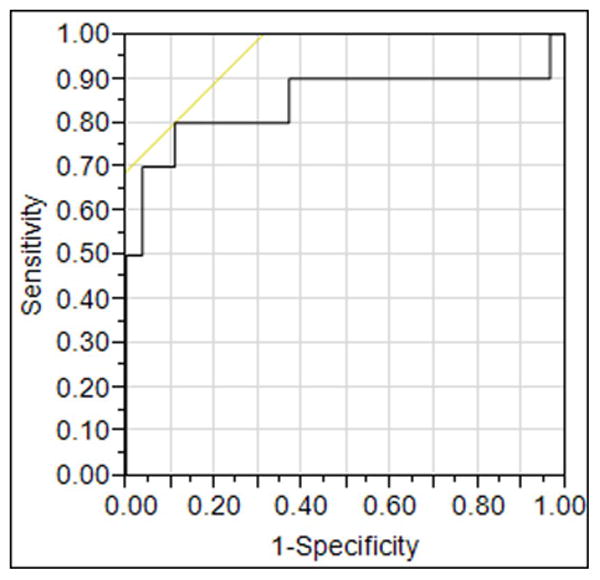
Decreased NAA/Cr levels in the posterior cingulate voxel differentiate MCI patients who progressed to DLB from those who progressed to AD with an area under the receiver operating characteristic curve (ROC) of 0.85 on logistic regression analysis (p<0.001).
Discussion
Findings of this study indicate that higher neuronal integrity marker NAA/Cr[21] in the posterior cingulate gyrus distinguishes patients with MCI who progress to DLB from those who progress to AD dementia within an average period of 18 months. However, we did not find statistically significant differences in metabolite ratios from the occipital and frontal lobe voxels when comparing MCI patients who progressed to DLB, AD dementia or were stable.
Our findings are consistent with prior studies showing that patients with DLB are characterized by higher NAA/Cr levels in the posterior cingulate gyrus compared to patients with AD dementia[22]. Preserved NAA/Cr in patients with DLB[22] and in patients with MCI who progress to DLB within 18 months, is consistent with the finding of preserved cortical neuronal density in patients with DLB at autopsy[23, 24]. In contrast, AD dementia is characterized by neurofibrillary tangle pathology and neurodegeneration in the posterior cingulate gyrus early in the disease course[25]. Therefore, our current finding of lower NAA/Cr levels in patients with MCI who progressed to AD dementia compared to those who progressed to DLB is an indicator of early neurodegeneration in the posterior cingulate gyri of patients with prodromal AD dementia but not of patients with prodromal DLB.
Patients with MCI who progress to DLB may have a variety of cognitive domain-specific impairment pattern or MCI subtype, with the attention/executive and visuospatial domains most frequently impaired [26, 27]. Therefore, we did not separate patients with MCI into amnestic and non-amnestic subtypes due to the small sample size of MCI patients who progressed to DLB. However, our findings are in line with a previous report that decreased NAA/Cr ratio in the posterior cingulate gyri differentiates amnestic MCI who progress to AD dementia from those who progress to other dementias[28].
DLB is characterized by relatively preserved posterior cingulate gyrus metabolism on FDG PET, which can be visualized on FDG PET images as the “cingulate island sign”[29]. The relative preservation of posterior cingulate cortex metabolism in DLB has also shown high diagnostic accuracy when differentiating patients with DLB from those with AD dementia with a specificity of near 100% and sensitivity of 62-82%[29]. Although FDG PET findings in MCI patients who progress to DLB are unknown at this time, posterior cingulate hypometabolism is typically present in early AD dementia[30], amnestic MCI[31, 32] and APOE ε4 positive individuals who are at a higher risk for AD dementia[33]. Preserved posterior cingulate gyrus NAA/Cr ratio in patients with MCI who progressed to DLB in the current study is consistent with the relative integrity of posterior cingulate gyrus metabolism observed on FDG PET studies in patients with DLB [29].
We did not observe differences in Cho/Cr or in mI/Cr ratios among the MCI groups. The presence of a trend of higher frontal Cho/Cr levels in MCI patients who progressed to DLB compared to those who progressed to AD dementia raises the possibility of limited power in the current study. Further investigation with a sample that is larger and followed for a longer period of time may help to clarify whether this trend has any relevance. Elevated mI/Cr ratio is associated with β-amyloid load on PET in cognitively normal elderly[34], and is typically elevated in MCI and AD dementia[35]. Although higher mI/Cr ratio in the posterior cingulate voxel increases the risk of MCI in cognitively normal elderly[36], higher mI/Cr ratio is not associated with the risk of dementia in patients with MCI[37]. Patients with DLB also have elevated mI/Cr ratio in the posterior cingulate voxel likely due to higher β-amyloid load in patients with DLB compared to cognitively normal older adults[38, 39]. Thus, finding similar mI/Cr ratios in patients with MCI who progressed to AD dementia, DLB or were stable may be expected. Because β-amyloid load on PET tends to plateau once it reaches a certain threshold[40], it is possible that the mI/Cr ratio that is associated with β-amyloid load also plateaus in patients with MCI who progress to dementia[13].
In comparison to AD dementia, individuals with DLB and those with MCI who eventually develop DLB are disproportionately affected by visual-spatial perceptual impairments and up to 75% of DLB patients experience visual hallucinations[5],[41, 42]. SPECT and FDG PET studies have demonstrated occipital visual association area hypoperfusion and hypometabolism respectively in patients with DLB which distinguishes them from patients with AD dementia[43, 44]. In the current study, we did not find any metabolite changes in the occipital voxel in patients with MCI who progressed to DLB compared to those who progressed to AD dementia. There may be two explanations for this unexpected finding: 1) Metabolic abnormalities exist in the occipital lobes of patients with prodromal DLB but MRS at 1.5T scanners with limited metabolite measures is not sensitive enough to these abnormalities; 2) Metabolic abnormalities in the occipital lobes appear later in the disease course after patients with MCI progress to DLB[45]. Investigations of FDG PET metabolic abnormalities in patients with MCI who are at an increased risk for DLB are warranted, to clarify the temporal course of occipital lobe metabolic changes in DLB.
A limitation of our study is a relatively small sample size with an average of 18 months of follow-up, which did not allow performing time-to-event analysis. It is likely that some of the MCI patients classified as MCI stable had prodromal AD dementia or DLB at baseline, but with an average follow-up of 18 months, we were unable to differentiate these individuals. Second, diagnosis of DLB and AD were not confirmed at autopsy nor was amyloid PET imaging available in these subjects. Thus patients with DLB may have had additional AD pathology that was not manifest clinically. Presence of additional pathologies may have increased the variability of MRS metabolite ratios and weakened the power of differentiation of MCI/DLB and MCI/AD groups from MCI-stable using MRS biomarkers.
Current advances in development of disease-modifying treatments for AD, and the responsiveness to acetylcholinesterase inhibitor treatment and marked neuroleptic sensitivity of DLB patients generate a need for non-invasive surrogate markers for identification of AD and DLB pathologies early in the disease course for treatment planning [46]. In a prospectively followed sample of patients with MCI, NAA/Cr ratio measured with MRS from the posterior cingulate voxel differentiated patients with MCI who progressed to DLB from those who progressed to AD dementia. Identifying the pathologic underpinnings of MRS metabolite changes associated with AD and DLB will be critical for validating MRS as an early non-invasive imaging marker for differential diagnosis of these dementia syndromes at the prodromal stage. The value of imaging along with clinical features in predicting progression to DLB needs further investigation.
Acknowledgments
Supported by Paul Beeson Career Development Awards in Aging K23AG030935, NIH-R01AG040042, R01AG11378, P50AG44170, P50AG16574, U01AG06786, the Mangurian Foundation, and Robert H. and Clarice Smith and Abigail van Buren Alzheimer Disease Research Program.
Abbreviations
- MCI
mild cognitive impairment
- DLB
Dementia with Lewy bodies
- AD
Alzheimer's disease
- 1HMRS
proton magnetic resonance spectroscopy
References
- 1.Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, Smith GE, Jack CR., Jr Mild cognitive impairment: ten years later. Arch Neurol. 2009;66:1447–55. doi: 10.1001/archneurol.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knopman DS. Alzheimer disease biomarkers and insights into mild cognitive impairment. Neurology. 2013;80:978–80. doi: 10.1212/WNL.0b013e31828728ac. [DOI] [PubMed] [Google Scholar]
- 3.Morris JC. Revised criteria for mild cognitive impairment may compromise the diagnosis of Alzheimer disease dementia. Arch Neurol. 2012;69:700–08. doi: 10.1001/archneurol.2011.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–79. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferman T, Smith G, Pankratz V, Kantarci K, Boeve B, Graff-Radford N, Uitti R, Wszolek Z, Van Gerpen J, Pedraza O, Knopman D, Dickson D, Petersen R. Non-amnestic mild cognitive impairment progresses to dementia with Lewy Bodies. American Academy of Neurology 65th Annual Meeting; Mar 16-23, 2013; San Diego, CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujishiro H, Nakamura S, Kitazawa M, Sato K, Iseki E. Early detection of dementia with Lewy bodies in patients with amnestic mild cognitive impairment using 123I-MIBG cardiac scintigraphy. J Neurol Sci. 2012;315:115–19. doi: 10.1016/j.jns.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Molano J, Boeve B, Ferman T, Smith G, Parisi J, Dickson D, Knopman D, Graff-Radford N, Geda Y, Lucas J, Kantarci K, Shiung M, Jack C, Silber M, Pankratz VS, Petersen R. Mild cognitive impairment associated with limbic and neocortical Lewy body disease: a clinicopathological study. Brain. 2010;133:540–56. doi: 10.1093/brain/awp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jack CR, Jr, Knopman DS, Weigand SD, Wiste HJ, Vemuri P, Lowe V, Kantarci K, Gunter JL, Senjem ML, Ivnik RJ, Roberts RO, Rocca WA, Boeve BF, Petersen RC. An operational approach to National Institute on Aging-Alzheimer's Association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71:765–75. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jack CR, Jr, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Lowe V, Kantarci K, Bernstein MA, Senjem ML, Gunter JL, Boeve BF, Trojanowski JQ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Knopman DS. Shapes of the trajectories of 5 major biomarkers of Alzheimer disease. Arch Neurol. 2012;69:856–67. doi: 10.1001/archneurol.2011.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boeve BF. REM sleep behavior disorder: Updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann N Y Acad Sci. 2010;1184:15–54. doi: 10.1111/j.1749-6632.2009.05115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boeve BF. Mild cognitive impairment associated with underlying Alzheimer's disease versus Lewy body disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S41–4. doi: 10.1016/S1353-8020(11)70015-3. [DOI] [PubMed] [Google Scholar]
- 12.Kantarci K. Proton MRS in mild cognitive impairment. J Magn Reson Imaging. 2013;37:770–77. doi: 10.1002/jmri.23800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kantarci K, Weigand SD, Petersen RC, Boeve BF, Knopman DS, Gunter J, Reyes D, Shiung M, O'Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Jack CR., Jr Longitudinal 1H MRS changes in mild cognitive impairment and Alzheimer's disease. Neurobiol Aging. 2007;28:1330–39. doi: 10.1016/j.neurobiolaging.2006.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minoshima S, Foster NL, Sima AA, Frey KA, Albin RL, Kuhl DE. Alzheimer's disease versus dementia with Lewy bodies: cerebral metabolic distinction with autopsy confirmation. Ann Neurol. 2001;50:358–65. doi: 10.1002/ana.1133. [DOI] [PubMed] [Google Scholar]
- 15.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 16.McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 17.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 18.The Unified Parkinson's Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003;18:738–50. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- 19.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–70. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 20.American Academy of Sleep Medicine. Diagnostic and Coding Manual. Chicago: 2005. International Classification of Sleep Disorders 2. [Google Scholar]
- 21.Benarroch EE. N-acetylaspartate and N-acetylaspartylglutamate: neurobiology and clinical significance. Neurology. 2008;70:1353–57. doi: 10.1212/01.wnl.0000311267.63292.6c. [DOI] [PubMed] [Google Scholar]
- 22.Kantarci K, Petersen RC, Boeve BF, Knopman DS, Tang-Wai DF, O'Brien PC, Weigand SD, Edland SD, Smith GE, Ivnik RJ, Ferman TJ, Tangalos EG, Jack CR., Jr 1H MR spectroscopy in common dementias. Neurology. 2004;63:1393–98. doi: 10.1212/01.wnl.0000141849.21256.ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez-Isla T, Growdon WB, McNamara M, Newell K, Gomez-Tortosa E, Hedley-Whyte ET, Hyman BT. Clinicopathologic correlates in temporal cortex in dementia with Lewy bodies. Neurology. 1999;53:2003–09. doi: 10.1212/wnl.53.9.2003. [DOI] [PubMed] [Google Scholar]
- 24.Yokota O, Tsuchiya K, Uchihara T, Ujike H, Terada S, Takahashi M, Kimura Y, Ishizu H, Akiyama H, Kuroda S. Lewy body variant of Alzheimer's disease or cerebral type lewy body disease? Two autopsy cases of presenile onset with minimal involvement of the brainstem. Neuropathology. 2007;27:21–35. doi: 10.1111/j.1440-1789.2006.00736.x. [DOI] [PubMed] [Google Scholar]
- 25.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 26.Ferman TJ, Smith GE, Boeve BF, Graff-Radford NR, Lucas JA, Knopman DS, Petersen RC, Ivnik RJ, Wszolek Z, Uitti R, Dickson DW. Neuropsychological differentiation of dementia with Lewy bodies from normal aging and Alzheimer's disease. Clin Neuropsychol. 2006;20:623–36. doi: 10.1080/13854040500376831. [DOI] [PubMed] [Google Scholar]
- 27.Mori E, Shimomura T, Fujimori M, Hirono N, Imamura T, Hashimoto M, Tanimukai S, Kazui H, Hanihara T. Visuoperceptual impairment in dementia with Lewy bodies. Arch Neurol. 2000;57:489–93. doi: 10.1001/archneur.57.4.489. [DOI] [PubMed] [Google Scholar]
- 28.Fayed N, Davila J, Oliveros A, Castillo J, Medrano JJ. Utility of different MR modalities in mild cognitive impairment and its use as a predictor of conversion to probable dementia. Acad Radiol. 2008;15:1089–98. doi: 10.1016/j.acra.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Lim SM, Katsifis A, Villemagne VL, Best R, Jones G, Saling M, Bradshaw J, Merory J, Woodward M, Hopwood M, Rowe CC. The 18F-FDG PET cingulate island sign and comparison to 123I-beta-CIT SPECT for diagnosis of dementia with Lewy bodies. J Nucl Med. 2009;50:1638–45. doi: 10.2967/jnumed.109.065870. [DOI] [PubMed] [Google Scholar]
- 30.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurology. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 31.Kantarci K, Senjem ML, Lowe VJ, Wiste HJ, Weigand SD, Kemp BJ, Frank AR, Shiung MM, Boeve BF, Knopman DS, Petersen RC, Jack CR., Jr Effects of age on the glucose metabolic changes in mild cognitive impairment. AJNR Am J Neuroradiol. 2010;31:1247–53. doi: 10.3174/ajnr.A2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosconi L, Perani D, Sorbi S, Herholz K, Nacmias B, Holthoff V, Salmon E, Baron JC, De Cristofaro MT, Padovani A, Borroni B, Franceschi M, Bracco L, Pupi A. MCI conversion to dementia and the APOE genotype: a prediction study with FDG-PET. Neurology. 2004;63:2332–40. doi: 10.1212/01.wnl.0000147469.18313.3b. [DOI] [PubMed] [Google Scholar]
- 33.Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–58. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 34.Kantarci K, Lowe V, Przybelski SA, Senjem ML, Weigand SD, Ivnik RJ, Roberts R, Geda YE, Boeve BF, Knopman DS, Petersen RC, Jack CR., Jr Magnetic resonance spectroscopy, beta-amyloid load, and cognition in a population-based sample of cognitively normal older adults. Neurology. 2011;77:951–58. doi: 10.1212/WNL.0b013e31822dc7e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kantarci K, Jack CR, Jr, Xu YC, Campeau NG, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Kokmen E, Tangalos EG, Petersen RC. Regional metabolic patterns in mild cognitive impairment and Alzheimer's disease: A 1H MRS study. Neurology. 2000;55:210–17. doi: 10.1212/wnl.55.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kantarci K, Weigand S, Przybelski S, Preboske G, Pankratz S, Vemuri P, Senjem M, Murphy M, Gunter J, Ivnik R, Roberts R, Boeve B, Rocca W, Knopman D, Petersen R, Jack C. MRI and MRS Predictors of Mild Cognitive Impairment in a Population–based Sample. Neurology. 2013;81:1–8. doi: 10.1212/WNL.0b013e31829a3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kantarci K, Weigand SD, Przybelski SA, Shiung MM, Whitwell JL, Negash S, Knopman DS, Boeve BF, O'Brien PC, Petersen RC, Jack CR., Jr Risk of dementia in MCI: combined effect of cerebrovascular disease, volumetric MRI, and 1H MRS. Neurology. 2009;72:1519–25. doi: 10.1212/WNL.0b013e3181a2e864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kantarci K, Yang C, Schneider JA, Senjem ML, Reyes DA, Lowe VJ, Barnes LL, Aggarwal NT, Bennett DA, Smith GE, Petersen RC, Jack CR, Jr, Boeve BF. Antemortem amyloid imaging and beta-amyloid pathology in a case with dementia with Lewy bodies. Neurobiol Aging. 2012;33:878–85. doi: 10.1016/j.neurobiolaging.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edison P, Rowe CC, Rinne JO, Ng S, Ahmed I, Kemppainen N, Villemagne VL, O'Keefe G, Nagren K, Chaudhury KR, Masters CL, Brooks DJ. Amyloid load in Parkinson's disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. J Neurol Neurosurg Psychiatry. 2008;79:1331–38. doi: 10.1136/jnnp.2007.127878. [DOI] [PubMed] [Google Scholar]
- 40.Jack CR, Jr, Wiste HJ, Lesnick TG, Weigand SD, Knopman DS, Vemuri P, Pankratz VS, Senjem ML, Gunter JL, Mielke MM, Lowe VJ, Boeve BF, Petersen RC. Brain beta-amyloid load approaches a plateau. Neurology. 2013;80:890–96. doi: 10.1212/WNL.0b013e3182840bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stavitsky K, Brickman AM, Scarmeas N, Torgan RL, Tang MX, Albert M, Brandt J, Blacker D, Stern Y. The progression of cognition, psychiatric symptoms, and functional abilities in dementia with Lewy bodies and Alzheimer disease. Arch Neurol. 2006;63:1450–56. doi: 10.1001/archneur.63.10.1450. [DOI] [PubMed] [Google Scholar]
- 42.Ala TA, Yang KH, Sung JH, Frey WH. Hallucinations and signs of parkinsonism help distinguish patients with dementia and cortical Lewy bodies from patients with Alzheimer's disease at presentation: a clinicopathological study. J Neurol Neurosurg Psychiatry. 1997;62:16–21. doi: 10.1136/jnnp.62.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasquier J, Michel BF, Brenot-Rossi I, Hassan-Sebbag N, Sauvan R, Gastaut JL. Value of (99m)Tc-ECD SPET for the diagnosis of dementia with Lewy bodies. Eur J Nucl Med Mol Imaging. 2002;29:1342–48. doi: 10.1007/s00259-002-0919-x. [DOI] [PubMed] [Google Scholar]
- 44.Fujishiro H, Iseki E, Kasanuki K, Murayama N, Ota K, Suzuki M, Sato K. Glucose hypometabolism in primary visual cortex is commonly associated with clinical features of dementia with Lewy bodies regardless of cognitive conditions. Int J Geriatr Psychiatry. 2012;27:1138–46. doi: 10.1002/gps.2836. [DOI] [PubMed] [Google Scholar]
- 45.Graff-Radford J, Boeve BF, Murray ME, Ferman TJ, Tosakulwong N, Lesnick TG, Maroney-Smith M, Senjem ML, Gunter J, Smith GE, Knopman DS, Jack CR, Jr, Dickson DW, Petersen RC, Kantarci K. Regional proton magnetic resonance spectroscopy patterns in dementia with Lewy bodies. Neurobiol Aging. 2014 doi: 10.1016/j.neurobiolaging.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Graff-Radford J, Boeve BF, Pedraza O, Ferman TJ, Przybelski S, Lesnick TG, Vemuri P, Senjem ML, Smith GE, Knopman DS, Lowe V, Jack CR, Jr, Petersen RC, Kantarci K. Imaging and acetylcholinesterase inhibitor response in dementia with Lewy bodies. Brain. 2012;135:2470–7. doi: 10.1093/brain/aws173. [DOI] [PMC free article] [PubMed] [Google Scholar]


