Abstract
Objective
Few studies evaluate factors that influence menstrual cycle length (MCL) during the menopausal transition (MT), a life-stage during which very long cycles become more likely to occur. The objective of this paper was to assess how BMI and race/ethnicity, factors associated with MCL in young women, influence MCL during the MT.
Methods
SWAN Menstrual Calendar Substudy data were available from 3 sites (southeastern Michigan, Los Angeles, and northern California) including African-American, White, Chinese, and Japanese women. Self-recorded monthly menstrual calendars with end of the month questions on hormone therapy use and smoking were collected from 1996–2006. Height and weight were measured at annual study visits. We used quantile regression to model MCL at the 25th, 50th, 75th, and 90th percentiles with bootstrap sampling to construct 95% confidence intervals. Models evaluated MCL with time indexed to the start of the MT (n=963) and to the final menstrual period (n=431).
Results
During the MT, increases in MCL occurred mostly in the right tail of the distribution, reflecting a lengthening of long menstrual cycles not of the median MCL. After adjusting for smoking, education, physical activity, and time, Chinese and Japanese women had 1–6 day longer MCLs as compared to White women. Obese women had 1–5 day longer MCLs as compared to non-obese women.
Conclusions
As occurs in younger women, menstrual characteristics during the MT are influenced by race/ethnicity and obesity. The long menstrual cycles characteristic of the MT are longer in obese women and in Chinese and Japanese women.
Keywords: menstrual cycle, menopause, race/ethnicity, body mass index, Chinese, Japanese
INTRODUCTION
The Stages of Reproductive Aging Workshop + 10 (STRAW+10) updated criteria for staging reproductive age and clarified the menstrual bleeding criteria for the early and late menopausal transition (MT). 1 However, few data are available that evaluate factors associated with population differences in menstrual cycle characteristics during this reproductive life-stage. Prior studies of younger women have documented that menstrual cycle length (MCL) is influenced by race/ethnicity 2–4 and by body mass index (BMI) 4–8. BMI also influences levels and trajectories of change in follicle-stimulating hormone (FSH) and estradiol (E2) during the MT. 9 The few extant menstrual calendar studies documenting women’s menstrual experience during the MT, with one exception include only White women. 10
The purpose of the present study was to assess the relationships of race/ethnicity and BMI with MCL among a multi-racial/ethnic cohort of midlife women in the Study of Women’s Health Across the Nation (SWAN). We used quantile regression to evaluate the pattern of MCL during the MT given the increased frequency of very long cycles. Linear regression is often used to examine mean MCL, however an assumption of this modeling technique is that MCL’s are normally distribution. However, the distribution of MCL in perimenopausal women is right-skewed 11, so that the median is a more informative about central tendency than the mean. Quantile regression allows examination of factors that influence the median as well as other quantiles of the MCL distribution. Furthermore, quantile regression allows us to identify factors that influence the median independently from factors that influence other sections of the distribution 12, including the important right-tail. Another benefit of quantile regression is that the coefficients are interpreted similar to linear regression with differences between groups quantified in days providing more clinically useful information than an odds ratio estimated from a logistic regression model.
METHODS
Study design and population
This analysis included women from the three SWAN sites --southeastern Michigan, Los Angeles, and northern California. While participants at all seven sites filled out menstrual calendars, women at these three study sites participated in a more comprehensive menstrual calendar substudy, which included additional end of the month questions including questions on current smoking and physical activity. The design of the main cohort study has been previously described. 13 Briefly, following a cross-sectional screening survey to assess eligibility, 1498 women were enrolled into the cohort study from the three substudy sites. Cohort eligibility criteria included age 42–52 years, self-designation as a member of a targeted racial/ethnic group, residence in the geographic area of one of the three clinic sites, the ability to speak English, Cantonese, or Japanese, ability to give verbal consents, an intact uterus, at least one menstrual period and no use of reproductive hormones in the previous 3 months, and not pregnant or lactating. Each site recruited White women and women from one specified minority group (African Americans in southeastern Michigan, Japanese in Los Angeles, and Chinese in northern California). Institutional Review Boards at each study site approved the protocol and all participants provided written, informed consent.
SWAN began enrollment in 1996 and annual follow-up visits have been conducted since then. Each visit consisted of interviewer-administered and self-administered questionnaires on a broad range of topics, including menstrual characteristics, socio-demographic characteristics, lifestyle, and medical history. Participants underwent physical assessments which included a blood draw and measurement of height and weight.
A self-administered menstrual calendar component began in 1996 and continued through 2006, corresponding to the tenth annual follow-up visit. Participants filled out menstrual calendars daily to capture days when any spotting or bleeding occurred. On the last day of the month, women indicated if no bleeding occurred and answered questions about hormone use and gynecological procedures which could affect bleeding, as well as cigarette use and physical exercise. Women were asked to maintain the monthly calendar for two years after they last bleed.
The sequence of women’s MCLs was assessed. MCL was calculated using World Health Organization bleeding definitions 14 adapted by the ReSTAGE Collaboration for application to perimenopausal women 15. A menstrual cycle consists of a bleeding episode and a subsequent bleed free interval of at least 3 days. Onset of the early MT was defined using the STRAW+10 definitions. 1 It is defined as the onset of a persistent difference of at least 7 days in the length of consecutive menstrual cycles. Persistence is defined as a re-occurrence within the next 10 observed cycles. The onset of early MT is defined by the date of the start of the menstrual cycle in the first pair of cycles that differ in length by 7 days. The final menstrual period (FMP) was defined as the first day of the bleeding episode followed by at least 12 months of amenorrhea. In order to identify where in the MT a menstrual cycle occurred, two definitions of time were used, time since the MT and time until the FMP.
Hormone use, including menopausal hormone therapy, oral contraceptives, or chemotherapy, was assessed monthly. For months with missing hormone information, menstrual cycles were coded as untreated if a woman never reported hormone therapy use in the study, if the menstrual cycle occurred before the first report of hormone therapy use, or if the menstrual cycle occurred in a year where no hormone therapy use was reported in the monthly calendars or annual interview.
At each annual visit, height was measured without shoes using either a metric folding wooden ruler or measuring tape, or a fixed stadiometer and weight was measured without shoes, and in light indoor clothing, using a portable digital scale or a digital or balance beam. For each menstrual cycle, weight was linearly interpolated between the last annual visit and the next annual visit. BMI, calculated as weight in kilograms divided by height in meters squared, was categorized as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2) overweight (25.0–29.9 kg/m2), or obese (≥30.0 kg/m2).
At each annual interview women were asked whether they had been diagnosed with uterine fibroids or diabetes since the last visit or were taking medications for diabetes. Serum glucose levels were also measured at baseline, and annual visits 1 and 3–7. A woman was considered diabetic if she indicated she was diagnosed with diabetes, was taking diabetes medication, or had a fasting serum glucose level of ≥126 mg/dl. For each of these two medical conditions, six months were subtracted from the annual visit date when a woman first indicated she had the condition and all menstrual cycles on or after this date were considered to have the condition.
Women were asked whether they smoked at least one cigarette a day or a total of 30 cigarettes in the last month, and positive responses were considered current cigarette use for that month. If a woman never indicated she was a smoker, all her menstrual cycles were considered non-smoking. Women were asked if they participated in moderate to vigorous physical activity, the average number of times a week they participated and the average minutes they participated each time. Average total hours of physical activity/week were calculated for each month. For menstrual cycles that covered more than one month, the highest average total hours of physical activity/week was used.
Race/ethnicity was self-defined and categorized as African American, Chinese or Chinese American, Japanese or Japanese American, or White. Highest education attained (high school graduate /GED or less than high school versus at least some college) and marital status (single, married, or separated, widowed, or divorced) were assessed at baseline. Economic strain was assessed at baseline with the question “how hard is it to pay for basics?” and was categorized as very hard, somewhat hard, or not hard. Prior use of female hormones (yes/no), which did not include oral contraceptives, was also assessed at baseline.
Statistical analysis
Of the 1498 women who were enrolled, the 1320 (88.2%) who had at least one untreated menstrual cycle with menstrual cycle-level covariate information available were included in this analysis. Menstrual cycles were excluded if women were using hormones, hormone use could not be ruled out, or the first cycle post hormone use. Women were censored at time of hysterectomy, bilateral oophorectomy, or chemotherapy. Pregnancy and post pregnancy cycles were excluded as were cycles with missing covariate information. Baseline demographics were compared for women included and excluded in the analyses. Pearson’s chi square or Fisher's exact tests and Student's t tests were calculated as appropriate.
To examine how race/ethnicity, BMI and other covariates influenced central tendency and variance of cycle length, quantile regression was used to model MCL at the 25th, 50th, 75th, and 90th percentiles. Quantile regression coefficients are interpreted similarly to linear regression coefficients of a linear regression. For example, the coefficients of a categorical predictor represent the difference in MCL in days for the relevant percentile between one category and the reference group, adjusted for all other covariates in the model. To reflect better the standard error in our repeated measures data, bootstrap sampling with 500 repetitions was conducted to construct 95% confidence intervals (CI). To handle within-woman correlation, bootstrap sampling was based on samples of women with all their eligible menstrual cycles included.
Two sets of quantile regression models were evaluated. First we included 963 women with 37,288 cycles in whom we observed the start of the MT during the calendar substudy and examined factors associated with cycle length after the onset of the MT, indexing the time covariate to the start of the MT. In addition to race/ethnicity and BMI, we evaluated as covariates factors known to be associated with MCL in younger women. Covariates were included in final models if we had evidence of an adjusted association with the 90th percentile, using the same model for each of the four percentiles of interest. Time since the start of the MT was added to the multivariate model using a natural cubic spline that contained knots at 2, 3, 4, 5, and 6 years. Statistical interaction between time since the start of the MT and the other covariates was examined graphically.
The second set of models included 431 women with 18,305 cycles who had a final menstrual period (FMP) in the menstrual calendar data. To compare the FMP adjusted models with the start of the MT adjusted models, similar covariates were included in both sets of models. Time until the FMP was added to the models using a natural cubic spline, with knots at -5, -4,-3,-2, and -1 years until the FMP. There were 356 women had both the start of the MT observed as well as their FMP. Therefore these women were included in both sets of models. In order to examine the association with study site, multivariate models were run among White women with study site as a variable instead of race/ethnicity.
Baseline demographics were analyzed using SAS 9.2 (Cary, NC). Quantile regression was conducted using the quantreg package in R 2.13.1.12
RESULTS
Of the 1320 eligible women, 963 (73.0%) had onset of the early MT and 431 (32.7%) had their FMP identified in the menstrual calendars. Additionally, 19 (1.4%) had a hysterectomy, 233 (17.7%) began using hormones and had no further untreated menstrual cycles observed, and 637 (48.3%) withdrew from the study before their FMP was identified. In 356 women, we observed both the onset of the early MT and their FMP in the calendar. African American women comprised a higher percentage of those in whom we did not observe onset of the early MT (30.0%) than of those in whom we did (13.4%). They also comprised a higher percentage of women in whom we did not observe their FMP observed (22.1%) compared to women in whom we did (9.3) (Table 1).
Table 1.
Baseline Demographics of 1320 Women in SWAN Menstrual Calendar by Eligibility
| Start of Transition Observed n=963 |
NO Start Observed n=357 |
FMP Observed n=431 |
NO FMP Observed n=889 |
|
|---|---|---|---|---|
| Age at Screener years, Mean (STD) | 45.6 (2.6) | 46.0 (2.9) | 46.4 (2.6) | 45.4 (2.7) |
| n (%) | n(%) | n (%) | n(%) | |
| Study Site | ||||
| Michigan | 256 (26.6) | 174 (48.7) | 89 (20.7) | 341 (38.4) |
| Oakland | 331 (34.4) | 101 (28.3) | 153 (35.5) | 279 (31.4) |
| Los Angeles | 376 (39.0) | 82 (23.0) | 189 (43.9) | 269 (20.2) |
| Race/Ethnicity | ||||
| African-American | 129 (13.4) | 107 (30.0) | 40 (9.3) | 196 (22.1) |
| Chinese | 184 (19.1) | 48 (13.4) | 94 (21.8) | 138 (15.5) |
| Japanese | 212 (22.0) | 50 (14.0) | 123 (28.5) | 139 (15.6) |
| White | 438 (45.5) | 152 (42.6) | 173 (40.4) | 416 (46.8) |
| Education | ||||
| Less than High School | 39 (4.1) | 27 (7.6) | 23 (5.3) | 43 (4.8) |
| High School Grad | 151 (15.7) | 68 (19.0) | 74 (17.2) | 145 (16.3) |
| Some College/Vocation | 319 (33.1) | 136 (38.1) | 132 (30.6) | 323 (36.3) |
| College Graduate | 240 (24.9) | 56 (15.7) | 104 (24.1) | 192 (21.6) |
| Post College | 214 (22.2) | 70 (19.6) | 98 (22.7) | 186 (20.9) |
| Marital Status | ||||
| Single | 125 (13.0) | 47 (13.2) | 58 (13.5) | 114 (12.8) |
| Married | 690 (71.7) | 221 (62.1) | 314 (72.9) | 597 (67.2) |
| Separated | 27 (2.8) | 17 (4.8) | 9 (2.1) | 35 (3.9) |
| Widowed | 15 (1.6) | 9(2.5) | 8 (1.9) | 16 (1.8) |
| Divorced | 106 (11.0) | 62 (17.4) | 42 (9.7) | 126 (14.2) |
| How Hard Is It To Pay For Basics | ||||
| Very Hard | 55 (5.7) | 36 (10.1) | 15 (3.5) | 76 (8.6) |
| Somewhat Hard | 243 (25.2) | 106 (29.7) | 110 (25.5) | 239 (26.9) |
| Not Hard | 665 (69.1) | 215 (60.2) | 306 (71.0) | 574 (64.6) |
| Body Mass Index, kg/m2 | ||||
| Underweight (<18.5) | 32 (3.4) | 10 (2.9) | 18 (4.3) | 24 (2.7) |
| Normal (18.5 −24.9) | 554 (58.3) | 163 (46.7) | 254 (60.2) | 463 (52.7) |
| Overweight (25.0 −29.9) | 184 (19.4) | 67 (19.2) | 72 (17.1) | 179 (20.4) |
| Obese(≥ 30.0) | 181 (19.0) | 109 (31.2) | 78 (18.5) | 212 (24.2) |
| Baseline Smoking Status | ||||
| Never | 639 (67.2) | 197 (55.8) | 281 (67.1) | 555 (62.7) |
| Past | 194 (20.4) | 83 (23.5) | 91 (21.7) | 186 (21.0) |
| Current | 118 (12.4) | 73 (20.7) | 47 (11.2) | 144 (16.3) |
| Ever Taken Hormones Prior to Studya | 118 (12.3) | 36 (10.1) | 42 (9.8) | 112 (12.6) |
Does not include oral contraceptive pills
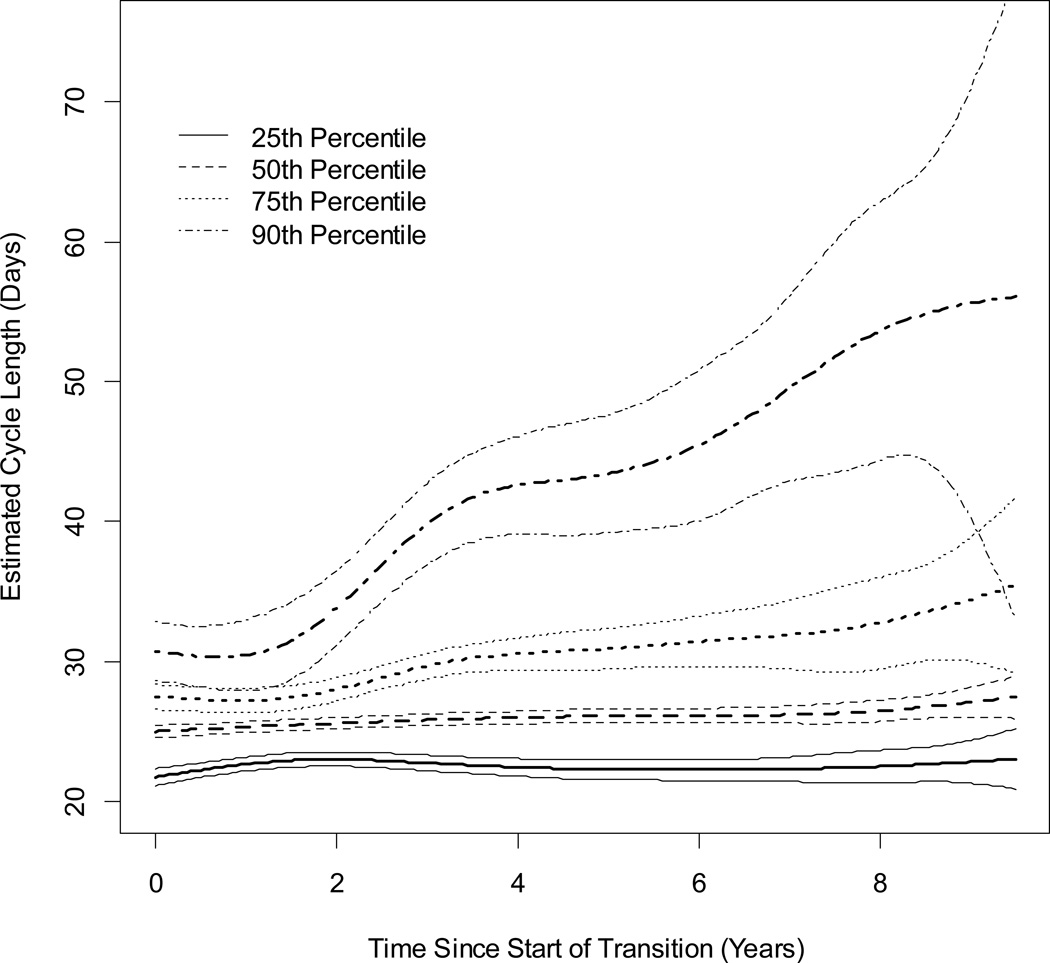
Estimated MCL and 95% confidence interval by time since the start of the MT is displayed in Figure 1 for women who were White, normal or underweight, who at the start of the MT were aged <46.25 years, did not smoke, had at least some post high school education, and had no moderate or physical activity during the average week. At approximately 2 years after the start of the MT, the MCL distribution began to widen, as seen by increases at the 75th and 90th percentiles. From 2 to 8 years after the early MT, MCLs at the 90th percentile increased sharply. At approximately 7.5 years after the start of the MT, the variance of estimated MCL increased for each of the four percentiles, suggesting greater variability of MCL as women approached the FMP.
Figure 1.
Estimated Cycle Length and 95% Confidence Intervals by Time Since the Start of Transition From Multivariate Quantile Regressions for Four Percentiles: 25th, 50th, 75th and 90th.
Natural cubic spline of time since the start of transition contained 5 knots at 2, 3, 4, 5, and 6 years. Estimated cycle lengths are for women who were White, normal or underweight, had an age at the start of the transition <46.25 years, did not smoke, had at least some post high school education, and did not have any moderate or physical activity during the average week. Sample size decreases as time since the start of the transition increases.
Multivariable quantile regression models for the 25th, 50th, 75th, and 90th percentile of MCLs since the start of the MT are given in Table 2. Associations were adjusted for all factors listed in the table as well as time since the start of the MT. Quantile regression models are interpreted similar to linear regression models. For example, the intercept for the 75th percentile, 27.06 days was the estimated MCL at the start of the MT for women in the reference category (women who were white, normal/underweight, who at the start of the MT were aged <46.25 years, did not smoke, had at least some post high school education, and had no moderate or physical activity during the average week). The adjusted difference in MCL at the 75th percentile among women were older at the start of the MT (> 46.25 years) was 3.01 days. The adjusted difference in MCL at the 75th percentile was 2.15 days longer for obese women, 1.56 days longer for smokers, and 1.27 days longer for women with only a high school education or less. Notably women who were older at the start of the menopausal transition (> 46.25 years) had longer cycle lengths at the 50th, 75th, and 90th percentiles compared to women who started the MT at a younger age. Chinese and Japanese women had longer MCLs at all percentiles by 0.82–5.69 days than White women. Overweight women had longer MCLs than normal/underweight women at the 25th and 50th percentiles, and obese women had longer MCLs than normal/underweight women at all percentiles by 1.45 to 4.94 days. Current smoking and having only a high school education or less was associated with longer MCLs at the 75th and 90th percentiles. Hours of moderate or vigorous physical activity/week were associated with longer MCLs at only the 90th percentile. When added to the multivariable model, diabetes was associated with longer MCL only at the 50th percentile (0.70 days, 95% CI: 0.03, 1.36). Uterine fibroids or previous hormone use during the study were not associated with MCL. We found no evidence of statistical interaction between time since the start of the MT and the other covariates. Among White women, study site was not associated with MCL when entered into the multivariate model.
Table 2.
Adjusted Menstrual Cycle Length Differences in Days Among Women Since Start of the Menopausal Transition, by Percentile
| Percentile Effect |
25th day/s (95%CI) |
50th Median day/s (95%CI) |
75th day/s (95%CI) |
90th day/s (95%CI) |
|---|---|---|---|---|
| Intercept | 21.69 (21.06, 22.32) | 24.95 (24.42, 25.48) | 27.06 (26.13, 27.98) | 30.67 (28.49, 32.85) |
| Above Median Age at Transition (46.25 years) | 0.07(−0.35, 0.48) | 0.64 (0.24, 1.03) | 3.01 (2.12, 3.90) | 11.36 (9.11, 13.61) |
| Race/Ethnicity | ||||
| African-American | 0.01 (−0.74, 0.74) | 0.48 (−0.19, 1.15) | 1.10 (−0.40, 2.60) | 0.25 (−2.77, 3.28) |
| Chinese | 1.81 (1.21, 2.41) | 1.28 (0.82, 1.74) | 2.25 (1.07, 3.43) | 5.69 (2.34, 9.04) |
| Japanese | 1.02 (0.41, 1.63) | 0.82 (0.34, 1.31) | 1.19 (0.19, 2.20) | 3.30 (0.53, 6.06) |
| White | Referent | Referent | Referent | Referent |
| Body Mass Index, kg/m2 | ||||
| Normal weight (≤24.9) | Referent | Referent | Referent | Referent |
| Overweight (25.0–29.9) | 0.72 (0.18, 1.25) | 0.62 (0.16, 1.08) | 0.96 (−0.06, 1.97) | 1.60 (−0.88, 4.08) |
| Obese (≥ 30) | 1.70 (1.06, 2.34) | 1.45 (0.95, 1.95) | 2.15 (1.07, 3.23) | 4.94 (2.08, 7.80) |
| Current Smoker | 0.15 (−0.47, 0.77) | 0.34 (−0.19, 0.88) | 1.56 (0.21, 2.91) | 3.93 (0.88, 6.97) |
| High School Graduate or Less Education | 0.15 (−0.30, 0.61) | 0.22 (−0.18, 0.63) | 1.27 (0.07, 2.48) | 3.39 (0.47, 6.30) |
| Every hour of moderate or vigorous physical activity per week | 0.01 (−0.07, 0.08) | 0.01(−0.04, 0.06) | 0.11 (−0.10, 0.32) | 0.74 (0.09, 1.38) |
All associations are adjusted for all factors listed, and time since the start of the menopausal transition.
Each percentile is a separate multivariable model which contained 37,288 observations from 963 women.
Results are interpreted similar to linear regression.
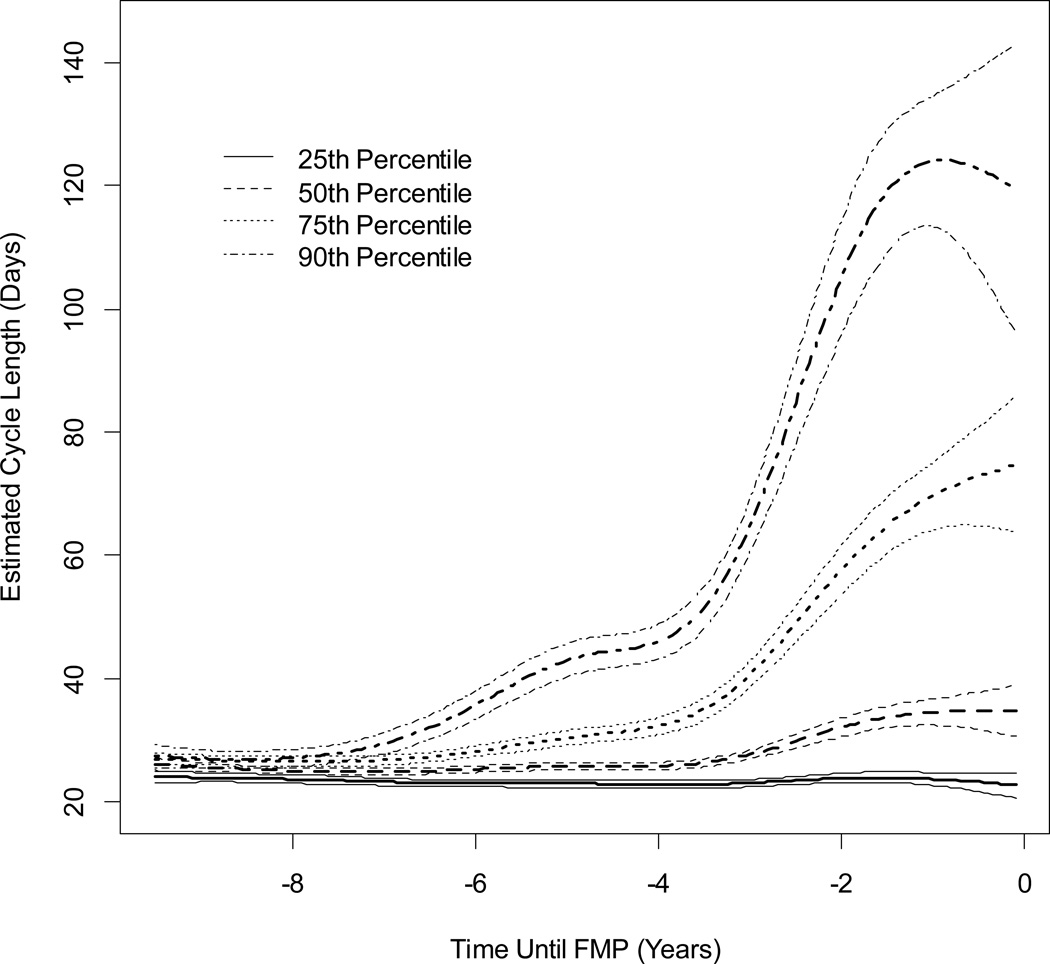
Estimated MCL and 95% confidence interval by time until the FMP is shown in Figure 2, for women who were White, normal or underweight, did not smoke, had at least some post high school education, and had no moderate or physical activity during the average week. When observed from this time perspective, at approximately 7.5 years prior to the FMP, the distribution of MCLs began to increase as shown by the increase in the 90th percentile. The steepest increase in MCL was seen between 4 and 1.5 years prior to the FMP. In the last 2 years prior to the FMP, the variance of estimated MCL increased for each of the four percentiles.
Figure 2.
Estimated Cycle Length 95% Confidence Intervals by Time Until FMP From Multivariable Quantile Regressions for Four Percentiles: 25th, 50th, 75th and 90th.
Natural cubic spline of time until FMP contained 5 knots at -5, -4,-3,-2, and -1 years. FMP is at time 0. Estimated cycle lengths are for women who were White, normal or underweight, did not smoke, had at least some post high school education, and did not have any moderate or physical activity during the average week. Sample size decreases as time moves farther from the FMP.
Relationships between factors of interest and MCL in the time until the FMP models (Table 3) were similar to those observed in the time since the start of the MT models. However, African-American women had longer MCLs than White women at the 50th and 75th percentile. Neither current smoking nor education was associated with any of the percentiles. Again, no statistical interactions were observed between time until the FMP and the other covariates. Among White women, women at Oakland had longer MCLs at the 25th (1.32 days, 95%CI=0.02, 2.63), 50th (1.20 days, 95%CI= 0.16, 2.24), and 75th (1.71 days, 95%CI=0.14, 3.29) percentiles as compared to women at Michigan.
Table 3.
Adjusted Menstrual Cycle Length Differences in Days Among Women Until the FMP by Percentile
| Percentile Effect |
25th day/s (95%CI) |
50th Median day/s (95%CI) |
75th day/s (95%CI) |
90th day/s (95%CI) |
|---|---|---|---|---|
| Intercept | 24.47 (23.31, 25.62) | 26.09 (25.12, 27.06) | 26.96 (25.97, 27.95) | 27.43 |
| Race/Ethnicity | ||||
| African-American | 0.18 (−1.32, 1.67) | 1.58 (0.18, 2.97) | 2.32 (0.39, 4.24) | 3.21 (−0.72, 7.15) |
| Chinese | 1.14 (0.42, 1.87) | 1.18 (0.49, 1.86) | 1.52 (0.44, 2.60) | 3.32 (0.65, 6.00) |
| Japanese | 1.00 (0.38, 1.62) | 1.02 (0.46, 1.58) | 1.29 (0.29, 2.28) | 2.15 (−0.25, 4.55) |
| White | Referent | Referent | Referent | Referent |
| Body Mass Index, kg/m2 | ||||
| Normal weight (≤24.9) | Referent | Referent | Referent | Referent |
| Overweight (25.0–29.9) | 1.00 (0.40, 1.60) | 0.95 (0.40, 1.50) | 1.14 (−0.01, 2.28) | 1.68 (−0.74, 4.10) |
| Obese (≥ 30) | 1.01 (0.35, 1.66) | 1.13 (0.42, 1.85) | 1.93 (0.85, 3.01) | 3.77 (1.12, 6.43) |
| Current Smoker | 0.02 (−0.94, 0.98) | 0.25 (−0.63, 1.13) | 0.45 (0.77, 1.68) | 0.84 (2.02, 3.70) |
| High School Graduate or Less Education | 0.01 (−0.49, 0.52) | −0.08 (−0.60, 0.45) | −0.25 (1.14, 0.63) | −0.19 (−2.20, 1.81) |
| Every hour of moderate or vigorous physical activity per week | 0.00 (−0.08, 0.08) | 0.05 (−0.04, 0.15) | 0.22 (0.02, 0.42) | 0.97 (0.29, 1.66) |
All associations are adjusted for all factors listed and time until FMP.
Multivariable models contained 18,305 observations from 431 women.
Results are interpreted similar to linear regression.
DISCUSSION
This paper is one of the first to assess whether demographic, lifestyle and medical factors that have been identified as influencing menstrual characteristics in adolescent and young adult women also influence MCL as women transition through to menopause, particularly the length of the long cycles characteristic of this life stage. Quantile regression facilitated understanding of how race/ethnicity and BMI influenced both the median length of menstrual cycles and the length of cycles in the tails of the distribution. Notably, women who start the transition at older ages experienced consistently longer cycles. Chinese and Japanese women had consistently longer MCLs across the distribution of menstrual cycle lengths as compared to White women, as did obese women compared to normal weight women. Increased moderate to vigorous physical activity lengthened the menstrual cycle only at the right tail of the distribution, that is only the length of the longest cycles. Current cigarette smoking also tended to increase MCL in the right tail only.
Increases in MCL, as women progress through the MT, were mostly limited to the upper percentiles, with the median unaffected until about 3 years prior to the FMP, when a small increase was seen. MCL in the right tail began to increase approximately 2 years after the start of the MT or 7 years prior to the FMP. In a prior analysis from the same cohort 9, FSH levels indexed to the FMP rose during a similar timeframe. In an analysis of TREMIN data, an historic cohort with menstrual calendars maintained across the entire lifespan, the average MCL began to increase rapidly starting at four years prior to the FMP.16 In the present study, we found that menstrual cycles became highly variable in the two years prior to the FMP as seen by the increase in the 95% CI across all four percentiles. The Melbourne Midlife Women’s Health Project reported a similar result; MCL variability was the highest during the last 17 menstrual cycles prior to the FMP 17, as was the rate of FSH increase in SWAN 9.
Available evidence suggests that menstrual cycle characteristics differ by race/ethnicity. The Semiconductor Health Study reported that Asian women had MCLs that were approximately two days longer than White women. 3 A similar result was reported by the Women’s Reproductive Health Study. 4 In the present study, Chinese and Japanese women had longer MCLs than White women, suggesting that these East Asian women may have longer MCLs throughout their reproductive lifespan.
We found obese women had longer MCLs than normal weight women across the distribution of MCL, consistent with prior studies. In the baseline analysis of the SWAN Daily Hormone Study, which utilized information from one menstrual cycle at the first follow-up visit, a higher percentage of women who were overweight or obese had a MCL of 33 or more days compared with normal weight women.18 Higher BMI has been associated with longer MCLs in women aged 17–19 years 5 and women aged 20–40 years 4, 6–8. However, not all studies report an association between MCL and BMI. 3, 19, 20 Obesity has been associated with circulating levels of FSH and E2 in SWAN and other studies of the MT. 9, 21
Several studies have demonstrated an association between physical activity and longer MCL. In athletes, less frequent menstrual cycles have been reported. 22, 23 Among adolescent girls 2 and young adult women 5, 24, 25, physical activity increased mean MCL. We found that physical activity was associated with longer MCL in the right tail of the distribution, but not at the median.
Current cigarette smoking was associated with longer MCL in the upper percentiles in the model indexed to the onset of the early MT but not in the model indexed to the FMP. Prior studies examining cigarette use have not found an association with MCL 3, 24–26, or have found that smoking is associated with shorter MCLs 6, 7, 27. Smokers have an earlier age at menopause than non-smokers 28 29–34, and a shorter duration of perimenopause 35, thus the longer cycles exhibited by smokers when indexed to the start of the MT, may reflect the shorter duration of perimenopause in these women.
This study had some important limitations. The mean age of women eligible for SWAN was 45.7 years; it is possible that the onset of the MT occurred before enrollment into the study for some women. Age-eligible women who were in the late stage of the MT or who had already experienced their FMP were excluded. Women with younger ages at FMP may have different menstrual cycle characteristics. 36 In the cross-sectional study, from which the SWAN cohort study was selected, African-American women were more likely to have had a hysterectomy and thus less likely to be enrolled into the cohort study. 28 In the current analysis, African-American women were less likely to have their FMP observed thus observed associations in the time until FMP model may have been due to differential loss to follow-up.
CONCLUSION
In conclusion, we found that increases in MCL during the MT, particularly during the early transition, occur mostly in the right tail, reflecting a greater propensity for longer menstrual cycles, not a lengthening of the median MCL. Greater variability of the longest menstrual cycles was observed during the 2 years prior to the FMP. Chinese, Japanese, and obese women as well as women who began their transition at older ages experienced consistently longer MCLs throughout the MT. Women with longer mean menstrual cycles during their reproductive years have been shown to have a later age at menopause, and women with shorter mean MCLs have demonstrated an earlier age at menopause. 35 These results help explain the variability in women’s reports of their transition experience and may help women and clinicians better predict the types of change in MCL they are likely to experience during the MT. Further investigation into the relationship between MCL in the transition period and duration of the early and late menopausal transition may facilitate prediction of expected age at FMP. As little is known about the transition experience of women with polycystic ovarian syndrome, investigation of MCL patterns among women with this condition is warranted.
ACKNOWLEDGEMENTS
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Winifred Rossi 2012; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, Co-PI 2001 – 2012; Maria Mori Brooks Co-PI 2012 -- present; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
SOURCES OF FUNDING
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495).
The first author was also supported by the ReSTAGE collaboration which had grant support from the NIA (Grant AG 021543) and the University of Michigan Rackham Graduate School.
Footnotes
CONFLICTS OF INTEREST: The authors report no conflict of interest.
DISCLAMER: The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH), National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH).
References
- 1.Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97(4):1159–1168. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harlow SD, Campbell B, Lin X, Raz J. Ethnic differences in the length of the menstrual cycle during the postmenarcheal period. Am J Epidemiol. 1997;146(7):572–580. doi: 10.1093/oxfordjournals.aje.a009316. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Gold EB, Lasley BL, Johnson WO. Factors affecting menstrual cycle characteristics. Am J Epidemiol. 2004;160(2):131–140. doi: 10.1093/aje/kwh188. [DOI] [PubMed] [Google Scholar]
- 4.Waller K, Swan SH, Windham GC, Fenster L, Elkin EP, Lasley BL. Use of urine biomarkers to evaluate menstrual function in healthy premenopausal women. Am J Epidemiol. 1998;147(11):1071–1080. doi: 10.1093/oxfordjournals.aje.a009401. [DOI] [PubMed] [Google Scholar]
- 5.Harlow SD, Matanoski GM. The association between weight, physical activity, and stress and variation in the length of the menstrual cycle. Am J Epidemiol. 1991;133(1):38–49. doi: 10.1093/oxfordjournals.aje.a115800. [DOI] [PubMed] [Google Scholar]
- 6.Kato I, Toniolo P, Koenig KL, et al. Epidemiologic correlates with menstrual cycle length in middle aged women. Eur J Epidemiol. 1999;15(9):809–814. doi: 10.1023/a:1007669430686. [DOI] [PubMed] [Google Scholar]
- 7.Rowland AS, Baird DD, Long S, et al. Influence of medical conditions and lifestyle factors on the menstrual cycle. Epidemiology. 2002;13(6):668–674. doi: 10.1097/00001648-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Symons JP, Sowers MF, Harlow SD. Relationship of body composition measures and menstrual cycle length. Ann Hum Biol. 1997;24(2):107–116. doi: 10.1080/03014469700004852. [DOI] [PubMed] [Google Scholar]
- 9.Randolph JF, Jr, Zheng H, Sowers MR, et al. Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J Clin Endocrinol Metab. 2011;96(3):746–754. doi: 10.1210/jc.2010-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harlow SD, Paramsothy P. Menstruation and the menopausal transition. Obstet Gynecol Clin North Am. 2011;38(3):595–607. doi: 10.1016/j.ogc.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lisabeth L, Harlow S, Qaqish B. A new statistical approach demonstrated menstrual patterns during the menopausal transition did not vary by age at menopause. J Clin Epidemiol. 2004;57(5):484–496. doi: 10.1016/j.jclinepi.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Koenker R. Quantile Regression. Cambridge University Press; 2005. [Google Scholar]
- 13.Sowers M, Crawford S, Sternfeld B, et al. SWAN: A Multicenter, Multiethnic, Community-Based Cohort Study of Women and the Menopausal Transition. In: RA L, J K, R M, editors. Menopause: Biology and Pathobiology. San Diego: Academic Press; 2000. pp. 175–188. [Google Scholar]
- 14.Rodriguez G, Faundes-Latham A, Atkinson LE. An approach to the analysis of menstrual patterns in the critical evaluation of contraceptives. Stud Fam Plann. 1976;7(2):42–51. [PubMed] [Google Scholar]
- 15.Harlow SD, Cain K, Crawford S, et al. Evaluation of four proposed bleeding criteria for the onset of late menopausal transition. J Clin Endocrinol Metab. 2006;91(9):3432–3438. doi: 10.1210/jc.2005-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrell RJ, Simon JA, Pincus SM, et al. The length of perimenopausal menstrual cycles increases later and to a greater degree than previously reported. Fertil Steril. 2006;86(3):619–624. doi: 10.1016/j.fertnstert.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 17.Taffe JR, Dennerstein L. Menstrual patterns leading to the final menstrual period. Menopause. 2002;9(1):32–40. doi: 10.1097/00042192-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Santoro N, Lasley B, McConnell D, et al. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: The Study of Women's Health across the Nation (SWAN) Daily Hormone Study. J Clin Endocrinol Metab. 2004;89(6):2622–2631. doi: 10.1210/jc.2003-031578. [DOI] [PubMed] [Google Scholar]
- 19.Lin HT, Lin LC, Shiao JS. The impact of self-perceived job stress on menstrual patterns among Taiwanese nurses. Ind Health. 2007;45(5):709–714. doi: 10.2486/indhealth.45.709. [DOI] [PubMed] [Google Scholar]
- 20.Messing K, Saurel-Cubizolles MJ, Bourgine M, Kaminski M. Menstrual-cycle characteristics and work conditions of workers in poultry slaughterhouses and canneries. Scand J Work Environ Health. 1992;18(5):302–309. doi: 10.5271/sjweh.1572. [DOI] [PubMed] [Google Scholar]
- 21.Freeman EW, Sammel MD, Lin H, Gracia CR. Obesity and reproductive hormone levels in the transition to menopause. Menopause. 2010;17(4):718–726. doi: 10.1097/gme.0b013e3181cec85d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dale E, Gerlach DH, Wilhite AL. Menstrual dysfunction in distance runners. Obstet Gynecol. 1979;54(1):47–53. doi: 10.1097/00006250-197907000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Torstveit MK, Sundgot-Borgen J. Participation in leanness sports but not training volume is associated with menstrual dysfunction: a national survey of 1276 elite athletes and controls. Br J Sports Med. 2005;39(3):141–147. doi: 10.1136/bjsm.2003.011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper GS, Sandler DP, Whelan EA, Smith KR. Association of physical and behavioral characteristics with menstrual cycle patterns in women age 29–31 years. Epidemiology. 1996;7(6):624–628. doi: 10.1097/00001648-199611000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Sternfeld B, Jacobs MK, Quesenberry CP, Jr, Gold EB, Sowers M. Physical activity and menstrual cycle characteristics in two prospective cohorts. Am J Epidemiol. 2002;156(5):402–409. doi: 10.1093/aje/kwf060. [DOI] [PubMed] [Google Scholar]
- 26.Hornsby PP, Wilcox AJ, Weinberg CR. Cigarette smoking and disturbance of menstrual function. Epidemiology. 1998;9(2):193–198. [PubMed] [Google Scholar]
- 27.Windham GC, Elkin EP, Swan SH, Waller KO, Fenster L. Cigarette smoking and effects on menstrual function. Obstet Gynecol. 1999;93(1):59–65. doi: 10.1016/s0029-7844(98)00317-2. [DOI] [PubMed] [Google Scholar]
- 28.Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153(9):865–874. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 29.Cooper GS, Sandler DP, Bohlig M. Active and passive smoking and the occurrence of natural menopause. Epidemiology. 1999;10(6):771–773. [PubMed] [Google Scholar]
- 30.Dorjgochoo T, Kallianpur A, Gao YT, et al. Dietary and lifestyle predictors of age at natural menopause and reproductive span in the Shanghai Women's Health Study. Menopause. 2008;15(5):924–933. doi: 10.1097/gme.0b013e3181786adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardy R, Kuh D, Wadsworth M. Smoking, body mass index, socioeconomic status and the menopausal transition in a British national cohort. Int J Epidemiol. 2000;29(5):845–851. doi: 10.1093/ije/29.5.845. [DOI] [PubMed] [Google Scholar]
- 32.Kaczmarek M. The timing of natural menopause in Poland and associated factors. Maturitas. 2007;57(2):139–153. doi: 10.1016/j.maturitas.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Kinney A, Kline J, Levin B. Alcohol, caffeine and smoking in relation to age at menopause. Maturitas. 2006;54(1):27–38. doi: 10.1016/j.maturitas.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Palmer JR, Rosenberg L, Wise LA, Horton NJ, Adams-Campbell LL. Onset of natural menopause in African American women. Am J Public Health. 2003;93(2):299–306. doi: 10.2105/ajph.93.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am. 2011;38(3):425–440. doi: 10.1016/j.ogc.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang X, Harlow SD, Elliott MR. Distinguishing 6 population subgroups by timing and characteristics of the menopausal transition. Am J Epidemiol. 2012;175(1):74–83. doi: 10.1093/aje/kwr276. [DOI] [PMC free article] [PubMed] [Google Scholar]