Abstract
Objectives
We investigated the incidence trends for adenocarcinoma (AC) of the cervix among the 20-44 age group in the United States and compared the observed birth cohort incidence patterns with the changing patterns of exposure to potential risk factors associated with AC of the cervix, such as infection with human papillomavirus (HPV), use of diethylstilbestrol (DES), obesity, and use of oral contraceptives.
Methods
Using data from the National Cancer Institute's Surveillance, Epidemiology, and End Results program for 1973-2010, we conducted age–period–cohort modeling to evaluate birth cohort patterns on incidence trends of AC of the cervix over time.
Results
The increase in incidence of AC of the cervix started among those born around the mid 1940s and accelerated up until around the mid 1960s birth cohort in both whites and in all races combined, regardless of the assumed period slope. There was a suggestion that the incidence rates of AC of the cervix slowed down after the 1975 birth cohort in both whites and all races combined.
Conclusion
DES was used by millions of women in the United States as a synthetic estrogen between the years 1940-1971. This time period of DES use among pregnant women parallels the observed birth cohort trends in our study, whereby a notable acceleration of AC of the cervix incidence rates was observed for those born in the mid 1940s through the mid 1975s. Thus, our results appear to suggest that in utero exposure to DES might be at least partly responsible for the observed incidence pattern of AC of the cervix as observed in this study.
Keywords: Adenocarcinoma, cervical cancer, incidence rate, age-period-cohort effect, Diethylstilbestrol use
Introduction
The overall cervical cancer incidence and mortality rates in the United States have decreased dramatically since Pap smear screening was introduced in the 1950s (Waxman, 2005). However, the two main histologic types of cervical cancer, squamous cell carcinoma (SCC) and adenocarcinoma (AC) of the cervix, have different incidence patterns. While SCC has been decreasing, AC of the cervix has been increasing in the past several decades (Mathew & George, 2009), and the increase of AC has been more pronounced among younger women (Zheng et al., 1996; Liu et al., 2001; Visioli et al., 2004; Wang et al., 2004). We have previously demonstrated a strong birth cohort effect to be largely responsible for the observed increasing incidence trends in AC of the cervix among women in the United States between 1973 and 1990 (Zheng et al., 1996). The increase in incidence for this subtype was particularly apparent in whites, reaching 4.2% per year for those born since 1935 (Zheng et al., 1996). Thereafter, several studies have explored the incidence trends of AC of the cervix using the United States Surveillance, Epidemiology, and End Results (SEER) population data, and have similarly suggested an increasing incidence of AC of the cervix over the last few decades (Wang et al., 2004; Adegoke et al., 2012; Simard et al., 2012). However, none of these studies conducted a birth cohort analysis while one study compared the incidence rates for SCC and AC between 1976-2005 in the U.S. using comparative age-period-cohort (APC) modeling (Reimers et al., 2009).
In order to understand the current trends and potential birth cohort pattern of AC of the cervix in the U.S., particularly among the age group between 20-44 that showed an increasing incidence pattern in the latest studies (Liu et al., 2001; Visioli et al., 2004; Wang et al., 2004), we conducted a birth cohort analysis and used standard APC modeling methods to explore whether a birth cohort effect may continue to explain the incidence of invasive AC of the cervix in the United States from 1973 – 2010. We further compared the observed birth cohort pattern of AC of the cervix with the changing patterns of exposure to potential risk factors associated with this subtype, such as infection with human papillomavirus (HPV) (Bosch et al., 2002), use of diethylstilbestrol (DES)(1976; Smith et al., 2012), obesity (Lacey et al., 2003), and use of oral contraceptives (OC) (Smith et al., 2003; Vaccarella et al., 2006).
Methods
Data Source
The current study was based on data from the SEER program, which was released in April 2013 (SEER*Stat Database: Incidence---SEER 9 Regs Research Data, Nov 2012 Sub (1973-2010), Single Ages to 85+, Katrina/Rita Population Adjustment). The SEER 9 data includes approximately 12% of the United States population. Based on the International Classification of Disease for Oncology, 3rd ed. (ICD-O-3), cervical cancer cases were classified into three histologic subtypes: SCC (ICD-O-3 8050-8130), AC (ICD-O-3 8140-8147, 8160-8162, 8180-8221, 8250-8506, 8520-8550, 8570-8573, 8940-8941) and adenosquamous (ICD-O-3 8560-8570).
A total of 44,021 newly diagnosed cervical cancer cases were reported to the nine registries of SEER from 1973 to 2010. Of them, 17,831 cases (∼41%) were among 20–44 year olds. 15,929 cases, excluding 173 cases with an unknown race, had available histologic subtype data for analysis. Among these, 12,420 (78%) cases were white race and 2,848 (18%) were AC. The AC type accounted for approximately 9% of invasive cervical cancer among whites in 1973, whereas it accounted for 33% of cervical cancer in 2010.
Data Analysis
Overall age-adjusted incidence rates for whites and all races combined as well as sex-specific age-adjusted incidence rates were calculated using SEER*Stat (8.0.4) from 1973 through 2010 for the 20-44 year old age group, with rates adjusted to the 2000 U.S. standard population. The joinpoint analysis was conducted using joinpoint version 4.0.4 by weighted least squares regression of the natural logarithms of the age-adjusted incidence rates. The data were also presented by calendar year and by cohort year of birth in order to explore the secular trends and the potential birth cohort patterns. The APC analysis based on a log-linear Poisson regression model was conducted for the 20-44 year old age group. Age, period, and cohort models were based on 5 five-year age intervals between 20 and 44, and eight time period intervals (1973-1974, 1975-1979, 1980-1984, 1985-1989, 1990-1994, 1995-2000, 2000-2004, 2005-2010). Because of the nonidentifiability problem (cohort = year − age), the independent effects of age, period, and cohort cannot be evaluated (Holford, 2003). However, the cohort effect can be evaluated by constraining the period effect to different assumptions (parameter values βp=0, -0.01, or 0.01), where βp = 0 represents a slope of zero, βp = -0.01 indicates that the period slope was decreasing, and βp = 0.01 denotes that the period slope was increasing during the study period. All models were fit using SAS (version 9.3). We only present the data for whites and for all the races combined since the sample size is not large enough to do data analysis separately for non-whites. The significance level was set at 0.05 for a two-sided test.
Results
A total of 2,848 AC of the cervix cases of known race were newly diagnosed between the ages of 20 and 44 from 1973 to 2010. Of these, 2,420 (84.0%) were white.
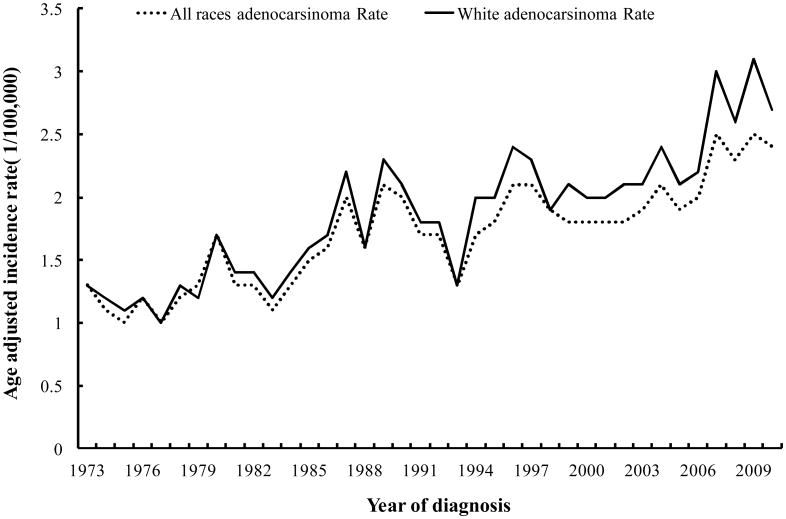
The age-adjusted incidence rates of cervical cancer for the 20-44 age groups are presented in Figure 1. For white cases, the age-adjusted incidence rates of AC of the cervix for the 20-44 year old age group were 1.3/100,000 in 1973 and increased to 2.7/100,000 in 2010. The joinpoint analysis showed an annual percent change of 2.2% (95% CI: 1.8%-2.7%) for whites between 1973 and 2010. The incidence trend of all races combined was similar to that of whites and increased from 1.3/100,000 in 1973 to 2.4/100,000 in 2010 with an annual percent change of 2.0% (95% CI: 1.6%-2.4%).
Figure 1.
Age- adjusted incidence rates of adenocarcinoma of the cervix for age group 20-44 only.
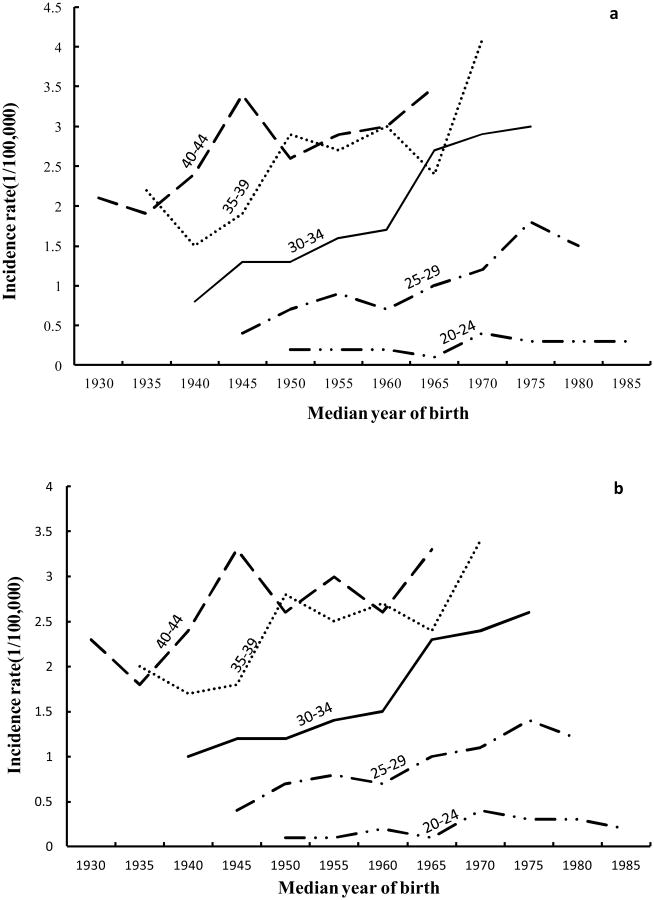
The age-specific incidence rates of AC of the cervix for 20-44 year olds by median year of birth are shown in Figure 2. For whites (Fig. 2a), the incidence rates of AC of the cervix showed a generally increasing trend for more recent birth cohorts for age groups between 20-44. The incidence rates of AC of the cervix for whites aged 30-34 had the largest increase from 0.8 cases per 100,000 in the 1940 birth cohort to 3.0 cases per 100,000 in the 1975 birth cohort. For both whites (Fig. 2a) and all racial groups combined (Fig. 2b), the birth cohort curves were similar, that is, there was a much sharper increase in rates after the 1960 birth cohort, but there was a suggestion of a decrease in rates after the 1975 birth cohort.
Figure 2.
Cohort age curves of adenocarcinoma of the cervix for age group 20-44 (a: whites, b: all races).
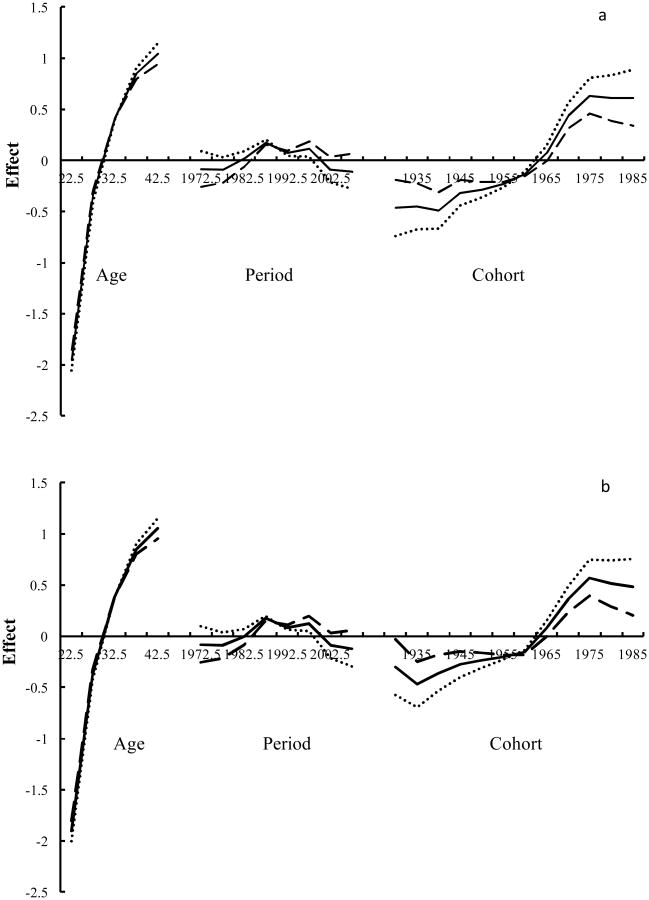
Figures 3a and 3b present the results from the APC modeling for whites and all races, respectively, using three different assumptions for the period slope (βp= 0, -0.01, or 0.01), where the solid line is based on an assumption without an overall period slope and the dotted line and long dashed line denote the period slope as -0.01 and 0.01 respectively. These results support what was observed from the univariate analyses, that is, the increase in AC of the cervix started among those born around the mid-1940s and accelerated in those born up until around the mid-1960s in both whites and all races, regardless of the assumed period slope. This analysis further suggested that the incidence rates began to slow down after the 1975 birth cohort in both whites and for all races combined (Figure 3a, 3b).
Figure 3.
Age-period-cohort modeling for adenocarcinoma of the cervix (a: whites, b: all races).
Discussion
The results from our current study show that the incidence rates of AC of the cervix have been increasing in both whites and in all races combined among young women in the United States. The results also show that the observed increase could be a birth cohort phenomenon. The observed increase in AC of the cervix started around the mid 1940 birth cohort and accelerated around the mid 1960 birth cohort before slowing down around the 1975 birth cohort.
While HPV is considered to be a necessary cause of cervical cancer, it is not a sufficient cause of this malignancy (Walboomers et al., 1999). Thus, the HPV cofactors may play an important role in the development of AC of the cervix (Castellsague et al., 2006). In utero exposure to DES has been consistently linked to clear cell adenocarcinoma (CCA) of the vagina and cervix in young women, with risks reported to be greater than twenty-fold (Herbst et al., 1971; Hatch et al., 1998) while two case reports suggested that DES exposure in utero might be also associated with the development of non-clear-cell adenocarcinoma (Townsend, 1996). DES is a potent synthetic estrogen that was prescribed to pregnant women between 1940 and 1971 to prevent spontaneous abortion and premature delivery, and related complications of pregnancy in the United States and Europe(IARC, 2012). The number of women exposed prenatally to DES worldwide is unknown, and most reports about DES use are from the United States (IARC, 2012). It was estimated that 5 to 10 million United States citizens received DES during pregnancy or were exposed to the drug in utero from the 1940s to the 1970s (Giusti et al., 1995), and most of the exposed women from the United States were born during 1948–65 (IARC, 2012). Interestingly, the time period of DES use among pregnant women in the United States paralleled the observed time interval (i.e., birth cohorts starting from the mid-1940s through the mid 1975s) with increasing birth cohort patterns in our study. These observations would seemingly suggest that in utero exposure to DES might be at least partly responsible for the observed incidence pattern of AC of the cervix, although we note that there were only 66 cases of CCA (∼2.8%) in the study and this particular subtype has been reported to have the strongest association with DES exposure in epidemiologic studies. It has been suggested that genomic instability may be an important mechanism of DES-induced carcinogenesis (Boyd et al., 1996) and animal experiments have suggested that DES disrupts TRP63 expression in mice and induces adenosis lesions in the cervix and vagina (Laronda et al., 2012).
With respect to other potential risk factors that may help to explain the trends observed in our study, previous studies have also linked long term OC use with an increased risk of AC of the cervix (Madeleine et al., 2001; Smith et al., 2003; Appleby et al., 2007). A pooled study of eight case-control studies of cervical cancer also indicated that OC use is a cofactor for AC of the cervix (Castellsague et al., 2006). OC pills were first approved for use in 1960 in the United States and it is the most widely used contraceptive method by white women, particularly women in their teens and 20s (Mosher WD, 2010; Jones J, 2012). The prevalence of OC pill use among women aged 15–44 years in 1982, 1995, 2002 and 2006-2008 was 15.6%, 17.3%, 18.9% and 17.3% respectively (Mosher WD, 2010). While OC use might be related to the risk of AC of the cervix, it could not explain why SCC has been decreasing (data not shown) during the time period of the study and why the observed birth cohort pattern of AC of the cervix has been increasing, since OC pills have been associated with all histological types of cervical cancer (Green et al., 2003).
Obesity and body fat distribution were recently associated with AC of the cervix (Lacey et al., 2003). The prevalence of adult obesity was reported to have increased between 1980 and 1999 (Flegal et al., 1998; Flegal et al., 2002) and has levelled off since 2000 in the United States among adult women (Flegal et al., 2012). This incidence pattern of AC of the cervix does not coincide with the time trend of obesity in the United States population since overall the incidence of AC of the cervix showed a continuous increase with an annual percent change of 2.0% after the year 2000. However, obesity together with other risk factors of cervical cancer, such as long term OC use, HPV infection, and tobacco smoking, as reviewed previously, could well be the reasons for the observed rise in cervical AC incidence in countries without history of DES use. A period effect (such as screening) also does not seem to be the major reason for the observed incidence pattern of AC of the cervix in this study since the increasing incidence rate of AC of the cervix started in the 1970s while the endocervical brush was added to cytologic screening in the early 1990s and the U.S. Food and Drug Administration (FDA) approved the first HPV test at the end of the 1990s. Also, the time interval may be insufficient for a benefit from the HPV vaccine for the observed decrease in rates for recent birth cohorts since the FDA approved Gardasil in 2006, the first preventive HPV vaccine (NCI., 2011).
Given the lower incidence rate and smaller number of AC of the cervix cases in other racial groups in this study, we did not attempt to analyze the birth cohort pattern for other racial groups as we did for whites. However, the observed birth cohort pattern in analyses that included all racial groups combined was quite similar to that of whites. A second limitation is that since this is an ecological study, we did not have information on the joint distribution between DES use, other risk factors, and risk of AC of the cervix.
In conclusion, our analysis of the incidence of AC of the cervix from 1973-2010 using data from SEER suggests that exposure to DES in utero might be partly responsible for the observed increase in AC of the cervix in the US population. Targeted screening could be beneficial for women born between the 1940s and 1970s who were affected by DES exposure in utero and a yearly exam has been recommended for DES Daughters, even after a hysterectomy or menopause (NCI., 2014).
Acknowledgments
This work was partly supported by Fogarty training grants D43TW 008323 and D43TW 007864-01 from the National Institutes of Health.
Footnotes
The authors declare that they have no conflict of interest.
References
- 1.Exposure in utero to diethylstilbestrol and related synthetic hormones. Association with vaginal and cervical cancers and other abnormalities. JAMA. 1976;236:1107–1109. doi: 10.1001/jama.1976.03270110011002. [DOI] [PubMed] [Google Scholar]
- 2.Adegoke O, Kulasingam S, Virnig B. Cervical cancer trends in the United States: a 35-year population-based analysis. J Womens Health (Larchmt) 2012;21:1031–1037. doi: 10.1089/jwh.2011.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appleby P, Beral V, Berrington de Gonzalez A, Colin D, Franceschi S, Goodhill A, Green J, Peto J, Plummer M, Sweetland S. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet. 2007;370:1609–1621. doi: 10.1016/S0140-6736(07)61684-5. [DOI] [PubMed] [Google Scholar]
- 4.Bosch FX, Lorincz A, Munoz N, Meijer CJLM, Shah KV. The causal relation between human papillomavirus and cervical cancer. Journal of Clinical Pathology. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyd J, Takahashi H, Waggoner SE, Jones LA, Hajek RA, Wharton JT, Liu FS, Fujino T, Barrett JC, McLachlan JA. Molecular genetic analysis of clear cell adenocarcinomas of the vagina and cervix associated and unassociated with diethylstilbestrol exposure in utero. Cancer. 1996;77:507–513. doi: 10.1002/(SICI)1097-0142(19960201)77:3<507::AID-CNCR12>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Castellsague X, Diaz M, de Sanjose S, Munoz N, Herrero R, Franceschi S, Peeling RW, Ashley R, Smith JS, Snijders PJ, Meijer CJ, Bosch FX. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. J Natl Cancer Inst. 2006;98:303–315. doi: 10.1093/jnci/djj067. [DOI] [PubMed] [Google Scholar]
- 7.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of Obesity and Trends in the Distribution of Body Mass Index Among US Adults, 1999-2010. Jama-J Am Med Assoc. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 8.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960-1994. Int J Obesity. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 9.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. Jama-J Am Med Assoc. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 10.Giusti RM, Iwamoto K, Hatch EE. Diethylstilbestrol revisited: a review of the long-term health effects. Ann Intern Med. 1995;122:778–788. doi: 10.7326/0003-4819-122-10-199505150-00008. [DOI] [PubMed] [Google Scholar]
- 11.Green J, Berrington de Gonzalez A, Sweetland S, Beral V, Chilvers C, Crossley B, Deacon J, Hermon C, Jha P, Mant D, Peto J, Pike M, Vessey MP. Risk factors for adenocarcinoma and squamous cell carcinoma of the cervix in women aged 20-44 years: the UK National Case-Control Study of Cervical Cancer. Brit J Cancer. 2003;89:2078–2086. doi: 10.1038/sj.bjc.6601296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatch EE, Palmer JR, Titus-Ernstoff L, Noller KL, Kaufman RH, Mittendorf R, Robboy SJ, Hyer M, Cowan CM, Adam E, Colton T, Hartge P, Hoover RN. Cancer risk in women exposed to diethylstilbestrol in utero. JAMA. 1998;280:630–634. doi: 10.1001/jama.280.7.630. [DOI] [PubMed] [Google Scholar]
- 13.Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971;284:878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- 14.Holford TR. Monitoring the Health of Populations Statistical Principles and Methods for Public Health Surveillance. Oxford Univerity Press; 2003. [Google Scholar]
- 15.IARC. Diethylstilbestrol 2012 [Google Scholar]
- 16.Jones J, M W, D K. Current contraceptive use in the United States, 2006–2010 and changes in patterns of use since 1995. National Health Statistics Reports. 2012 No. 60. [PubMed] [Google Scholar]
- 17.Lacey JV, Swanson CA, Brinton LA, Altekruse SF, Barnes WA, Gravitt PE, Greenberg MD, Hadjimichael OC, McGowan L, Mortel R, Schwartz PE, Kurman RJ, Hildesheim A. Obesity as a potential risk factor for adenocarcinomas and squamous cell carcinomas of the uterine cervix. Cancer. 2003;98:814–821. doi: 10.1002/cncr.11567. [DOI] [PubMed] [Google Scholar]
- 18.Laronda MM, Unno K, Butler LM, Kurita T. The development of cervical and vaginal adenosis as a result of diethylstilbestrol exposure in utero. Differentiation. 2012;84:252–260. doi: 10.1016/j.diff.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu S, Semenciw R, Mao Y. Cervical cancer: the increasing incidence of adenocarcinoma and adenosquamous carcinoma in younger women. CMAJ. 2001;164:1151–1152. [PMC free article] [PubMed] [Google Scholar]
- 20.Madeleine MM, Daling JR, Schwartz SM, Shera K, McKnight B, Carter JJ, Wipf GC, Critchlow CW, McDougall JK, Porter P, Galloway DA. Human papillomavirus and long-term oral contraceptive use increase the risk of adenocarcinoma in situ of the cervix. Cancer Epidem Biomar. 2001;10:171–177. [PubMed] [Google Scholar]
- 21.Mathew A, George PS. Trends in incidence and mortality rates of squamous cell carcinoma and adenocarcinoma of cervix--worldwide. Asian Pac J Cancer Prev. 2009;10:645–650. [PubMed] [Google Scholar]
- 22.Mosher WD, J J. Use of contraception in the United States: 1982–2008. National Center for Health Statistics Vital Health Stat. 2010;23 [PubMed] [Google Scholar]
- 23.NCI. Fact Sheet Human Papillomavirus (HPV) Vaccines 2011 [Google Scholar]
- 24.NCI. DES Exposure: Questions and Answers 2014 [Google Scholar]
- 25.Reimers LL, Anderson WF, Rosenberg PS, Henson DE, Castle PE. Etiologic heterogeneity for cervical carcinoma by histopathologic type, using comparative age-period-cohort models. Cancer Epidemiol Biomarkers Prev. 2009;18:792–800. doi: 10.1158/1055-9965.EPI-08-0965. [DOI] [PubMed] [Google Scholar]
- 26.Simard EP, Naishadham D, Saslow D, Jemal A. Age-specific trends in black-white disparities in cervical cancer incidence in the United States: 1975-2009. Gynecol Oncol. 2012;127:611–615. doi: 10.1016/j.ygyno.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 27.Smith EK, White MC, Weir HK, Peipins LA, Thompson TD. Higher incidence of clear cell adenocarcinoma of the cervix and vagina among women born between 1947 and 1971 in the United States. Cancer Cause Control. 2012;23:207–211. doi: 10.1007/s10552-011-9855-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith JS, Green J, Berrington de Gonzalez A, Appleby P, Peto J, Plummer M, Franceschi S, Beral V. Cervical cancer and use of hormonal contraceptives: a systematic review. Lancet. 2003;361:1159–1167. doi: 10.1016/s0140-6736(03)12949-2. [DOI] [PubMed] [Google Scholar]
- 29.Townsend DE. Primary non-clear cell adenocarcinoma of the vagina in older DES-exposed women. Gynecol Oncol. 1996;61:454. doi: 10.1006/gyno.1996.0175. [DOI] [PubMed] [Google Scholar]
- 30.Vaccarella S, Herrero R, Dai M, Snijders PJF, Meijer CJLM, Thomas JO, Anh PTH, Ferreccio C, Matos E, Posso H, de Sanjose S, Shin HR, Sukvirach S, Lazcano-Ponce E, Ronco G, Rajkumar R, Qiao YL, Munoz N, Franceschi S. Reproductive factors, oral contraceptive use, and human papillomavirus infection: Pooled analysis of the IARC HPV prevalence surveys. Cancer Epidem Biomar. 2006;15:2148–2153. doi: 10.1158/1055-9965.EPI-06-0556. [DOI] [PubMed] [Google Scholar]
- 31.Visioli CB, Zappa M, Ciatto S, Iossa A, Crocetti E. Increasing trends of cervical adenocarcinoma incidence in Central Italy despite Extensive Screening Programme, 1985-2000. Cancer Detect Prev. 2004;28:461–464. doi: 10.1016/j.cdp.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 33.Wang SS, Sherman ME, Hildesheim A, Lacey JV, Devesa S. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976-2000. Cancer. 2004;100:1035–1044. doi: 10.1002/cncr.20064. [DOI] [PubMed] [Google Scholar]
- 34.Waxman AG. Guidelines for cervical cancer screening: history and scientific rationale. Clin Obstet Gynecol. 2005;48:77–97. doi: 10.1097/01.grf.0000151590.08451.26. [DOI] [PubMed] [Google Scholar]
- 35.Zheng T, Holford TR, Ma Z, Chen Y, Liu W, Ward BA, Boyle P. The continuing increase in adenocarcinoma of the uterine cervix: a birth cohort phenomenon. Int J Epidemiol. 1996;25:252–258. doi: 10.1093/ije/25.2.252. [DOI] [PubMed] [Google Scholar]