Summary
Background
The formation of neutralizing antibodies (inhibitors) directed against human coagulation factor VIII (hFVIII) is a life-threatening pathogenic response that occurs in 20–30% of severe congenital hemophilia A patients and 0.00015% of remaining population (i.e. acquired hemophilia A). Interspecies amino acid sequence disparity among FVIII orthologs represents a promising strategy to mask FVIII from existing inhibitors while retaining procoagulant function. Evidence for the effectiveness of this approach exists in clinical data obtained for porcine FVIII products, which have demonstrated efficacy in the setting of congenital and acquired hemophilia.
Objectives
In the current study, recombinant (r) ovine FVIII (oFVIII), was evaluated for antigenicity and procoagulant activity in the context of human patient-derived and murine model-generated FVIII inhibitors.
Methods
The antigenicity of roFVIII was assessed using i) inhibitor patient plasma samples, ii) murine anti-FVIII MAbs, iii) immunized murine hemophilia A plasmas, and iv) an in vivo model of acquired hemophilia A
Results
Overall, roFVIII demonstrated reduced reactivity to, and inhibition by, anti-hFVIII immunoglobulin in patient plasmas. Additionally, several hFVIII epitopes were predicted and empirically shown not to exist within roFVIII. In a murine hemophilia A model designed to mimic clinical inhibitor formation, it was demonstrated that inhibitor titers to roFVIII were significantly reduced compared to the orthologous immunogens, rhFVIII or rpFVIII. Furthermore in a murine model of acquired hemophilia A, roFVIII administration conferred protection from bleeding following tail transection.
Conclusion
These data support the investigation of FVIII orthologs as treatment modalities in both the congenital and acquired FVIII inhibitor settings.
Keywords: Hemophilia A, congenital, Hemophilia A, acquired, Factor VIII, Antibody Specificity, Cross Reactions
Introduction
Factor VIII (FVIII) is a procofactor in the intrinsic pathway of blood coagulation. Deficiency of FVIII activity resulting from genetic mutation of the X-chromosome-linked F8 gene presents as a bleeding disorder, termed hemophilia A, that has a reported prevalence of 1 in 7,800 males [1]. Treatment consists of lifelong protein replacement via intravenous infusions of recombinant (r) or plasma-derived (pd) human (h) FVIII products. Upon repeated exposure, approximately 20–30% of severe hemophilia A patients develop inhibitory anti-hFVIII alloantibodies (inhibitors). In countries where replacement therapy is available, the immune response to hFVIII is the most significant complication affecting the management of patients with hemophilia A. Additionally, autoantibodies to hFVIII develop in non-hemophiliacs at a rate of 1.48/million/year producing an autoimmune condition termed acquired hemophilia A, which frequently results in life- or limb-threatening bleeding. [2–5]
On the molecular level, FVIII displays a domain structure A1-A2-B-ap-A3-C1-C2 where the A and C domains are defined by internal sequence homology and the heavy and light chains are separated by an activation peptide (ap) [6]. Although antibodies targeting each of the hFVIII domains can be found in patient plasmas, the A2 and C2 domains appear to contain the dominant immunogenic and inhibitory epitopes [7–9]. Epitopes, mechanisms of action, and kinetics have been defined for a large collection of anti-A2 and C2 domain murine monoclonal antibodies (MAbs) demonstrating that the murine hemophilia A model recapitulates many features of the anti-FVIII immune response observed in humans [10–12]. Recently, high resolution structural data of anti-hFVIII MAbs in complex with the FVIII C2 domain using small angle x-ray scattering, x-ray crystallography, and hydrogen-deuterium exchange mass spectrometry was obtained and has brought the understanding of inhibitor mechanism of action to the atomic level [13–15].
Treatment options for hemophilia A patients with inhibitors are limited in terms of availability and efficacy. For example, in Immune Tolerance Induction (ITI) studies, the frequent administration of FVIII product at doses as high as 200 IU/kg/day, is effective at eradicating inhibitors in up to 70% of patients [16]. However, due to FVIII product supply constraints and expense, ITI is not an option for the majority of patients with hemophilia A. Aside from eradicating inhibitors, acute and frequently life-threatening bleeding can be treated in this setting using FVIII bypassing agents (e.g. activated prothrombin complex concentrate or activated recombinant factor VII). Plasma-derived porcine FVIII (pd-pFVIII) products also have been utilized although they no longer are available. However, a recombinant pFVIII (rpFVIII) product is under clinical development for acquired hemophilia A. The rationale for use of pd- or rpFVIII products stems from the presence of non-conserved amino acid sequence differences that confer reduced antigenicity and inhibition. Furthermore, it has been shown that there are several species-specific differentials in non-immunological properties between rh- and rp-FVIII [17, 18] and preclinical evidence exists to support the benefit of utilizing FVIII orthologs, i.e. proteins from different species that evolved from a common ancestral gene, or hybrid FVIII molecules engineered to possess sequences from multiple orthologs, in gene therapy applications [19–23].
A naturally occurring ovine model of severe hemophilia A has been identified and the responsible genetic lesion and disease phenotype was characterized [24]. Additionally, the ovine FVIII (oFVIII) ortholog was generated in recombinant form thereby facilitating biochemical characterization. B-domain-deleted (BDD) recombinant ovine FVIII (roFVIII) has been shown to display greater specific activity, prolonged half-life following activation by thrombin, functionality in a hFVIII-deficient plasma bioassay and efficacy in a murine hemophilia A tail-transection bleeding model [25]. As the rationale for clinical use of pFVIII is based on reduced antigenicity achieved through differential amino acid sequence, herein we sought to investigate the potential therapeutic utility of roFVIII which also contains a distinct repertoire of non-conserved amino acids, but still possesses procoagulant function in human plasma. Outside of the B-domain, ovine and porcine FVIII share 86 and 83% amino acid identity to human FVIII, respectively [24, 26]. We hypothesized that roFVIII would be less antigenic than hfVIII in plasma from human patients and hemophilia A mice with inhibitors and that there would be inter-patient differentials in the antigenicity to each FVIII ortholog. To test these ideas, the antigenicity of roFVIII was assessed using i) inhibitor patient plasma samples, ii) a collection of well-characterized murine anti-FVIII MAbs that mimic human inhibitors {Healey, 2007 #552}[10–12], iii) plasmas from hemophilia A mice immunized with either rhFVIII or rpFVIII, and iv) an in vivo bleeding challenge assay designed to model acquired hemophilia A.
Materials and Methods
Materials
Pooled citrated normal plasma (FACT) and FVIII-deficient plasma were purchased from George King Biomedical (Overland Park, KS). Automated APTT reagent was purchased from Trinity Biotech (Wicklow, Ireland). Inhibitor patient plasmas were drawn and banked in accordance with Emory University IRB protocol no. IRB00006290. Acquired patient samples were generously donated by Dr. David Green (Feinberg School of Medicine of Northwestern University, Chicago, IL). Patients were selected for inclusion if inhibitor titers against human exceeded 5 BU/ml and sufficient plasma was available. Streptavidin-alkaline phosphate conjugate was purchased from Jackson Immuno Research (West Grove, PA). Goat anti-mouse IgG-alkaline phosphatase conjugate and alkaline phosphatase substrate kit (AP pNPP) was purchased from Bio-Rad (Hercules, CA). Dimethyl pimelimidate was purchased from Thermo Scientific (Rockford, IL) and Protein A/G Plus was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Domain specific monoclonal antibodies were generated and purified as previously described [12]. MAb ESH-8 was purchased from American Diagnostica (Stamford CT). MAbs 413 and CLB-Cag 9 were gifts from American Red Cross (Rockville, MD) and Dr. Jan Voorberg (Sanquin-AMC Lamdsteiner Laboratory, Amsterdam, The Netherlands). BDD roFVIII, rpFVIII, and rhFVIII were generated and purified as described previously [25, 27]. Full-length rhFVIII was a generous gift from Hemophilia of Georgia. OTII C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, Maine) and kept in accordance with Emory IACUC. Exon 16-disrupted hemophilia A mice have been previously described [28].
Inhibitor plasma and monoclonal competition ELISA
Competition ELISAs were performed as previously described [10, 11, 29]. Controls for each MAb against the A2 or C2 domain were replicated 11 and 16 times, respectively. Competition was defined as a reduction of signal greater than 2 standard deviations (SD) from the control mean. Only patient plasmas that displayed predominantly A2 and/or C2 specificity, as determined by homolog-scanning ELISA using human/porcine FVIII hybrids revealed (data not shown), were selected for competition ELISA analysis.
Cross-reactivity of inhibitor plasma and MAbs
An indirect ELISA was performed using plates containing adsorbed rhFVIII, rpFVIII, and roFVIII to which serial dilutions of patient plasma or MAbs were added followed by detection using goat anti-mouse AP-conjugated secondary antibody. ELISA titration curves were fitted to the 4-parameter logistic equation. The dilution of inhibitor plasma required to produce A405 of 0.3 was calculated by interpolation and compared across orthologs. The absorbance threshold was set as an arbitrary point in which colorimetric signal is approximately three times background while substrate remains in excess. MAb interactions that did not achieve an absorbance at 405 nm of 0.3 at the lowest dilution (10-fold molar excess) were designated as non-reactive or below the limit of detection.
FVIII inhibitor titer assays: patient plasma, mouse plasma, and MAbs
FVIII inhibitor titers against rhFVIII, rpFVIII, and roFVIII were measured using a modified Bethesda assay previously described [30]. For determination of the ortholog titers, pooled citrated FVIII-deficient plasma was combined with 0.8–1.2 units/ml of rpFVIII or roFVIII and buffered with 100 mM imidazole. Due to limited availability of plasma, not all patients could be fully screened. MAb inhibitor titer was calculated similarly using dilutions of MAbs at known concentrations and reported in BU/mg IgG.
Immunization of hemophilia A E16 F8−/− mice
Mice were immunized with BDD rhFVIII or rpFVIII as described previously [31]. Mice received six tail vein injections of 10 μg/kg FVIII at 7-day intervals followed by a final injection of 25 μg/kg FVIII two weeks after the sixth dose. Subsequently, terminal plasma collections were performed and these samples were used to determine inhibitor titer by modified Bethesda assay.
Murine acquired hemophilia A hemostatic challenge
In a blinded study, 8–12 week old C57BL/6 OTII mice received 10ug anti-A2 domain MAb 4A4 via intraperitoneal (IP) injection. After 15 minutes, the mice were administered 9 units of rFVIII or saline via intravenous tail vein injection. Hemostatic challenge was performed 2 hours after rFVIII administration by tail transection at 2mm diameter and total blood loss over 40 minutes was recorded as previously described [25, 32].
Anti-oFVIII MAb generation, and domain specificity immunoprecipitations
Three 9–12 week old E16 F8−/− mice were injected with 1μg roFVIII each diluted in 100μl saline via tail vein each week for 7 weeks. Inhibitor titers were taken at week 8 via ELISA and Bethesda assay as described above. Two weeks after the last injection, mice were administered 1.25 μg roFVIII via tail vein. Three days after final immunization, the mouse with the highest ELISA and Bethesda titer was sacrificed and MAbs were generated and purified as previously described [12]. The 9 resulting MAbs were used to immunoprecipitate roFVIII following activation by thrombin and visualized by SDS-PAGE as previously described [25].
Results
RoFVIII displays reduced IgG binding and inhibition in inhibitor patient plasmas
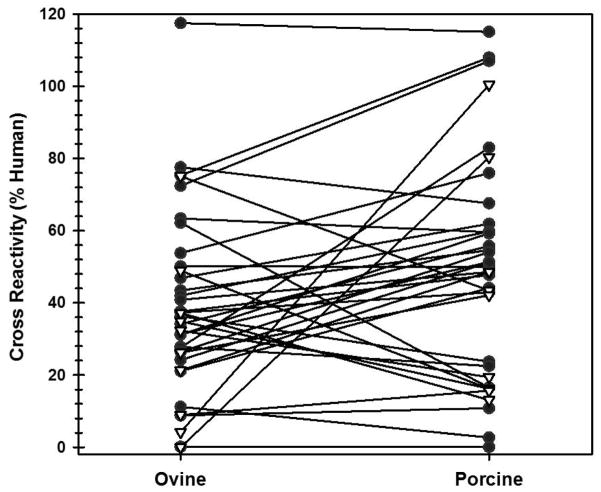
As an expansion of the ortholog approach to hemophilia A treatment in the context of FVIII inhibitors, roFVIII was investigated for binding to IgG present in inhibitor patient plasmas (i.e. antigenicity) and resistance to inhibition conferred by the same. An indirect ELISA-based screen of 26 congenital and 10 acquired hemophilia A patient plasmas demonstrated reduced reactivity of plasma IgG to both roFVIII and rpFVIII compared to hFVIII with median values of 35.6 and 49.3%, respectively (Figure 1A). No significant difference was observed between roFVIII and rpFVIII (P = 0.097; Mann-Whitney U test). Of the 36 plasmas tested, 32 displayed reduced reactivity to both roFVIII and rpFVIII and of these 22 demonstrated less reactivity to roFVIII compared to rpFVIII.
Figure 1. Antigenicity and inhibitor titers for inhibitor patient plasmas.
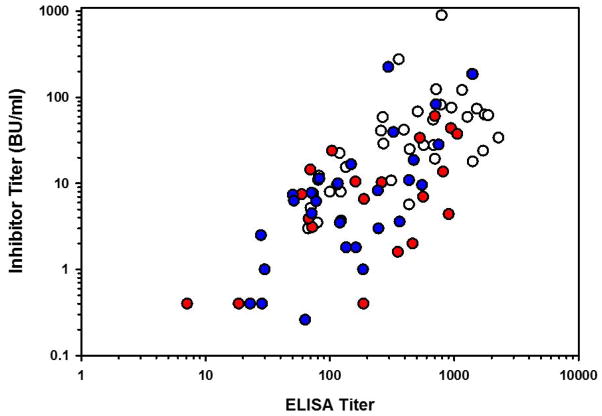
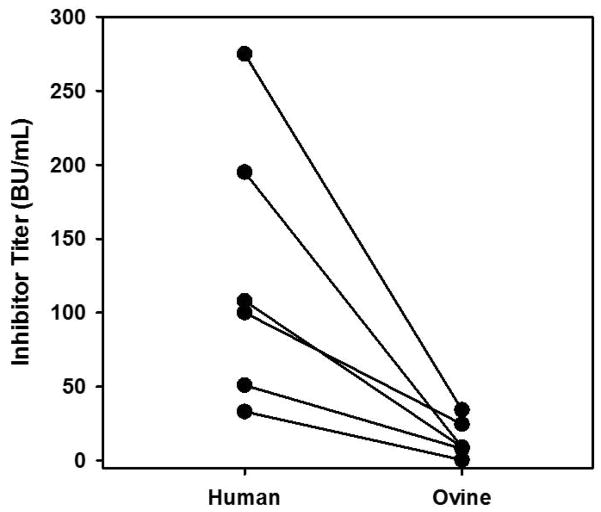
(A) An ELISA was performed on 26 congenital (black circle) and 10 acquired (white triangle) hemophilia A inhibitor patient plasmas using rhFVIII, roFVIII, or rpFVIII as the capture antigen. Data are presented as the relative cross-reactivity to that observed with rhFVIII. (B) The inhibitor titer of each patient plasma against hFVIII (white circle), rpFVIII (red circle), and roFVIII (blue circle) was measured by modified Bethesda assay as described in Methods. Due to limited plasma availability, triangles depict maximum/minimum approximations corresponding with their orientation. For example, an inverted triangle represents a value less than the position of the triangle on the y-axis. (C) Patient plasma ELISA versus inhibitor titers against human (white), porcine (red), and ovine (blue) FVIII orthologs were plotted and analyzed for correlation. Significant non-zero correlations were observed with P values of 0.0028, and 0.0003 for p-, and o-FVIII while P = 0.4913 for hFVIII.
To measure inhibitor titers, a modified Bethesda assay utilizing the three FVIII orthologs was implemented. This analysis revealed that inhibitory titers against both roFVIII and rpFVIII were statistically reduced compared to hFVIII (P < 0.05) although they were not distinguishable from each other (P > 0.05; Kruskal-Wallis One Way ANOVA) with median titers of 7.25 (roFVIII), 4.4 (rpFVIII), and 34 BU/mL (rhFVIII) (Figure 1B). Clinical experience shows that patients with inhibitor titers less than 5 often respond to high dose hFVIII replacement therapy while patients with inhibitor titers >10 BU/ml generally are not considered candidates for hFVIII infusion therapy [33]. Twenty-nine of the patient plasmas studied possessed inhibitor titers above 10 BU/mL against hFVIII and of those, 21 had <10 BU/mL titers against rpFVIII or roFVIII. Furthermore, 5 of the plasma samples assayed harbored comparatively lower titers against roFVIII than rpFVIII and 2 of these plasmas had titers >10 BU/ml against both rhFVIII and rpFVIII suggesting that roFVIII exclusively might be effective in certain populations of inhibitor patients. Due to limited availability of certain patient plasmas, 2 patient plasmas could not be tested for inhibitor titer and an additional sample (from patient 17) could not be tested for rpFVIII inhibitor titer. Significant correlations were observed between the ELISA and Bethesda titers determined for rpFVIII and roFVIII (P = 0.0028, and 0.0003, respectively, Student’s two-tailed t distribution), but no significant correlation was observed for rhFVIII (P = 0.4913; Figure 1C). Correlation coefficients for rhFVIII, rpFVIII, and roFVIII are 0.0145, 0.354, and 0.3827 respectively. These data demonstrate that hFVIII titers are not predictive of each other given that similar inhibitor titers spanned two orders of magnitude of ELISA titer. Inhibitor titers against rpFVIII or roFVIII were consistently refined within only one order of magnitude.
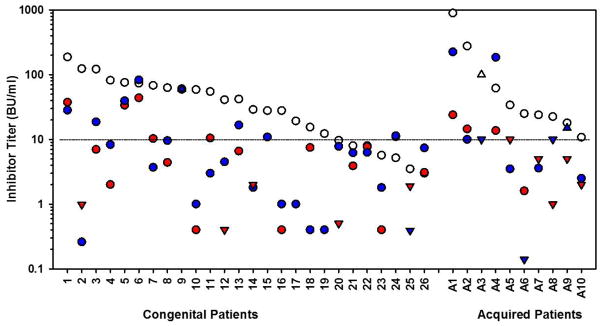
Distribution of A2 and C2 epitopes targeted by inhibitor patient plasmas
Inhibitor bank plasmas were screened for domain specificity by homolog-scanning ELISA incorporating single domain human/porcine hybrid molecules as described previously [12] (data not shown). Twenty patient plasmas of the initial 36 were shown to contain anti-hFVIII antibodies predominantly against the A2, C2, or both domains (Table 1). For 18/20 patients, there was sufficient plasma available to interrogate the targeted A2 and C2 epitopes by competition with panels of MAbs known to recognize non-overlapping epitopes in these domains (Figure 2) [10, 11]. Due to limited plasma availability, a single MAb was used to represent each inhibitor group. Additional A2 – A and C2 – BC MAbs were added because of their clinical prevalence and inhibitor potency and efficacy. Successful competition with at least one of the A2 and/or C2 domain targeting MAbs was demonstrated for 15/18 patient plasmas. Furthermore, at least one patient plasma competed with each group of MAbs within the C2 domain but only 3/7 groups within the A2 domain. Within the C2 domain, 12/14 patient plasmas competed with group BC MAbs followed by 8/14 with groups A and C, 4/14 with group B, and 3/14 with group AB. In contrast, only 3/7 groups of A2 domain targeting MAbs were competed by the patient plasmas. Overall, the A2 epitopes targeted by the patient plasmas appeared to overlap primarily with those targeted by A2 – A (6/10 plasmas) followed by A2 – E (2/10 plasmas) and then A2 – B (1/10 plasmas) with no overlap/competition observed with A2 – BCD, – C, – D or – DE MAbs. Of note, MAb groups A2 – A and C2 – BC contain the most potent inhibitory MAbs and are the most prevalent groups with which patient plasma ELISA competition was observed.
Table 1.
Inhibitor patient plasma Cross-reactivity and inhibitor titers of patient plasmas
| ELISA Cross Reactivity (%) | Bethesda Titer (BU/mL) | |||||
|---|---|---|---|---|---|---|
| Patient | Domain | Porcine | Ovine | Human | Porcine | Ovine |
| 10 | A2 | 3 | 11 | 59.0 | 0.4 | 1.0 |
| 14 | A2 | 50 | 50 | 29.0 | 2.0 | 1.8 |
| 19 | A2 | 23 | 28 | 12.3 | 0.4 | 0.4 |
| 20 | A2 | 17 | 62 | 9.7 | 0.5 | 7.8 |
| 1 | C2 | 76 | 54 | 187.6 | 37.5 | 28.3 |
| 3 | C2 | 49 | 41 | 119.5 | 7.0 | 18.7 |
| 5 | C2 | 56 | 34 | 76.0 | 33.8 | 39.6 |
| 7 | C2 | 51 | 24 | 68.4 | 10.3 | 3.7 |
| 9 | C2 | 55 | 43 | 59.3 | 60.7 | 60^ |
| 13 | C2 | 48 | 38 | 42.0 | 6.6 | 16.7 |
| 21 | C2 | 68 | 78 | 8.0 | 3.9 | 6.2 |
| 22 | C2 | 60 | 42 | 8.0 | 7.7 | 6.3 |
| 23 | C2 | 43 | 37 | 5.7 | 0.4 | 1.8 |
| 6 | A2 + C2 | 62 | 47 | 73.8 | 43.9 | 83.1 |
| 2 | A2 + C2 | 11 | 9 | 124.0 | 1.0 | 0.3 |
| 11 | A2 + C2 | 24 | 36 | 55.0 | 10.5 | 3.0 |
| 12 | A2 + C2 | 83 | 28 | 45.0 | 0.4 | 4.5 |
| 16 | A2 + C2 | 0 | 0 | 27.9 | 0.4 | 1.0 |
| 17 | A2 + C2 | 54 | 26 | 19.3 | NT | 1.0 |
| A1 | A2 + C2 | 13 | 37 | 900.0 | 24.0 | 225.0 |
NT: Not tested
Value shown is a minimal approximation
Figure 2. Identification of A2 and C2 domain epitopes targeted by patient plasmas.
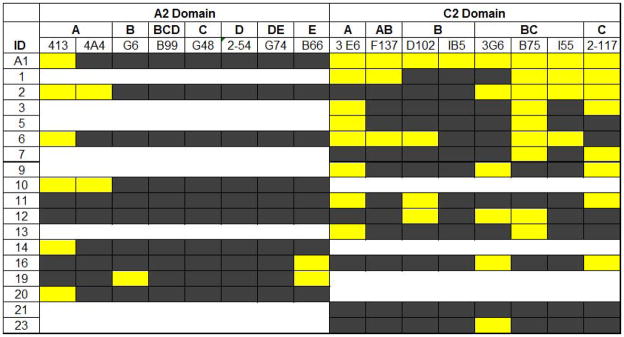
A competition ELISA was performed with anti-A2 and -C2 domain MAbs competing against human inhibitor IgG for binding to hFVIII. HFVIII first was blocked with patient plasma and then incubated with individual biotinylated MAbs. Patient ID is listed along the y-axis in descending order of the Bethesda titers measured for each sample and individual MAbs ID/group are listed across the top. Absence of competition is represented by black shading, and white shading designates data not determined. Competition is defined as a reduction of kinetic signal outside 2 standard deviations of control kinetic rates and is represented as yellow shading.
Inhibitory MAbs display reduced reactivity to orthologous FVIII molecules
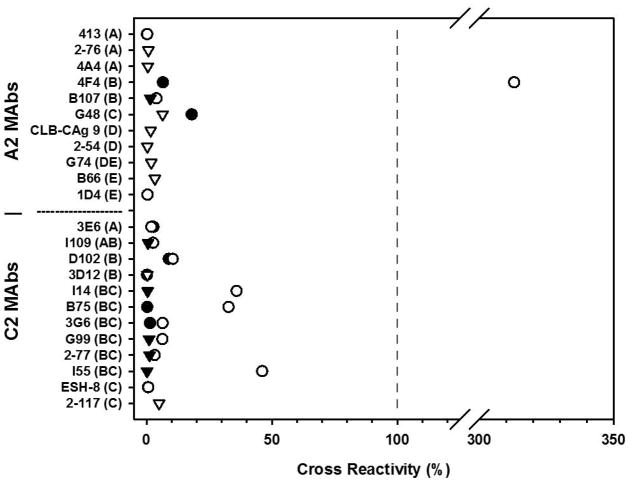
To determine if the reduced reactivity and inhibition of rpFVIII and roFVIII was due to the absence of inhibitory epitopes, we tested the ability of anti-hFVIII A2 and C2 domain specific MAbs known to possess varying inhibitor titers and kinetics to bind and inhibit roFVIII via indirect ELISA. Within the A2 domain, cross-reactivity only was observed with two MAbs, 4F4 and G48, representing inhibitor groups A2 – B and A2 – C, respectively (Figure 3). These antibodies previously were shown to have low human FVIII specific inhibitory activities of 330 and 5 BU/mg IgG respectively. To confirm that the diminished ELISA binding observed correlated with decreased inhibitor titers, specific inhibitory titers were measured for each MAb against roFVIII and rpFVIII (Table 2). Both MAb 4F4 and G48 demonstrated no detectable titer against roFVIII or rpFVIII. None of the high specific inhibitory activity hFVIII A2 domain targeting MAbs demonstrated cross-reactivity to either FVIII ortholog.
Figure 3. Reactivity of anti-hFVIII A2 and C2 domain MAbs with FVIII orthologs.
Panels of anti-A2 and -C2 domain targeting MAbs were assayed for cross-reactivity via indirect ELISA. Binding to roFVIII (white) and rpFVIII (black) is displayed as percent hFVIII binding as calculated by titration curve analysis. MAbs are listed on the y-axis by name with the inhibitor group classification in parenthesis. Triangles represent the maximal cross-reactivity percentages that could be determined experimentally with the available plasma.
Table 2.
Inhibitor titers of anti-human FVIII MAbs against ovine and porcine.
| Inhibitor | Domain | Kinetics | Group | HumanTiter (BU/mg) | Porcine Titer (BU/mg) | Ovine Titer (BU/mg) |
|---|---|---|---|---|---|---|
| 4A4 | A2 | I | A | 25,500 | < 8 | < 8 |
| 413 | A2 | I | A | 61,000 | < 1 | < 1 |
| 4F4 | A2 | I | B | 330 | < 1 | < 1 |
| B25 | A2 | I | C | 18 | < 4 | < 1 |
| 1D4 | A2 | I | E | 51,000 | 5 | 6 |
| G48 | A2 | II | C | * | * | * |
| 2–54 | A2 | II | D | **** | * | * |
| 3E6 | C2 | I | A | 11 | < 1 | < 4 |
| I109 | C2 | I | AB | 3,100 | < 1 | < 1 |
| D102 | C2 | I | B | 8,600 | 10 | 5 |
| 3D12 | C2 | I | B | 3,800 | < 1 | < 1 |
| I14 | C2 | II | BC | **** | * | ** |
| B75 | C2 | II | BC | * | * | * |
| 3G6 | C2 | II | BC | **** | * | * |
| G99 | C2 | II | BC | **** | * | * |
| 2–77 | C2 | II | BC | **** | * | * |
| I55 | C2 | II | BC | **** | * | * |
| ESH-8 | C2 | II | C | **** | * | * |
| 2–117 | C2 | II | C | * | * | * |
0–100 BU/mg;
100–1,000 BU/mg;
1,000–10,000 BU/mg;
> 10,000 BU/mg
Cross-reactivity within the C2 domain, however, revealed moderate roFVIII-specific cross-reactivity with 3 inhibitors, I14, B75, and I55, at 30 – 50% of hFVIII reactivity. MAbs I14, B75, and I55 are characterized by specific inhibitory activities of 44,000, <1, and 10,000 BU/mg IgG against hFVIII, respectively. Of these, only I14 possessed an inhibitor titer to roFVIII with a specific inhibitory activity between 100 – 1,000 BU/mg IgG. An additional 3 MAbs, D102, G99, and 3G6, cross-reacted with roFVIII at 5 – 15% of the hFVIII level however, inhibitor titers against the orthologs were again nominal. Cross-reactivity against rpFVIII was observed only with two MAbs, 2–117 and D102, and the percent reactivity was between 5 – 10% that of the reactivity to hFVIII.
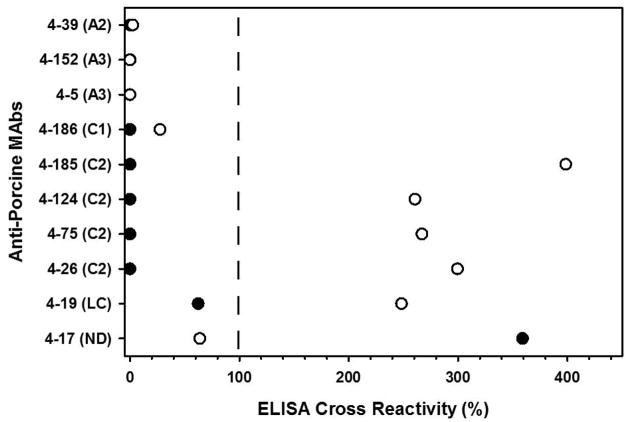
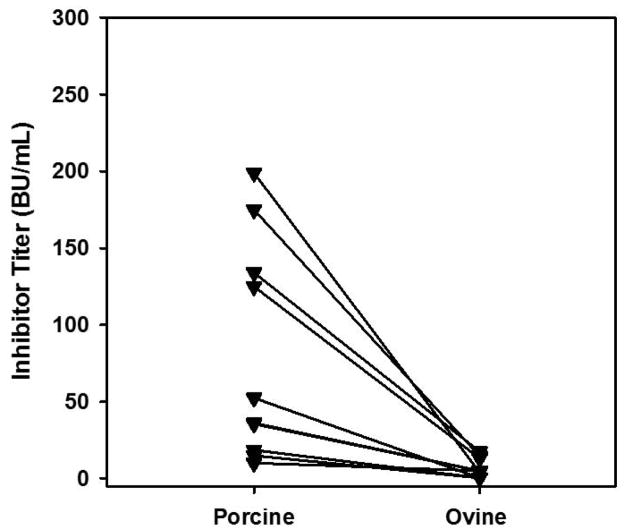
Due to the selection bias against identification of cross-reactive hFVIII C2 domain targeting MAbs, a similar pool of MAbs, this time generated against rpFVIII, were tested for cross-reactivity with rhFVIII and roFVIII. Ten MAbs with measurable inhibitor titers against rpFVIII were selected and tested by indirect ELISA. MAbs targeting the A1 or A2 domains of rpFVIII did not show any cross-reactivity to hFVIII or roFVIII, however all anti-rpFVIII light chain (A3 – C1 – C2) MAbs demonstrated cross-reactivity to roFVIII exceeding rpFVIII for all four C2 domain targeting MAbs (Figure 4). However, measurement of the specific inhibitory activities of these MAbs revealed near zero inhibition of either hFVIII or roFVIII (data not shown).
Figure 4. Reactivity of anti-rpFVIII MAbs with roFVIII.
Murine MAbs isolated from hemophilia A mice immunized with rpFVIII were screened for cross-reactivity to hFVIII (black) and roFVIII (white). The data presented are normalized to rpFVIII binding. MAbs are listed in the y-axis with the FVIII domain epitope specificity in parentheses. LC: Light chain; ND: not determined.
Pre-immunized hemophilia A mice display reduced inhibitor titer to roFVIII
To study the reactivity of anti-rFVIII immune plasma to roFVIII, samples from hemophilia A mice immunized with rhFVIII (n = 6) or rpFVIII (n = 10) were obtained from a previous study [31]. All mice displayed inhibitor titers to the specific immunogen of ≥10 BU/ml. However when tested for inhibition of roFVIII, all mice demonstrated reduced inhibitor titers against roFVIII as compared to the FVIII immunogen with mean reduction of 22 and 31 fold (P = 0.021 and 0.007, respectively for rhFVIII and rpFVIII; Paired t-test; Figure 5).
Figure 5. Inhibitor titers to FVIII ortholog in pre-immunized murine hemophilia A plasmas.
Mice were immunized with either rhFVIII (A) or rpFVIII (B), respectively. Following the 7th injection, plasma was collected via terminal cardiac puncture and assayed for inhibitor titer to the original immunogen and roFVIII via modified Bethesda assay. Lines connect the intramouse inhibitor titers recorded for the two FVIII orthologs. N = 6 and 10 respectively.
RoFVIII restores procoagulant function in an in vivo acquired hemophilia A model
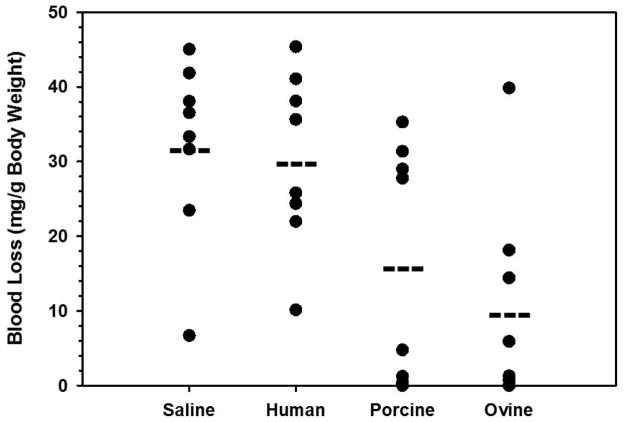
As each of the previous studies utilized in vitro surrogate assays for prediction of hemostatic function, an in vivo assay was developed to assess roFVIII functionality in vivo in a model of acquired hemophilia A. Autoimmunity against endogenous FVIII develops unpredictably in individuals resulting in a transient but rapid development of inhibitors. Although the hyper-immune state can correct without intervention, affected individuals are high risk for loss of life or limb and in these cases immune tolerance induction is not recommended. The high potency, type I kinetics and prevalence of A2-group A inhibitors in patient plasma provided support for their use for modeling this condition. Because C2 domain inhibitors possess type II kinetics, the residual activity even in saturating concentrations of inhibitor has empirically corrected a bleeding phenotype in mice. Therefore, F8+/+ mice were administered MAb 4A4 at levels empirically shown to completely inhibit endogenous murine FVIII and predicted to neutralize infused rhFVIII. Subsequently, each animal received 9 units of one rFVIII ortholog, to achieve near 100% normal murine FVIII activity levels, or saline only and then were challenged by tail transection bleeding assay. Wild type mice were selected to more accurately recapitulate the acquired disorder due to continuous biosynthesis and secretion of endogenous FVIII into circulation. Mean blood loss of roFVIII treated mice was significantly reduced compared to saline and rhFVIII (10.0, 32.1, and 30.3 mg/g body weight, respectively; P = 0.005 and 0.007 respectively, Student’s t-test) and no significant difference was observed between rpFVIII and roFVIII (P = 0.421, Mann Whitney U-test) (Figure 6). Administration of rhFVIII did not significantly reduce bleeding over saline only (P = 0.771; Student’s t-test).
Figure 6. In vivo testing of rFVIII orthologs in a murine model of acquired hemophilia A.
C57BL/6 mice were administered 10μg MAb 4A4 IP followed by 9 units rFVIII or saline only (n = 8). Blood loss over 40 minutes following tail transection was recorded. Dashes denote mean blood loss values for each experimental cohort.
RoFVIII produces high titer inhibitor titer mice with predominant A2 specificity
The previous findings of this study have all addressed the antigenicity of roFVIII with respect to existing anti-human or anti-porcine inhibitors. To address the issue of immunogenicity briefly, we sought to generate monoclonal antibodies against oFVIII. Following 8 injections of roFVIII, the paired ELISA/inhibitor titers of the 3 immunized F8−/− mice were 7,500/900, 2,500/71, and 700/35 (arbitrary units/BU per ml) respectively. Following purification of 9 anti-oFVIII MAbs, immunoprecipitation of activated roFVIII revealed a predominant A2 domain specificity with 6 of the 9 precipitating the A2 domain and 3 MAbs precipitating the cleaved light chain (cLC). Although there is no data regarding the inhibitory status of these MAbs, these data suggest that immunogenic regions within the A2 are highly conserved in oFVIII.
Discussion
The development of anti-hfVIII inhibitors remains the most challenging complication of FVIII replacement therapy, which otherwise is effective at achieving and maintaining hemostasis in individuals with hemophilia A. While the development of humoral immunity to a protein replacement product is not unique, the doses (2 – 4 μg/kg) of FVIII needed to elicit this response are comparatively low [34]. Although knowledge regarding the antigen uptake, presentation, costimulatory signals, and predisposing genetic factors associated with FVIII inhibitor development is rather sparse, recent studies have described the epitopes, tertiary structures, mechanism of inhibition, and frequency of inhibitors in both human patients and FVIII-immunized murine models of hemophilia A [10, 11, 13, 29, 31, 35]. Well in advance of these high-resolution molecular studies, FVIII had been crudely isolated from animal plasmas (e.g. bovine and porcine) [36] and shown to control bleeding in hemophilia A patients with and without FVIII inhibitors [37, 38]. The clinical successes demonstrated using these animal FVIII preparations in inhibitor patients (both congenital and acquired) supported the commercial development of a highly-purified pd-pFVIII product (Hyate:C, formerly Speywood/Ipsen) in 1980 [39]. A decade later, Lollar and colleagues cloned the pFVIII cDNA [26] and began defining the major inhibitory epitopes present in hFVIII, but lacking in pFVIII, using hybrid human/porcine FVIII constructs and inhibitor patient plasmas [8, 9, 40–42]. This work also provided a scientific foundation and supported the development of a commercial rpFVIII product (OBI-1, Baxter International Inc.). However outside of the rpFVIII (OBI-1) and original bovine plasma FVIII studies, no further development of the interspecies antigenicity differential has been pursued despite the obvious success of the approach, which is supported by the clinical utility of both pd- and r-pFVIII products.
Although the antigenicity and immunogenicity characteristics of FVIII orthologs largely have been ignored, their study has provided a platform for identifying structure/function relationships as well as interspecies differentials in biosynthetic, biochemical, and pharmacological properties that are thought to be exploitable for the rational design of improved rFVIII therapeutics and hemophilia A gene therapy applications. To date, FVIII orthologs from pigs, dogs, mice, monkeys (Dr. Pete Lollar, personal communication), and sheep have been generated and studied in recombinant form [25, 27, 43, 44]. Despite the interspecies differentials described above, each FVIII ortholog displays effective procoagulant activity in a human FVIII-deficient plasma bioassay and binds tightly to human von Willebrand factor, which is necessary for stabilization in plasma circulation. Furthermore, each of the FVIII orthologs displays unique biochemical properties in areas such as cellular secretion efficiency (rpFVIII > roFVIII > rhFVIII ≥ rmFVIII), decay rate following thrombin activation (rmFVIII > rpFVIII ≥ roFVIII > rhFVIII), and specific procoagulant activity (roFVIII > rpFVIII > rhFVIII > rmFVIII). The current study represents a continuation of this line of pursuit to identify FVIII sequences/molecules that can better address the clinical FVIII inhibitor problem.
Four key observations/findings were made in the current study. First, the inhibitor titers to roFVIII were significantly lower in most patient plasmas. Second, utilization of the ovine and porcine FVIII orthologs enabled further refinement of inhibitor epitopes within the A2 and C2 domains of hFVIII as well as determination of the inhibitor-epitope targeting frequencies within an existing patient population. Third, murine anti- rh- and rp-FVIII inhibitor plasmas both demonstrate lower inhibitor titers against roFVIII suggesting its potential utility as a tertiary treatment for patients with inhibitors formed against pFVIII in addition to hFVIII. Fourth, roFVIII retains procoagulant activity and restores hemostatic protection in vivo in an acquired A2 domain specific hemophilia A murine model. It was reported in a previous study that A2 domain – group A inhibitors map to residues 484–508 [8]. Alignment of human, porcine, and ovine FVIII A2 domains within this region reveals that oFVIII and pFVIII share non-conserved residues R484S, Y487H, R489G, and F501M. RoFVIII also contains unique residues at L491F and I508V. In the current study, it was observed that group A inhibitors are present within several patient plasmas and A2–A MAbs do not cross-react with, or inhibit, either rpFVIII or roFVIII. In contrast to the A2 domain findings, for patient plasmas that displayed inhibitory titers above 10 BU against roFVIII and rpFVIII, there tended to be an abundance of polyclonal anti-C2 domain IgG that are predicted to bind conserved functional epitopes. Thus it can be concluded that in the absence of a significant C2 domain inhibitor population, shared non-conserved residues within the A2 domains of rpFVIII and roFVIII are responsible for retained activity.
The present study provides evidence for reduced antigenicity of roFVIII in the context of human inhibitor patients. While we acknowledge that roFVIII displayed no overall significant difference from rpFVIII, this study demonstrates response to exogenous FVIII orthologs is not universal. Due to inter-patient variation in inhibitor epitope specificity as well as inter-species differentials in epitope targeting efficiency, it seems reasonable to conclude that there would be distinct advantages to having multiple FVIII ortholog-based products in the clinical hemophilia A armamentarium. The current results also support the general investigation of orthologous biomolecules not only as an approach to understanding structure/function, but also for the development of improved biotherapeutics. Given the ever-advancing push towards personalized medicine and the established clinical Bethesda assay for inhibitor detection, the case-by-case identification of the least antigenic and inhibited FVIII molecule may become the status quo.
Acknowledgments
This work was supported by grants from the National Institute of Health: C.B.D. – HL092179; C.B.D. and S.L.M. – HL112309, P.M.Z. – 5T32GM008602, and from Hemophilia of Georgia: S.L.M.
Footnotes
Authorship Contributions: P. M. Zakas, K. Vanijcharoenkarn and R. C. Markovitz designed and performed research, analyzed data, and co-wrote the manuscript; S. L. Meeks and C. B. Doering provided reagents, designed research, analyzed data, and co-wrote the manuscript.
Disclosure of Conflicts of Interest: The authors declare no competing financial interests.
References
- 1.Stonebraker JSB-MP, Soucie JM, Walker I, Brooker M. A study of variations in the reported haemophilia A prevalence around the world. Haemophilia. 2010;16:20–32. doi: 10.1111/j.1365-2516.2009.02127.x. [DOI] [PubMed] [Google Scholar]
- 2.Collins PWHS, Baglin TP, Dolan G, Hanley J, Makris M, Keeling DM, Liesner R, Brown SA, Hay CR UK Haemophilia Centre Doctors’ Organisation. Acquired hemophilia A in the United Kingdom: a 2-year national surveillance study by the United Kingdom Haemophilia Centre Doctors’ Organisation. Blood. 2007:109. doi: 10.1182/blood-2006-06-029850. [DOI] [PubMed] [Google Scholar]
- 3.Lusher JM, Arkin S, Abildgaard CF, Schwartz RS. Recombinant factor VIII for the treatment of previously untreated patients with hemophilia A. Safety, efficacy, and development of inhibitors. Kogenate Previously Untreated Patient Study Group. NEnglJMed. 1993;328:453–9. doi: 10.1056/NEJM199302183280701. [DOI] [PubMed] [Google Scholar]
- 4.Bray GL, Gomperts ED, Courter S, Gruppo R, Gordon EM, Manco-Johnson M, Shapiro A, Scheibel E, White G, III, Lee M. A multicenter study of recombinant factor VIII (recombinate): safety, efficacy, and inhibitor risk in previously untreated patients with hemophilia A. The Recombinate Study Group. Blood. 1994;83:2428–35. [PubMed] [Google Scholar]
- 5.Lusher JMLC, Kessler CM, Bedrosian CL. The safety and efficacy of B-domain deleted recombinant factor VIII concentrate in patients with severe haemophilia A. Haemophilia. 2003;9:38–49. doi: 10.1046/j.1365-2516.2003.00708.x. [DOI] [PubMed] [Google Scholar]
- 6.Gitschier J, Wood WI, Goralka TM, Wion KL, Chen EY, Eaton DH, Vehar GA, Capon DJ, Lawn RM. Characterization of the human factor VIII gene. Nature. 1984;312:326–30. doi: 10.1038/312326a0. [DOI] [PubMed] [Google Scholar]
- 7.Prescott RNH, Saenko EL, Scharrer I, Nilsson IM, Humphries JE, Hurst D, Bray G, Scandella D. The inhibitor antibody response is more complex in hemophilia A patients than in most nonhemophiliacs with factor VIII autoantibodies. Recombinate and Kogenate Study Groups. Blood. 1997;89:3663–71. [PubMed] [Google Scholar]
- 8.Healey JF, Lubin IM, Nakai H, Saenko EL, Hoyer LW, Scandella D, Lollar P. Residues 484–508 contain a major determinant of the inhibitory epitope in the A2 domain of human factor VIII. JBiolChem. 1995;270:14505–9. doi: 10.1074/jbc.270.24.14505. [DOI] [PubMed] [Google Scholar]
- 9.Barrow RT, Healey JF, Gailani D, Scandella D, Lollar P. Reduction of the antigenicity of factor VIII toward complex inhibitory antibody plasmas using multiply-substituted hybrid human/porcine factor VIII molecules. Blood. 2000;95:564–8. [PubMed] [Google Scholar]
- 10.Meeks SL, Healey JF, Parker ET, Barrow RT, Lollar P. Antihuman factor VIII C2 domain antibodies in hemophilia A mice recognize a functionally complex continuous spectrum of epitopes dominated by inhibitors of factor VIII activation. Blood. 2007;110:4234–42. doi: 10.1182/blood-2007-06-096842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Markovitz RCHJ, Parker ET, Meeks SL, Lollar P. The diversity of the immune response to the A2 domain of human factor VIII. Blood. 2013;121:2785–95. doi: 10.1182/blood-2012-09-456582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Healey JF, Parker ET, Barrow RT, Langley TJ, Church WR, Lollar P. The humoral response to human factor VIII in hemophilia A mice. J Thromb Haemost. 2007;5:512–9. doi: 10.1111/j.1538-7836.2007.02373.x. [DOI] [PubMed] [Google Scholar]
- 13.Walter JDWR, Brison CM, Cragerud RK, Healey JF, Meeks SL, Lollar P, Spiegel PC., Jr Structure of the factor VIII C2 domain in a ternary complex with 2 inhibitor antibodies reveals classical and nonclassical epitopes. Blood. 2013;122:4270–8. doi: 10.1182/blood-2013-08-519124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walter JDWR, Polozova MS, Pohlman J, Healey JF, Meeks SL, Lollar P, Spiegel PC., Jr Characterization and solution structure of the factor VIII C2 domain in a ternary complex with classical and non-classical inhibitor antibodies. J Biol Chem. 2013;288:9905–14. doi: 10.1074/jbc.M112.424564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sevy AMHJ, Deng W, Spiegel PC, Meeks SL, Li R. Epitope mapping of inhibitory antibodies targeting the C2 domain of coagulation factor VIII by hydrogen-deuterium exchange mass spectrometry. J Thromb Haemost. 2013 doi: 10.1111/jth.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hay CRDDIITS. The principal results of the International Immune Tolerance Study: a randomized dose comparison. Blood. 2012;119:1335–44. doi: 10.1182/blood-2011-08-369132. [DOI] [PubMed] [Google Scholar]
- 17.Doering CB, Healey JF, Parker ET, Barrow RT, Lollar P. High level expression of recombinant porcine coagulation factor VIII. J Biol Chem. 2002;277:38345–9. doi: 10.1074/jbc.M206959200. [DOI] [PubMed] [Google Scholar]
- 18.Doering CB, Healey JF, Parker ET, Barrow RT, Lollar P. Identification of porcine coagulation factor VIII domains responsible for high level expression via enhanced secretion. J Biol Chem. 2004;279:6546–52. doi: 10.1074/jbc.M312451200. [DOI] [PubMed] [Google Scholar]
- 19.Gangadharan B, Parker ET, Ide LM, Spencer HT, Doering CB. High-level expression of porcine factor VIII from genetically modified bone marrow-derived stem cells. Blood. 2006;107:3859–64. doi: 10.1182/blood-2005-12-4961. [DOI] [PubMed] [Google Scholar]
- 20.Ide LM, Gangadharan B, Chiang KY, Doering CB, Spencer HT. Hematopoietic stem-cell gene therapy of hemophilia A incorporating a porcine factor VIII transgene and nonmyeloablative conditioning regimens. Blood. 2007;110:2855–63. doi: 10.1182/blood-2007-04-082602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doering CB, Gangadharan B, Dukart HZ, Spencer HT. Hematopoietic stem cells encoding porcine factor VIII induce pro-coagulant activity in hemophilia A mice with pre-existing factor VIII immunity. Mol Ther. 2007;15:1093–9. doi: 10.1038/sj.mt.6300146. [DOI] [PubMed] [Google Scholar]
- 22.Dooriss KL, Denning G, Gangadharan B, Javazon EH, McCarty DA, Spencer HT, Doering CB. Comparison of Factor VIII Transgenes Bioengineered for Improved Expression in Gene Therapy of Hemophilia A. Hum Gene Ther. 2009;20:465–78. doi: 10.1089/hum.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doering CB, Denning G, Dooriss K, Gangadharan B, Johnston JM, Kerstann KW, McCarty DA, Spencer HT. Directed Engineering of a High-expression Chimeric Transgene as a Strategy for Gene Therapy of Hemophilia A. Mol Ther. 2009;17:1145–54. doi: 10.1038/mt.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porada CD, Sanada C, Long CR, Wood JA, Desai J, Frederick N, Millsap L, Bormann C, Menges SL, Hanna C, Flores-Foxworth G, Shin T, Westhusin ME, Liu W, Glimp H, Zanjani ED, Lozier JN, Pliska V, Stranzinger G, Joerg H, Kraemer DC, Almeida-Porada G. Clinical and molecular characterization of a re-established line of sheep exhibiting hemophilia A. J Thromb Haemost. 2010;8:276–85. doi: 10.1111/j.1538-7836.2009.03697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zakas PMGB, Almeida-Porada G, Porada CD, Spencer HT, Doering CB. Development and characterization of recombinant ovine coagulation factor VIII. PLoS One. 2012;7:e49481. doi: 10.1371/journal.pone.0049481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Healey JF, Lubin IM, Lollar P. The cDNA and derived amino acid sequence of porcine factor VIII. Blood. 1996;88:4209–14. [PubMed] [Google Scholar]
- 27.Doering CB, Healey JF, Parker ET, Barrow RT, Lollar P. High-level expression of recombinant porcine coagulation factor VIII. JBiolChem. 2002;277:38345–9. doi: 10.1074/jbc.M206959200. [DOI] [PubMed] [Google Scholar]
- 28.Bi L, Lawler AM, Antonarakis SE, High KA, Gearhart JD, Kazazian HH., Jr Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. NatGenet. 1995;10:119–21. doi: 10.1038/ng0595-119. [DOI] [PubMed] [Google Scholar]
- 29.Meeks SL, Healey JF, Parker ET, Barrow RT, Lollar P. Nonclassical anti-C2 domain antibodies are present in patients with factor VIII inhibitors. Blood. 2008;112:1151–3. doi: 10.1182/blood-2008-01-132639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barrow RT, Lollar P. Neutralization of antifactor VIII inhibitors by recombinant porcine factor VIII. J Thromb Haemost. 2006;4:2223–9. doi: 10.1111/j.1538-7836.2006.02135.x. [DOI] [PubMed] [Google Scholar]
- 31.Healey JF, Parker ET, Barrow RT, Langley TJ, Church WR, Lollar P. The comparative immunogenicity of human and porcine factor VIII in haemophilia A mice. Thromb Haemost. 2009;102:35–41. doi: 10.1160/TH08-12-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spencer HT, Denning G, Gautney RE, Dropulic B, Roy AJ, Baranyi L, Gangadharan B, Parker ET, Lollar P, Doering CB. Lentiviral vector platform for production of bioengineered recombinant coagulation factor VIII. Mol Ther. 2011;19:302–9. doi: 10.1038/mt.2010.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hay CR. The epidemiology of factor VIII inhibitors. Haemophilia. 2006;(Suppl 6):23–8. doi: 10.1111/j.1365-2516.2006.01362.x. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 34.Porter S. Human immune response to recombinant human proteins. J Pharm Sci. 2001;90:1–11. doi: 10.1002/1520-6017(200101)90:1<1::aid-jps1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 35.Meeks SL, Healey JF, Parker ET, Barrow RT, Lollar P. Non-classical anti-factor VIII C2 domain antibodies are pathogenic in a murine in vivo bleeding model. J Thromb Haemost. 2009;7:658–64. doi: 10.1111/j.1538-7836.2009.03299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bidwell E. The purification of antihaemophilic globulin from animal plasma. Br J Haematol. 1955;1:386–9. doi: 10.1111/j.1365-2141.1955.tb05527.x. [DOI] [PubMed] [Google Scholar]
- 37.Macfarlane RGMP, Witts LJ, Bidwell E, Biggs R, Fraenkel GJ, Honey GE, Taylor KB. Surgery in haemophilia; the use of animal antihaemophilic globulin and human plasma in thirteen cases. Lancet. 1957;273:251–9. doi: 10.1016/s0140-6736(57)90720-1. [DOI] [PubMed] [Google Scholar]
- 38.Biggs RMR. Treatment of Haemophilia and Other Coagulation Disorders. Oxford: John Wiley and Sons Ltd; 1966. [Google Scholar]
- 39.Kernoff PBTN, Lilley PA, Matthews KB, Goldman E, Tuddenham EG. Clinical experience with polyelectrolyte-fractionated porcine factor VIII concentrate in the treatment of hemophiliacs with antibodies to factor VIII. Blood. 1984;63:31–41. [PubMed] [Google Scholar]
- 40.Lollar P. Mapping factor VIII inhibitor epitopes using hybrid human/porcine factor VIII molecules. Haematologica. 2000;85:26–8. [PubMed] [Google Scholar]
- 41.Healey JF, Barrow RT, Tamim HM, Lubin IM, Shima M, Scandella D, Lollar P. Residues Glu2181-Val2243 contain a major determinant of the inhibitory epitope in the C2 domain of human factor VIII. Blood. 1998;92:3701–9. [PubMed] [Google Scholar]
- 42.Lollar P. Analysis of factor VIII inhibitors using hybrid human/porcine factor VIII. ThrombHaemost. 1997;78:647–51. [PubMed] [Google Scholar]
- 43.Doering C, Parker ET, Healey JF, Craddock HN, Barrow RT, Lollar P. Expression and characterization of recombinant murine factor VIII. Thromb Haemost. 2002;88:450–8. [PubMed] [Google Scholar]
- 44.Sabatino DEFC, Toso R, Santos A, Merricks EP, Kazazian HH, Jr, Nichols TC, Camire RM, Arruda VR. Recombinant canine B-domain-deleted FVIII exhibits high specific activity and is safe in the canine hemophilia A model. Blood. 2009;114:4562–5. doi: 10.1182/blood-2009-05-220327. [DOI] [PMC free article] [PubMed] [Google Scholar]