Summary
Objective
To investigate the prevalence of social exposure to a large, government-run ART program in rural South Africa.
Method
Clinical data on 6681 patients were matched with demographic data on a nearly complete cohort of 102,359 people residing in the program catchment area. We calculated the proportion of residents in the demographic surveillance area that were members of a household, or resided in a compound where someone had initiated ART or received pre-ART care.
Results
By January 2010, 3% of the population had initiated ART. However, 25% of the population shared household membership or resided in a compound with someone who had initiated ART; 40% shared household or living arrangements with people who had either initiated ART or were enrolled in pre-ART care.
Conclusion
Such high rates of social exposure suggest that large scale ART programs are likely to have profound spillover effects on social norms and economic welfare. These results also point to the opportunity to reach large numbers of people with other health and social services through existing ART programs.
Keywords: AIDS, South Africa, Antiretroviral therapy, Demography, Economics, Surveillance
Introduction
The widespread provision of life-saving antiretroviral treatment (ART) represents an unprecedented mobilization of resources and systems capacity for public health in settings of high HIV prevalence. In South Africa, an estimated 568,000 people were on ART by 2008, with a further 760,000 in need of treatment, according to Department of Health guidelines (Adam & Johnson 2010). With the government committed to universal rollout, over three million people are projected to be on ART by 2020 (Cleary & McIntyre 2010). In addition to its impacts on the health of people living with HIV, the implementation of large-scale treatment programs may have important spillover effects on households and communities. These may include changes in perceptions of HIV (Bechange et al. 2010, Cassell et al. 2006, Kennedy et al. 2007, Stolte et al. 2004) and attitudes towards people living with the virus (Wolfe et al. 2008); improvements in labor productivity (Thirumurthy et al. 2005, Rosen et al. 2008, Larson et al. 2008), consumption (Beard et al. 2009, Chhagan et al. 2008, Kaler et al. 2010, Wagner et al. 2009), and human capital investment (Graff Zivin et al. 2009); and perceptions of the health system itself. Although there is growing evidence of social spillover effects, hardly anything is known about the prevalence of social exposure to ART programs in HIV-hyperendemic populations. This study seeks to fill this gap.
In this paper, we describe the evolution of social exposure to a government-run ART program in a poor, rural population in northern KwaZulu-Natal, South Africa. The Hlabisa HIV Care and Treatment Programme is a highly decentralized ART program, implemented by nurses at public health clinics (Houlihan et al. 2010, Mutevedzi et al. 2010). Established in 2004, the program had initiated over 13,500 people on ART by 2010. We define social exposure as shared membership of a household, or residency in a compound (a group of one or more households) with someone who has either initiated ART or enrolled in pre-ART monitoring and care. These close-range measures of social exposure are particularly salient, given the high levels of social and economic integration within households and compounds. Further, patients in the treatment program were explicitly encouraged to disclose their status to other household members and to seek social support.
This analysis makes use of a novel data opportunity: the ability to match clinical data from an ART treatment program with a nearly complete population cohort residing in the program catchment area. We match clinical enrolment dates for 6681 patients in the Hlabisa HIV Care and Treatment Programme with longitudinal data on the living arrangements and household memberships of 102,359 people in the Africa Centre for Health and Population Studies (Africa Centre) demographic surveillance area (DSA). We calculate the proportion of this population that shared household membership or resided in the same compound as someone who had initiated ART. We also estimate the prevalence of co-residence and co-membership with people who enrolled in pre-ART monitoring and care. We describe the evolution of social exposure to the ART program since its inception in 2004 and discuss implications for future research and intervention.
Methods
Study population
The study population consists of all people who resided in the Africa Centre DSA at any point between 01 January 2004 and 30 June 2010. The Africa Centre is a research initiative funded by the Wellcome Trust (www.africacentre.com). Since 2004, the Africa Centre has collected longitudinal demographic data on 102,359 people who have resided in a 438 km2 area of Umkhanyakude District. Data on household composition, place of residence, migration, and vital status of members are collected every six months, with household response rates of > 99% (Tanser et al. 2008). The population of the DSA is mostly rural, and quite poor (Tanser et al. 2008), with high rates of unemployment and government assistance (Ardington et al. 2009). More than 20% of adults are HIV positive, with over half of women, ages 25–29, living with HIV (Tanser et al. 2008).
Hlabisa HIV Treatment and Care Programme
As part of the 2004 national treatment rollout, the South African Department of Health and the Africa Centre collaborated to launch an HIV care and treatment program in Hlabisa sub-district, in northern KwaZulu-Natal. The Hlabisa HIV Care and Treatment Programme is supported financially by the Presidential Emergency Programme for AIDS Relief (PEPFAR), via the United States Agency for International Development. The first patients were initiated in late 2004 at the Hlabisa referral hospital, with subsequent roll-out to all 17 primary health care clinics in the subdistrict (Houlihan et al. 2010). The program is primarily implemented by nurses in a highly decentralized model of care delivery, appropriate for the rural setting (Mutevedzi et al. 2010). To date, more than 13,500 patients have been initiated on ART in the Hlabisa Care and Treatment Programme. The Africa Centre DSA lies in the southern third of the Hlabisa health services catchment area, and it is estimated that 40% of patients in the Hlabisa HIV Care and Treatment Programme reside in the DSA (Houlihan et al. 2010). In 2008, it was estimated that 21% of HIV-infected individuals had initiated ART (Cooke et al. 2010); by 2010, the proportion was likely much higher. As a result of the ART program, AIDS related mortality in the DSA decreased by 40% between 2003 and 2006 (Herbst et al. 2009).
The ART program adheres to Department of Health guidelines regarding eligibility and treatment regimens. Adults are initiated on ART for WHO stage IV HIV disease or a CD4+ lymphocyte count of ≤ 200 cells/μl. Patients attend treatment education and counseling sessions, in which they are encouraged to disclose their status to household members and identify supporters to help them adhere. CD4 counts are monitored semi-annually for both those on treatment and those not yet eligible; viral load is measured annually (Houlihan et al. 2010). Maintenance in the program is quite high, with only 3.7% of patients lost to follow-up in the first 12 months (Mutevedzi et al. 2010).
Data
Patient records are kept at health clinics, but are regularly entered into the Africa Centre's ART Evaluation and Monitoring System (ARTemis) database (Houlihan et al. 2010). Patients provided written informed consent to use their clinical records for anonymous research. Ethics approval for data collection, linkage, and use was obtained from the Biomedical Research and Ethic Committee of the University of KwaZulu-Natal, in agreement with the Research Office of the KwaZulu-Natal Department of Health.
In this paper, we focus on two operational indicators: date of initiation on ART and date of earliest CD4 count obtained at the time of the first positive HIV test. Any individual who ever initiated ART was classified as an ART initiator from the date of initiation. Individuals who accessed the Hlabisa Care and Treatment Programme and had a CD4 count, but had not yet initiated treatment, were classified as enrollees in pre-ART monitoring and care from the date of the first CD4 count until the date of ART initiation or the end of follow up. For both ART initiators and pre-ART enrollees, we take the date of first CD4 count as a proxy for the date when an individual first accessed the Care and Treatment Programme. In cases where ART patients did not have a date for first CD4 count recorded (n = 26), or were reported to have had their first CD4 count after initiating therapy (n = 158), we use the date of ART initiation as the date of the first CD4 count. CD4 counts for non-initiators were not reported prior to 2007.
Data in ARTemis were linked with demographic cohort data from the Africa Centre Demographic Information System (ACDIS). Individuals were matched by their unique South African identification number, or by first name, surname, age, and sex. We were able to match 6681 patients in the Hlabisa HIV Care and Treatment Programme with individuals in the ACDIS database who met our eligibility criterion of having resided in the DSA at some point between 1 January 2004 and 30 June 2010. With such strict requirements for matching, the probability that a patient was mistakenly identified as a DSA resident (Type-I error) is likely very small. However, some patients who resided in the DSA could not be matched (Type-II error), for reasons such as data entry errors or use of different names in different settings. Previous analysis (conducted in 2009) found that 26% of patients who reported living within the DSA could not be matched to ACDIS (Cooke et al. 2010). Since this analysis, Africa Centre data management staff have worked to identify additional patients within ACDIS. Nevertheless, we expect Type-II error to be substantially higher than the negligible Type-I error, and estimates of social exposure reported in this paper should be viewed as lower bounds.
Of the 6681 patients matched for this analysis, 3825 had initiated ART; the remainder were patients with a CD4 count who had not yet initiated ART by the end of follow-up. Data on dates of ART initiation and first CD4 count were merged with demographic data on dates of all household residency episodes, household membership episodes, and bounded structure residency episodes. We defined enrolment in the ART program as carrying forward indefinitely from the date of ART initiation or the first CD4 count, until that patient exits the DSA, through out-migration or death.
Measures of Social Exposure to ARTWe measure four concepts of social exposure to the ART program, based on shared household membership or residence in a compound with someone who has initiated ART or enrolled in pre-ART monitoring and care. These concepts derive from the complex multilevel structure of the ACDIS data, with individuals nested in households and bounded structures, and households nested in bounded structures (Table 1). Due to high rates of labor migration, many individuals in rural KwaZulu Natal reside apart from other household members, but maintain close social ties, for example, visiting on weekends or during holidays. To capture important differences in social and geographic proximity, the Africa Centre distinguishes between household membership and residence in a bounded structure such as a “compound” or “homestead”, which may contain several households. Individuals may be members of multiple households in different places, but reside at only one bounded structure (Tanser et al. 2008, Africa Centre 2008). During the period of study, DSA residents shared household membership with 8.49 - 9.45 people, and resided in a bounded structure with 5.65 - 6.02 people, where these numbers represent the range of averages calculated semiannually during the study period (Table 1). In addition to these concepts, we defined “household of residence” as the household of which the individual is a member, which resides in the same bounded structure as the individual herself.
Table 1.
Household memberships and living arrangements of DSA residents
| Variable | Mean (low) | Mean (high) |
|---|---|---|
| Households per bounded structure | 1.04 | 1.11 |
| Households per individual | 1.06 | 1.07 |
| Individuals per household | 6.53 | 7.22 |
| Household co-members per individual | 8.49 | 9.45 |
| Individuals per bounded structure | 6.65 | 7.02 |
| Bounded structure co-residents per individual | 5.65 | 6.02 |
Note: data are high and low means for the thirteen semiannual cross-sections, Jan 2004 – Jun 2010
For each of these membership/residence concepts, we created variables at the level of the bounded structure and household, indicating the dates when there was someone living in that household/bounded structure who had initiated ART or had a CD4 count. We then merged these social exposure variables with individual level membership and residence data, creating the following four measures of social exposure:
co-residence in a household with someone who has initiated ART;
co-membership of a household with someone who has initiated ART;
co-residence in a bounded structure with someone who has initiated ART; and
any co-membership or co-residence affiliation with someone who has initiated ART.
These four measures were also defined for co-membership or co-residence with someone who had accessed the program as either an ART initiator or as a pre-ART enrollee, as indicated by the date of the person’s first CD4 count. In all cases, individuals who had accessed the program or initiated ART were counted among the “exposed”, so long as they resided in the DSA during the period of interest. We report the above measures as accumulated, rather than cross-sectional, exposures, due to substantive interest in “ever-exposure” to the ART program.
Data analysis
Prevalence of each social exposure was evaluated every six months, from 01 January 2004 through 30 June 2010. Numerators were the number of individuals exposed to the ART program by co-residence or co-membership, as defined above, at any point in the previous six months. Denominators included all individuals residing in a household within the DSA at any point in the previous six months. Due to births, deaths, external migration, internal migration, and changing household affiliations, denominators were not constant over time, with values ranging from 63,328 to 65,078. At any given time, approximately one third of the population under surveillance was not resident in the DSA. Since we report prevalence rates based on a nearly complete (>99%) sample of the underlying population of interest (Tanser et al. 2008), confidence intervals are extremely narrow and are not reported. Stata/SE 11.1 (StataCorp, LP; College Station, Texas) was used for all data analysis.
Results
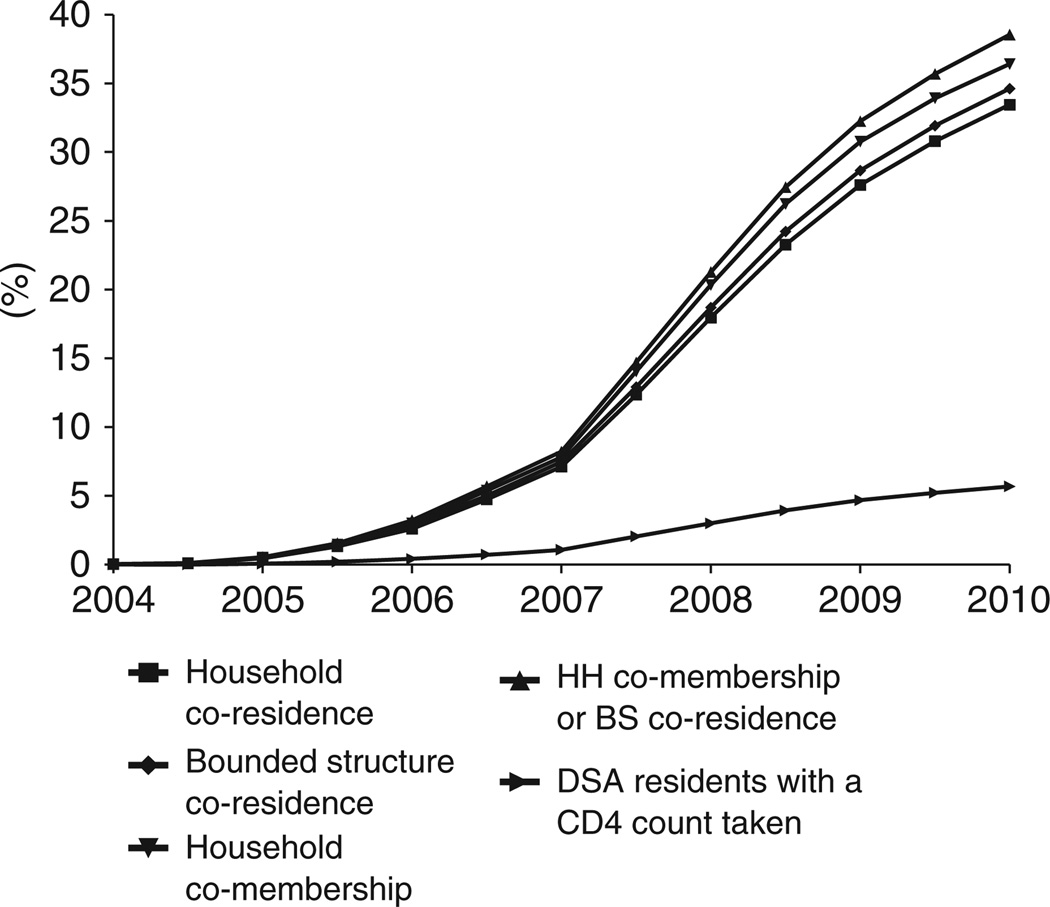
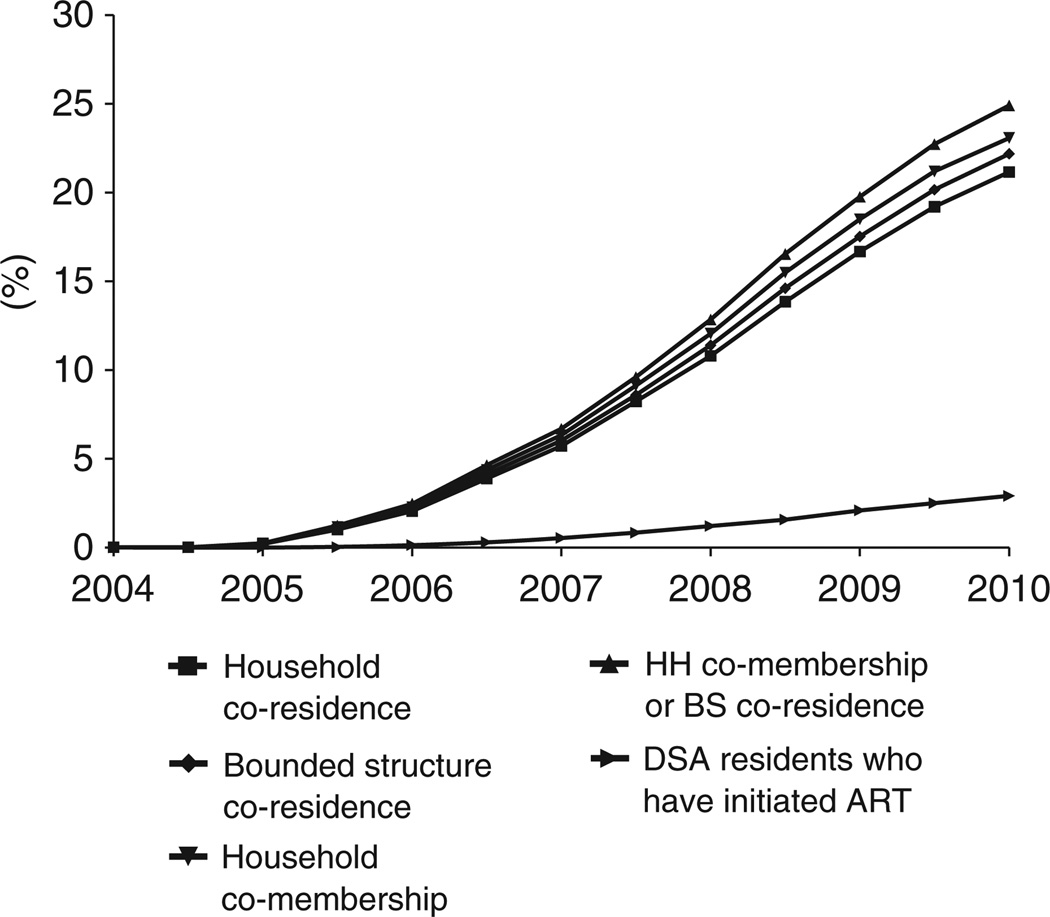
Figures 1 and 2 portray the evolution of social exposure to the ART program. Figure 1 displays rates of ever co-residence and ever co-membership with patients who have accessed the program and have had a CD4 count taken. Figure 2 presents the same measures for social exposure to ART initiators. Starting from zero exposure to ART in 2004, there has been a continuous and remarkably smooth expansion of social exposure to the ART program. By 1 January 2010, 25% of individuals in the DSA had been members of households or residents in a bounded structure in which someone was on ART. A full 39% of individuals in the DSA had shared membership of a household or residence in a bounded structure with someone who had accessed the HIV Care and Treatment Programme as either an ART initiator or pre-ART enrollee. We also present on each graph the percent of DSA residents who have accessed the program or initiated ART, providing a point of comparison for the trends in social exposure. In January 2004, for every individual who had initiated ART, 7.0 other individuals were “socially exposed” through shared household membership or compound of residence; by 2010, this number had declined to 6.0. (About 10% of the ART patients with which DSA residents shared household membership lived outside the DSA and were not included in these calculations.)
Figure 1.
Social exposure to someone who has either initiated ART or enrolled in pre-ART monitoring and care.
Figure 2.
Social exposure to someone who has initiated ART.
The graphs also show a clear ordinal ranking by type of social exposure. Household co-memberships consistently yield higher rates of social exposure than bounded structure co-residence. These findings are in line with our descriptive statistics, which indicate that on average, individuals are linked to more people through household memberships than through compound of residence. The dominance of household memberships may also be driven by the higher probability of accessing the ART program among people who are members of multiple households (RR = 1.23 - 1.42 for each additional household membership, p < .0001).
Discussion
This paper has described the evolution of social exposure to a government ART program in a large population cohort in rural South Africa with high HIV prevalence. Close social and geographical ties, at the level of household membership and compound of residence, link large proportions of the population to the ART program. By January 2010, 39% of the population had been members of households or resided in a compound with someone who had accessed the program; 25% of the population had shared household membership or resided with someone who had initiated ART. Comparing these numbers with the number of people who had accessed the program or initiated ART and were in the sample in June 2010, we observe that for every person who had initiated ART, about six had been exposed “socially” through shared household membership or residence in the same compound.
Like HIV itself, antiretroviral treatment is becoming a feature of everyday life for a large proportion of people in the DSA. Such high levels of social exposure suggest that ART roll-out may have widespread social, economic, and behavioural effects, not just on individual patients, but also on their households, compounds, and the larger communities in which they live. Economic spillover effects of ART are likely to include: improved labor productivity of people on ART (Thirumurthy et al. 2005, Rosen et al. 2008, Larson et al. 2008); lower care-giving burdens for other household members; lower health expenditure on treatment for opportunistic infections (Nachega et al. 2010); lower labor supply and increased school attendance of children in the household (Thirumurthy et al. 2005, Graff Zivin et al. 2009); and improved household consumption, e.g. leading to better nutritional status of children (Graff Zivin et al. 2009). In South Africa, ART programs also connect people who are unable to work with government disability grants and other sources of assistance (Chhagan et al. 2008, Nattrass 2005).
Social and behavioral effects may include increased understanding and communication about HIV and sexual risk; lower HIV stigma and improved social position of PLWHA (Wolfe et al. 2008); higher HIV testing rates (Mfundisi et al. 2005); and a reduction in fatalism and greater investment in the future (Fortson 2011). Large scale ART roll-out may also affect attitudes towards the health system and the ability of government to deliver needed services. The effect of ART on HIV risk behaviors is theoretically ambiguous. Some scholars have emphasized the potential for “disinhibition” as the consequences of HIV infection are perceived to be less severe (Cassell et al. 2006, Stolte et al. 2004); however, studies in sub-Saharan Africa have found little evidence of disinhibition (Bechange et al. 2010, Kennedy et al. 2007). Rather, ART initiation has been shown to reduce unprotected sex among initiators (Goldstein et al. 2010) and among non-spousal household members of initiators (Bechange et al. 2010). In addition to these behavior pathways, ART's effect on viral loads and longevity impact HIV transmission directly. Further research is necessary to elucidate the full range of spillover effects from ART programs; however our study illustrates that any such effects will have large population level impacts.This analysis also suggests an excellent opportunity to reach people with HIV services and other public health interventions. In settings of high HIV prevalence, household networks provide opportunities to recruit people living with HIV/AIDS into care and treatment and to reach many people with primary prevention programs. ART programs could also be a vehicle for prevention, surveillance, and clinical recruitment of patients with multi-drug resistant tuberculosis, cancer, diabetes, and other chronic diseases which require early detection and long term management. Finally, social workers at ART programs can link households to government grants and other resources to improve child nutrition and other aspects of household welfare.
Already, people on ART are engaging members of their households by disclosing their HIV status and seeking social support for ART adherence, as recommended by South Africa's National Treatment Guidelines (Bärnighausen et al. 2011; National Department of Health 2004). Women surveyed in the Hlabisa Programme disclosed their HIV status to an average of four close family members and friends; men disclosed to 3.4 people (Peoples & Bärnighausen 2008). Through the household networks of people on ART, large numbers of people could be reached with health and other social services.
The levels of social exposure reported in this paper are based on nearly complete population reporting (>99%) and are very precise (Tanser et al. 2008). However, there remain sources of uncertainty in our estimates of social exposure to ART. In particular, it is possible that we have not captured all individuals who have accessed the Hlabisa HIV Care and Treatment Programme. As described in the methods section, some patients who resided in the DSA could not be matched to ACDIS (Type-II error). Other DSA residents may have sought care in the private sector (Adam & Johnson 2010) or accessed government ART programs outside Hlabisa sub-district. However, it is unlikely that the proportion of patients accessing ART through the private sector is higher than a few percent (personal communication, Dr. Kevi Naidu, Clinical Programme Leader, Hlabisa HIV Care and Treatment Programme, 12 April 2011). Still others may have shared household membership with someone who accessed the Hlabisa Programme but resided outside the DSA between 1 January 2004 and 30 June 2010. Finally, clinical reporting of CD4 counts among pre-ART patients in the Hlabisa Care and Treatment Programme may be incomplete. Each of these factors suggests that our estimates of social exposure should be interpreted as lower bounds.
The rollout of life-saving HIV treatment has already touched the lives of a quarter of residents in this rural community of KwaZulu-Natal, through shared household membership or co-residence with someone who has initiated ART. Such high levels of social exposure to life-prolonging AIDS treatment are likely to have profound social, economic, and behavioral effects, and may also affect the course of the HIV epidemic itself. Future research on the spillover effects of ART should acknowledge the widespread impacts of such effects in HIV endemic populations. Further, high levels of social exposure to ART suggest a promising avenue for health systems to reach large populations with preventive and therapeutic health services through existing vertical ART programs (Bärnighausen et al. 2011).
Acknowledgements
This research was supported by the National Institute of Child Health and Human Development. Funding for the Africa Centre's Demographic Surveillance Information System was received from the Wellcome Trust, UK. The Hlabisa HIV Care and Treatment Programme has received support from the United States Agency for International Development and the President's Emergency Program for AIDS Relief.
References
- Adam MA, Johnson LF. Estimation of adult antiretroviral treatment coverage in South Africa. South African Medical Journal. 2010;99:661–667. [PubMed] [Google Scholar]
- Africa Centre. Africa Centre Demographic Information System (ACDIS) Fieldwork Training Manual, Africa Centre for Health and Population Studies. Somkhele, South Africa; 2008. [Google Scholar]
- Ardington C, Case A, Hosegood V. Labor supply responses to large social transfers: longitudinal evidence from South Africa. American Economic Journal: Applied Economics. 2009;1:22–48. doi: 10.1257/app.1.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bärnighausen T, Bloom DE, Humair S. Going horizontal - are recent shifts in U.S. international funding of global health interventions advisable? 2011 doi: 10.1056/NEJMp1014255. (submitted). [DOI] [PubMed] [Google Scholar]
- Bärnighausen T, Chaiyachati K, Chimbindi N, et al. Interventions to increase antiretroviral adherence in sub-Saharan Africa: a systematic review of evaluation studies. 2011 doi: 10.1016/S1473-3099(11)70181-5. (submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard J, Feeley F, Rosen S. Economic and quality of life outcomes of antiretroviral therapy for HIV/AIDS in developing countries: a systematic literature review. AIDS Care. 2009;21:1343–1356. doi: 10.1080/09540120902889926. [DOI] [PubMed] [Google Scholar]
- Bechange S, Bunnell R, Awor A, et al. Two-year follow-up of sexual behavior among HIV-uninfected household members of adults taking antiretroviral therapy in Uganda: no evidence of disinhibition. AIDS and Behavior. 2010;14:816–823. doi: 10.1007/s10461-008-9481-2. [DOI] [PubMed] [Google Scholar]
- Cassell MM, Halperin DT, Shelton JD, et al. Risk compensation: the Achilles' heel of innovations in HIV prevention? British Medical Journal. 2006;332:605–607. doi: 10.1136/bmj.332.7541.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhagan V, Luiz J, Mohapi L, McIntyre J, Martinson N. The socioeconomic impact of antiretroviral treatment on individuals in Soweto, South Africa. Health Sociology Review. 2008;17:95–105. [Google Scholar]
- Cleary S, McIntyre D. Financing equitable access to antiretroviral treatment in South Africa. BMC Health Services Research. 2010;10:S2. doi: 10.1186/1472-6963-10-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke G, Tanser F, Bärnighausen T, et al. Population uptake of antiretroviral treatment through primary care in rural South Africa. BMC Public Health. 2010:10. doi: 10.1186/1471-2458-10-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortson J. Mortality risk and human capital investment: The impact of HIV/AIDS in Sub-Saharan Africa. Review of Economics and Statistics, 2011;93:1–15. [Google Scholar]
- Goldstein M, Graff Zivin J, Habyarimana J, et al. Behvioral responses of patients in AIDS treatment programs: sexual behavior in Kenya. 2010 doi: 10.1515/1558-9544.1230. Working Paper. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff Zivin J, Thirumurthy H, Goldstein M. AIDS treatment and intrahousehold resource allocation: children's nutrition and schooling in Kenya. Journal of Public Economics. 2009;93:1008–1015. doi: 10.1016/j.jpubeco.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst AJ, Cooke GS, Bärnighausen T, et al. Adult mortality and antiretroviral treatment roll-out in rural KwaZulu-Natal, South Africa. Bulletin of the World Health Organization. 2009;87:754–762. doi: 10.2471/BLT.08.058982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlihan CF, Bland RM, Mutevedzi PC, et al. Cohort profile: Hlabisa HIV Treatment and Care Programme. International Journal of Epidemiology. 2010;39:351–360. doi: 10.1093/ije/dyp402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaler A, Alibhai A, Kipp W, et al. “Living by the hoe” in the age of treatment: perceptions of household well-being after antiretroviral treatment among family members of persons with AIDS. AIDS Care. 2010;22:509–519. doi: 10.1080/09540120903220287. [DOI] [PubMed] [Google Scholar]
- Kennedy C, O’Reilly K, Medley A, et al. The impact of HIV treatment on risk behaviour in developing countries: a systematic review. AIDS Care. 2007;19:707–720. doi: 10.1080/09540120701203261. [DOI] [PubMed] [Google Scholar]
- Larson BA, Fox MP, Rosen S, et al. Early effects of antiretroviral therapy on work performance: preliminary results from a cohort study of Kenyan agricultural workers. AIDS. 2008;22:421–425. doi: 10.1097/QAD.0b013e3282f3cc0c. [DOI] [PubMed] [Google Scholar]
- Mfundisi C, Chiranjan N, Rodrigues C, et al. Availability of antiretroviral therapy is associated with increased uptake of HIV testing services. South African Medical Journal. 2005;95:483–485. [PubMed] [Google Scholar]
- Mutevedzi PC, Lessells RJ, Heller T, et al. Scale-up of a decentralized HIV treatment programme in rural KwaZulu-Natal, South Africa: does rapid expansion affect patient outcomes? Bulletin of the World Health Organization. 2010;88:593–600. doi: 10.2471/BLT.09.069419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachega JB, Leisegang R, Bishai D, et al. Association of antiretroviral therapy adherence and health care costs. Annals of Internal Medicine. 2010;152:18–25. doi: 10.7326/0003-4819-152-1-201001050-00006. [DOI] [PubMed] [Google Scholar]
- National Department of Health, South Africa. National Antiretroviral Treatment Guidelines. First Edition. South Africa: Jacana; 2004. [Google Scholar]
- Nattrass N. Trading off income and health? AIDS and the Disability Grant in South Africa. Journal of Social Policy. 2005;35:3–19. [Google Scholar]
- Peoples A, Bärnighausen T. A descriptive assessment of disclosure among ART patients in rural South Africa. Poster presentation at the 3rd Annual Global Health Symposium; 5 November, 2008; University of Michigan, Ann Arbor, USA. 2008. [Google Scholar]
- Rosen S, Ketlhapile M, Sanne I, et al. Differences in normal activities, job performance and symptom prevalence between patients not yet on antiretroviral therapy and patients initiating therapy in South Africa. AIDS. 2008;22:S131–S139. doi: 10.1097/01.aids.0000327634.92844.91. [DOI] [PubMed] [Google Scholar]
- Stolte IG, Dukers NHTM, Geskus RB, et al. Homosexual men change to risky sex when perceiving less threat of HIV/AIDS since availability of highly active antiretroviral therapy: a longitudinal study. AIDS. 2004;18:303–309. doi: 10.1097/00002030-200401230-00021. [DOI] [PubMed] [Google Scholar]
- Tanser F, Hosegood V, Bärnighausen T, et al. Cohort profile: Africa Centre Demographic Information System (ACDIS) and population-based HIV survey. International Journal of Epidemiology. 2008;37:956–962. doi: 10.1093/ije/dym211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumurthy H, Graff Zivin J, Goldstein MP. The economic impact of AIDS treatment: labor supply in western Kenya. Journal of Human Resources. 2005;43:511–552. [PMC free article] [PubMed] [Google Scholar]
- Wagner G, Ryan G, Huynh A, et al. A qualitative analysis of the economic impact of HIV and antiretroviral therapy on individuals and households in Uganda. AIDS Patient Care and STDs. 2009;23:793–798. doi: 10.1089/apc.2009.0028. [DOI] [PubMed] [Google Scholar]
- Wolfe WR, Weiser SD, Leiter K, et al. The impact of universal access to antiretroviral therapy on HIV stigma in Botswana. American Journal of Public Health. 2008;98:1865–1871. doi: 10.2105/AJPH.2007.122044. [DOI] [PMC free article] [PubMed] [Google Scholar]