Abstract
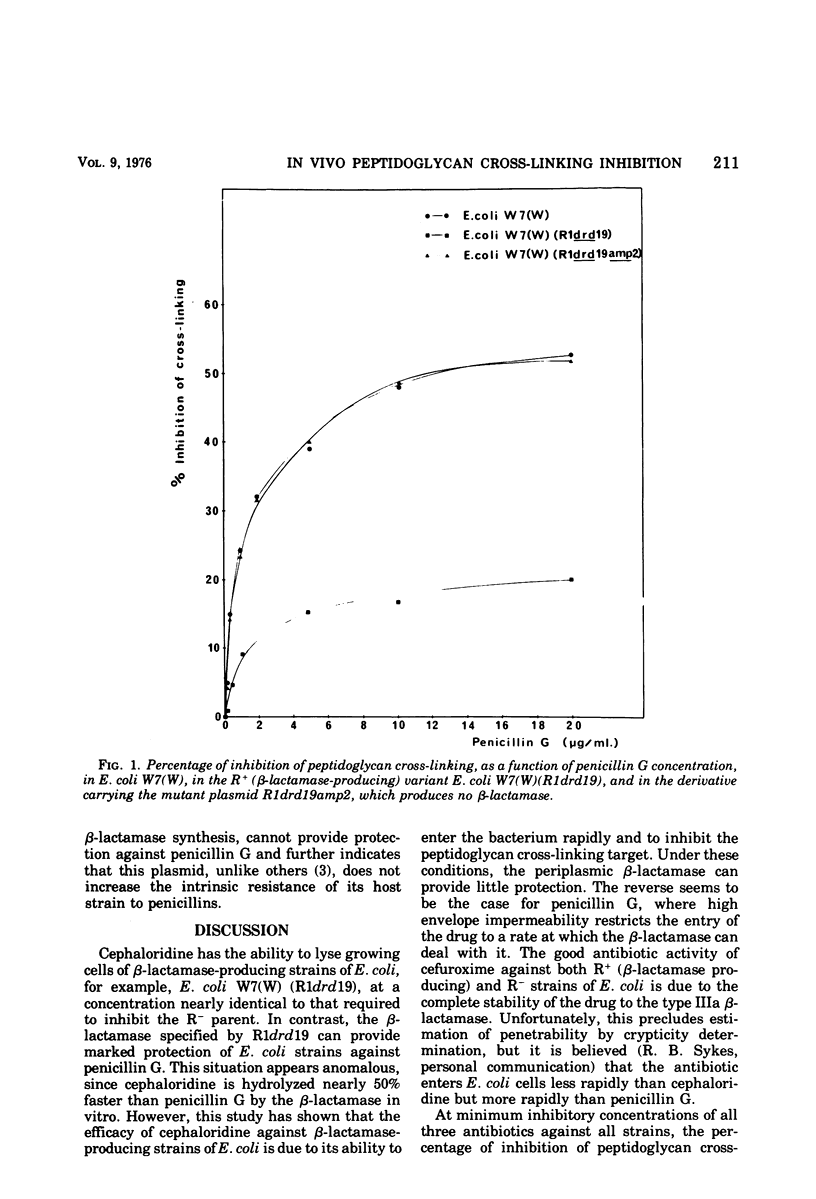
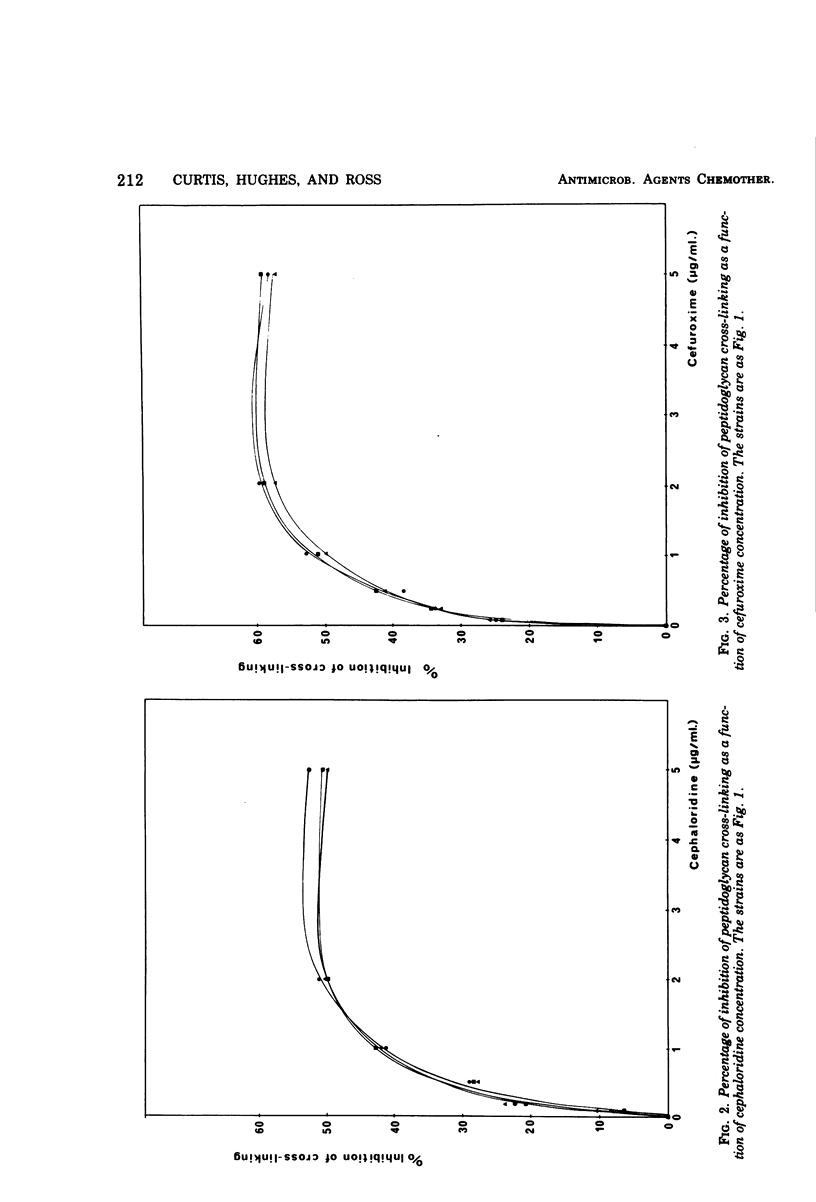
The degree of peptidoglycan cross-linking has been studied in growing cells of a Dap− Lys− auxotroph of Escherichia coli K-12 by following the incorporation of [3H]diaminopimelic acid into the lysozyme digestion products of crude, isolated peptidoglycan. The percentage of inhibition of cross-linking increases with increasing concentrations of penicillin G, cephaloridine, and cefuroxime. When the R factor R1drd 19 was introduced into the strain by conjugation, it was found that the type IIIa, β-lactamase specified by the plasmid was able to protect the cross-linking target against inhibition by penicillin G but not against cephaloridine, even though the β-lactamase hydrolyzes this substrate 50% faster than penicillin G. Cefuroxime, which is completely resistant to hydrolysis by the type IIIa β-lactamase, inhibited the peptidoglycan cross-linking target in both the R+ and R− variants of the assay strain. A mutant plasmid, R1drd19amp2, which specified no type IIIa β-lactamase synthesis, could not provide protection of the cross-linking target against penicillin G. The significance of these results, in relation to the ability of the antibiotics to pass the permeability barrier of the bacterial envelope, is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun V., Bosch V., Hantke K., Schaller K. Structure and biosynthesis of functionally defined areas of the Escherichia coli outer membrane. Ann N Y Acad Sci. 1974 May 10;235(0):66–82. doi: 10.1111/j.1749-6632.1974.tb43257.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Gnirke H., Henning U., Rehn K. Model for the structure of the shape-maintaining layer of the Escherichia coli cell envelope. J Bacteriol. 1973 Jun;114(3):1264–1270. doi: 10.1128/jb.114.3.1264-1270.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis N. A., Richmond M. H. Effect of R-factor-mediated genes on some surface properties of Escherichia coli. Antimicrob Agents Chemother. 1974 Dec;6(6):666–671. doi: 10.1128/aac.6.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann R., Höltje J. V., Schwarz U. Targets of penicillin action in Escherichia coli. Nature. 1972 Feb 25;235(5339):426–429. doi: 10.1038/235426a0. [DOI] [PubMed] [Google Scholar]
- Henning U., Rehn K., Braun V., Höhn B. Cell envelope and shape of Escherichia coli K12. Properties of a temperature-sensitive rod mutant. Eur J Biochem. 1972 Apr 24;26(4):570–586. doi: 10.1111/j.1432-1033.1972.tb01800.x. [DOI] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Glycopeptide transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reactions. Proc Natl Acad Sci U S A. 1966 Mar;55(3):656–663. doi: 10.1073/pnas.55.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiryo T., Strominger J. L. Penicillin-resistant temperature-sensitive mutants of Escherichia coli which synthesize hypo- or hyper-cross-linked peptidoglycan. J Bacteriol. 1974 Feb;117(2):568–577. doi: 10.1128/jb.117.2.568-577.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn A. M., Meynell G. G., Meynell E., Datta N. Sex pili and the classification of sex factors in the enterobacteriaceae. Nature. 1967 Oct 28;216(5113):343–346. doi: 10.1038/216343a0. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. Staphylococcal penicillinase and the new penicillins. Biochem J. 1962 May;83:229–235. doi: 10.1042/bj0830229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRET C. J. Iodometric assay of penicillinase. Nature. 1954 Nov 27;174(4439):1012–1013. doi: 10.1038/1741012a0. [DOI] [PubMed] [Google Scholar]
- Pollock J. J., Nguyen-Distèche M., Ghuysen J. M., Coyette J., Linder R., Salton M. R., Kim K. S., Perkins H. R., Reynolds P. Fractionation of the DD-carboxypeptidase-transpeptidase activities solubilized from membranes of Escherichia coli K12, strain 44. Eur J Biochem. 1974 Feb 1;41(3):439–446. doi: 10.1111/j.1432-1033.1974.tb03285.x. [DOI] [PubMed] [Google Scholar]
- Richmond M. H., Curtis N. A. The interplay of beta-lactamases and intrinsic factors in the resistance of gram-negative bacteria to penicillins and cephalosporins. Ann N Y Acad Sci. 1974 May 10;235(0):553–568. doi: 10.1111/j.1749-6632.1974.tb43290.x. [DOI] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]



