Abstract
Catatonia is one of the main symptoms of anti-N-Methyl-D-aspartate receptor (NMDAR) encephalitis. However, it is unknown whether metabolic changes observed with 18F-Fluorodeoxyglucose positron-emission tomography (FDG-PET) are correlated with the severity of the catatonic symptoms and clinical course. Three patients with anti-NMDAR encephalitis showing variable degrees of catatonia were performed with FDG-PET scans during the acute and recovery phase. PET findings showed hypermetabolism in the frontotemporoparietal regions and bilateral basal ganglia in the patient with mild catatonia, but more widespread hypermetabolic regions including the thalamus and brainstem were observed in the patients with more severe catatonia. Follow-up PET scans in one patient showed mild hypermetabolism in the right basal ganglia that correlated with mild rigidity and tonic posturing in the left extremities. Extent of cerebral metabolic changes correlates with the severity of catatonia accompanied by behavioural, motor, autonomic, and breathing abnormalities in anti-NMDAR encephalitis.
Keywords: NMDA, Encephalitis, PET, Catatonia, Metabolism, Autoimmune encephalitis
Introduction
Anti-N-Methyl-D-aspartate receptor (NMDAR) encephalitis is a recently described form of autoimmune encephalitis that has characteristic clinical features that include psychosis, dyskinesia, encephalopathy, seizures, and autonomic dysfunctions.1–3 As one of the main symptom in anti-NMDAR encephalitis, catatonia presents with motor and behaviour abnormalities that include alterations in thought, mood, and vigilance. The common signs of catatonia are posturing, rigidity, mutism, and negativism.4 Half of anti-NMDAR encephalitis patients do not display any abnormalities in brain magnetic resonance image (MRI) exams and the other half only show nonspecific changes in grey and white matter.5
There are only a few studies that assess anti-NMDAR encephalitis with 18F-Fluorodeoxyglucose positron-emission tomography (FDG-PET) scans. These studies found reduced glucose metabolism in temporal cortex regions6 and global hypometabolism with a prominent focal intense hypermetabolic lesion in the right cerebellar cortex.3 In addition, a recent FDG-PET study reported an increased cerebral metabolism gradient along the frontotemporal to occipital axis that correlates with disease severity as assessed by a modified Rankin Scale.7 However, no studies have correlated metabolic changes in PET with detailed clinical symptoms such as the severity of catatonia and rigidity.
Recently we experienced three serologically confirmed anti-NMDAR encephalitis with FDG-PET scan who presented with variable degrees of catatonia symptoms such as behavioural, motor, autonomic, and breathing abnormalities. We hereby report clinical course and PET findings with anti-NMDAR encephalitis
Case
Summary of clinical history
Three patients were admitted to the Asan Medical Centre in Seoul, Korea between 2011 and 2012. Clinical findings in all three patients were summarized in Table 1. These patients initially presented with marked agitation, psychotic behaviour, dyskinesis, and intermittent seizures. Seizures were observed in two patients (Patients 2 and 3) and disappeared 2–3 weeks after symptom onset. After admission, these patients showed varied degrees of catatonia symptoms such as behavioural, motor, autonomic, and breathing abnormalities. The patients were diagnosed with anti-NMDAR encephalitis after antibodies to NMDARs were found in their serum and cerebrospinal fluid (CSF).
Table 1.
Summary of the clinical characteristics of all three patients
| Patient 1 (22/F) | Patient 2 (30/F) | Patient 3 (17/F) | |
|---|---|---|---|
| Initial presentation | Intermittent involuntary movement on the Lt hand, blepharospasm, irrelevant speech, anxious, irritability, visual hallucination | Anxiousness, Lt head deviation and Lt hand tonic seizure, irritable, violent behavior, decreased verbal output, visual hallucination | Anxiety, focal seizure on the Lt U/E and face, psychotic behavior, visual hallucination |
| Clinical symptoms at initial PET | Catatonia, Lt U/E abnormal movement, tonic posture (Lt>Rt), irrelevant speech, mutism (−) obey command (+) | Catatonia, rigidity (Lt tonic posture), mutism (+), obey command (−), autonomic dysfunction (mild fever, tachycardia) | Catatonia, rigidity, tonic posture (Lt>Rt), opisthotonic posture, orofacial-tongue dyskinesia, mutism, obey command (−), autonomic dysfunction (mild fever, tachycardia, tachypnea, intermittent hypoventilation) |
| MRI finding | Hyperintense signal on the bilateral hemispheres | Normal | Diffuse hyperintense lesions involving cingulate gyrus and multifocal high signal intensities in bilateral cortices, more prominent on the right hemisphere |
| CSF findings | WBC 4/mm3 | WBC 6/mm3 | WBC 2/mm3 |
| Anti-NMDA Ab | Positive in CSF and serum | Positive in serum and negative in CSF | Positive in CSF and Serum |
| Underlying tumor | No underlying tumor | No underlying tumor | No underlying tumor |
| Treatment | Methylprednisolone | Methylprednisolone, electroconvulsive treatment | Methylprednisolone, intravenous immunoglobulin, electroconvulsive treatment |
| Outcome | Normalized | Normalized | Mild left U/E tonic movement |
F, frontal; Lt, left; PET, positron-emission tomography; U/E, upper extremities; Rt, right; MRI, magnetic resonance image; CSF, cerebrospinal fluid; WBC, white blood cell; NMDA, N-methyl-D-aspartate; Ab, antibody.
During this acute phase of the encephalitis, all three patients had catatonia with rigidity and tonic posturing in their extremities that was more severe on the left side. The catatonic symptoms were relatively mild in Patient 1, were moderate in Patient 2, and were severe in Patient 3 (Table 1). Clinical improvements were obtained after methyl-prednisolone therapy in Patient 1, after methyl-prednisolone with electroconvulsive therapy (ECT) in Patient 2, and after methyl-prednisolone, immunoglobulin, and ECT in Patient 3.
FDG-PET image were acquired 6–7 weeks after the onset of clinical symptoms (acute phase) and follow-up PET studies were performed at 11–23 weeks from the onset of symptoms (recovery phase; Patient 1 at 11 weeks, Patient 2 at 23 weeks, and Patient 3 at 21 weeks). None of the patients had clinical seizures the day before the PET scans.
To compare the brain area of metabolic change, the brain images were normalised with the Statistical Parametric Mapping software package (SPM2) with a 12 mm full width at half maximum (FWHM). For single-subject analyses, the scaled FDG-PET brain image of a patient was compared voxel-by-voxel with corresponding images from the age-matched normal control groups. One-sided, two sample t-tests for detecting areas of hypermetabolism and hypometabolism was done with false discovery rate (FDR) <0.05 in the acute phase (multiple comparisons with voxel size > 100) and uncorrected p< 0.001 in the recovery phase (multiple comparisons with voxel size >100).
Results of PET findings
The clinical symptoms and PET findings from the three patients are summarised in Table 2. The PET images in all three patients showed the various extent of increased metabolism in the right frontotempoparietal regions including the right orbitofrontal cortex, in the right insular cortex, in the bilateral basal ganglia (more prominent on the right side), in the cerebellum, and in the left frontal cortex depending on the severity of catatonia. No increase was observed in the thalamus or brainstem in Patient 1 (Fig. 1A). By contrast, increased metabolism was observed in the brainstem and right thalamus of Patients 2 (Fig. 1B) and 3 (Fig. 1C), with the increase being more prominent in Patient 3. Widespread decreased metabolism in the bilateral occipital cortex and the various extent of hypometabolism in the frontal lobe was observed in all three patients. This hypometabolism was more extensive in Patient 2 and 3 compared to Patient 1.
Table 2.
Brain areas that displayed metabolic changes in the acute and recovery phases and correlation with catatonic symptoms and clinical improvements
| Patient 1 (22/F) | Patient 2 (30/F) | Patient 3 (17/F) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Initial (6 wk) | Follow-up (11 wk) | Initial (7 wk) | Follow-up (23 wk) | Initial (6 wk) | Follow-up (21 wk) | ||
| Increase PET metabolism | Rt F, T, P, Rt BG, Rt Insular, Lt BG, Lt Cbll, Lt F | Normal | Rt F, T, Rt BG, Rt Tm, Rt insular, Lt BG, Lt T | Normal | Rt T, Rt BG, Rt Tm, Rt OF, Rt Insular, Lt F, Lt BG, Lt Cbll, brainstem | Rt BG | |
| Decreased PET metabolism | Both occipital, Lt sup. F | Normal | Both occipital, Lt F | Normal | Both occipital, Lt F, Lt angular G | Normal | |
| Clinical symptoms | Cognitive function | Anxiety, irritability, psychotic symptoms, obey command (+) | Normal | Mute, echolalia, intermittent obey command (−) | Normal | Mute, echolalia, obey command (−) | Normal |
| Motor symptom | Catatonia, Lt U/E tonic posture (Lt>Rt) | Normal | Catatonia, tonic posture rigidity (Lt>Rt), mild autonomic dysfunction | Normal | Catatonia, rigidity, tonic posture (Lt>Rt), opisthtonic posture, orofacio-tongue dyskinesia, cataplexy, autonomic dysfunction, breath abnormality | Intermittent Lt tonic rigidity | |
| Visual symptom | Visual hallucination | Normal | Visual hallucination | Normal | Visual hallucination | Normal | |
F, frontal; wk, weeks from the onset of symptoms; PET, positron-emission tomography; Rt, right; T, temporal; P, parietal; BG, basal ganglia; Lt, left; Cbll, cerebellum; Tm, thalamus; OF, orbitofrontal; sup., superior; G, gyrus; U/E, upper extremities.
Figure 1.
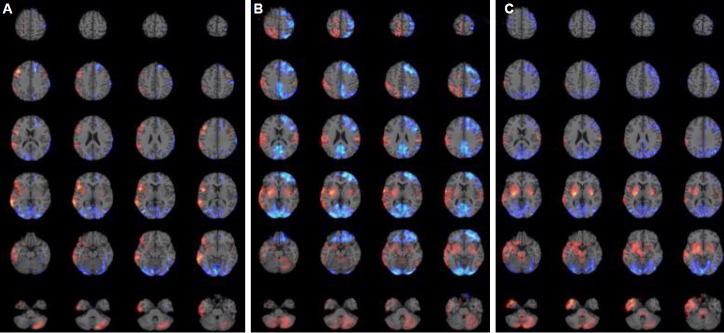
PET findings of acute phase in three patients with anti-NMDAR encephalitis. Increased metabolism is expressed as red color and decreased metabolism as blue color. (A) Patient 1; increased metabolism in the right frontotemporoparietal regions including the right orbitofrontal cortex, in the right insular cortex, in the bilateral basal ganglia, and in the left cerebellum. Decreased metabolism in the bilateral occipital areas and in the left frontal area. (B) Patient 2; increased metabolism in the right frontotemporoparietal regions including the right orbitofrontal cortex, in the bilateral basal ganglia, in the left parietal cortex, in the bilateral amygdala, in the right insular cortex, in the right thalamus, and in the bilateral cerebellum. Decreased metabolism in the bilateral occipital areas and in the left frontal and parietal areas. (C) Patient 3; increased metabolism in the right frontotemporoparietal regions including the right orbitofrontal cortex, in the bilateral basal ganglia, in the left parietal cortex, in the bilateral amygdala, in the right insular cortex, in the bilateral cerebellum, in the right thalamus, and in the brainstem. Decreased metabolism in the bilateral occipital areas, in the right frontal area, and bilateral parietal areas. PET, positronemission tomography; NMDAR, N-Methyl-D-aspartate receptor.
Follow-up PET showed normal finding in two patients (Patient 1 and 2) who did not show catatonia or tonic rigidity, but, showed mild increased metabolism in the right basal ganglia in Patient 3 who showed mild intermittent rigidity and tonic posturing in the left extremities without behavioural abnormalities (Fig. 2).
Figure 2.
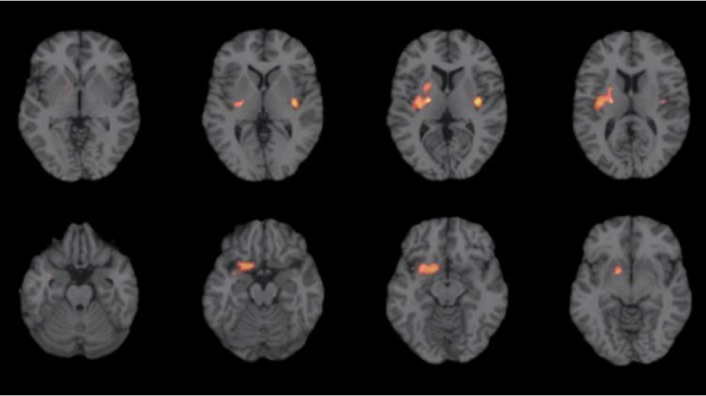
PET findings in follow-up period in patient 3. Mild intermittent rigidity and tonic posturing in her left extremities without behavioural abnormalities correlated with mild increased metabolism in the right basal ganglia. PET, positron-emission tomography.
Discussion
The results in our patients revealed that the extent of the metabolic changes in the PET images was well correlated with the severities of catatonia accompanied by behavioural and motor abnormalities. Hypermetabolism was observed in the frontotemporoparietal regions and bilateral basal ganglia in the mild catatonic patient, whereas the severe catatonic patient displayed more widespread and intense metabolic changes in the above mentioned areas and hypermetabolism in the thalamus and brainstem. These results were consistent with previous reports, which found that only right frontolateral hypermetabolism or occipital hypometabolism is observed in less severe cases7 and that wider metabolic changes in severe cases.8 But, until now there have been no reports about brainstem involvement in severe cases.
Anti-NMDAR antibodies cause a selective and reversible decrease in surface NMDARs, which results in variable clinical symptoms that depend on the extent of the decrease.5 Decreased NMDAR provokes frontostriatal syndrome through inactivation of gamma-aminobutyric acid (GABA) ergic neurons, orofacial and limb movements through disinhibition of a brainstem central pattern generator, breathing abnormalities through direct effects on the medullary-pontine respiratory network, and autonomic manifestations such as hypersalivation, hypertension, hyperthermia, and cardiac dysrhythmia through decreased regulation of dopaminergic, cholinergic, and noradrenergic systems.5 The extent of the affected brain areas by anti-NMDAR antibodies was well correlated with the severity of clinical symptoms and with the extent of hypermetabolic changes in our cases.
Although catatonia may be caused by GABAergic abnormalities in the orbitofrontal cortex that lead to dysfunctional dopaminergic interactions between the motor/premotor cortices and the subcortical basal ganglia,9,10 the pathogenesis and anatomical substrates of catatonia remain poorly defined. The behavioural, emotional, and affective symptoms may correlate with dysfunctions in orbitofrontal cortical activity. Furthermore, the motor symptoms may be mediated by dysregulation of cortical-subcortical circuits between the motor/pre-motor cortices and the basal ganglia.9 In addition, alterations in the brainstem may underlie the severe vegetative symptoms.9 In this study, Patient 1 displayed mild catatonic symptoms such as irrelevant speech, and tonic rigidity without displaying mutism, autonomic abnormalities, and breathing abnormalities. These findings were correlated with increased metabolism in frontotemporoparietal regions and in both basal ganglia without hypermetabolism in the thalamus or the brainstem. Patients 2 and 3 displayed more severe catatonic symptoms that included mutism, rigidity, tonic posturing, and autonomic dysfunctions, demonstrating diffuse hypermetabolism in the above mentioned areas and in the thalamus, and in the brainstem. Patient 3 showed more severe motor symptoms such as opisthotonic posture, orolingual dyskinesia, and breathing abnormalities than Patient 2. The most prominent difference between Patients 2 and 3 was that Patient 3 showed increased brainstem hypermetabolism. In contrast, the extent of the hypometabolic changes was not markedly different among these three patients even though Patient 2 and 3 slowed slightly wide hypometablic changes compared to Patient 1. Taken together, these data suggest that the severity of catatonic symptoms may be associated with the extent of increased metabolism rather than decreased metabolism.
Interestingly, Patient 3 showed intermittent tonic rigidity in her left extremities without any behavioural abnormalities during the follow-up period. The follow-up PET images showed mild hypermetabolism in the right basal ganglia. These findings suggest that tonic rigidity in catatonia is likely caused by hypermetabolism in the contralateral basal ganglia even though the statistical threshold of the second PET study was lower than the first study at acute phase. A previous study reported a patient with marked limb rigidity that showed basal ganglia hypermetabolism.11 In addition, reduced N-acetylasparatate was observed in the basal ganglia of a patient in the acute phase of anti-NMDAR encephalitis.12 The results of these studies are consistent with our findings.
In conclusion, the results of the present cases revealed that the extent of cerebral hypermetabolic changes correlates with the severity of catatonia accompanied by behavioural, motor, autonomic, and breathing abnormalities in anti-NMDAR encephalitis patients. The behavioural abnormalities were correlated with diffuse fronto-tempro-parietal, especially orbitofrontal, hypermetabolism, and tonic rigidity was correlated with hypermetabolism in the basal ganglia. In addition, hypermetabolism in the brainstem may provoke more severe clinical symptoms such as hypoventilation and autonomic dysfunctions.
Acknowledgments
This work was supported by the National Research Foundation of Korea grant founded by the MSIP (No. 2013R1A2A2A040-15925).
Footnotes
Conflicts of interest
The authors have no conflicts of interest.
References
- 1.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–8. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wandinger KP, Saschenbrecker S, Stoecker W, Dalmau J. Anti-NMDA-receptor encephalitis: a severe, multistage, treatable disorder presenting with psychosis. J Neuroimmunol. 2011;231:86–91. doi: 10.1016/j.jneuroim.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Maqbool M, Oleske DA, Huq AH, Salman BA, Khodabakhsh K, Chugani HT. Novel FDG-PET findings in anti-NMDA receptor encephalitis: a case based report. J Child Neurol. 2011;26:1325–8. doi: 10.1177/0883073811405199. [DOI] [PubMed] [Google Scholar]
- 4.Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry. 2003;160:1233–41. doi: 10.1176/appi.ajp.160.7.1233. [DOI] [PubMed] [Google Scholar]
- 5.Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pillai SC, Gill D, Webster R, Howman-Giles R, Dale RC. Cortical hypometabolism demonstrated by PET in relapsing NMDA receptor encephalitis. Pediatr Neurol. 2010;43:217–20. doi: 10.1016/j.pediatrneurol.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 7.Leypoldt F, Buchert R, Kleiter I, et al. Fluorodeoxyglucose positron emission tomography in anti-N-methyl-D-aspartate receptor encephalitis: distinct pattern of disease. J Neurol Neurosurg Psychiatry. 2012;83:681–6. doi: 10.1136/jnnp-2011-301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohr BC, Minoshima S. F-18 fluorodeoxyglucose PET/CT findings in a case of anti-NMDA receptor encephalitis. Clin Nucl Med. 2010;35:461–3. doi: 10.1097/RLU.0b013e3181db4d4a. [DOI] [PubMed] [Google Scholar]
- 9.Northoff G. Catatonia and neuroleptic malignant syndrome: psychopathology and pathophysiology. J Neural Transm. 2002;109:1453–67. doi: 10.1007/s00702-002-0762-z. [DOI] [PubMed] [Google Scholar]
- 10.Kaestner F, Mostert C, Behnken A, et al. Therapeutic strategies for catatonia in paraneoplastic encephalitis. World J Biol Psychiatry. 2008;9:236–40. doi: 10.1080/15622970701459802. [DOI] [PubMed] [Google Scholar]
- 11.Maeder-Ingvar M, Prior JO, Irani SR, Rey V, Vincent A, Rossetti AO. FDG-PET hyperactivity in basal ganglia correlating with clinical course in anti-NDMA-R antibodies encephalitis. J Neurol Neurosurg Psychiatry. 2011;82:235–6. doi: 10.1136/jnnp.2009.198697. [DOI] [PubMed] [Google Scholar]
- 12.Kataoka H, Dalmau J, Taoka T, Ueno S. Reduced N-acetylaspartate in the basal ganglia of a patient with anti-NMDA receptor encephalitis. Mov Disord. 2009;24:784–6. doi: 10.1002/mds.22167. [DOI] [PubMed] [Google Scholar]


