Abstract
Cefepime-induced neurotoxicity, including nonconvulsive status epilepticus, has been reported especially in patients with renal impairment. However, focal nonconvulsive status epilepticus is very rare and cefepime-induced aphasic status epilepticus proven by electroencephalography have never been reported to our knowledge. We present an interesting case of aphasic status epilepticus mimicking acute stroke in a patient treated by cefepime.
Keywords: Cefepime, Aphasic status epilepticus
Introduction
Cefepime is a broad-spectrum fourth-generation cephalosporin with enhanced coverage against Gram-positive and Gram-negative bacteria.1 Cefepime is predominantly cleared from the body by renal secretion, therefore the elimination half-life is increased in patients with renal impairment.2 Cefepime-induced neurotoxicity, including nonconvulsive status epilepticus (NCSE), has been reported especially in patients with renal impairment. We present a rare case of aphasic status epilepticus (SE) mimicking acute stroke in a patient with nephrotic syndrome and urinary tract infection treated by cefepime.
Case
A 36-year-old right-handed man was admitted to internal medicine department with 3 months history of generalized edema and decreased urine output. He was diagnosed as nephrotic syndrome and urinary tract infection. Because his urine culture demonstrated Pseudomonas aeruginosa, intravenous cefepime 2 g every 8 hours was initiated. His creatinine clearance calculated by Modification of Diet in Renal Disease (MDRD) formula was 49 mL/min/1.73 m2, suggesting mild renal impairment. He was consulted to neurology department for acute onset global aphasia and right hemiplegia during admission. His mental status was alert and he did not have any convulsive movements. Although he had a past medical history of intracerebral hemorrhage (ICH) due to left middle cerebral artery (MCA) aneurysmal rupture about 7 years ago, he did not have any language difficulties, hemiplegia, and seizures. At first, under the suspicion of acute stroke, an emergent brain magnetic resonance imaging (MRI) with angiography was done. However, there were no acute lesions in brain imaging, except old encephalomalacia in left fronto-temporal area and clipping of left MCA aneurysm (Fig. 1). There was no significant alteration of perfusion status on perfusion-weighted MRI. Although right hemiplegia recovered completely within a few hours, motor aphasia was persistent. After ruling out acute stroke, we performed electroencephalography (EEG) due to the persistent aphasia. EEG revealed continuous 2–3 Hz rhythmic spike-and-waves in left hemisphere, especially in fronto-temporal area (Fig. 2A), with clinical ictal phenomena (aphasia) during the EEG patterns, compatible with the working clinical criteria for NCSE with focal onset.3 Because he has been treated with high dose of cefepime since 2 days ago from the onset of neurological symptoms, we suspected cefepime-induced NCSE, particularly aphasic SE. Cefepime was withdrawn after the diagnosis of NCSE. His EEG (Fig. 2B) and clinical symptoms made a gradual recovery within 3 days after the discontinuation of cefepime. Follow-up brain diffusion-weighted MRI after 5 days from symptom onset also did not show the acute lesions.
Figure 1.
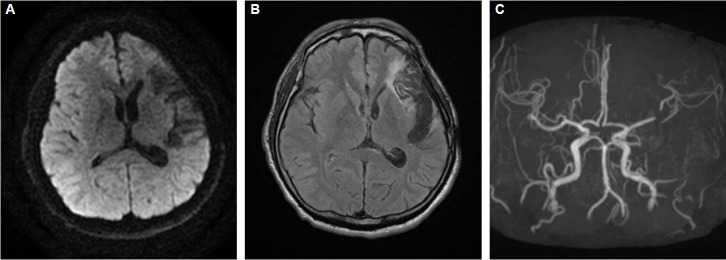
Brain magnetic resonance imaging and angiography at the onset of symptoms. Diffusion-weighted images (A) revealed no acute lesions. Fluid-attenuated inversion recovery images (B) showed old encephalomalacia in left fronto-temporal area and there was a trace of previous clipping of left MCA bifurcation aneurysm on angiography (C). MCA, middle cerebral artery.
Figure 2.
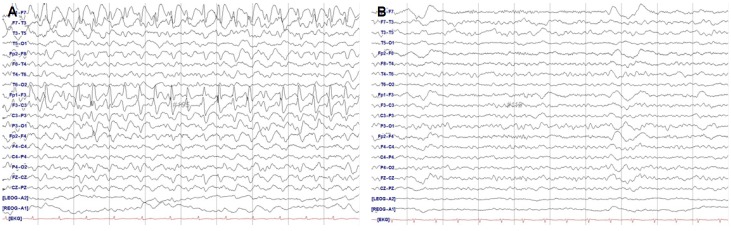
Electroencephalography at the onset of neurologic symptoms (A) and 4 days after the withdrawal of cefepime (B). Rhythmic spike-and-waves in the anterior left hemisphere (A) resolved after the withdrawal of cefepime (B).
Discussion
Various antibiotics have been reported to be associated with SE, and these drugs representatively include third and fourth generation cephalosporins and quinolones.4 Patients with prior central nervous system (CNS) disease, advanced age, and particularly renal impairment may be vulnerable.2,4–7 Although there have been various case reports and series of cefepime-induced neurotoxicity, including NCSE, aphasic SE itself is a rare phenomenon, and cefepime-induced aphasic SE proven by EEG has never been reported to our knowledge.
Although our patient had a past medical history of ICH, there were no acute lesions in brain imaging and he has never experienced a seizure before the administration of cefepime. Besides his neurologic symptoms and EEG abnormalities completely recovered after the discontinuation of cefepime. Therefore the diagnosis of cefepime-induced NCSE was more relevant than acute stroke or simple post-stroke seizures.
Our case was a high-risk patient having renal impairment and prior CNS disease simultaneously. Renal impairment may increase the concentration of cefepime in the serum, cerebrospinal fluid, and brain tissue.2,6 In addition, the increased permeability of blood-brain barrier (BBB) and decreased albumin binding of antibiotics in the presence of uremia, as well as the presence of endogenous uremic toxins might increase a patient’s vulnerability to neurotoxicity.6 Our patient’s brain, especially the left hemisphere, might be particularly vulnerable because BBB was locally disrupted by the prior ICH. The most widely accepted theory on the pathogenesis of seizures induced by beta-lactam antibiotics including cefepime involves the interference or inhibition of gamma-aminobutyric acid (GABA) binding to GABAA receptors.6 GABAA receptor inhibition can lead to increased neuronal excitability which can cause SE. The focal-aphasic-SE was thought to be the result of this hyperexcitability in the vulnerable brain area in our case. Therefore focal NCSE can be induced by cefepime in the presence of prior CNS disease, although the most of previous reports of cefepime-induced NCSE delineated generalized epileptiform discharges on EEG with decreased consciousness.
In conclusion, as the use of cefepime is increasing, clinicians should be aware of various cefepime-induced neurotoxicity. When encountered with unexplained neurologic deficits, EEG should be considered to diagnose NCSE in in patients receiving cefepime especially with renal impairment and prior CNS disease. Discontinuation of the drug is necessary to treat NCSE.
Acknowledgments
This work was supported by Inha University research grant (INHA-50479).
References
- 1.Yahav D, Paul M, Fraser A, Sarid N, Leibovici L. Efficacy and safety of cefepime: a systematic review and meta-analysis. Lancet Infect Dis. 2007;7:338–48. doi: 10.1016/S1473-3099(07)70109-3. [DOI] [PubMed] [Google Scholar]
- 2.Thabet F, Al Maghrabi M, Al Barraq A, Tabarki B. Cefepime-induced nonconvulsive status epilepticus: case report and review. Neurocritical Care. 2009;10:347–51. doi: 10.1007/s12028-008-9166-8. [DOI] [PubMed] [Google Scholar]
- 3.Beniczky S, Hirsch LJ, Kaplan PW, et al. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia. 2013;54(Suppl 6):28–9. doi: 10.1111/epi.12270. [DOI] [PubMed] [Google Scholar]
- 4.Misra UK, Kalita J, Chandra S, Nair PP. Association of antibiotics with status epilepticus. Neurol Sci. 2013;34:327–31. doi: 10.1007/s10072-012-1001-5. [DOI] [PubMed] [Google Scholar]
- 5.Garces EO, Andrade de Anzambuja MF, da Silva D, Bragatti JA, Jacoby T, Thome Saldanha F. Renal failure is a risk factor for cefepime-induced encephalopathy. J Nephrol. 2008;21:526–34. [PubMed] [Google Scholar]
- 6.Chow KM, Hui AC, Szeto CC. Neurotoxicity induced by beta-lactam antibiotics: from bench to bedside. Eur J Clin Microbiol Infect Dis. 2005;24:649–53. doi: 10.1007/s10096-005-0021-y. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Rodriguez JE, Barriga FJ, Santamaria J, et al. Nonconvulsive status epilepticus associated with cephalosporins in patients with renal failure. Am J Med. 2001;111:115–9. doi: 10.1016/s0002-9343(01)00767-7. [DOI] [PubMed] [Google Scholar]


