Abstract
Objective
Regulators of peri-implant bone loss in diabetic patients appears to involve multiple risk factors that have not been clearly elucidated. This study was conducted to explore putative local etiologic factors on implant bone loss in relation to type 2 diabetes mellitus, including clinical, microbial, salivary biomarker, and psychosocial factors.
Materials and Methods
Thirty-two subjects (divided into type 2 diabetes mellitus and non-diabetic controls), having at least one functional implant and 6 teeth, were enrolled in a one-year longitudinal investigation. Analyses of clinical measurements and standardized intra-oral radiographs, saliva and serum biomarkers (via protein arrays for 20 selected markers) and plaque biofilm (via qPCR for 8 periodontal pathogens) were performed at baseline and 1 year. In addition, the subjects were asked to respond to questionnaires to assess behavioral and psychosocial variables.
Results
There was a significant increase from baseline to 1 year in the probing depth of implants in the diabetes group (1.95mm to 2.35mm, p=0.015). The average radiographic bone loss during the study period marginally increased at dental implants compared to natural teeth over the study period (0.08mm vs. 0.05mm; p=0.043). The control group harbored higher levels of T. denticola at their teeth at baseline (p=0.046) and the levels of the pathogen increased significantly over time around the implants of the same group (p=0.003). Salivary osteoprotegerin (OPG) levels were higher in the diabetes group than the control group at baseline only; in addition, the salivary levels of IL-4, IL-10, and OPG associated with host defense were significantly reduced in the diabetes group (p=0.010, p=0.019, and p=0.024) while controls showed an increase in the salivary OPG levels (p=0.005). For psychosocial factors, there were not many significant changes over the observation period, except for some findings related to coping behaviors at baseline.
Conclusions
The study suggests that the clinical, microbiological, salivary biomarker, and psychosocial profiles of dental implant patients with type 2 diabetes who are under good metabolic control and regular maintenance care are very similar to those of non-diabetic individuals. Future studies are warranted to validate the findings in longer-term and larger clinical trials (ClinicalTrials.gov # NCT00933491).
Keywords: diabetes mellitus, dental implants, microbiology, salivary diagnostics, psychosocial indicator, alveolar bone loss
Introduction
The application of implant therapy in dentistry has offered viable solutions for the rehabilitation of edentulism, and systematic reviews have reported high survival rates for implant-supported restorations in partially-edentulous and well-maintained patients (Jung, et al. 2008, Pjetursson, et al. 2007, Tomasi, et al. 2008). However, the use of implants is not without complications. Among the biological complications that can affect implants after the initial integration phase, peri-implant diseases hold a key position, and particularly peri-implantitis is a major cause of progressive implant bone loss (Lindhe & Meyle 2008). The prevalence of peri-implantitis has been reported to range between 0-14.4% (Berglundh, et al. 2002) and is expected to increase considering the widespread implementation of implant therapy. Despite the prevalence of the disease, very limited information is available concerning the local and systemic risk factors that affect the preservation of peri-implant bone support. A systematic analysis of the literature concluded that while a history of periodontitis, poor oral hygiene, and smoking are strongly associated with peri-implant disease, there is insufficient evidence with respect to the effect of diabetes on peri-implant health (Heitz-Mayfield 2008).
Diabetes mellitus is a metabolic disorder expressed through different forms. Type 2 diabetes accounts for approximately 90-95% of the patients with the disease (Association 2011). Epidemiological records indicate that 25.8 million United States citizens have diabetes and that about 7 million of these patients remain undiagnosed (U.S. Department of Health and Human Services 2011). The global disease burden is also expected to rise in the future (Wild, et al. 2004). As opposed to the role of diabetes for peri-implant diseases, substantial evidence exists to support that diabetes is a true risk factor for periodontitis, affecting the prevalence, severity, and the extent of periodontal disease (Loe 1993, Mealey & Oates 2006, Soskolne & Klinger 2001, Taylor 2001, Taylor & Borgnakke 2008). In the context of a complex, multi-factorial disease such as periodontitis, diabetes is only one of the risk indicators (Genco 1996). Research also showed that psychosocial factors such as stress, depression, and certain types of negative coping behaviors may contribute as putative risk factors for periodontal deterioration as well (Peruzzo, et al. 2007).
Considering the complexities of the pathogenesis of periodontal disease, single-level risk assessment models cannot always accurately predict disease progression (Kornman 2008, Offenbacher, et al. 2008, Laine, et al. 2013). For this reason, multivariate analyses have been introduced that often combine clinical and other markers of disease activity (Lamster, et al. 1994, Lang & Tonetti 2003, Page, et al. 2002). Recently, salivary diagnostics have offered promising panels of biomarkers for monitoring disease progression (Taba, et al. 2005). Composite risk assessment incorporating clinical, biochemical (serum- and saliva-derived) as well as microbiological risk factors can characterize patient signatures predicting disease progression or stability (Kinney, et al. 2011).
In view of the above, this study was designed to: (i) longitudinally evaluate partially-edentulous patients affected by type 2 diabetes mellitus with functional dental implants to determine clinical and psychosocial risk factors for progressive alveolar bone resorption; and (ii) evaluate salivary and serum-derived biomarkers as well as putative periodontal pathogens for their ability to predict alveolar bone loss.
Material and Methods
Subjects and study design
The investigation was approved by the University of Michigan Medical Sciences Institutional Review Board and was registered with the National Institutes of Health clinical trials registry (ClinicalTrials.gov: NCT00933491). Only respondents who gave their written informed consent and met the inclusion criteria participated in this project. The cohort group consisted of subjects, over 40 years of age, who were in good general health and possessed at least 6 natural teeth and at least 1 implant in function for a minimum of 6 months. Subjects were excluded if they were medically unstable and had any of the following conditions: life expectancy of less than 5 years; history of chronic systemic illness or infection; history of blood dyscracias; history of oral cancer or non-healing lesion; history of cancer treatment within 12 months; or diagnoses of osteoporosis, osteopenia, or any bone malformations/defects/diseases. Subjects with active oral infection such as rampant caries or periodontitis as well as pregnant women were also excluded. Subjects were assigned to either the type 2 diabetes/test group or non-diabetes/control group. Participants were considered for the diabetes group if they presented with a self-reported diagnosis and management of type 2 diabetes. The control group included non-diabetic individuals as determined by medical history, lab test values and medical consultation, if needed.
Study timeline and procedures
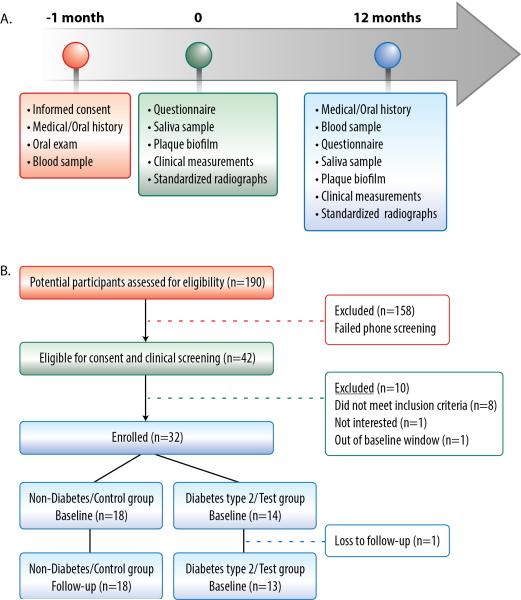
The baseline visit was completed within one month from the screening visit at the Michigan Center for Oral Health Research (MCOHR), while a follow-up visit was completed 12 months after the baseline appointment. Figure 1a illustrates the timeline, patient flow, and the conducted procedures.
Figure 1.
A. At the screening visit (-1 month), participant eligibility was assessed. The baseline visit (0) for the enrolled subjects was scheduled within 1 month from the screening visit and involved the described study procedures. The follow-up visit (12 months) was scheduled 12 months after the baseline within a window of 6 weeks. Similar procedures to the baseline appointment were performed with the addition of blood sampling. B. Thirty-two subjects were enrolled in the study, 18 in the control group and 14 in the test group. All participants completed the investigation with the exception of one subject in the diabetes group.
Clinical measurements
All teeth except for third molars were examined for periodontal measures by one of non-masked, calibrated examiners (TJO or NT) during the baseline and follow-up visits. Clinical parameters including free gingival margin level (FGM), probing depth (PD), clinical attachment level (CAL) and bleeding on probing (BOP) were measured at six sites per tooth and implant. Dichotomous scale indices of plaque accumulation (PI) and exudate (Exud) were also recorded as previously described (Haffajee, et al. 1983). The measurements were performed with the use of metal and plastic, 15-mm, calibrated periodontal probes (PCP-UNC 15, Hu-Friedy Manufacturing Co, Chicago, IL) for teeth and implants, respectively. In cases where changes had occurred between the baseline and the 12-month visit, such as tooth extraction or replacement of a restoration, the involved teeth as well as the adjacent surfaces of their neighboring teeth/implants were excluded from the analysis.
Standardized radiographs and analysis
Standardized periapical digital radiographs (Schick Technologies, Long Island City, NY, USA) were taken in the posterior dentition of all participants using a parallel technique. In cases where implants were placed in the anterior regions of the mouth, standardized periapical digital radiographs were taken as well. The radiographs were standardized with the use of bite registration material and an aluminum step wedge of known density (Duckworth, et al. 1983) while the same settings were used (63 kV, 8 mA, 0.1 s) (PLANMECA Intra DC, Finland). Linear bone measurements were taken on the mesial and distal surfaces of each tooth and implant. Reproducible reference points where used such as the cementoenamel junction, the apical border of a restoration or the implant crown-abutment junction for the determination of alveolar bone height at baseline and at 12 months. The radiographs were taken by one examiner (NT) and were analyzed by the same trained and calibrated examiner in a masked, random order with the use of a computer software measurement tool (Emago®, Oral Diagnostic Systems, Amsterdam, Netherlands). In cases where changes had occurred between the baseline and the 12-month visit such as, tooth extraction or replacement of a restoration the involved teeth as well as the adjacent surfaces of their neighboring teeth/implants were excluded from the analysis. The same applied if unrestored implants were identified.
Serum and saliva biomarkers
Twenty mL of whole blood sample was collected from each subject at the screening and 1 year follow-up visit. Once collected, samples were allowed to clot at room temperature for 30 minutes and then were centrifuged for 15 min at 2600 rpm. Serum was stored at -80°C until analysis. Likewise, unstimulated whole saliva was collected at the baseline and follow-up visit by passive drooling into sterile plastic tubes from all participants (Mandel & Wotman 1976). The collection was completed as soon as 2 ml whole saliva was collected or 15 minutes of sampling time had elapsed. Subsequently, the samples were placed on ice, supplemented with a proteinase inhibitor combination of 1% aprotinin and 0.5% phenylmethylsulphonylfluoride and finally aliquotted prior to storage at −80°C (Ramseier, et al. 2009). The following biomarkers were analyzed for both the serum and saliva samples: IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, TNF-α, INF-γ, CRP, MIP-1α, MIP-1β, MMP-1, MMP-2, MMP-8, MMP-9, TIMP-1, TIMP-2, osteoprotegerin (OPG), adiponectin and procalcitonin (ProCT). Protein biomarker levels were determined by a custom human array-based multiplex sandwich ELISA system (Quantibody® Custom Array, RayBiotech, Inc, Norcross, GA, USA), as previously reported (Ramseier, et al. 2009).
Microbial plaque collection and analysis
Sub-gingival plaque biofilm was harvested from the mesiobuccal surface of implants and their adjacent teeth at the baseline and follow-up visit. The area was dried with a gentle blast of air and the supra-gingival/supra-mucosal plaque was carefully removed. A sterile Gracey curette was inserted apically until resistance was felt at the base of the sulcus/pocket. The operator (TJO or NT) then initiated one working stroke upward against the tooth/implant collecting the sample. A plastic Gracey curette was used for plaque sampling around implants. The sample was immediately placed into labeled vials containing 500 μl of stabilizing buffer to prevent mRNA degradation (RNA Protect™, Ambion, Austin, TX) and was shaken for 10 seconds. The vial was closed and then vortexed for 30 seconds. Samples were stored at 4°C until sent to the laboratory for analysis. The detection of Aggregatibacter actinomycetemcomitans, Campylobacter rectus, Fusobacterium nucleatum, Prevotella intermedia, Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola and Candida albicans was evaluated by qPCR, as previously described (Mullally, et al. 2000). The percentage of the total flora for each species was calculated by dividing the number of target organisms by the total number of bacteria as determined by qPCR using 16S rRNA primers that reacted with all bacterial species. Data were presented per group separately for teeth and implants.
Questionnaire for behavioral and psychosocial factors
A questionnaire was developed to measure the respondents’ background characteristics (i.e., gender, age, ethnicity/race, employment status, educational background and financial information) as well as several behavioral and psychosocial risk factors, such as smoking, alcohol consumption, depression, stress and coping styles. To measure depression, stress, and coping styles, standardized and validated scales, namely the Center of Epidemiological Studies Depression Scale (CESD) (Radloff 1977), the Perceived Stress Scale (PSS) (Cohen S 1988), and the Brief COPE (Carver 1997) were used, respectively.
Examiner training and calibration for clinical and radiographic measurements
The two clinical examiners (TJO, NT) completed inter- and intra-examiner calibration sessions held at the beginning of the study with the participation of a gold standard examiner (JK). The two examiners demonstrated at least 83% of CAL measurements within 1 mm of each other with a 95% confidence interval of (0.74, 0.90) and at least 96% of PD measurements within 1 mm of each other with a 95% confidence interval of (0.90, 0.99). The examiner who performed the radiographic analysis (NT) completed inter- and intra- examiner calibrations sessions held at the beginning and at the end of the analysis with the participation of a gold standard examiner. The inter-examiner Pearson's correlation coefficient was at least 0.975 with a mean difference 0.16 and a 95% confidence interval of (0.05, 0.27). The intra-examiner Pearson's correlation coefficient was at least 0.992 with a mean difference 0.10 and a 95% confidence interval of (0.04, 0.16).
Statistical plan and analysis
The clinical, radiographic, biomarker and microbial data were averaged within each subject at each time point, separately for teeth and implants where available. The biomarker data were also log-transformed before averaging to promote normality. Average values per group were calculated with their respective standard errors or deviations. Categorical data were compared between groups at each study visit using chi-square tests. For continuous data, the following comparisons were performed: (i) comparisons between groups separately for teeth and implants at each time point (ii) comparisons within each group over time independently for teeth and implants (iii) comparisons of the differences noted between the average values around implants and the average values around natural teeth. Significance of comparisons (i) was based upon a two-sample t-test, and significance of comparisons (ii), and (iii) was based on a paired t-test and repeated-measures ANOVA for dependent variables. Repeated measurement MANOVA was used for the Brief-COPE scores. A p-value less than 0.05 was considered statistically significant. Due to the exploratory nature of this study, no adjustment was made to p-values for multiple comparisons. Normality was not calculated in this study due to the small sample size of the patient population.
Results
A total of 190 individuals were screened for eligibility, and 32 subjects were enrolled (18 in the non-diabetes/control group and 14 in the diabetes type 2/test group). One patient of the test group did not return for the follow-up visit (Figure 1b). One patient of the control group could not provide an adequate amount of whole saliva for analysis in any of the study visits. Table 1 presents the background characteristics of the study groups at baseline. Nine males and nine females were in the control group while seven males and seven females were in the diabetes group. The mean ages of the participants were 64 ± 8.1 years for the control and 65 ± 8.9 years for the test group. The groups differed significantly only at their mean HbA1C levels (5.7% vs. 7.1%; p=0.001). The difference between groups was also significant at the 12-month visit. However, no significant intragroup changes occurred over the study period (data not shown).
Table 1.
Patient Demographics
| Control | Diabetes | p-value | |
|---|---|---|---|
| Gender: | |||
| - Male | 9 | 7 | 0.639 |
| - Female | 9 | 7 | |
| Age (years): | |||
| Mean ± SD | 64± 8.1 | 65 ± 8.9 | 0.746 |
| Median | 66.5 | 66.5 | |
| Range | 48-75 | 51-80 | |
| Ethnicity/race: | |||
| - European American | 18 | 11 | |
| - African American | 0 | 2 | 0.119 |
| - Asian American | 0 | 1 | |
| HbA1c (%): | |||
| Mean ± SD | 5.7 ± 0.27 | 7.1 ± 1.16 | 0.001 |
| Median | 5.7 | 6.7 | |
| Range | 5.2-6.2 | 5.5-9.5 | |
| Diabetes duration (years): | |||
| Mean ± SD | NA | 9.2± 6.9 | NA |
| Median | 7.8 | ||
| Range | 1-25 | ||
| Teeth - Implants | |||
| Mean | 22.1 - 2.3 | 22.6 - 1.9 | 0.51 |
| Median | 23.0-2.0 | 25.0-1.5 | |
| Range | (11-27) - (1-6) | (7-27) - (1-6) | |
SD: Standard deviation
NA: Not applicable
Table 2 provides an overview of the clinical data at teeth and implants for both groups. No statistically significant differences were noted between the groups both at baseline and follow-up when teeth and implants were compared independently. In the diabetes group only, a significant increase in the mean probing depth around implants between the baseline and follow-up visit was observed (1.95 ± 0.17 mm vs. 2.35 ± 0.18 mm, p=0.015). There was a significant trend noted that the mean probing depth around implants was statistically greater than around teeth, both at baseline (2.01 ± 0.17 mm vs. 1.58 ± 0.07 mm, p<0.001) and at 12 months (2.20 ± 0.21 mm vs. 1.53 ± 0.06 mm, p<0.001) in the control group and both at baseline (1.95 ± 0.17 mm vs. 1.62 ± 0.06 mm, p<0.001) and at 12 months (2.35 ± 0.18 mm vs. 1.56 ± 0.05 mm, p<0.001) in the test group, respectively. Regarding the mean clinical attachment level, the mean attachment level around implants was higher than around teeth, both at baseline (p=0.002) and follow-up (p=0.001) visits in both groups. When bleeding upon probing was considered, statistically higher scores were observed around implants compared to teeth in both groups at both time points (p<0.001). In radiographic linear bone levels, both groups exhibited a statistically significant increase around the teeth over the study period. The mean values at the baseline and 1 year visits were 2.71 ± 0.14 mm and 2.76 ± 0.14 mm (p=0.029) for the control group and 2.59 ± 0.15 mm and 2.65 ± 0.15 mm (p=0.004) for the diabetes group, respectively. In the control group, the mean change (gain) that occurred around implants during the study visits was significantly different from the mean change (loss) that occurred around teeth (-0.08 ± 0.12 mm vs. 0.06 ± 0.03 mm, p=0.043).
Table 2.
Clinical and Radiographic Measures at Teeth and Implants
| Teeth | Implants | ||||||
|---|---|---|---|---|---|---|---|
| Index | Group | Baseline | 12-months | Δ | Baseline | 12-months | Δ |
| PD (mm) | Control | 1.58 ± 0.07 | 1.53 ± 0.06 | −0.05 ± 0.06 | 2.01 ± 0.17b | 2.20 ± 0.21b | 0.19 ± 0.17 |
| Diabetes | 1.62 ± 0.06 | 1.56 ± 0.05 | −0.06 ± 0.05 | 1.95 ± 0.17b | 2.35 ± 0.18a, b | 0.40 ± 0.15 | |
| CAL (mm) | Control | 1.38 ± 0.11 | 1.46 ± 0.10 | 0.08 ± 0.06 | 0.62 ± 0.06b | 0.56 ± 0.06c | −0.06 ± 0.06 |
| Diabetes | 1.58 ± 0.20 | 1.66 ± 0.17 | 0.08 ± 0.06 | 0.61 ± 0.07b | 0.67 ± 0.06b | 0.05 ± 0.07 | |
| BOP (0/1) | Control | 0.32 ± 0.03 | 0.29 ± 0.03 | −0.03 ± 0.03 | 0.62 ± 0.06b | 0.56 ± 0.06b | −0.06 ± 0.06 |
| Diabetes | 0.25 ± 0.04 | 0.26 ± 0.04 | 0.01 ± 0.03 | 0.52 ± 0.08b | 0.67 ± 0.06b | 0.15 ± 0.08 | |
| PI (0/1) | Control | 0.23 ± 0.03 | 0.20 ± 0.03 | −0.03 ± 0.03 | 0.20 ± 0.06 | 0.12 ± 0.04 | −0.08 ± 0.06 |
| Diabetes | 0.29 ± 0.05 | 0.29 ± 0.05 | 0.00 ± 0.07 | 0.10 ± 0.04b | 0.13 ± 0.06 | 0.03 ± 0.07 | |
| RBL (mm) | Control | 2.71 ± 0.14 | 2.76 ± 0.14a | 0.05 ± 0.03 | 2.62 ± 0.18 | 2.54 ± 0.15 | −0.08 ± 0.12c |
| Diabetes | 2.59 ± 0.15 | 2.65 ± 0.15a | 0.06 ± 0.02 | 2.50 ± 0.19 | 2.69 ± 0.17 | 0.19 ± 0.12 | |
Values are means +/− SEM. Δ: Change; PD: Probing depth; CAL: Clinical attachment level; BOP: Bleeding on probing; PI: Plaque index; RBL: Radiographic bone level; SE: Standard error
Significant difference within group over time (p<0.05)
Significant difference between teeth and implants within group at same visit (p<0.05)
Significant difference between the changes that occurred within the control group over time when teeth and implants were compared (p<0.05)
In the biomarker analysis, no major differences were identified between the two groups (Table 3, Figure 2). However, the salivary levels of IL-4 and IL-10 in the diabetes group showed a statistically significant reduction at 12 months when compared to the baseline visit (0.34 ± 0.08 vs. 0.14 ± 0.06 log10pg/ml, p=0.010) and 0.43 ± 0.11 vs. 0.16 ± 0.09 log10pg/ml, p=0.019) for IL-4 and IL-10, respectively. The salivary OPG levels in the control group revealed a statistically significant increase from the baseline to follow-up (2.59 ± 0.12 vs. 2.87 ± 0.13 log10pg/ml, p=0.005), while in the diabetes group, a significant reduction occurred during the study period (2.92 ± 0.13 vs. 2.79 ± 0.16 log10pg/ml, p=0.024). The two groups also differed significantly at the baseline level of salivary OPG, with the diabetes group exhibiting higher levels than the control group subjects (2.92 ± 0.13 log10pg/ml vs. 2.59 ± 0.12 log10pg/ml; p=0.050). For serum biomarkers, there were no notable findings, except MMP-1 levels being higher in the control group than the test group at baseline (data not shown).
Table 3.
Salivary Protein Biomarkers for Diabetic and Control Patients
| Biomarker log10(pg/mL) | Group | Baseline | 12-months |
|---|---|---|---|
| IL-2 | Control | 1.31 ± 0.21 | 1.06 ± 0.22 |
| Diabetes | 0.76 ± 0.22 | 0.85 ± 0.28 | |
| IL-6 | Control | 1.42 ± 0.18 | 1.41 ± 0.18 |
| Diabetes | 1.41 ± 0.18 | 1.21 ± 0.25 | |
| IL-8 | Control | 3.20 ± 0.03 | 3.21 ± 0.04 |
| Diabetes | 3.24 ± 0.05 | 3.17 ± 0.07 | |
| TNF-α | Control | 1.68 ± 0.24 | 1.89 ± 0.13 |
| Diabetes | 1.24 ± 0.31 | 1.44 ± 0.28 | |
| INF-γ | Control | 1.05 ± 0.16 | 0.92 ± 0.20 |
| Diabetes | 0.93 ± 0.18 | 0.69 ± 0.23 | |
| CRP | Control | 3.01 ± 0.29 | 2.82 ± 0.30 |
| Diabetes | 2.93 ± 0.32 | 2.99 ± 0.30 | |
| MIP-1α | Control | 2.74 ± 0.20 | 2.81 ± 0.15 |
| Diabetes | 2.85 ± 0.28 | 2.71 ± 0.30 | |
| MIP-1β | Control | 0.86 ± 0.11 | 0.83 ± 0.10 |
| Diabetes | 0.81 ± 0.13 | 0.88 ± 0.15 | |
| MMP-1 | Control | 3.95 ± 0.15 | 4.07 ± 0.11 |
| Diabetes | 4.01 ± 0.11 | 4.09 ± 0.17 | |
| MMP-2 | Control | 2.70 ± 0.16 | 2.51 ± 0.23 |
| Diabetes | 2.24 ± 0.24 | 1.94 ± 0.39 | |
| MMP-9 | Control | 4.26 ± 0.04 | 4.29 ± 0.04 |
| Diabetes | 4.32 ± 0.05 | 4.24 ± 0.07 | |
| TIMP-2 | Control | 3.90 ± 0.02 | 3.88 ± 0.03 |
| Diabetes | 3.92 ± 0.04 | 3.90 ± 0.06 | |
| ProCT | Control | 0.88 ± 0.23 | 1.08 ± 0.20 |
| Diabetes | 1.33 ± 0.23 | 0.94 ± 0.25 |
Values are means +/− SE: Standard error
Figure 2.
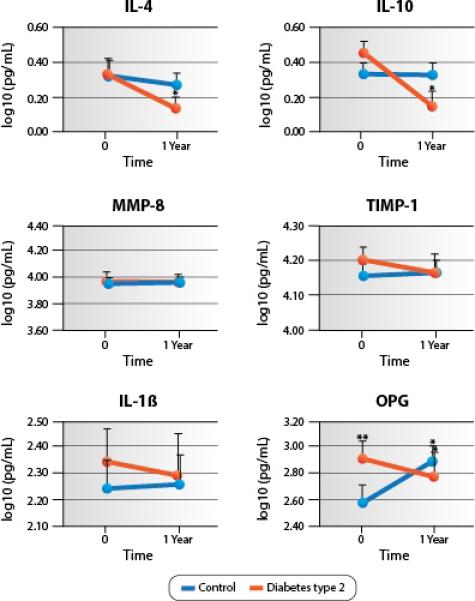
Levels of selected salivary biomarkers over the study period. The levels of IL-4, IL-10, and OPG were significantly (*) reduced between baseline and follow-up visits in the diabetes group (p<0.05); on the other hand, the levels of OPG were significantly (*) increased from the baseline to the follow-up visit in the control group (p<0.05). The levels of OPG were significantly (**) different between groups at baseline (p<0.05).
Microbial analysis demonstrated no significant differences between the groups both at the baseline and follow-up, even when teeth and implants were considered separately (Table 4). The only exceptions were related to the levels of T. denticola; at the baseline visit, higher mean levels of the bacterium were noted at teeth in the control group as compared to the diabetes group (0.71 ± 0.12 vs. 0.44 ± 0.06 , p=0.046). Moreover, a statistically significant increase from the baseline to the follow-up visit was observed in the mean levels of T. denticola in the control group around implant sites (0.45 ± 0.08 vs. 0.78 ± 0.10 %, p=0.003).
Table 4.
Pathogens Identified at Tooth and Implant Sites in Diabetic and Control Patients
| Teeth only | Implants only | ||||
|---|---|---|---|---|---|
| Species | Group | Baseline | 12-months | Baseline | 12-months |
| T.f. (%) | Control | 1.24 ± 0.21 | 1.99±0.36 | 1.56 ± 0.28 | 1.62 ± 0.31 |
| Diabetes | 1.25 ± 0.14 | 1.24 ± 0.26 | 1.62 ± 0.37 | 1.61 ± 0.24 | |
| T.d. (%) | Control | 0.71 ± 0.12a | 0.73 ± 0.09 | 0.45 ± 0.08 | 0.78 ± 0.10b |
| Diabetes | 0.44 ± 0.06 | 0.62 ± 0.10 | 0.45 ± 0.10 | 0.54 ± 0.09 | |
| P.g. (%) | Control | 0.88 ± 0.13 | 0.82 ± 0.09 | 0.81 ± 0.10 | 0.98 ± 0.09 |
| Diabetes | 0.76 ± 0.09 | 0.87 ± 0.17 | 0.57 ± 0.12 | 0.76 ± 0.11 | |
| C.r. (%) | Control | 1.85 ± 0.37 | 1.38 ± 0.19 | 1.67 ± 0.39 | 1.51 ± 0.30 |
| Diabetes | 1.30 ± 0.33 | 1.82 ± 0.41 | 1.67 ± 0.33 | 1.20 ± 0.35 | |
| F.n. (%) | Control | 2.21 ± 0.32 | 2.17 ± 0.28 | 2.33 ± 0.35 | 2.46 ± 0.53 |
| Diabetes | 2.12 ± 0.30 | 2.43 ± 0.49 | 2.26 ± 0.40 | 2.04 ± 0.41 | |
| P.i. (%) | Control | 1.87 ± 0.25 | 1.54 ± 0.18 | 1.66 ± 0.25 | 1.87 ± 0.19 |
| Diabetes | 1.31 ± 0.15 | 1.67 ± 0.24 | 1.51 ± 0.27 | 1.55 ± 0.29 | |
| A.a. (%) | Control | 1.64 ± 0.18 | 1.76 ± 0.16 | 1.65 ± 0.15 | 1.71 ± 0.19 |
| Diabetes | 1.40 ± 0.14 | 1.78 ± 0.20 | 1.33 ± 0.12 | 1.38 ± 0.15 | |
| C.a. (%) | Control | 1.69 ± 0.18 | 1.74 ± 0.17 | 1.63 ± 0.16 | 1.80 ± 0.24 |
| Diabetes | 1.60 ± 0.21 | 1.73 ± 0.22 | 1.53 ± 0.18 | 1.70 ± 0.26 | |
Values are means +/− SE: Standard error; T.f.: Tannerella forsythia; T.d.: Treponema denticola; P.g.: Porphyromonas gingivalis; C.r.: Campylobacter rectus; F.n.: Fusobacterium nucleatum; P.i.: Prevotella intermedia; A.a.: Aggregatibacter actinomycetemcomitans; C.a.: Candida albicans
Significant difference between groups at baseline (p<0.05)
Significant difference within group over time (p<0.05)
The two groups did not differ in their oral health-related behaviors both at the baseline and follow-up (Table 5). In the average stress and depression scores both at baseline and follow-up appointments, no differences were noted between the two groups, and no changes occurred longitudinally as well within each group (Table 6). Concerning the patients’ coping styles, the data showed that the test group scored significantly higher compared to the control group on the “religion” coping domain (2.7 vs. 1.8; p=0.040) and on the “self-blame” domain (1.8 vs. 1.4; p=0.043) at baseline and significantly lower on the “venting” subscale (1.3 vs. 1.7; p=0.049) at follow-up.
Table 5.
Behavioral Factors in Diabetic and Control Patients (Mean)
| Behavioral responses: | Control | Diabetes |
p (time)
p (t × d) |
|---|---|---|---|
| Baseline Follow-up | Baseline Follow-up | ||
| How often do you brush your teeth?1: | 4.67 4.50 |
4.54 4.38 |
0.22 0.96 |
| How often do you floss your teeth?1: | 3.78 3.83 |
3.23 3.23 |
0.88 0.88 |
| How often do you drink alcohol?1: | 2.28 2.33 |
2.31 2.31 |
0.83 0.83 |
| Do you smoke? | p-value | ||
|---|---|---|---|
| Yes (Baseline) | 1 | 2 | 0.40 |
| Cigarettes | 1 | 2 | |
| Cigars | 0 | 0 | |
| Yes (12 months) | 1 | 0 | 0.58 |
| Cigarettes | 0 | 0 | |
| Cigars | 1 | 0 | |
t × d, time × diabetes (effect of time on diabetes status)
The answers were given on a scale with 1 = Never, 2= once a month, 3= once a week 4= more than once a week, and 5= every day.
Table 6.
Psychosocial Factors in Diabetic and Control Patients
| Control | Diabetes | |
|---|---|---|
| Baseline (Mean ± SD) Follow-up (Mean ± SD) |
Baseline (Mean ± SD) Follow-up (Mean ± SD) |
|
| PSS1 | 2.0 ± 0.48 | 2.0 ± 0.37 |
| 2.1 ± 0.27 | 2.3 ± 0.60 | |
| CESD2 | 1.2 ± 0.26 | 1.3 ± 0.27 |
| 1.3 ± 0.26 | 1.3 ± 0.22 | |
| Brief COPE3 | ||
| Self-distraction | 1.9 ± 1.01 | 1.6 ± 0.49 |
| 1.7 ± 0.71 | 1.8 ± 0.88 | |
| Active coping | 2.3 ± 0.97 | 2.3 ± 1.01 |
| 2.6 ± 0.87 | 2.5 ± 0.96 | |
| Denial | 1.3 ± 0.67 | 1.2 ± 0.37 |
| 1.1 ± 0.27 | 1.2 ± 0.22 | |
| Substance use | 1.1 ± 0.37 | 1.1 ± 0.29 |
| 1.1 ± 0.37 | 1.0 ± 0.00 | |
| Use of emotional support | 2.0 ± 1.0 | 2.3 ± 0.99 |
| 2.0 ± 0.92 | 2.2 ± 0.69 | |
| Use of instrumental support | 1.8 ± 0.96 | 2.0 ± 0.87 |
| 1.8 ± 0.96 | 2.0 ± 0.87 | |
| Behavioral disengagement | 1.5 ± 1.43 | 1.4 ± 0.66 |
| 1.2 ± 0.49 | 1.1 ± 0.28 | |
| Venting | 1.8 ± 0.91 | 1.8 ± 0.64 |
| 1.7 ± 0.55 | a1.3 ± 0.43 | |
| Positive reframing | 2.4 ± 1.06 | 2.6 ± 1.04 |
| 2.3 ± 0.96 | 2.2 ± 0.85 | |
| Planning | 2.3 ± 1.20 | 2.3 ± 0.77 |
| 2.3 ± 1.05 | 2.3 ± 1.16 | |
| Humor | 2.2 ± 1.13 | 1.6 ± 0.77 |
| 2.0 ± 1.04 | 1.4 ± 0.68 | |
| Acceptance | 2.1 ± 0.93 | 2.7 ± 1.17 |
| 2.2 ± 0.97 | 2.3 ± 0.99 | |
| Religion | 1.8 ± 1.06 | a2.7 ± 1.19 |
| 2.1 ± 1.0 | b2.3 ± 1.10 | |
| Self-blame | 1.4 ± 0.51 | a1.8 ± 0.72 |
| 1.5 ± 0.66 | 1.8 ± 0.78 | |
SD: Standard deviation; repeated measurement ANOVAs used for the dependent variables PSS and CESD, and repeated measurementMANOVA for the Brief-COPE scores.
The answers range from 1 = never to 5 = always
The answers range from 1 = hardly ever, 2 = some of the time, 3 = most of the time
The answers range from 1 = I haven't been doing this at all, 2 = a little, 3 = medium amount, and 4 = a lot
Significant difference between groups (p<0.05)
Significant difference within group over time (p<0.05)
Discussion
Despite the high predictability of implant therapy, little is known regarding the role of systemic conditions, such as diabetes type 2, on the long-term prognosis of osseointegrated implants. To the best of our knowledge, this feasibility study was the first that attempted to elucidate differences in the clinical behavior of both implants and teeth in patients with diabetes type 2, comparing them to those of non-diabetes controls, in a longitudinal perspective. Moreover, the study explored the microbiological, proteomic and psychosocial profiles of the two groups as potential explanatory variables for any identified differences.
The one-year changes in the attachment levels that characterized the two groups around their teeth correlate well with the mean annual rates of disease progression as noted in other longitudinal studies (Ismail, et al. 1990, Schatzle, et al. 2003). Specifically, in our study, the mean attachment loss for both groups over the study period was equal to 0.08 mm, which is very similar to the 0.05 mm mean annual attachment loss found in a cohort of Norwegians with regular access to professional dental care over a period of 26 years (Schatzle, Loe, Lang, Heitz-Mayfield, Burgin, Anerud & Boysen 2003). When it comes to radiographic bone changes around teeth over time, both groups showed a mean change of 0.06 mm that also corresponds to the findings of other prospective investigations (Lavstedt, et al. 1986, Norderyd, et al. 1999, Paulander, et al. 2004). Both groups presented with a significant increase in the radiographic bone level between baseline and follow-up visits. However, this did not exceed the margin of statistical error and it was accompanied by similar changes in the clinical attachment levels. Interestingly, the diabetes group in this study did not show significantly higher disease progression rates as evaluated by changes in the mean clinical attachment level or mean radiographic bone level. This finding contradicts the results of classic prospective studies conducted in diabetic populations in which the diabetic individuals presented with higher levels of attachment and bone loss compared to their healthy counterparts (Nelson, et al. 1990, Novaes, et al. 1996, Taylor, et al. 1998). However, certain aspects differentiate our study from the aforementioned investigations. Firstly, the previous studies were performed in populations with higher levels of periodontal disease at baseline and followed a model of untreated, natural disease progression during the observation period, while in our study the subjects were periodontally stable and received prophylaxis at least twice, as required by the study protocol. Secondly, the level of diabetes control in our sample could be regarded as good given that the mean HbA1C level was approximately 7.0% and did not change during the study period. In fact, only 3 subjects at screening and 2 subjects at 12 months presented with a value higher than 8%. It has been postulated that metabolic control correlates with periodontal health status (Taylor & Borgnakke 2008).
The comparison of the two groups in peri-implant changes failed to identify significant differences as well. The mean probing depth of diabetic subjects around implants increased significantly between the two visits; however, this change was not followed by significant alterations in the attachment or radiographic bone levels. Considering that no differences existed around teeth, as explained above, this appears to be a biologically acceptable finding. In the same vein, there is no other report indicating certain differences in the mean annual bone changes around implants of diabetic and non-diabetic patients. Tawil and associates did not find any significant differences in the mean peri-implant bone loss between diabetic and non-diabetic subjects after following them for a period of 1-12 years (Tawil, et al. 2008). The latter investigation is relevant because it included type 2 diabetes patients with mainly good and fairly good control (mean HbA1C=7.2%) under regular maintenance, a profile that is very similar to our study population. Besides, the importance of periodontal maintenance for the stability of peri-implant tissues was confirmed by a 5-year follow-up study that showed that the absence of preventive maintenance in individuals with pre-existing peri-implant mucositis was associated with a high incidence of peri-implantitis (Costa, et al. 2012).
When evaluating the salivary proteomic profiles of the two groups, there were significantly higher salivary OPG levels in the test group at baseline. Costa and associates have reported higher salivary OPG levels in diabetics, irrespective of their periodontal status when compared to non-diabetic controls (Costa, et al. 2010). This may be in line with the finding that increased concentrations have been also identified in the serum of diabetic individuals (O'Sullivan, et al. 2010). The hypothesis that has been proposed is that OPG is released by the vascular system as a putative compensatory mechanism to prevent further vascular damage or is alternatively induced by other inflammatory mechanisms and mediators (Schoppet, et al. 2003). When analyzing the proteomic markers within each group between the two time points, it was observed that some significant changes took place. The mean levels of OPG in the diabetes group were reduced both at whole saliva as well at serum. The opposite occurred in the control group, where a significant increase was noted in the whole saliva levels. The above changes are difficult to justify biologically taking into account the metabolic status of the groups solely, as the latter did not change significantly over time. However, we have to acknowledge that the observed changes were based on two time points; therefore, we could not detect the fluctuations that might had taken place during the whole year. Similarly, it appeared that the salivary levels of IL-4 and IL-10 of the diabetes group and the serum levels of the IL-10 in the control group were reduced significantly over time. Considering that the clinical and metabolic status of the groups remained unchanged during the study period, we can only speculate that other factors such as the ones described above are responsible for this effect.
In microbiological aspects, no major differences were noted either between the groups or within the groups at the two study visits. This is not surprising because previous investigations have reported similar findings (Collin, et al. 1998, Yuan, et al. 2001). In the study by Yuan and colleagues, certain species (A. actinomycetemcomitans, P. gingivalis, E. corrodens, T. denticola, and C. albicans) were detected in a sample of 246 healthy and diabetes type 2 adults with the use of PCR. Both healthy and diseased sites were sampled and the results demonstrated that the prevalence of the above pathogens was similar in both groups. In our study, the subjects had stable periodontal and peri-implant health, with the majority of the sites being shallow as reflected by the recorded clinical measures. The sampled sites both around teeth and implants harbored typical pathogens. However, diabetes did not appear to be an important modifying factor. This finding can be attributed to the fact that all participants were receiving regular dental care with periodontal prophylaxis at least twice during the study period. Our study is the first to report that the subgingival peri-implant flora of well maintained, type 2 diabetic subjects does not contain higher concentrations of certain pathogens compared to non-diabetic individuals. The study also confirmed that in partially edentulous patients the microbial ecology does not majorly differ between teeth and implants, a finding that has been documented in the literature (Leonhardt, et al. 1993, Oringer, et al. 1998, van Winkelhoff, et al. 2000). Interestingly, the control group harbored higher levels of T. denticola around their teeth at baseline and the levels of the pathogen increased significantly over time around the implants of the same group. Yet, the above findings cannot be related to any specific clinical changes that occurred during the study period as no disease progression took place in the control group.
When comparing the two groups with regards to their stress and depression scores, no significant differences were observed at any of the time points or during the study period. A meta-analysis reported that the prevalence of depression was significantly higher in patients with type 2 diabetes compared with those without [17.6 vs. 9.8%, OR = 1.6, 95%, confidence interval (CI) 1.2–2.0] (Ali, et al. 2006). In fact, two out of the ten included studies applied the same instrument that we used and confirmed the effect. However, we have to note that the meta-analysis was based on cross-sectional studies only. Therefore, causality could not be implied. In addition, several confounding factors may have influenced the effect. A study examining the relationship between diabetes and depressive symptoms assessed through CESD in a large, racially diverse cohort in the U.S. found that demographics, lifestyle behaviors, antidepressant use, and BMI were actually more strongly associated with depressive symptoms than having a diabetes diagnosis (Osborn, et al. 2011).
The two study groups differed in specific domains of coping strategies; the test group used a combination of problem-based and emotional-based strategies namely, religion and self-blame to a greater extent when coping with daily strains compared to the control group at baseline. Interestingly, over time the diabetes group responded with a significant reduction in the religion-based coping style score. At the follow-up appointment, the control group demonstrated a higher ability based on the responses to cope with their strains by venting compared to the diabetes group. Overall, both groups used problem-based strategies most strongly such as positive reframing, active coping, planning, religion, acceptance and humor. This finding is consistent with the findings of a study that was conducted with diabetic patients in Turkey (Tuncay, et al. 2008). Moreover, the total mean scores for the different domains in both groups appear to be low, indicating that the participants were not dealing with significant stressful life events.
We believe that there are several limitations and strengths in our study. Similar to other hypothesis-generating studies, it has limited power to fully address any of the investigated outcomes. Even though the two groups appear to be statistically balanced at baseline we encountered challenges in the recruitment of diabetes patients, which led to a discrepancy in the number of participants in the two groups. Moreover, we performed analyses based on two time points only over a one-year period. Despite most longitudinal studies are based on annual or biannual examinations, there is a possibility that we could not identify some of the episodic effects of diabetes, considering that fluctuations of metabolic control are not uncommon. The same applies to the proteomic and microbial data. Even though the harvesting techniques for saliva and serum were standardized, it has been reported that many factors can influence the biomarker concentration, such as the time of collection, hormonal circadian rhythms, diet, smoking, and medications. Moreover, the type of implant design and surface, the type of surgery and technical aspects of the supra-structure could not be standardized because of the nature of the study. The impact of the above on implant bone loss has been suggested, known to take place mainly during the first six months to one year of function. In order to minimize the role of this modifier, only functional implants of 6 months or more were included in the study. All implants but one were in function for more than 1 year (data not shown). An aspect that may have implications for the generalizability of our study results relates to the profile of our diabetes population. For the most part, the participants were well controlled and compliant with their dental appointments, belonged to a specific age range, and were partially edentulous. Our study presents with certain strengths; to the best of our knowledge, this was the first investigation designed in a prospective manner with specific criteria evaluating the effect of diabetes both on teeth and implants. It also offered the opportunity to elucidate whether the presence of disease modified the response of teeth and implants differently within the same host. The selected methodology was very comprehensive permitting a multivariate assessment and comparison of the two groups in terms of their clinical, microbial, proteomic, behavioral and psychosocial profiles. The complexity of periodontal and peri-implant bone loss is not fully understood. This study differs from previous investigations that focused only on crude endpoints, such as implant loss. Salivary diagnostics is an emerging field and holds a potential in the risk assessment of oral bone loss, especially in patients with systemic implications, such as diabetes. In addition, the role of psychosocial measures was analyzed for the first time in diabetic individuals with dental implants. The applied methods have been previously validated and offer a standardized way to evaluate the groups longitudinally.
In conclusion, the study results suggest that the clinical, microbiological, salivary biomarker and psychosocial profiles of dental implant patients with type 2 diabetes who are under good metabolic control and regular maintenance care are very similar to those of non-diabetic individuals. Future studies are warranted to validate the findings in longer-term and larger clinical trials.
ACKNOWLEDGEMENTS
The study was supported by a pilot grant from the Michigan Center for Oral Health Research and the Michigan Institute for Clinical and Health Research. This study was also supported by NIH/NCRR UL-RR00042. The authors appreciate the administrative and clinical help of the staff members of the Michigan Center for Oral Health Research Karen Gardner, Anna Galloro, Lea Franco, Hilye Pittman, Tina Huffman and Alice Liu. We appreciate the assistance of Jim Sugai, Min Oh and Dr. Bina Oh during the saliva analysis as well as the contribution of Dr. Lindsay Rayburn- Holman in the radiographic analysis.
Footnotes
The authors report no conflict of interest.
REFERENCES
- Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with type 2 diabetes: A systematic review and meta-analysis. Diabetic medicine : a journal of the British Diabetic Association. 2006;23:1165–1173. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- Association, A. D. Standards of medical care in diabetes--2011. Diabetes care. 2011;34(Suppl 1):S11–61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglundh T, Persson L, Klinge B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. Journal of clinical periodontology. 2002;29(Suppl 3):197–212. doi: 10.1034/j.1600-051x.29.s3.12.x. discussion 232-193. [DOI] [PubMed] [Google Scholar]
- Carver CS. You want to measure coping but your protocol's too long: Consider the brief cope. Int J Behav Med. 1997;4:92–100. doi: 10.1207/s15327558ijbm0401_6. [DOI] [PubMed] [Google Scholar]
- Cohen S WG. Perceived stress in a probability sample of the united states. In: Spacapan S, Oskamp S, editors. The social psychology of health. Sage; Newbury Park, CA.: 1988. pp. 31–67. [Google Scholar]
- Collin HL, Sorsa T, Meurman JH, Niskanen L, Salo T, Ronka H, Konttinen YT, Koivisto AM, Uusitupa M. Salivary matrix metalloproteinase (mmp-8) levels and gelatinase (mmp-9) activities in patients with type 2 diabetes mellitus. Journal of periodontal research. 2000;35:259–265. doi: 10.1034/j.1600-0765.2000.035005259.x. [DOI] [PubMed] [Google Scholar]
- Collin HL, Uusitupa M, Niskanen L, Kontturi-Narhi V, Markkanen H, Koivisto AM, Meurman JH. Periodontal findings in elderly patients with non-insulin dependent diabetes mellitus. Journal of periodontology. 1998;69:962–966. doi: 10.1902/jop.1998.69.9.962. [DOI] [PubMed] [Google Scholar]
- Costa FO, Takenaka-Martinez S, Cota LO, Ferreira SD, Silva GL, Costa JE. Peri-implant disease in subjects with and without preventive maintenance: A 5-year follow-up. Journal of clinical periodontology. 2012;39:173–181. doi: 10.1111/j.1600-051X.2011.01819.x. [DOI] [PubMed] [Google Scholar]
- Costa PP, Trevisan GL, Macedo GO, Palioto DB, Souza SL, Grisi MF, Novaes AB, Jr., Taba M., Jr. Salivary interleukin-6, matrix metalloproteinase-8, and osteoprotegerin in patients with periodontitis and diabetes. Journal of periodontology. 2010;81:384–391. doi: 10.1902/jop.2009.090510. [DOI] [PubMed] [Google Scholar]
- Derosa G, D'Angelo A, Tinelli C, Devangelio E, Consoli A, Miccoli R, Penno G, Del Prato S, Paniga S, Cicero AF. Evaluation of metalloproteinase 2 and 9 levels and their inhibitors in diabetic and healthy subjects. Diabetes & metabolism. 2007;33:129–134. doi: 10.1016/j.diabet.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Duckworth JE, Judy PF, Goodson JM, Socransky SS. A method for the geometric and densitometric standardization of intraoral radiographs. J Periodontol. 1983;54:435–440. doi: 10.1902/jop.1983.54.7.435. [DOI] [PubMed] [Google Scholar]
- Genco RJ. Current view of risk factors for periodontal diseases. J Periodontol. 1996;67:1041–1049. doi: 10.1902/jop.1996.67.10.1041. [DOI] [PubMed] [Google Scholar]
- Haffajee AD, Socransky SS, Goodson JM. Comparison of different data analyses for detecting changes in attachment level. Journal of clinical periodontology. 1983;10:298–310. doi: 10.1111/j.1600-051x.1983.tb01278.x. [DOI] [PubMed] [Google Scholar]
- Heitz-Mayfield LJ. Peri-implant diseases: Diagnosis and risk indicators. J Clin Periodontol. 2008;35:292–304. doi: 10.1111/j.1600-051X.2008.01275.x. [DOI] [PubMed] [Google Scholar]
- Heitz-Mayfield LJ. Peri-implant diseases: Diagnosis and risk indicators. Journal of Clinical periodontology. 2008;35:292–304. doi: 10.1111/j.1600-051X.2008.01275.x. [DOI] [PubMed] [Google Scholar]
- Ismail AI, Morrison EC, Burt BA, Caffesse RG, Kavanagh MT. Natural history of periodontal disease in adults: Findings from the tecumseh periodontal disease study, 1959-87. Journal of dental research. 1990;69:430–435. doi: 10.1177/00220345900690020201. [DOI] [PubMed] [Google Scholar]
- Jung RE, Pjetursson BE, Glauser R, Zembic A, Zwahlen M, Lang NP. A systematic review of the 5-year survival and complication rates of implant-supported single crowns. Clin Oral Implants Res. 2008;19:119–130. doi: 10.1111/j.1600-0501.2007.01453.x. [DOI] [PubMed] [Google Scholar]
- Kanjirath PP, Kim SE, Inglehart MR. Diabetes and oral health: The importance of oral health-related behavior. Journal of Dental Hygiene : JDH / American Dental Hygienists' Association. 2011;85:264–272. [PubMed] [Google Scholar]
- Kinney JS, Morelli T, Braun T, Ramseier CA, Herr AE, Sugai JV, Shelburne CE, Rayburn LA, Singh AK, Giannobile WV. Saliva/pathogen biomarker signatures and periodontal disease progression. J Dent Res. 2011;90:752–758. doi: 10.1177/0022034511399908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klokkevold PR, Han TJ. How do smoking, diabetes, and periodontitis affect outcomes of implant treatment? The International journal of oral & maxillofacial implants. 2007;22(Suppl):173–202. [PubMed] [Google Scholar]
- Kornman KS. Mapping the pathogenesis of periodontitis: A new look. J Periodontol. 2008;79:1560–1568. doi: 10.1902/jop.2008.080213. [DOI] [PubMed] [Google Scholar]
- Kumar MS, Vamsi G, Sripriya R, Sehgal PK. Expression of matrix metalloproteinases (mmp-8 and -9) in chronic periodontitis patients with and without diabetes mellitus. Journal of periodontology. 2006;77:1803–1808. doi: 10.1902/jop.2006.050293. [DOI] [PubMed] [Google Scholar]
- Lamster IB, Smith QT, Celenti RS, Singer RE, Grbic JT. Development of a risk profile for periodontal disease: Microbial and host response factors. J Periodontol. 1994;65:511–520. doi: 10.1902/jop.1994.65.5s.511. [DOI] [PubMed] [Google Scholar]
- Laine ML, Moustakis V, Koumakis L, Potamias G, Loos B. Modeling susceptibility to periodontitis. J Dent Res. 2013;92:45–50. doi: 10.1177/0022034512465435. [DOI] [PubMed] [Google Scholar]
- Lang NP, Tonetti MS. Periodontal risk assessment (pra) for patients in supportive periodontal therapy (spt). Oral Health Prev Dent. 2003;1:7–16. [PubMed] [Google Scholar]
- Lavstedt S, Bolin A, Henrikson CO. Proximal alveolar bone loss in a longitudinal radiographic investigation. Ii. A 10-year follow-up study of an epidemiologic material. Acta Odontologica Scandinavica. 1986;44:199–205. doi: 10.3109/00016358608997721. [DOI] [PubMed] [Google Scholar]
- Leonhardt A, Adolfsson B, Lekholm U, Wikstrom M, Dahlen G. A longitudinal microbiological study on osseointegrated titanium implants in partially edentulous patients. Clinical oral implants research. 1993;4:113–120. doi: 10.1034/j.1600-0501.1993.040301.x. [DOI] [PubMed] [Google Scholar]
- Lewandowski KC, Banach E, Bienkiewicz M, Lewinski A. Matrix metalloproteinases in type 2 diabetes and non-diabetic controls: Effects of short-term and chronic hyperglycaemia. Archives of medical science : AMS. 2011;7:294–303. doi: 10.5114/aoms.2011.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindhe J, Meyle J. Peri-implant diseases: Consensus report of the sixth european workshop on periodontology. J Clin Periodontol. 2008;35:282–285. doi: 10.1111/j.1600-051X.2008.01283.x. [DOI] [PubMed] [Google Scholar]
- Loe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329–334. [PubMed] [Google Scholar]
- Mandel ID, Wotman S. The salivary secretions in health and disease. Oral sciences reviews. 1976:25–47. [PubMed] [Google Scholar]
- Mealey BL, Oates TW. Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77:1289–1303. doi: 10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- Mullally BH, Dace B, Shelburne CE, Wolff LF, Coulter WA. Prevalence of periodontal pathogens in localized and generalized forms of early-onset periodontitis. Journal of periodontal research. 2000;35:232–241. doi: 10.1034/j.1600-0765.2000.035004232.x. [DOI] [PubMed] [Google Scholar]
- Nelson RG, Shlossman M, Budding LM, Pettitt DJ, Saad MF, Genco RJ, Knowler WC. Periodontal disease and niddm in pima indians. Diabetes care. 1990;13:836–840. doi: 10.2337/diacare.13.8.836. [DOI] [PubMed] [Google Scholar]
- Norderyd O, Hugoson A, Grusovin G. Risk of severe periodontal disease in a swedish adult population. A longitudinal study. Journal of clinical periodontology. 1999;26:608–615. doi: 10.1034/j.1600-051x.1999.260908.x. [DOI] [PubMed] [Google Scholar]
- Novaes AB, Jr., Gutierrez FG, Novaes AB. Periodontal disease progression in type ii non-insulin-dependent diabetes mellitus patients (niddm). Part i--probing pocket depth and clinical attachment. Brazilian dental journal. 1996;7:65–73. [PubMed] [Google Scholar]
- O'Sullivan EP, Ashley DT, Davenport C, Devlin N, Crowley R, Agha A, Thompson CJ, O'Gorman D, Smith D. Osteoprotegerin and biomarkers of vascular inflammation in type 2 diabetes. Diabetes/metabolism research and reviews. 2010;26:496–502. doi: 10.1002/dmrr.1109. [DOI] [PubMed] [Google Scholar]
- Offenbacher S, Barros SP, Beck JD. Rethinking periodontal inflammation. J Periodontol. 2008;79:1577–1584. doi: 10.1902/jop.2008.080220. [DOI] [PubMed] [Google Scholar]
- Oringer RJ, Palys MD, Iranmanesh A, Fiorellini JP, Haffajee AD, Socransky SS, Giannobile WV. C-telopeptide cross-links (ICTP) and periodontal pathogens associated with endosseous oral impalnts. Clinical oral implants research. 1998;9:365–373. doi: 10.1034/j.1600-0501.1996.090602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn CY, Patel KA, Liu J, Trott HW, Buchowski MS, Hargreaves MK, Blot WJ, Cohen SS, Schlundt DG. Diabetes and co-morbid depression among racially diverse, low-income adults. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2011;41:300–309. doi: 10.1007/s12160-010-9241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RC, Krall EA, Martin J, Mancl L, Garcia RI. Validity and accuracy of a risk calculator in predicting periodontal disease. J Am Dent Assoc. 2002;133:569–576. doi: 10.14219/jada.archive.2002.0232. [DOI] [PubMed] [Google Scholar]
- Papazafiropoulou A, Perrea D, Moyssakis I, Kokkinos A, Katsilambros N, Tentolouris N. Plasma levels of mmp-2, mmp-9 and timp-1 are not associated with arterial stiffness in subjects with type 2 diabetes mellitus. Journal of diabetes and its complications. 2010;24:20–27. doi: 10.1016/j.jdiacomp.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Paulander J, Axelsson P, Lindhe J, Wennstrom J. Intra-oral pattern of tooth and periodontal bone loss between the age of 50 and 60 years. A longitudinal prospective study. Acta Odontologica Scandinavica. 2004;62:214–222. doi: 10.1080/00016350410001630. [DOI] [PubMed] [Google Scholar]
- Peruzzo DC, Benatti BB, Ambrosano GM, Nogueira-Filho GR, Sallum EA, Casati MZ, Nociti FH., Jr. A systematic review of stress and psychological factors as possible risk factors for periodontal disease. J Periodontol. 2007;78:1491–1504. doi: 10.1902/jop.2007.060371. [DOI] [PubMed] [Google Scholar]
- Pjetursson BE, Bragger U, Lang NP, Zwahlen M. Comparison of survival and complication rates of tooth-supported fixed dental prostheses (fdps) and implant-supported fdps and single crowns (scs). Clinical oral implants research 18 Suppl. 2007;3:97–113. doi: 10.1111/j.1600-0501.2007.01439.x. [DOI] [PubMed] [Google Scholar]
- Radloff The ces-d scale: A self-report depression scale for research in the general population. Applied Psychosocial Measurement. 1977:385–405. [Google Scholar]
- Ramseier CA, Kinney JS, Herr AE, Braun T, Sugai JV, Shelburne CA, Rayburn LA, Tran HM, Singh AK, Giannobile WV. Identification of pathogen and host-response markers correlated with periodontal disease. Journal of Periodontology. 2009;80:436–446. doi: 10.1902/jop.2009.080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzle M, Loe H, Lang NP, Heitz-Mayfield LJ, Burgin W, Anerud A, Boysen H. Clinical course of chronic periodontitis. Iii. Patterns, variations and risks of attachment loss. Journal of clinical periodontology. 2003;30:909–918. doi: 10.1034/j.1600-051x.2003.00401.x. [DOI] [PubMed] [Google Scholar]
- Schoppet M, Sattler AM, Schaefer JR, Herzum M, Maisch B, Hofbauer LC. Increased osteoprotegerin serum levels in men with coronary artery disease. The Journal of clinical endocrinology and metabolism. 2003;88:1024–1028. doi: 10.1210/jc.2002-020775. [DOI] [PubMed] [Google Scholar]
- Soskolne WA, Klinger A. The relationship between periodontal diseases and diabetes: An overview. Annals of Periodontology. 2001;6:91–98. doi: 10.1902/annals.2001.6.1.91. [DOI] [PubMed] [Google Scholar]
- Taba M, Jr., Kinney J, Kim AS, Giannobile WV. Diagnostic biomarkers for oral and periodontal diseases. Dent Clin North Am. 2005;49:551–571. vi. doi: 10.1016/j.cden.2005.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawil G, Younan R, Azar P, Sleilati G. Conventional and advanced implant treatment in the type ii diabetic patient: Surgical protocol and long-term clinical results. The International journal of oral & maxillofacial implants. 2008;23:744–752. [PubMed] [Google Scholar]
- Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: An epidemiologic perspective. Annals of periodontology / the American Academy of Periodontology. 2001;6:99–112. doi: 10.1902/annals.2001.6.1.99. [DOI] [PubMed] [Google Scholar]
- Taylor GW, Borgnakke WS. Periodontal disease: Associations with diabetes, glycemic control and complications. Oral Diseases. 2008;14:191–203. doi: 10.1111/j.1601-0825.2008.01442.x. [DOI] [PubMed] [Google Scholar]
- Taylor GW, Borgnakke WS. Periodontal disease: Associations with diabetes, glycemic control and complications. Oral Dis. 2008;14:191–203. doi: 10.1111/j.1601-0825.2008.01442.x. [DOI] [PubMed] [Google Scholar]
- Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, Pettitt DJ. Non-insulin dependent diabetes mellitus and alveolar bone loss progression over 2 years. Journal of periodontology. 1998;69:76–83. doi: 10.1902/jop.1998.69.1.76. [DOI] [PubMed] [Google Scholar]
- Tomasi C, Wennstrom JL, Berglundh T. Longevity of teeth and implants -a systematic review. J Oral Rehabil 35 Suppl. 2008;1:23–32. doi: 10.1111/j.1365-2842.2007.01831.x. [DOI] [PubMed] [Google Scholar]
- Tuncay T, Musabak I, Gok DE, Kutlu M. The relationship between anxiety, coping strategies and characteristics of patients with diabetes. Health and quality of life outcomes. 2008;6:79. doi: 10.1186/1477-7525-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, C. f. D. C. a. P. National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the united states. Centers for disease control and prevention; Atlanta, GA.: 2011. 2011. [Google Scholar]
- van Winkelhoff AJ, Goene RJ, Benschop C, Folmer T. Early colonization of dental implants by putative periodontal pathogens in partially edentulous patients. Clinical oral implants research. 2000;11:511–520. doi: 10.1034/j.1600-0501.2000.011006511.x. [DOI] [PubMed] [Google Scholar]
- Westfelt E, Rylander H, Blohme G, Jonasson P, Lindhe J. The effect of periodontal therapy in diabetics. Results after 5 years. Journal of clinical periodontology. 1996;23:92–100. doi: 10.1111/j.1600-051x.1996.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Yuan K, Chang CJ, Hsu PC, Sun HS, Tseng CC, Wang JR. Detection of putative periodontal pathogens in non-insulin-dependent diabetes mellitus and non-diabetes mellitus by polymerase chain reaction. Journal of Periodontal Research. 2001;36:18–24. doi: 10.1034/j.1600-0765.2001.90613.x. [DOI] [PubMed] [Google Scholar]