Abstract
Background
Propylthiouracil (PTU) and methimazole (MMI) are anti-thyroid drugs used to treat hyperthyroidism. Despite the widespread use of PTU and MMI during pregnancy, modest clinical data and little animal data are available on the teratogenic potential of these drugs.
Methods
We evaluated the teratogenicity of in utero exposure to PTU or MMI in mice and rats. First, pregnant C57Bl/6 mice were treated daily with PTU (10 mg/kg or 100 mg/kg), MMI (2 mg/kg or 20 mg/kg), or vehicle from gestation day (GD) 6–16. GD 18 fetuses were evaluated for gross and histopathological abnormalities. Next, pregnant Sprague-Dawley rats were treated daily with PTU (50 mg/kg or 100 mg/kg), MMI (10 mg/kg or 20 mg/kg) or vehicle from GD 6–19, followed by evaluation for gross and histopathological abnormalities at GD 20.
Results
In mice treated with PTU or MMI, no significant histopathological abnormalities or external gross malformations, and no adverse effects on placental weight, litter size, resorption rates, or fetal weight were observed at GD18. In rats, no adverse effects on litter size, placental weights, or maternal body weights were observed with either PTU- or MMI- treatment. PTU (50 mg/kg and 100 mg/kg), and MMI (10 mg/kg) treatment resulted in a decrease in crown-rump length in rat fetuses but no external gross malformations or histopathological abnormalities were observed.
Conclusion
We did not observe either gross external malformations or histopathological malformations in mice or rats treated long-term with high doses of PTU or MMI during pregnancy.
Keywords: anti-thyroid drugs, Propylthiouracil, methimazole, developmental toxicity
INTRODUCTION
Graves’ disease is an autoimmune condition caused by immunoglobulin activation of the thyroid-stimulating hormone receptor and is the most common cause of hyperthyroidism in pregnancy (Benavides et al., 2012; El Baba and Azar, 2012; Mestman, 1997; Neale and Burrow, 2004). The incidence of hyperthyroidism in the general population is about 2–4 cases per 10,000 individuals, and the incidence of hyperthyroidism in childbearing women is about 4–5 per 10,000 in United States. Based on these data, it is estimated that 4,000–8,000 women will have Graves’ disease during pregnancy each year.
Untreated hyperthyroidism or Graves’ disease during pregnancy may have serious adverse consequences on the mother and fetus (Abalovich et al., 2007; Chan and Mandel, 2007; Rivkees and Mandel, 2011). The maternal risks of untreated Graves’ disease include hypertension, congestive heart failure, thyroid storm, and pre-eclampsia (Benavides et al., 2012; Glinoer, 1997). The adverse effects of Graves’ disease on the fetus include growth retardation, preterm delivery, miscarriage and death (Glinoer, 1997; Laurberg et al., 2009). Thus, it is imperative that hyperthyroidism be properly treated during gestation.
Anti-thyroid drugs (ATD), radioactive iodine (131I), or surgical excision of the thyroid gland are the major treatment options for controlling hyperthyroidism. During pregnancy, radioactive iodine treatment is contraindicated and surgery is recommended only when the hyperthyroid condition is refractory to anti-thyroid drugs (Am et al., 2007; Chan and Mandel, 2007). Thus, anti-thyroid drugs are the treatment of choice for active Graves’ disease during gestation. In the United States, propylthiouracil (PTU) is the drug-of-choice during the first trimester of pregnancy (Aaboe et al., 2011). In Europe and many other countries, methimazole (MMI) or carbamazole are used to treat Graves’ disease during pregnancy (Chattaway and Klepser, 2007).
Birth defects have been reported in children born to mothers taking anti-thyroid drugs, with MMI generally reported to have more teratogenic potential (Abalovich et al., 2007; Bahn et al., 2009). In utero exposure to MMI is associated with congenital malformations including aplasia cutis, omphalocele, and choanal atresia (Clementi et al., 2010; Yoshihara et al., 2012). PTU treatment during pregnancy is associated with low birth weight and situs inversus (Clementi et al., 2010). Human epidemiology studies of this issue are few (Bahn et al., 2009). Most recently, it has been shown that both PTU and MMI may be associated with a low rate of birth defects in humans (Andersen et al., 2013; Clementi et al., 2010; Yoshihara et al., 2012).
There has been some investigation of the effects of ATDs in animal models. Studies of E9.5 to E11.5 rat embryos cultured with high doses of MMI revealed abnormal head morphology and absence of neural tube closure (Stanisstreet et al., 1990). Treatment of rabbits and guinea pigs with PTU late in embryogenesis led to thyroid enlargement, but no congenital anomalies (Krementz et al., 1957). Treatment of rats, mice and rabbits late in embryogenesis did not cause congenital anomalies (Calikoglu et al., 1996; Goldey et al., 1995; Zolconski et al., 1964). More recently, we observed that administration of PTU during organogenesis caused delayed neural tube closure in E10.5 murine embryos, but these defects were not seen at the end of gestation (Benavides et al., 2012). In frog embryos, very early exposure to PTU results in heterotaxy defects (van Veenendaal et al., 2013). Despite widespread ATD use in pregnancy, formal studies of teratogenicity, similar to those required by the US Food and Drug Administration (ICH S5(R2)), have yet to be performed (Wise, 2013).
To address the teratogenic potential of PTU and MMI, we evaluated the developmental toxicity of these drugs in mice and rats. For this analysis, pregnant mice and rats were tested during embryonic and fetal stages. Per guidelines for such teratogenicity studies, doses were well in excess of recommended clinical doses (Wise, 2013).
MATERIALS AND METHODS
Animals
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at Yale University. All animal research was conducted at Yale University and concluded before corresponding author moved to the University of Florida, College of Medicine.
Mice
C57Bl/6 mice were obtained from Charles River Laboratories (Wilmington, MA, USA) and housed in a temperature-controlled room with a 12-hour light cycle and access to standard mouse chow and water ab libitum during the entire experiment including breeding. For breeding, 1–2 female mice were paired with one male mouse. Females were checked for copulation plugs every morning. When a copulation plug was seen, the female was separated from the male, and that day was designated as gestation day (GD) 0. Pregnant mice were euthanized on GD 18 by CO2 asphyxiation followed by cervical dislocation and whole blood collection by terminal cardiac puncture. Serum was stored at −70°C for measurement of T4 levels. Data regarding litter size were collected and resorption rates were evaluated by examining uterus for presence of remnants of fetus under dissecting microscope. Fetuses were euthanized by inducing hypothermia by submerging the fetus in cold physiological saline solution. Fetuses were harvested and fixed in Bouin’s solution for histopathological analysis. Placentas and fetuses were weighed and all fetuses were examined for gross malformations.
Rats
Male and female Sprague-Dawley (SD) rats were obtained from Charles River Laboratories and housed in a temperature-controlled room with a 12-hour light cycle and access to standard rat chow and water ab libitum during the entire experiment including breeding. For breeding, one female rat was paired with one male rat. Females were checked for copulation plugs every morning. When a copulation plug was found, that day was designated gestation day 0 (GD 0). Pregnant rats were weighed on GD 0, 6, 9, 12, 15, 18 and 20. Pregnant rats were euthanized on GD 20, and whole blood was collected by terminal cardiac puncture. Serum was stored at −70°C for measurement of T4 levels. Maternal and fetal parameters including litter size, resorption rates, viability, weights of the dam, gravid uterus, placenta and fetuses were measured. Fetuses were euthanized and tissues were collected for histopathological evaluations. Crown-rump lengths of all individual fetuses were measured and all fetuses were examined for gross malformations. Fetuses from each pregnant dam were euthanized by inducing hypothermia and fixed in Bouin’s solution for histopathological analysis.
Anti-Thyroid Drug Treatments
PTU and MMI (Sigma-Aldrich, St. Louis, MO) were dissolved in water and administered by oral gavage. Per standard toxicology protocols, the doses were multiples of those used clinically. Clinically, the dose range of PTU is 2–15 mg/kg and 0.2–1 mg/kg for MMI (Cooper, 2005).
In the first study, pregnant mice were randomly assigned to one of five different treatment groups by selecting a number at random from a container that corresponds to one of the five treatments. The treatment groups are 10 mg/kg PTU, 100 mg/kg PTU, 2 mg/kg MMI, 20 mg/kg MMI, or vehicle (control). Mice were treated daily from GD 6 to GD 16, and fetuses were collected on GD 18.
In the second study, pregnant rats were assigned to one of five different treatment groups: vehicle, 50 mg/kg PTU, 100 mg/kg PTU, 10 mg/kg MMI, or 20 mg/kg MMI from GD 6 to GD 19. Fetuses were collected on GD 20 and evaluated for gross malformations.
T4 Level Measurements
To evaluate circulating thyroxine (T4) levels, blood was collected from adults, and serum was isolated and stored at −70°C. Serum thyroxine (T4) levels were measured using commercially available Mouse/Rat Thyroxine solid phase competitive ELISA kit (GenWay Biotech Inc., San Diego, CA) according to manufacturer’s instructions. Briefly, 10 µl of serum samples, standards, and diluted T4 enzyme conjugate were added to the wells coated with anti-T4 polyclonal antibody. Upon the addition of the substrate, absorbance was measured at 450 nm, and a standard curve was generated and used to calculate serum T4 levels.
Histopathological Analysis
Fetuses were fixed in Bouin’s Fixative for 1–2 weeks, dissected and trimmed for routine processing, embedding, sectioning, and staining by hematoxylin and eosin (HE) using routine methods. All mouse and rat tissue samples were analyzed grossly and by light microscopy for malformations or histopathological changes blind to experimental manipulation. Samples were scored for the presence or absence of defects by a trained pathologist, as noted in Table 1.
Table 1.
Results of histopathological evaluation of GD 18 mouse fetuses.
| Histology Finding | Vehicle | MMI (20 mg/kg) |
MMI (2 mg/kg) |
PTU (100 mg/kg) |
PTU (10 mg/kg) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of effected (%) |
Total | No. of effected (%) |
Total | No. of effected (%) |
Total | No. of effected (%) |
Total | No. of effected (%) |
Total | |
| Placenta: | ||||||||||
| Hemorrhage | 2 (6) | 33 | 1 (4) | 23 | 0 (0) | 13 | 0 (0) | 25 | 0 (0) | 12 |
| Edema | 4 (12) | 33 | 2 (9) | 23 | 2 (15) | 13 | 0 (0) | 25 | 1 (8) | 12 |
| Limbs: | ||||||||||
| Subcutaneous hemorrhage | 2 (6) | 33 | 6 (26) | 23 | 4 (31) | 13 | 0 (0) | 34 | 2 (17) | 12 |
| Hemorrhage in the rear leg | 1 (3) | 33 | 0 (0) | 23 | 0 (0) | 13 | 0 (0) | 34 | 0 (0) | 12 |
| Hemorrhage in elbow | 1 (3) | 33 | 0 (0) | 23 | 0 (0) | 13 | 0 (0) | 34 | 1 (8) | 12 |
| Head: | ||||||||||
| Soft tissue edema | 0 (0) | 33 | 0 (0) | 23 | 1 (8) | 13 | 0 (0) | 34 | 0 (0) | 9 |
| Blood/ fluid in nasal cavity | 6 (18) | 33 | 0 (0) | 23 | 0 (0) | 13 | 2 (6) | 34 | 1 (11) | 9 |
| Blood in the oral cavity | 1 (3) | 33 | 0 (0) | 23 | 0 (0) | 13 | 0 (0) | 34 | 0 (0) | 9 |
| Cleft palate | 1 (3) | 33 | 0 (0) | 23 | 0 (0) | 13 | 0 (0) | 34 | 0 (0) | 9 |
| Brain: | ||||||||||
| Dilated lateral ventricles | 1 (3) | 33 | 0 (0) | 23 | 0 (0) | 13 | 0 (0) | 34 | 0 (0) | 9 |
| Fluid in the lateral ventricles | 2 (6) | 33 | 0 (0) | 23 | 0 (0) | 13 | 1 (3) | 34 | 0 (0) | 9 |
| Hemorrhage/ fluid in meninges | 1 (3) | 33 | 1 (4) | 23 | 0 (0) | 13 | 1 (3) | 34 | 0 (0) | 9 |
| Blood in corpus collosum | 1 (3) | 33 | 0 (0) | 23 | 0 (0) | 13 | 0 (0) | 34 | 0 (0) | 9 |
| Thorax: | ||||||||||
| Hemorrhage in the pericardium | 16 (48) | 33 | 10 (43) | 23 | 4 (31) | 13 | 11 (32) | 34 | 6 (60) | 10 |
| Laryngeal edema (minimal) | 1 (3) | 33 | 0 (0) | 23 | 0 (0) | 13 | 0 (0) | 34 | 0 (0) | 10 |
| Peri-tracheal edema | 0 (0) | 33 | 0 (0) | 23 | 1 (8) | 13 | 0 (0) | 34 | 0 (0) | 10 |
| Hemorrhage in neck | 1 (3) | 33 | 0 (0) | 23 | 0 (0) | 13 | 0 (0) | 34 | 0 (0) | 10 |
| Hemorrhage in thymus | 1 (3) | 33 | 0 (0) | 23 | 0 (0) | 13 | 1 (3) | 34 | 0 (0) | 10 |
| Fluid in the pleural cavity | 0 (0) | 33 | 0 (0) | 23 | 1 (8) | 13 | 0 (0) | 34 | 0 (0) | 10 |
| Abdomen: | ||||||||||
| Hemorrhage at the umbilicus | 3 (9) | 33 | 2 (9) | 23 | 1 (8) | 13 | 4 (12) | 34 | 3 (30) | 10 |
| Foci of hemorrhage in urinary bladder | 4 (12) | 33 | 0 (0) | 23 | 0 (0) | 13 | 0 (0) | 34 | 0 (0) | 10 |
| Hemorrhage and/ necrosis in liver | 2 (6) | 33 | 1 (4) | 23 | 0 (0) | 13 | 0 (0) | 34 | 0 (0) | 10 |
| Edema in urinary bladder | 1 (3) | 33 | 0 (0) | 23 | 0 (0) | 13 | 0 (0) | 34 | 0 (0) | 10 |
| Fluid in pleural cavity | 1 (3) | 33 | 0 (0) | 23 | 0 (0) | 13 | 0 (0) | 34 | 0 (0) | 10 |
| Mineral in the kidney | 1 (3) | 33 | 0 (0) | 23 | 0 (0) | 13 | 0 (0) | 34 | 0 (0) | 10 |
| Kidney: MF tubular mineralization (minimal) | 0 (0) | 33 | 11 (48) | 23 | 2 (15) | 13 | 1 (3) | 34 | 0 (0) | 10 |
| Body: whole congestion | 0 (0) | 33 | 1 (4) | 23 | 0 (0) | 13 | 0 (0) | 34 | 0 (0) | 10 |
| Blood in abdominal cavity | 0 (0) | 33 | 1 (4) | 23 | 1 (8) | 13 | 1 (3) | 34 | 0 (0) | 10 |
| Fibrin near the colon | 0 (0) | 33 | 0 (0) | 23 | 0 (0) | 13 | 0 (0) | 34 | 1 (10) | 10 |
Statistical Analysis
Data are presented as mean ± SE per litter. Difference between different treatments groups were compared using one-way ANOVA using GraphPad Prism software version 6.0 (GraphPad Software Inc., La Jolla, CA, USA). The Chi-squared test was used to determine significance of histopathological malformations. Statistical significance was set at p ≤ 0.05.
RESULTS
Effects of in utero anti-thyroid drug treatment on fetal mice
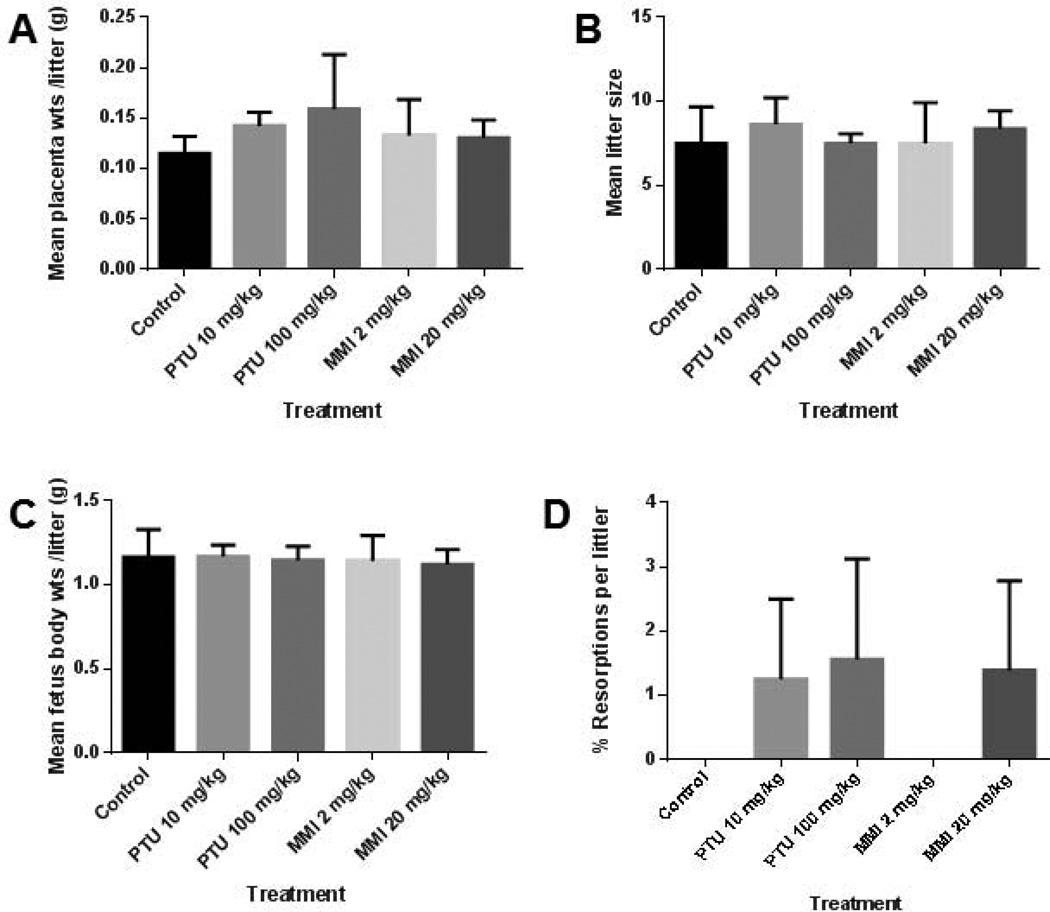
Six to nine mice were treated in each group and 45–74 embryos were examined per group. In the pregnant mice treated from GD 6–16 and examined at GD 18, no significant differences were observed in placental weights, mean littler size, resorption rates, or fetal weight among vehicle-, PTU- or MMI-treated mice (Fig. 1). Only one dead fetus was observed among all 5 treatment groups; that fetus belonged to the 2 mg/kg MMI group. No external gross malformations were observed in either PTU or MMI treated group. PTU and MMI treatment did not induce any significant histopathological abnormalities in the fetuses or in the placental tissue examined (Table 1).
Figure 1.
In utero PTU and MMI treatment had no effect on placental and fetal weights or on litter size and resorption rates in mice. Dams were treated with vehicle, PTU, or MMI from GD 6–16 and examined at GD18. No significant differences were detected among vehicle-, PTU- or MMI- treated mice in A) placental weight, B) litter size, C) fetal weight, or D) resorption rate per litter. Fetuses from dams exposed to vehicle (n = 6 litters), PTU 50 mg/kg (n = 8 litters), PTU 100 mg/kg (n = 8 litters), MMI 2 mg/kg (n = 10 litters), or MMI 20 mg/kg (n = 8 litters) were examined.
Effects of in utero anti-thyroid drug treatment on fetal rats
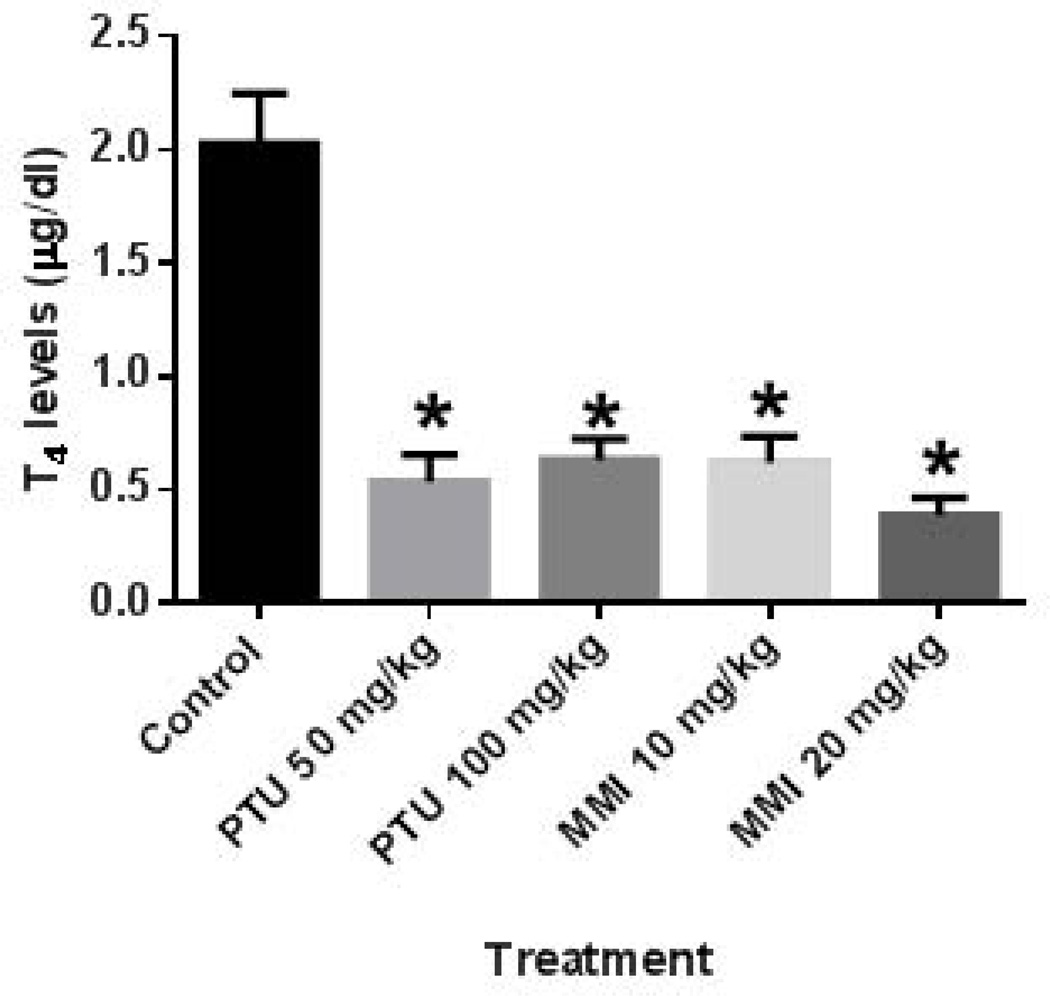
For these studies, 9–11 dams were treated per group, and 107–151 fetuses were examined per group. To assess thyroid hormone levels of the rat dams treated from GD 6–19, serum samples from dams at GD 20 were examined. Examination of the T4 levels indicated that all four anti-thyroid drug treatments lowered the circulating T4 levels in the treated dams (Fig. 2).
Figure 2.
PTU and MMI reduced serum T4 levels in rat dams treated during pregnancy. T4 serum levels were examined in pregnant rat dams at GD 20 following treatment from GD 6–19. All anti-thyroid drug treatments significantly reduced serum T4 levels. Dams were treated with vehicle (n = 9), 50 mg/kg PTU (n = 11), 100 mg/kg PTU (n = 8), 10 mg/kg MMI (n = 10), and 20 mg/kg MMI (n = 10). *p ≤ 0.0001.
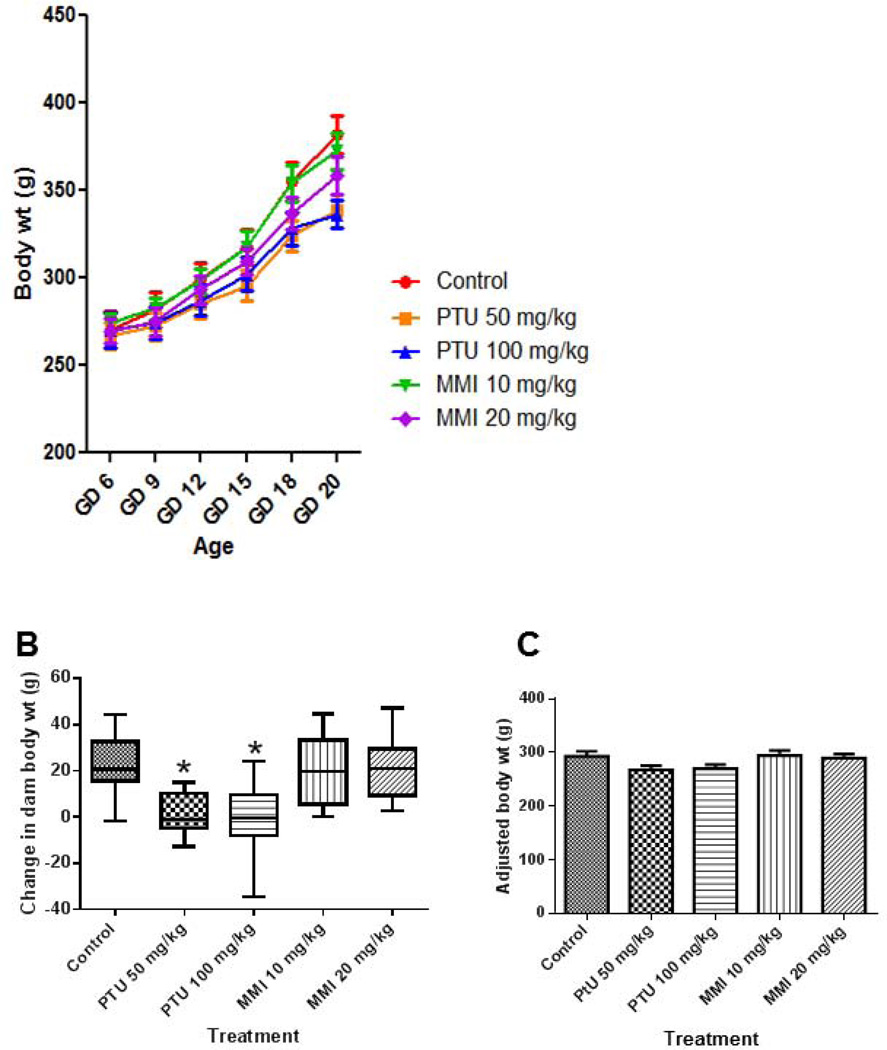
In pregnant rats treated from GD 6–19 and examined at GD 20, dam weights at GD 0, 6, 9, 12, 18 were not significantly different among vehicle-, PTU- or MMI-treated animals (Fig. 3). At GD 20, dams exposed to either 50 mg/kg PTU or 100 mg/kg PTU had significantly lower body weights than vehicle-treated dams (Fig. 3A). Dams treated with PTU (50 mg/kg or 100 mg/kg) had significantly less weight gain compared to the vehicle control group (Fig. 3B). Adjusted dam body weights were not different among treatment groups indicating that the change in body weight is from the gravid uterus weight (Fig. 3C).
Figure 3.
PTU treatment induced lower body weights at GD 20 in rat dams. Dam weights were measured at GD 0, 6, 9, 12, 15, 18, and 20 in dams treated from GD 6–19 with vehicle, PTU, or MMI. A) Dam body weights were measured during pregnancy and at GD 20 they were significantly lower in PTU treated dams compared to vehicle controls. B) Changes in dam body weight from GD 6 to GD 20 revealed that PTU treated dams gained less weight over the treatment period. C) Examining the adjusted body weights (total dam weight-gravid uterus weight) showed no difference in weight among all treatment groups. Pregnant rats were treated with vehicle (n = 9), 50 mg/kg PTU (n = 11), 100 mg/kg PTU (n = 8), 10 mg/kg MMI (n = 10), and 20 mg/kg MMI (n = 10). *p ≤ 0.0001.
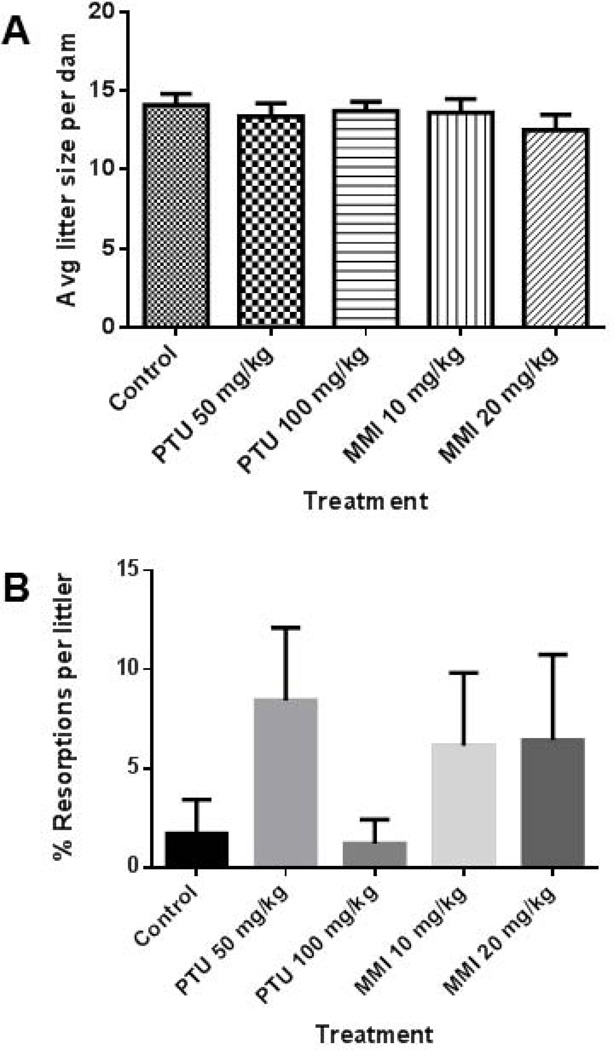
Mean litter size did not differ among the vehicle, PTU, or MMI groups (Fig. 4A).
Figure 4.
No effects on litter size or resorption rates were observed with PTU or MMI treatment in rats. Litters of rat dams treated from GD 6–19 were examined on GD 20. A) Mean litter sizes and B) percent resorption rates per litter were measured and no significant differences were detected among the treatment groups. Rat dams were treated with vehicle (n = 9), 50 mg/kg PTU (n = 11), 100 mg/kg PTU (n = 8), 10 mg/kg MMI (n = 10), and 20 mg/kg MMI (n = 10).
No significant differences in resorption rates were detected among the vehicle, PTU, or MMI groups (Figure 4B). No dead fetuses were observed in either vehicle or anti-thyroid drug groups except the 10 mg/kg MMI group, where one dead fetus was observed.
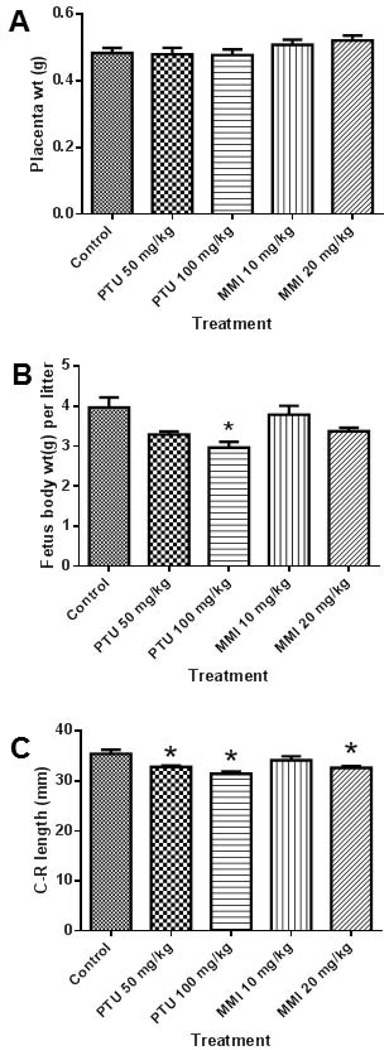
Examination of individual fetuses revealed no gross malformations from PTU or MMI treatment. No significant differences were observed in placenta weight among the vehicle-, PTU-, or MMI-treated groups (Figure 5A). Evaluation of the fetuses from rats revealed that fetal body weights were lower in the 100 mg/kg PTU treated group compared to the vehicle group (Fig. 5B). The fetal body weights of the MMI (10 or 20 mg/kg) and 50 mg/kg PTU groups were not significantly different compared to the vehicle control group (Fig. 5B). Fetal crown-rump length in both PTU-treated groups (50 mg/kg or 100 mg/kg) and one MMI group (20 mg/kg) were less than the vehicle control group (Fig. 5C).
Figure 5.
In utero PTU treatment caused a slight decrease in crown-rump lengths in rat fetuses. Fetal tissues from rat dams treated with vehicle, PTU, or MMI from GD 6–19 were analyzed at GD 20. A) No effects on placenta weights were observed with PTU or MMI treatment. B) The higher dose of PTU (100 mg/kg) resulted in lower fetal weights. C) Both PTU (50 and 100 mg/kg) and the higher MMI (20 mg/kg) dose significantly reduced the mean crown-rump lengths of fetuses compared to vehicle controls. Rat dams were treated with vehicle (n = 9), 50 mg/kg PTU (n = 11), 100 mg/kg PTU (n = 8), 10 mg/kg MMI (n = 10), and 20 mg/kg MMI (n = 10). *p ≤ 0.005.
DISCUSSION
Despite the use of anti-thyroid drugs during pregnancies for decades, the teratogenic potential of these compounds remains to be defined (Bahn et al., 2009). Few studies have evaluated the developmental toxicity of PTU and MMI in animal models, and there has not been formal evaluation of prolonged in utero exposure to anti-thyroid drugs on fetuses (Bahn et al., 2009). We now report that after examining hundreds of embryos exposed to high doses of PTU or MMI in utero, neither PTU nor MMI were associated with gross morphological malformations nor histopathological abnormalities in two rodent species.
In mice, PTU and MMI had no adverse effects on placental weight, litter size, resorption rates, or body weights of fetuses after maternal treatment from GD 6–16. Histopathological evaluations of GD 18 fetuses did not reveal any significant abnormalities with either PTU or MMI treatment.
In rat studies, exposure to PTU and MMI did not have adverse effects on litter size, placental weight, or maternal body weight from GD 6 to GD 18. However, at GD 20, fetuses from dams treated with PTU (100 mg/kg) had lower body weight compared to controls. Both doses of PTU and the higher dose of MMI adversely affected the crown-rump length of fetuses, indicating that these drugs may cause intrauterine growth restriction at high doses.
In humans, the notion as to whether MMI or PTU are teratogenic is controversial (Bahn et al., 2009). Reports of birth defects associated with MMI are generally limited to case reports noting aplasia cutis and scalp defects (Bahn et al., 2009; Laurberg and Andersen, 2014). On the basis of these reports, coupled with a paucity of reports describing birth defects in offspring born to mothers taking PTU, PTU was recommended as the treatment of choice during pregnancy by a consensus panel in 2007 (Abalovich et al., 2007).
Data disputing the notion that MMI is teratogenic, however, comes from human epidemiological studies. Several studies involving large numbers of treated humans did not observe adverse effects on the fetus that could be attributed to either PTU or MMI (Browne et al., 2009; Momotani et al., 1984; Van Dijke et al., 1987). Rather, the hyperthyroid state was thought to play a causative role. Thus, when a potential risk of liver injuries related to PTU use became recently apparent (Rivkees and Mattison, 2009a; Rivkees and Mattison, 2009b), it was recommended that PTU use be restricted to the first trimester and MMI used thereafter (Bahn et al., 2009). It was also noted that additional human studies of this issue were needed along with formal toxicological analyses (Bahn et al., 2009).
Delayed neurodevelopmental was reported in several human case reports associated with MMI treatment (Clementi et al., 1999; Greenberg, 1987; Wilson et al., 1998). In animal studies, it has been shown that MMI effects brain development, impairs learning and causes deficits in long term memory (Hosseini et al., 2010; Liu et al., 2010; Weller et al., 1996). However the effect is linked to hypothyroidism rather that the direct effect of MMI.
Additional studies of the teratogenic protocol of PTU or MMI has taken place over the past two years. In a case-controlled study of birth defects risk, PTU was found to be associated with congenital heart defects and MMI was associated with omphaloceles (Clementi et al., 2010; Yoshihara et al., 2012). In a study of children born to mothers on PTU or MMI during pregnancy, in Japan, aplasia cutis congenita, omphalocele, omphalomesenteric duct anomalies, and esophageal atresia were seen in babies born to mothers treated with MMI (Yoshihara et al., 2012). In a larger study in the US of women with hyperthyroidism, birth defects were generally not observed with either PTU or MMI, although there may have been a slight increase in the risk of congenital heart disease in children treated with anti-thyroid drugs (Korelitz et al., 2013). Most recently, Andersen reported a low level of birth defects in infants born to mothers taking PTU or MMI (Andersen et al., 2013).
In more recent animal studies on this issue, mice treated over a narrow developmental window from E8.5-E10.5, were observed to have delayed neural tube closure, but these defects were not seen at the end of pregnancy (Benavides et al., 2012). Xenopus embryos can be treated with drugs in vitro at very early embryonic stages unlike mammalian embryos, which are unable to be treated this early because fertilized ovum are inside the oviducts. When Xenopus embryos were treated very early during embryogenesis with MMI, no adverse effects were observed (van Veenendaal et al., 2013). Yet, PTU-treated embryos manifested heterotaxy defects, and PTU was shown to directly adversely affect ciliary movement (van Veenendaal et al., 2013).
In our study, we did not observe either fetal loss or gross fetal malformations with PTU or MMI in two rodent species. Considering these data along with human epidemiological data, we are moving to a more clear understanding of the risks of these drugs during pregnancy. Collectively, these data suggest that if there is a risk of either PTU or MMI on the developing embryo, these risks are very small. Furthermore, there is little support for the notion that MMI is teratogenic whereas PTU is not. However, more comprehensive studies that include skeletal examinations with larger sample size may be required to rule out the adverse effects of these anti-thyroid drugs during pregnancy.
Acknowledgments
Grant Support: NIH grant #1R01HD065200-01 (SAR)
REFERENCES
- Aaboe J, Bliddal H, Alkjaer T, Boesen M, Henriksen M. The influence of radiographic severity on the relationship between muscle strength and joint loading in obese knee osteoarthritis patients. Arthritis. 2011;2011:571519. doi: 10.1155/2011/571519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abalovich M, Amino N, Barbour LA, Cobin RH, De Groot LJ, Glinoer D, Mandel SJ, Stagnaro-Green A. Management of thyroid dysfunction during pregnancy and postpartum: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2007;92(8 Suppl):S1–S47. doi: 10.1210/jc.2007-0141. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Olsen J, Wu CS, Laurberg P. Birth defects after early pregnancy use of antithyroid drugs: A danish nationwide study. J Clin Endocrinol Metab. 2013;98(11):4373–4381. doi: 10.1210/jc.2013-2831. [DOI] [PubMed] [Google Scholar]
- Bahn RS, Burch HS, Cooper DS, Garber JR, Greenlee CM, Klein IL, Laurberg P, McDougall IR, Rivkees SA, Ross D, Sosa JA, Stan MN. The role of propylthiouracil in the management of graves' disease in adults: Report of a meeting jointly sponsored by the american thyroid association and the food and drug administration. Thyroid. 2009;19(7):673–674. doi: 10.1089/thy.2009.0169. [DOI] [PubMed] [Google Scholar]
- Benavides VC, Mallela MK, Booth CJ, Wendler CC, Rivkees SA. Propylthiouracil is teratogenic in murine embryos. PLoS One. 2012;7(4):e35213. doi: 10.1371/journal.pone.0035213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne ML, Rasmussen SA, Hoyt AT, Waller DK, Druschel CM, Caton AR, Canfield MA, Lin AE, Carmichael SL, Romitti PA, Prevention NBD. Maternal thyroid disease, thyroid medication use, selected birth defects in the national birth defects prevention study. Birth Defects Res A. 2009;85(7):621–628. doi: 10.1002/bdra.20573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calikoglu AS, Gutierrez-Ospina G, D'Ercole AJ. Congenital hypothyroidism delays the formation and retards the growth of the mouse primary somatic sensory cortex (s1) Neuroscience letters. 1996;213(2):132–136. doi: 10.1016/0304-3940(96)12836-6. [DOI] [PubMed] [Google Scholar]
- Chan GW, Mandel SJ. Therapy insight: Management of graves' disease during pregnancy. Nature clinical practice. Endocrinology & metabolism. 2007;3(6):470–478. doi: 10.1038/ncpendmet0508. [DOI] [PubMed] [Google Scholar]
- Chattaway JM, Klepser TB. Propylthiouracil versus methimazole in treatment of graves' disease during pregnancy. The Annals of pharmacotherapy. 2007;41(6):1018–1022. doi: 10.1345/aph.1H535. [DOI] [PubMed] [Google Scholar]
- Clementi M, Di Gianantonio E, Cassina M, Leoncini E, Botto LD, Mastroiacovo P. Treatment of hyperthyroidism in pregnancy and birth defects. J Clin Endocrinol Metab. 2010;95(11):E337–E341. doi: 10.1210/jc.2010-0652. [DOI] [PubMed] [Google Scholar]
- Clementi M, Di Gianantonio E, Pelo E, Mammi I, Basile RT, Tenconi R. Methimazole embryopathy: Delineation of the phenotype. Am J Med Genet. 1999;83(1):43–46. [PubMed] [Google Scholar]
- Cooper DS. Antithyroid drugs. N Engl J Med. 2005;352(9):905–917. doi: 10.1056/NEJMra042972. [DOI] [PubMed] [Google Scholar]
- El Baba KA, Azar ST. Thyroid dysfunction in pregnancy. International journal of general medicine. 2012;5:227–230. doi: 10.2147/IJGM.S27009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinoer D. The regulation of thyroid function in pregnancy: Pathways of endocrine adaptation from physiology to pathology. Endocrine reviews. 1997;18(3):404–433. doi: 10.1210/edrv.18.3.0300. [DOI] [PubMed] [Google Scholar]
- Goldey ES, Kehn LS, Rehnberg GL, Crofton KM. Effects of developmental hypothyroidism on auditory and motor function in the rat. Toxicology and applied pharmacology. 1995;135(1):67–76. doi: 10.1006/taap.1995.1209. [DOI] [PubMed] [Google Scholar]
- Greenberg F. Choanal atresia and athelia: Methimazole teratogenicity or a new syndrome? Am J Med Genet. 1987;28(4):931–934. doi: 10.1002/ajmg.1320280419. [DOI] [PubMed] [Google Scholar]
- Hosseini M, Hadjzadeh MA, Derakhshan M, Havakhah S, Rassouli FB, Rakhshandeh H, Saffarzadeh F. The beneficial effects of olibanum on memory deficit induced by hypothyroidism in adult rats tested in morris water maze. Archives of pharmacal research. 2010;33(3):463–468. doi: 10.1007/s12272-010-0317-z. [DOI] [PubMed] [Google Scholar]
- Korelitz JJ, McNally DL, Masters MN, Li SX, Xu Y, Rivkees SA. Prevalence of thyrotoxicosis, anti-thyroid medication use, complications among pregnant women in the united states. 2013 doi: 10.1089/thy.2012.0488. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krementz ET, Hooper RG, Kempson RL. The effect on the rabbit fetus of the maternal administration of propylthiouracil. Surgery. 1957;41(4):619–631. [PubMed] [Google Scholar]
- Laurberg P, Andersen SL. Antithyroid drug use in early pregnancy and birth defects. Time windows of relative safety and high risk? European journal of endocrinology / European Federation of Endocrine Societies. 2014 doi: 10.1530/EJE-14-0135. [DOI] [PubMed] [Google Scholar]
- Laurberg P, Bournaud C, Karmisholt J, Orgiazzi J. Management of graves' hyperthyroidism in pregnancy: Focus on both maternal and foetal thyroid function, and caution against surgical thyroidectomy in pregnancy. European journal of endocrinology / European Federation of Endocrine Societies. 2009;160(1):1–8. doi: 10.1530/EJE-08-0663. [DOI] [PubMed] [Google Scholar]
- Liu D, Teng W, Shan Z, Yu X, Gao Y, Wang S, Fan C, Wang H, Zhang H. The effect of maternal subclinical hypothyroidism during pregnancy on brain development in rat offspring. Thyroid. 2010;20(8):909–915. doi: 10.1089/thy.2009.0036. [DOI] [PubMed] [Google Scholar]
- Mestman JH. Hyperthyroidism in pregnancy. Clinical obstetrics and gynecology. 1997;40(1):45–64. doi: 10.1097/00003081-199703000-00007. [DOI] [PubMed] [Google Scholar]
- Momotani N, Ito K, Hamada N, Ban Y, Nishikawa Y, Mimura T. Maternal hyperthyroidism and congenital malformation in the offspring. Clin Endocrinol (Oxf) 1984;20(6):695–700. doi: 10.1111/j.1365-2265.1984.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Neale D, Burrow G. Thyroid disease in pregnancy. Obstetrics and gynecology clinics of North America. 2004;31(4):893–905. xi. doi: 10.1016/j.ogc.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Rivkees SA, Mandel SJ. Thyroid disease in pregnancy. Horm Res Paediatr 76 Suppl. 2011;1:91–96. doi: 10.1159/000329186. [DOI] [PubMed] [Google Scholar]
- Rivkees SA, Mattison DR. Ending propylthiouracil-induced liver failure in children. N Engl J Med. 2009a;360(15):1574–1575. doi: 10.1056/NEJMc0809750. [DOI] [PubMed] [Google Scholar]
- Rivkees SA, Mattison DR. Propylthiouracil (ptu) hepatoxicity in children and recommendations for discontinuation of use. Int J Pediatr Endocrinol. 2009b;2009:132041. doi: 10.1155/2009/132041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisstreet M, Herbert LC, Pharoah PO. Effects of thyroid antagonists on rat embryos cultured in vitro. Teratology. 1990;41(6):721–729. doi: 10.1002/tera.1420410609. [DOI] [PubMed] [Google Scholar]
- Van Dijke C, Heydendael R, De Kleine M. Methimazole, carbimazole, and congenital skin defects. Ann Intern Med. 1987;106(1):60–61. doi: 10.7326/0003-4819-106-1-60. [DOI] [PubMed] [Google Scholar]
- van Veenendaal NR, Ulmer B, Boskovski MT, Fang XF, Khokha MK, Wendler CC, Blum M, Rivkees SA. Embryonic exposure to propylthiouracil disrupts left-right patterning in xenopus embryos. Faseb J. 2013;27(2):684–691. doi: 10.1096/fj.12-218073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller A, Rozin A, Rigler O, Sack J. Neurobehavioral development of neonatal rats after in-utero hypothyroxinemia: Efficacy of prenatal thyroxine treatment. Early human development. 1996;46(1–2):63–76. doi: 10.1016/0378-3782(96)01742-2. [DOI] [PubMed] [Google Scholar]
- Wilson LC, Kerr BA, Wilkinson R, Fossard C, Donnai D. Choanal atresia and hypothelia following methimazole exposure in utero: A second report. Am J Med Genet. 1998;75(2):220–222. doi: 10.1002/(sici)1096-8628(19980113)75:2<220::aid-ajmg21>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Wise LD. The ich s5(r2) guideline for the testing of medicinal agents. Methods Mol Biol. 2013;947:1–11. doi: 10.1007/978-1-62703-131-8_1. [DOI] [PubMed] [Google Scholar]
- Yoshihara A, Noh J, Yamaguchi T, Ohye H, Sato S, Sekiya K, Kosuga Y, Suzuki M, Matsumoto M, Kunii Y, Watanabe N, Mukasa K, Ito K. Treatment of graves' disease with antithyroid drugs in the first trimester of pregnancy and the prevalence of congenital malformation. J Clin Endocrinol Metab. 2012;97(7):2396–2403. doi: 10.1210/jc.2011-2860. [DOI] [PubMed] [Google Scholar]
- Zolconski A, Heinrath T, Rzucidlo Z. Effect of methimazole on the development of rabbit fetuses. Ginekol Pol. 1964;35:593–596. [PubMed] [Google Scholar]