Abstract
Background
Fibromyalgia is a common chronic pain condition that has a significant impact on quality of life and often leads to disability. To date, there have been few well-controlled trials assessing the utility of nonpharmacological treatment modalities such as transcutaneous electrical nerve stimulation (TENS) in the management of pain and improvement in function in individuals with fibromyalgia.
Objectives
The purpose of this study will be to complete a long-term, multicenter study to assess the effects of TENS in women with fibromyalgia.
Design
This will be a phase II randomized, double-blind, placebo-controlled, multicenter clinical trial.
Participants
Three hundred forty-three participants with fibromyalgia will be recruited for this study.
Intervention
Participants will be randomly assigned to 1 of 3 groups: the intervention (TENS), placebo, or no treatment. After completing the randomized period, all participants will receive the intervention for 1 month. The participants will be asked to use TENS at the highest tolerable level for at least 2 hours daily during physical activity.
Measurements
The primary outcome will be pain with movement, with secondary outcomes assessing functional abilities, patient-reported outcomes, and quantitative sensory testing.
Limitations
Because having participants refrain from their typical medications is not practical, their usage and any change in medication use will be recorded.
Conclusions
The results of this study will provide some of the first evidence from a large-scale, double-blind, placebo-controlled trial on the effectiveness of TENS on pain control and quality-of-life changes in patients with fibromyalgia.
The American College of Rheumatology (ACR) classifies fibromyalgia as a clinical syndrome defined by chronic widespread muscular pain and tenderness.1 The cause of fibromyalgia is unknown, but people with fibromyalgia show sensitization of central nervous system pain pathways, measured by lower pain thresholds, enhanced temporal summation, and reduced diffuse noxious inhibitory controls (DNIC).2–4 Evidence suggests that individuals who develop fibromyalgia may have genetic polymorphisms associated with increased sensitivity to experimental pain.5,6 Furthermore, the marked increase in prevalence of fibromyalgia in people with other painful rheumatic diseases such as osteoarthritis, rheumatoid arthritis, and lupus suggests that inflammatory or mechanical pain may sensitize pain transmission pathways.7 Fibromyalgia, similar to other pain conditions, is more common in women than in men.7
Pain associated with fibromyalgia interferes with daily function, work, and social activities, resulting in a decreased quality of life.8 In addition, people with fibromyalgia have a significant amount of fatigue and a fear of movement.9 These 2 associated factors further contribute to the reduction in physical function and quality of life. Several pharmacologic treatments have been approved by the US Food and Drug Administration for management of fibromyalgia. However, the effect sizes of these drugs are moderate, and better outcomes are achieved when drug treatment is combined with physical modalities, especially aerobic exercise. Indeed, exercise interventions alone have effect sizes similar to those of drugs, although a significant barrier to their use is poor adherence.10,11 Poor adherence is thought to be related to pain during exercise and post-exertional malaise.12–14 A reduction in pain during exercise would reduce fear of movement and ultimately result in increased physical function and quality of life. Thus, one of the main treatments for patients with fibromyalgia must focus on pain relief during exercise to allow the person to benefit from improved fitness and to function more effectively both at home and at work.
One potential treatment is the use of transcutaneous electrical nerve stimulation (TENS), which is a modality used by health professionals that delivers electrical stimulation through the skin for pain control. Basic science studies show that TENS activates descending inhibitory pathways from the midbrain and brain stem to inhibit excitability of nociceptive neurons in the spinal cord.15–18 Based on the mechanism of TENS, one could argue that it is best used for conditions such as fibromyalgia with a strong component of central pain amplification or loss of inhibition. Furthermore, a previous TENS study showed that TENS is more effective in reducing pain with movement compared with resting pain.19 Thus, TENS should reduce pain most effectively when there is ongoing noxious stimulation that includes physical activity or exercise. As TENS reduces central excitability and increases central inhibition of pain, this treatment addresses the mechanisms thought to be central to the pain of people with fibromyalgia.
Although TENS is effective for several pain conditions such as osteoarthritis, chronic musculoskeletal pain, and postoperative pain,20–22 its effectiveness in treatment of people with fibromyalgia is virtually unknown. Furthermore, there is a general thought among clinicians that because fibromyalgia pain is widespread, TENS would be ineffective in this population. To initially test if TENS was effective in decreasing the pain associated with fibromyalgia, we performed a preliminary study examining the effectiveness of a single treatment with high-frequency TENS.23 The group receiving active TENS showed a reduction in pain during movement, but not resting pain, compared with placebo. Thus, TENS may decrease pain associated with fibromyalgia, particularly during exercise and physical activity. This decrease in pain is expected to increase function and improve quality of life.
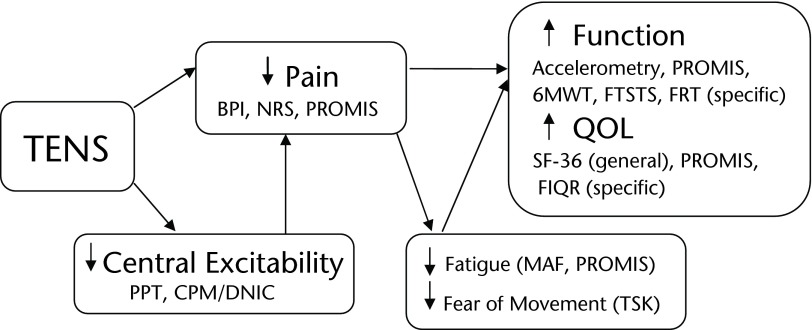
We hypothesize that application of TENS to women with fibromyalgia will reduce movement-related pain and reduce central excitability by restoring central inhibition and that these decreases in pain and central excitability will reduce fatigue and fear of movement, thereby improving function and quality of life (Fig. 1). The Fibromyalgia Activity Study with TENS (FAST) had 4 specific aims to test our hypothesis. The first aim of the study is to test the efficacy of repeated use of TENS on movement-related pain in women with fibromyalgia with random assignment to 3 treatment groups: standard care, placebo TENS, and active TENS. A second aim will test if movement-pain reduction by TENS results in a concomitant decrease in fear of movement, fatigue, resting pain, and use of rescue analgesic medications and an increase in function and quality of life. Outcome measures will include physical function by directly assessing daily activity with an accelerometer, as well as performing specific functional tasks. Our third aim will determine if active TENS alters pain processing in women with fibromyalgia and if improvement in clinical symptoms correlates with normalization of pain-processing physiology. We will evaluate change in these physiologic parameters in responders versus nonresponders as assessed clinically. Lastly, a fourth aim is to assess if the Patient Reported Outcomes Measurement Information System (PROMIS) is a useful instrument for assessing outcomes in women with fibromyalgia by comparing the PROMIS modules with symptom domains measured by other instruments validated for use in fibromyalgia clinical trials and by determining the performance of PROMIS in the definition of responders.
Figure 1.
Conceptual model for effectiveness of transcutaneous electrical nerve stimulation (TENS), showing the expected outcomes and mechanisms by which those outcomes will be assessed. A combination of functional tests, pain physiology assessments, and patient-reported outcomes will be used to provide a complete picture of the effectiveness of the use of TENS in patients with fibromyalgia. BPI=Brief Pain Inventory, NRS=numeric rating scale, PROMIS=Patient Reported Outcomes Measurement Information System, SF-36=36-Item Short-Form Health Survey, FIQR=Revised Fibromyalgia Impact Questionnaire, TSK=Tampa Scale of Kinesiophobia, 6MWT=Six-Minute Walk Test, FTSTS=Five-Times-Sit-to-Stand Test, PPT=pressure pain threshold, CPM/DNIC=conditioned pain modulation (diffuse noxious inhibitory control), MAF=Multidimensional Assessment of Fatigue, QOL=quality of life, FRT=Functional Reach Test.
Method
Study Design
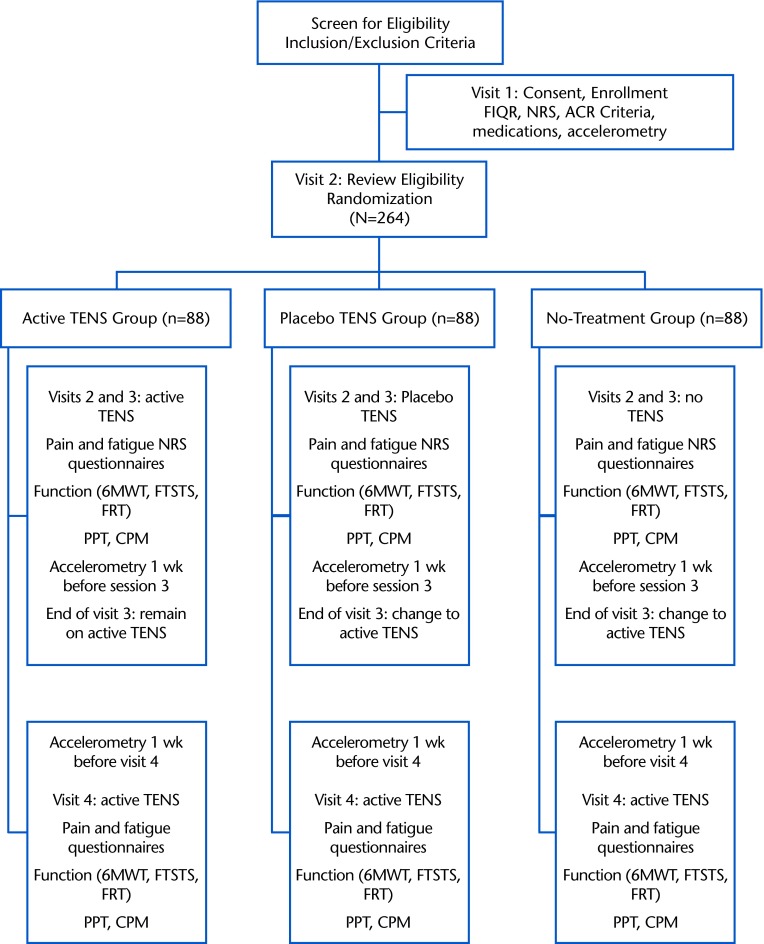
This is a phase II randomized, double-blind, placebo-controlled, multicenter clinical trial, version 1, that began in July 2013. The initial phase of the study will randomly allocate participants to receive active TENS, placebo TENS, or standard care (no TENS). Randomization will be stratified by site and opioid use. After participating in the 1-month random assignment, all participants will receive active TENS for 1 month. The participants will make 4 visits to the clinic. Figure 2 provides a flowchart for the study.
Figure 2.
Flowchart for the study showing the breakdown of what occurs during each visit per each group (active transcutaneous electrical nerve stimulation [TENS], placebo TENS, and no treatment). FIQR=Revised Fibromyalgia Impact Questionnaire, NRS=numeric rating scale, ACR=American College of Rheumatology, 6MWT=Six-Minute Walk Test, FTSTS=Five-Times-Sit-to-Stand Test, FRT=Functional Reach Test, PPT=pressure pain threshold, CPM=conditioned pain modulation.
Participants
Participants will be recruited from the University of Iowa and Vanderbilt University and surrounding communities. The research protocol has been reviewed and approved by the institutional review boards of both universities. Prior to enrolling into the study, participants will provide their written informed consent. Participants will be consented and enrolled by the research assistant on the first visit. The consent will review study purpose, procedures, risks and benefits, what to do if injured as a result of the study as well as give the opportunity for questions regarding the study. Confidentiality of participant information will be password protected and stored indefinitely with the University of Iowa and Vanderbilt University. We will enroll a total of 343 participants in the study; we expect that less than 4% of them will not meet the inclusion criteria, resulting in approximately 330 individuals randomized into 1 of 3 groups. With an attrition rate of 20%, we expect 264 participants to complete the study, with 88 individuals per treatment group.
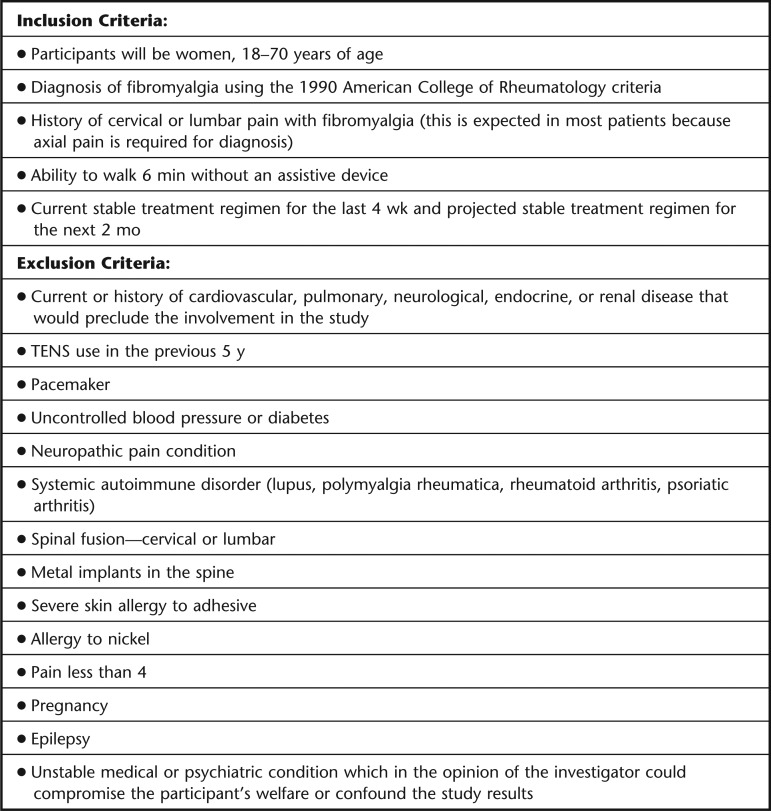
All participants will receive standard care and will be on a stable medical management plan prior to enrolling. Participants will not be asked to refrain from their current treatment plan but will be asked not to change their current treatments during the 2-month trial period. The use of “as needed” rescue medications will be recorded and compared as a secondary outcome measure for the study. We also will record the current treatment plan, including medications, exercise, and psychological management for each participant. Specific inclusion and exclusion criteria for entrance into the study are detailed in Figure 3.
Figure 3.
Inclusion and exclusion criteria for entrance into the transcutaneous electrical nerve stimulation (TENS) trial for patients with fibromyalgia.
Outcome Measures
Primary outcome.
The primary outcome measure will be pain intensity during movement after all treatment sessions.
Secondary outcomes.
The secondary outcome measures will be: (1) fear of movement, (2) function, (3) fatigue, (4) pressure pain threshold, (5) conditioned pain modulation, (6) analgesic medication use, (7) quality of life, and (8) activity levels using accelerometry.
Measurement Instruments
Pain and fatigue measures.
Pain and fatigue will be measured at rest and during movement activities using a pain numeric rating scale (NRS), which consists of a 0–10 vertical line. The anchors for pain will be “no pain” and “worst pain imaginable,”24 and the anchors for fatigue will be “no fatigue” and “worst fatigue imaginable.”
Multidimensional questionnaires.
Understanding the impact of TENS on quality of life and function is an important factor in translating the decreases in pain observed during TENS to a more global effect of the treatment. The questionnaires and details of visit implementation are listed in Table 1.
Table 1.
Measurements to Be Taken, Including Instrument to Be Used, Visit on Which Measurement Will Take Place, Construct and General Information, and Reliability, Validity, and References for the Instrumentsa
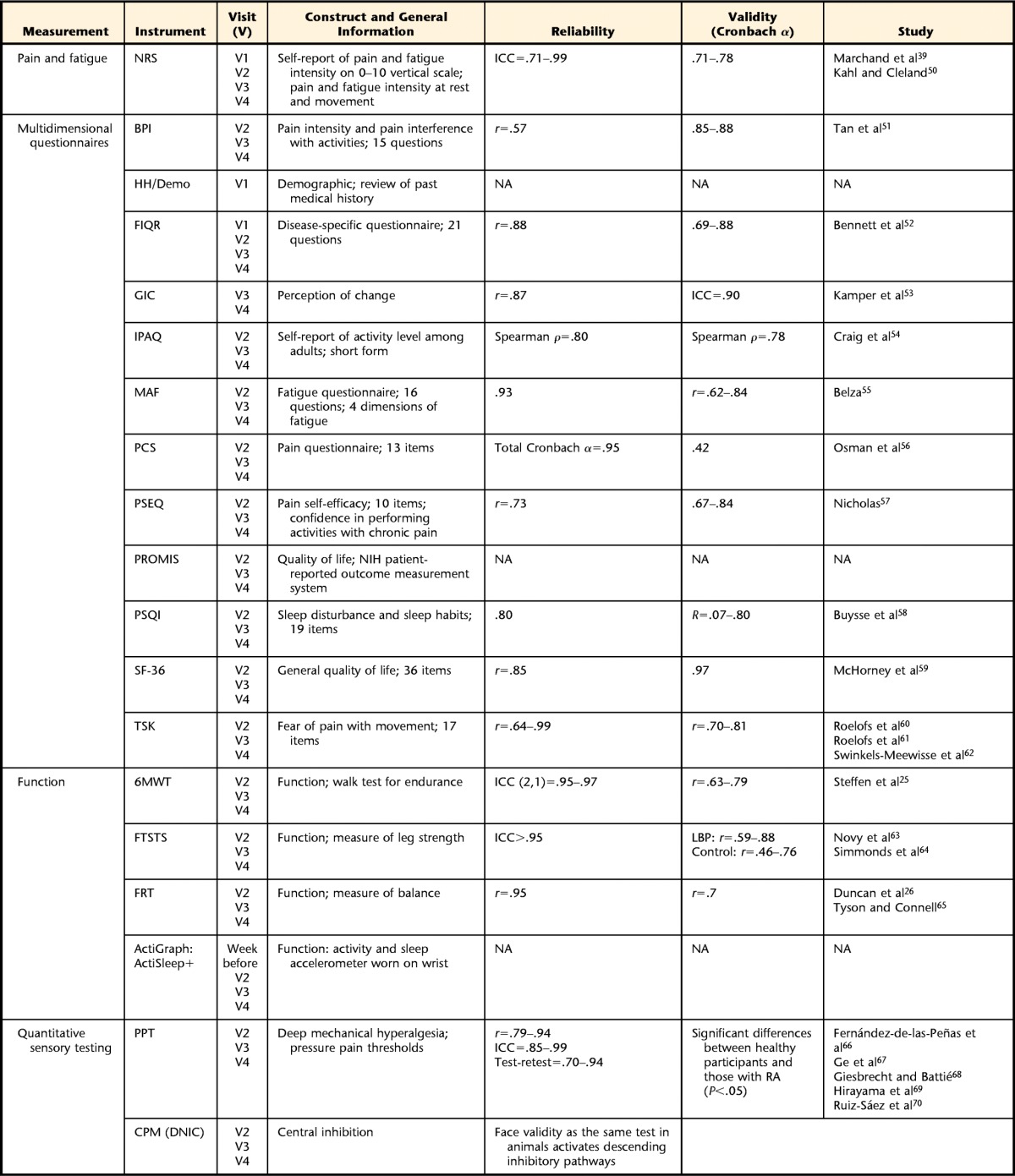
NRS=numeric rating scale, ICC=intraclass correlation coefficient, BPI=Brief Pain Inventory, HH/Demo=Health History/Demographic Questionnaire, NA=not applicable, FIQR=Revised Fibromyalgia Impact Questionnaire, IPAQ=International Physical Activity Questionnaire, MAF=Multidimensional Assessment of Fatigue, PCS=Pain Catastrophizing Scale, PSEQ=Pain Self-Efficacy Questionnaire, PROMIS=Patient Reported Outcomes Measurement Information System, NIH=National Institutes of Health, PSQI=Pittsburgh Sleep Quality Index, SF-36=36-Item Short-Form Health Survey, TSK=Tampa Scale of Kinesiophobia, 6MWT=Six-Minute Walk Test, FTSTS=Five-Times-Sit-to-Stand Test, FRT=Functional Reach Test, PPT=pressure pain threshold, CPM (DNIC)=conditioned pain modulation (diffuse noxious inhibitory control).
Function measures.
Direct measures of function will be done before and during TENS on visits 2, 3, and 4; details are shown in Table 1. The Six-Minute Walk Test (6MWT) measures the maximum distance a person can walk in 6 minutes25; we also will rate pain during this test. The Five-Times-Sit-to-Stand Test (FTSTS) measures the time it takes to come from a sitting to a standing position 5 times.25 The Functional Reach Test (FRT) measures the distance reached using the upper extremity (UE) as a measure of UE function.26 Accelerometry (ActiSleep+, ActiGraph, Pensacola, Florida) will be used to measure daily activity levels, and participants will wear the accelerometer for 1 week, at 3 intervals, before and during the intervention.
Quantitative sensory testing.
Pressure pain threshold (PPT) of the cervical, lumbar, and anterior tibialis muscle region will be measured with a pressure algometer (AlgoMed, 1-cm2 tip, Medoc Ltd, Ramat Yishai, Israel) applied at a rate of 40 kPa/s. We also will record PPT in distant sites on the lower leg to assess the widespread effects of TENS. Conditioned pain modulation (CPM), also known as diffuse noxious inhibitory controls (DNIC), will be completed by placing the left lower leg in an ice water bath and measuring change in PPT over the right lower leg. These measures; therefore, will examine the effects of TENS on reducing pain excitability by assessing pain thresholds and on restoring endogenous inhibition by examining CPM. Our prior study with a single treatment of TENS in fibromyalgia showed increases in pain thresholds at the site of application and distant to the site of application, as well as increases in endogenous inhibition,23 suggesting TENS has the capability of modulating pain physiology.
Randomization/allocation/blinding.
Upon enrollment into the study, participants will be randomly assigned to 1 of 3 groups: (1) active TENS, (2) placebo TENS, and (3) standard care. All assessments will be done by a separate person (outcome assessor) from the person allocating and applying the TENS (TENS allocator) to further control for blinding. Thus, the participants will be blinded to the TENS treatment (active or placebo TENS), and the outcome assessor will be blinded to all groups. Randomization will be stratified by site (University of Iowa versus Vanderbilt University) and opioid status (opioid use versus nonopioid use). Stratification by opioid status was done because TENS uses endogenous opioids to produce analgesia,15,16,27 and previous studies have shown that low-frequency TENS is ineffective in opioid-tolerant animals and people.28,29 The randomization schedule will be created by the study biostatistician, viewed and updated by the TENS allocator, and implemented in permuted blocks of 4 and 6 using SAS v9.2 PROC PLAN (SAS Institute Inc, Cary, North Carolina) to ensure adequate distribution of all groups across the collection period. The TENS allocator will not be blinded to the treatment, as he or she will administer the TENS session and will be responsible for discussion with the participants between visits and with those in the standard care group.
TENS intervention.
Transcutaneous electrical nerve stimulation is a nonpharmacological treatment that applies electrical current through the skin for pain control. The device is portable and allows the individuals to self-administer the treatment at home. We will use the Empi Select TENS unit (Empi Inc/DJO Global, Vista, California), which uses an asymmetrical biphasic alternating current. There are a number of parameters that can be modulated on the unit, including frequency of stimulation (1–150 Hz), pulse duration (10–250 microseconds), and intensity (0–60 mA). We will apply TENS using: (1) a modulating frequency between 10 and 100 Hz to prevent potential analgesic tolerance, as previously shown in a preclinical study,30 and (2) the highest tolerable intensity, as prior studies have shown that intensity is critical to effective relief of pain and that the higher the intensity, the greater the analgesia.22,31–33 Participants will be given a target output number based on a TENS trial test done during the first visit.
The placebo TENS unit will look exactly like the active TENS unit. The placebo TENS unit delivers current for 45 seconds, ramping to 0 in the last 15 seconds. This new transient placebo TENS device has recently been validated against a traditional placebo TENS device (nonworking unit) and an active TENS device by our laboratory in healthy controls and individuals with pain (osteoarthritis and fibromyalgia).32 Furthermore, there is no analgesic effect with the new transient placebo TENS device, similar to standard placebo care, and the active TENS unit shows greater increases in pain thresholds compared with the placebo TENS unit.32 Participants will be given a target number near sensory threshold defined during the TENS tolerance test on visit 1.
For the standard care group, participants will receive no TENS treatment but will receive a quiet rest period of 30 minutes instead of TENS during laboratory testing days. This 30-minute quiet rest period is done to blind the assessor to treatment group. Furthermore, during laboratory testing days, the standard care group will have electrodes placed on both the cervicothoracic spine and lumbar spine, and these electrodes will be attached to a TENS unit that is not turned on. This placement of electrodes and TENS unit on visits 2 and 3 again is done to blind the outcomes assessor to treatment group on clinic testing days. The participants will know they are not receiving a treatment and will be told not to discuss this fact with the outcomes assessor. The standard care group will not receive a TENS unit for home use. All other procedures will be the same. This group, therefore, will control for effect of placebo and for repeated testing. The standard care group is a critical group to assess for potential differences due primarily to placebo effects. This strategy of a standard care or no-treatment group is commonly used in clinical trials of nonpharmacological treatments. For example, exercise trials rarely use a placebo and only compare with a standard care group, whereas studies on acupuncture or manual therapy will often include placebo and standard care for comparison.34
Participants will be blinded to the active and placebo TENS treatments. The outcomes assessor will be blinded to all 3 groups. The TENS allocator will not be blinded to the treatment, as he or she will administer the TENS session and will be available throughout the study to answer questions regarding TENS. Assessment of blinding will be accomplished by asking both the outcomes assessor and the participant if they thought the individual received the active TENS, the placebo TENS, or do not know. Our previous studies showed that we can blind the active placebo units for the therapists, as they are unable to identify the unit.32,35 We also know that we can adequately blind the placebo unit that will be used in this study,32,36,37 as study participants identified it correctly only 60% of the time, which is no different from chance.32 In contrast, we could not adequately blind the standard (inactive) placebo TENS unit where they were able to identify it correctly 79% of the time, which is a significant difference from chance.32 Blinding to the placebo is critical to examine nonspecific effects, and double blinding of the assessor and participants removes potential expectation biases.
For the proposed study, we specifically chose to do a 1-month blinded trial of TENS and to follow this trial with a second 1-month trial where all participants will receive active TENS. We chose the 1-month trial with the realization that TENS is not a “cure” for fibromyalgia but rather an adjunct treatment aimed at reducing pain so that functional levels will be increased. Our preliminary data showed changes in movement pain with a single treatment, suggesting that TENS produces an immediate effect and could improve function by reducing movement pain.23 Based on prior studies in other populations,38,39 1 month is an adequate length of time to examine for cumulative effects, to examine if there are increases in physical activity during TENS usage, and to examine for potential changes in pain. A study of people with knee osteoarthritis by Cheing and Hui-Chan40 supports the notion that TENS given with an exercise program not only reduces pain but also increases function. However, the study was underpowered, with fewer than 20 participants per group (62 participants for 4 groups), and the results were difficult to interpret. In the proposed study, we will add a second month where all participants will receive the active treatment; thus, some individuals will receive 2 months of active TENS. Giving everyone an active treatment allows us to improve recruitment and retention in the study and gives us additional information that can be used to examine the placebo and active treatment groups in more detail.
Because TENS is used over a long period of time, the effect of prolonged utilization needs to be assessed. A TENS unit is typically given to the patient to take home for daily use.
Transcutaneous electrical nerve stimulation is thought to be more effective during or immediately following treatment,27 and there is a cumulative effect of repeated TENS.27,38 Therefore, the study was designed to test the long-term effects of TENS as well as to test the effects of TENS during a treatment session. We will test effects of a single treatment of TENS measured before and during application on the second, third, and fourth visits. This approach will allow us to see acute effects of TENS during treatment, long-term effects of daily use of TENS, and cumulative effects of TENS.
Home instruction and home use will be done on visit 2. As we have observed a reduction in pain with movement during TENS,23 we will instruct participants to use the TENS unit for a minimum of 2 hours per day while physically active (eg, doing chores, walking, exercising). Furthermore, we will instruct participants that they can break up the 2-hour block of time but that 30 minutes is the minimal effective time for which the unit must be worn. They will be allowed to use the unit more if they wish and will record usage on a home-record log. Usage also will be monitored directly on the TENS unit, which will allow us to examine the total time, number of sessions, and average duration of each session. Home use will occur for 4 weeks after visit 2, and all participants will receive active TENS for 4 weeks between visits 3 and 4. Weekly telephone calls between visits will be made by the TENS allocator to ensure proper use and answer study-related questions.
Statistical Analysis
We will use intention-to-treat analysis to examine treatment effect. For the primary aim, we will use a linear mixed model analysis for repeated measures, with treatment group, time (visit 3 − visit 2), and treatment group × time interaction as fixed effects. A post hoc pair-wise comparison of mean change among all 3 treatment groups will be conducted, with P values adjusted using Bonferroni correction. Potential confounders include age, race, ethnicity, educational attainment, marital status, TENS dose or intensity, and medication intake, and rescue medications will be included as covariates as appropriate. All data will be analyzed using SAS 9.5 or higher, and data with P values of <.05 will be considered statistically significant. More details on statistical analysis for aim 1 and a detailed analysis for aims 2 through 4 are included in Table 2.
Table 2.
Description of the Statistical Analysis to Take Place Showing What Measures Will Be Analyzed for Each Specific Aim of the Study and What the Statistical Plan Is for the Analysis of the Data by Aima
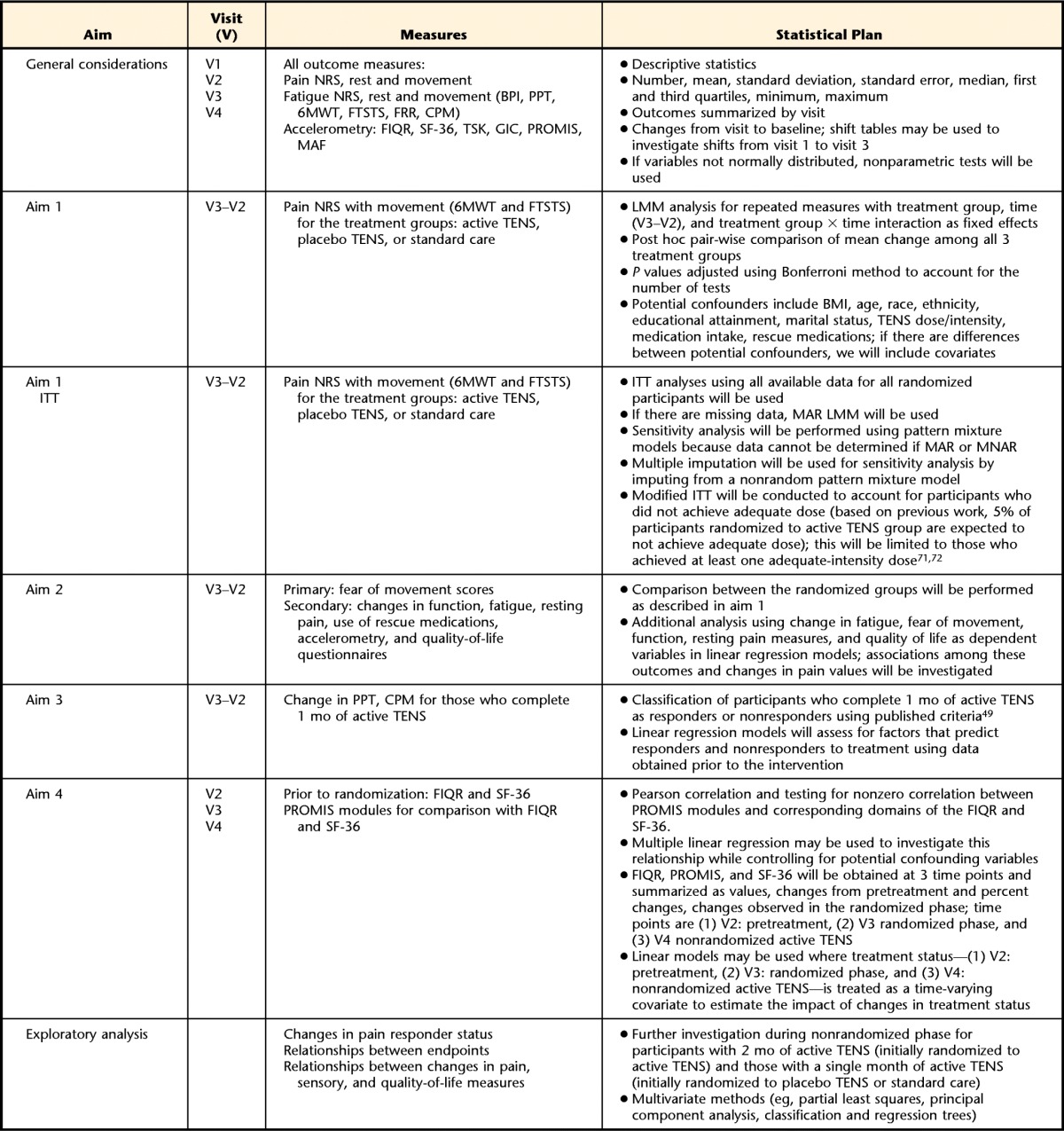
NRS=numeric rating scale, BPI=Brief Pain Inventory, FIQR=Revised Fibromyalgia Impact Questionnaire, MAF=Multidimensional Assessment of Fatigue, PROMIS=Patient Reported Outcomes Measurement Information System, SF-36=36-Item Short-Form Health Survey, TSK=Tampa Scale of Kinesiophobia, GIC=global impression of change, ITT=intention to treat, LMM=linear mixed model analysis, BMI=body mass index, TENS=transcutaneous electrical nerve stimulation, MAR=missing at random, MNAR=missing not at random, 6MWT=Six-Minute Walk Test, FTSTS=Five-Times-Sit-to-Stand Test, FRT=Functional Reach Test, PPT=pressure pain threshold, CPM=conditioned pain modulation.
Role of Funding Source
The funding for this study was provided by National Institute of Arthritis Musculoskeletal and Skin Diseases research grants UM1 AR063381 and R34 AR060278. The study funder did not have a role in the design, implementation, analysis, interpretation of results, or the decision as to if and where to publish papers.
Discussion
This clinical trial aims to examine the efficacy of TENS on pain in people with fibromyalgia, particularly pain during movement. Transcutaneous electrical nerve stimulation is the application of electrical current to the skin for pain control, and the TENS unit is a portable device for nonpharmacological pain management that patients can self-administer. Fibromyalgia affects many aspects of a patient's function and quality of life. Furthermore, patients with fibromyalgia have a fear of movement and significant fatigue, which further affects their function and quality of life.8,9,41 If treatments can be aimed at reducing pain during movement, we expect to decrease fear of movement, and this decreased fear of movement would be associated with an increase in physical activity. The increase in physical activity should then result in reductions in resting pain and fatigue and in improvements in function and quality of life. Previous studies showed that increasing physical activity by 30 minutes per day was sufficient to decrease pain in people with fibromyalgia.42,43 Further repetitive treatment with TENS reduces pain at rest in a cumulative manner in people with musculoskeletal pain such as low back pain.38,39 Thus, we expect initial decreases in movement-related pain but subsequent decreases in resting pain after 1 month of treatment.
Although TENS has been used for the treatment of pain for decades, only recently have high-quality placebo-controlled trials been implemented to examine its effects. However, most studies of TENS examine effects on pain, predominantly at rest. Furthermore, effectiveness of TENS remains controversial but may depend on many factors, including adequate stimulation parameters and outcome measures.22,32,44 For example, prior work showed that pain during function is more likely to be reduced by TENS and that there is minimal effect on pain at rest.19 It is also clear that pain is not the only indicator of effectiveness, and clinical trials with pharmaceutical agents in people with fibromyalgia also assess effects on function, quality of life, and other associated symptoms such as fatigue or sleep.45–48 Therefore, we will apply these same principles by examining not only whether TENS decreases pain with movement, but also whether this decrease in pain translates to improved function and quality of life.
This clinical trial will uniquely examine whether TENS affects pain physiology and whether improvements in clinical symptoms correlate with normalization of pain-processing physiology. The findings will allow us a scientific rationale for the effectiveness of TENS in the patient with fibromyalgia and will validate prior animal studies. Our preliminary studies strongly suggest that we will see changes in pain with movement, function, and pain physiology. In addition, we are assessing the utility of using the PROMIS modules in the assessment of the pain and other symptoms of fibromyalgia by validating its performance against disease- and domain-specific instruments used in fibromyalgia clinical trials. Lastly, we will evaluate the performance of the PROMIS modules compared with other measurement strategies as components of the ACR FM30 responder criteria.49
Footnotes
All authors provided concept/idea/study design. Dr Noehren, Dr Dailey, Dr Rakel, Dr Vance, Dr Crofford, and Dr Sluka provided writing. Dr Zimmerman provided data analysis. Dr Noehren, Dr Dailey, Dr Rakel, Dr Crofford, and Dr Sluka provided project management. Dr Crofford and Dr Sluka provided fund procurement, participants, and facilities/equipment. Dr Noehren, Dr Dailey, Dr Vance, and Dr Zimmerman provided consultation (including review of manuscript before submission).
Executive Committee: Project Director/Principal Investigator: Dr Sluka; Site Directors: Dr Rakel (Principal Investigator, University of Iowa) and Dr Crofford (Principal Investigator, Vanderbilt University); Study Statistician: Dr Zimmerman; Project Coordinator, University of Iowa: Dr Dailey; and National Institute of Arthritis and Musculoskeletal and Skin Diseases Program Officer, Dr William Tonkins.
Steering Committee: Project Director: Dr Sluka; Site Directors: Dr Rakel (Principal Investigator, University of Iowa) and Dr Crofford (Principal Investigator, Vanderbilt University); Study Coordinators: Dr Dailey (University of Iowa) and Dr Leon Darghosian (Vanderbilt University); Consultant: Dr Noehren (University of Kentucky); and Study Statistician: Dr Zimmerman (University of Iowa).
This study was approved by the institutional review boards of the University of Iowa and Vanderbilt University.
The funding of this study was provided by the National Institute of Arthritis and Musculoskeletal and Skin Diseases research grants UM1 AR063381 and R34 AR060278.
This trial is registered at ClinicalTrials.gov (trial number: NCT01888640).
References
- 1. Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken). 2010;62:600–610. [DOI] [PubMed] [Google Scholar]
- 2. Staud R, Cannon RC, Mauderli AP, et al. Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain. 2003;102:87–95. [DOI] [PubMed] [Google Scholar]
- 3. Staud R, Robinson ME, Vierck CJ, Jr, Price DD. Diffuse noxious inhibitory controls (DNIC) attenuate temporal summation of second pain in normal males but not in normal females or fibromyalgia patients. Pain. 2003;101:167–174. [DOI] [PubMed] [Google Scholar]
- 4. Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain. 1997;70:41–51. [DOI] [PubMed] [Google Scholar]
- 5. Cohen H, Buskila D, Neumann L, Ebstein RP. Confirmation of an association between fibromyalgia and serotonin transporter promoter region (5- HTTLPR) polymorphism, and relationship to anxiety-related personality traits. Arthritis Rheum. 2002;46:845–847. [DOI] [PubMed] [Google Scholar]
- 6. Buskila D. Developments in the scientific and clinical understanding of fibromyalgia. Arthritis Res Ther. 2009;11:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clauw DJ. Fibromyalgia: an overview. Am J Med. 2009;122(12 suppl):S3–S13. [DOI] [PubMed] [Google Scholar]
- 8. Arnold LM, Crofford LJ, Mease PJ, et al. Patient perspectives on the impact of fibromyalgia. Patient Educ Couns. 2008;73:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burwinkle T, Robinson JP, Turk DC. Fear of movement: factor structure of the Tampa Scale of Kinesiophobia in patients with fibromyalgia syndrome. J Pain. 2005;6:384–391. [DOI] [PubMed] [Google Scholar]
- 10. Busch AJ, Barber KA, Overend TJ, et al. Exercise for treating fibromyalgia syndrome. Cochrane Database Syst Rev. 2007;4:CD003786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mease PJ, Clauw DJ, Arnold LM, et al. Fibromyalgia syndrome. J Rheumatol. 2005;32:2270–2277. [PubMed] [Google Scholar]
- 12. Kosek E, Ekholm J, Hansson P. Modulation of pressure pain thresholds during and following isometric contraction in patients with fibromyalgia and in healthy controls. Pain. 1996;64:415–423. [DOI] [PubMed] [Google Scholar]
- 13. Dobkin PL, Da Costa D, Abrahamowicz M, et al. Adherence during an individualized home based 12-week exercise program in women with fibromyalgia. J Rheumatol. 2006;33:333–341. [PubMed] [Google Scholar]
- 14. Staud R, Robinson ME, Price DD. Isometric exercise has opposite effects on central pain mechanisms in fibromyalgia patients compared to normal controls. Pain. 2005;118:176–184. [DOI] [PubMed] [Google Scholar]
- 15. Sluka KA, Deacon M, Stibal A, et al. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. J Pharmacol Exp Ther. 1999;289:840–846. [PubMed] [Google Scholar]
- 16. Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS). J Pharmacol Exp Ther. 2001;298:257–263. [PubMed] [Google Scholar]
- 17. Ma YT, Sluka KA. Reduction in inflammation-induced sensitization of dorsal horn neurons by transcutaneous electrical nerve stimulation in anesthetized rats. Exp Brain Res. 2001;137:94–102. [DOI] [PubMed] [Google Scholar]
- 18. DeSantana JM, Da Silva LF, De Resende MA, Sluka KA. Transcutaneous electrical nerve stimulation at both high and low frequencies activates ventrolateral periaqueductal grey to decrease mechanical hyperalgesia in arthritic rats. Neuroscience. 2009;163:1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rakel B, Frantz R. Effectiveness of transcutaneous electrical nerve stimulation on postoperative pain with movement. J Pain. 2003;4:455–464. [DOI] [PubMed] [Google Scholar]
- 20. Osiri M, Welch V, Brosseau L, et al. Transcutaneous electrical nerve stimulation for knee osteoarthritis. Cochrane Database Syst Rev. 2000;4:CD002823. [DOI] [PubMed] [Google Scholar]
- 21. Johnson M, Martinson M. Efficacy of electrical nerve stimulation for chronic musculoskeletal pain: a meta-analysis of randomized controlled trials. Pain. 2007;130:157–165. [DOI] [PubMed] [Google Scholar]
- 22. Bjordal JM, Johnson MI, Ljunggreen AE. Transcutaneous electrical nerve stimulation (TENS) can reduce postoperative analgesic consumption: a meta-analysis with assessment of optimal treatment parameters for postoperative pain. Eur J Pain. 2003;7:181–188. [DOI] [PubMed] [Google Scholar]
- 23. Dailey DL, Rakel BA, Vance CG, et al. Transcutaneous electrical nerve stimulation reduces pain, fatigue and hyperalgesia while restoring central inhibition in primary fibromyalgia. Pain. 2013;154:2554–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Turk DC, Melzack R. Handbook of Pain Assessment. New York, NY: Guilford Press; 1992. [Google Scholar]
- 25. Steffen TM, Hacker TA, Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys Ther. 2002;82:128–137. [DOI] [PubMed] [Google Scholar]
- 26. Duncan PW, Weiner DK, Chandler J, Studenski S. Functional reach: a new clinical measure of balance. J Gerontol. 1990;45:M192–M197. [DOI] [PubMed] [Google Scholar]
- 27. Sluka KA, Bjordal JM, Marchand S, Rakel BA. What makes transcutaneous electrical nerve stimulation work: making sense of the mixed results in the clinical literature. Phys Ther. 2013;93:1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chandran P, Sluka KA. Development of opioid tolerance with repeated transcutaneous electrical nerve stimulation administration. Pain. 2003;102:195–201. [DOI] [PubMed] [Google Scholar]
- 29. Leonard G, Cloutier C, Marchand S. Reduced analgesic effect of acupuncture-like TENS but not conventional TENS in opioid-treated patients. J Pain. 2011;12:213–221. [DOI] [PubMed] [Google Scholar]
- 30. DeSantana JM, Santana-Filho VJ, Sluka KA. Modulation between high- and low-frequency transcutaneous electric nerve stimulation delays the development of analgesic tolerance in arthritic rats. Arch Phys Med Rehabil. 2008;89:754–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moran F, Leonard T, Hawthorne S, et al. Hypoalgesia in response to transcutaneous electrical nerve stimulation (TENS) depends on stimulation intensity. J Pain. 2011;12:929–935. [DOI] [PubMed] [Google Scholar]
- 32. Rakel B, Cooper N, Adams HJ, et al. A new transient sham TENS device allows for investigator blinding while delivering a true placebo treatment. J Pain. 2010;11:230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pantaleao MA, Laurino MF, Gallego NL, et al. Adjusting pulse amplitude during transcutaneous electrical nerve stimulation (TENS) application produces greater hypoalgesia. J Pain. 2011;12:581–590. [DOI] [PubMed] [Google Scholar]
- 34. Friedman LM, Furberg CD, DeMets DL. Fundamentals of Clinical Trials. 3rd ed New York, NY: Springer; 1998. [Google Scholar]
- 35. Vance CG, Rakel BA, Blodgett NP, et al. Effects of transcutaneous electrical nerve stimulation on pain, pain sensitivity, and function in people with knee osteoarthritis: a randomized controlled trial. Phys Ther. 2012;92:898–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liebano RE, Rakel B, Vance CG, et al. An investigation of the development of analgesic tolerance to TENS in humans. Pain. 2011;152:335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cowan S, McKenna J, McCrum-Gardner E, et al. An investigation of the hypoalgesic effects of TENS delivered by a glove electrode. J Pain. 2009;10:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marchand S, Charest J, Li J, et al. Is TENS purely a placebo effect: a controlled study on chronic low back pain. Pain. 1993;54:99–106. [DOI] [PubMed] [Google Scholar]
- 39. Cheing GL, Hui-Chan CW. Repeated applications of transcutaneous electrical nerve stimulatoin (TENS) produce cumulative effects on chronic clinical pain but not acute experimental pain in chronic low back pain patients. In: Proceedings from the 8th World Congress of the International Association for the Study of Pain; August 17–22, 1996; Seattle, Washington. [Google Scholar]
- 40. Cheing GL, Hui-Chan CW. Would the addition of TENS to exercise training produce better physical performance outcomes in people with knee osteoarthritis than either intervention alone? Clin Rehabil. 2004;18:487–497. [DOI] [PubMed] [Google Scholar]
- 41. Aaron LA, Buchwald D. Chronic diffuse musculoskeletal pain, fibromyalgia and co-morbid unexplained clinical conditions. Best Pract Res Clin Rheumatol. 2003;17:563–574. [DOI] [PubMed] [Google Scholar]
- 42. Fontaine KR, Conn L, Clauw DJ. Effects of lifestyle physical activity on perceived symptoms and physical function in adults with fibromyalgia: results of a randomized trial. Arthritis Res Ther. 2010;12:R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fontaine KR, Conn L, Clauw DJ. Effects of lifestyle physical activity in adults with fibromyalgia: results at follow-up. J Clin Rheumatol. 2011;17:64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. DeSantana JM, Walsh DM, Vance C, et al. Effectiveness of transcutaneous electrical nerve stimulation for treatment of hyperalgesia and pain. Curr Rheumatol Rep. 2008;10:492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Arnold LM, Russell IJ, Diri EW, et al. A 14-week, randomized, double-blinded, placebo-controlled monotherapy trial of pregabalin in patients with fibromyalgia. J Pain. 2008;9:792–805. [DOI] [PubMed] [Google Scholar]
- 46. Goldenberg DL, Clauw DJ, Palmer RH, et al. Durability of therapeutic response to milnacipran treatment for fibromyalgia: results of a randomized, double-blind, monotherapy 6-month extension study. Pain Med. 2010;11:180–194. [DOI] [PubMed] [Google Scholar]
- 47. Mease PJ, Clauw DJ, Gendreau RM, et al. The efficacy and safety of milnacipran for treatment of fibromyalgia: a randomized, double-blind, placebo-controlled trial [erratum in: J Rheumatol. 2009;36:661]. J Rheumatol. 2009;36:398–409. [DOI] [PubMed] [Google Scholar]
- 48. Arnold LM, Clauw DJ, Wohlreich MM, et al. Efficacy of duloxetine in patients with fibromyalgia: pooled analysis of 4 placebo-controlled clinical trials. Prim Care Companion J Clin Psychiatry. 2009;11:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Arnold LM, Williams DA, Hudson JI, et al. Development of responder definitions for fibromyalgia clinical trials. Arthritis Rheum. 2012;64:885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kahl C, Cleland J. Visual analogue scale, numeric rating scale and the McGill Pain Questionnaire: an overview of psychometric properties. Phys Ther Rev. 2005;10:123–128. [Google Scholar]
- 51. Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the brief pain inventory for chronic nonmalignant pain. J Pain. 2004;5:133–137. [DOI] [PubMed] [Google Scholar]
- 52. Bennett RM, Friend R, Jones KD, et al. The Revised Fibromyalgia Impact Questionnaire (FIQR): validation and psychometric properties [erratum in: Arthritis Res Ther. 2009;11:415]. Arthritis Res Ther. 2009;11:R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kamper SJ, Maher CG, Mackay G. Global rating of change scales: a review of strengths and weaknesses and considerations for design. J Man Manip Ther. 2009;17:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Craig CL, Marshall AL, Sjostrom M, et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. [DOI] [PubMed] [Google Scholar]
- 55. Belza BL. Comparison of self-reported fatigue in rheumatoid arthritis and controls. J Rheumatol. 1995;22:639–643. [PubMed] [Google Scholar]
- 56. Osman A, Barrios FX, Gutierrez PM, et al. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J Behav Med. 2000;23:351–365. [DOI] [PubMed] [Google Scholar]
- 57. Nicholas MK. The Pain Self-Efficacy Questionnaire: taking pain into account. Eur J Pain. 2007;11:153–163. [DOI] [PubMed] [Google Scholar]
- 58. Buysse DJ, Reynolds CF, III, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 59. McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36), III: tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. [DOI] [PubMed] [Google Scholar]
- 60. Roelofs J, Goubert L, Peters ML, et al. The Tampa Scale for Kinesiophobia: further examination of psychometric properties in patients with chronic low back pain and fibromyalgia. Eur J Pain. 2004;8:495–502. [DOI] [PubMed] [Google Scholar]
- 61. Roelofs J, Sluiter JK, Frings-Dresen MH, et al. Fear of movement and (re)injury in chronic musculoskeletal pain: evidence for an invariant two-factor model of the Tampa Scale for Kinesiophobia across pain diagnoses and Dutch, Swedish, and Canadian samples. Pain. 2007;131:181–190. [DOI] [PubMed] [Google Scholar]
- 62. Swinkels-Meewisse EJ, Swinkels RA, Verbeek AL, et al. Psychometric properties of the Tampa Scale for Kinesiophobia and the Fear-Avoidance Beliefs Questionnaire in acute low back pain. Man Ther. 2003;8:29–36. [DOI] [PubMed] [Google Scholar]
- 63. Novy DM, Simmonds MJ, Lee CE. Physical performance tasks: what are the underlying constructs? Arch Phys Med Rehabil. 2002;83:44–47. [DOI] [PubMed] [Google Scholar]
- 64. Simmonds MJ, Olson SL, Jones S, et al. Psychometric characteristics and clinical usefulness of physical performance tests in patients with low back pain. Spine (Phila Pa 1976). 1998;23:2412–2421. [DOI] [PubMed] [Google Scholar]
- 65. Tyson SF, Connell LA. How to measure balance in clinical practice: a systematic review of the psychometrics and clinical utility of measures of balance activity for neurological conditions. Clin Rehabil. 2009;23:824–840. [DOI] [PubMed] [Google Scholar]
- 66. Fernández-de-las-Peñas C, Alonso-Blanco C, Cleland JA, et al. Changes in pressure pain thresholds over C5–C6 zygapophyseal joint after a cervicothoracic junction manipulation in healthy subjects. J Manipulative Physiol Ther. 2008;31:332–337. [DOI] [PubMed] [Google Scholar]
- 67. Ge HY, Nie H, Madeleine P, et al. Contribution of the local and referred pain from active myofascial trigger points in fibromyalgia syndrome. Pain. 2009;147:233–240. [DOI] [PubMed] [Google Scholar]
- 68. Giesbrecht RJ, Battié MC. A comparison of pressure pain detection thresholds in people with chronic low back pain and volunteers without pain. Phys Ther. 2005;85:1085–1092. [PubMed] [Google Scholar]
- 69. Hirayama J, Yamagata M, Ogata S, et al. Relationship between low-back pain, muscle spasm and pressure pain thresholds in patients with lumbar disc herniation. Eur Spine J. 2006;15:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ruiz-Sáez M, Fernández-de-las-Peñas C, Blanco CR, et al. Changes in pressure pain sensitivity in latent myofascial trigger points in the upper trapezius muscle after a cervical spine manipulation in pain-free subjects. J Manipulative Physiol Ther. 2007;30:578–583. [DOI] [PubMed] [Google Scholar]
- 71. Carpenter J, Kenward MG. Multiple Imputation and Its Application. West Sussex, United Kingdom: John Wiley & Sons Ltd; 2013. [Google Scholar]
- 72. Molenberghs G, Kenward MG. Missing Data in Clinical Studies. West Sussex, United Kingdom: John Wiley & Sons Ltd; 2007. [Google Scholar]