Abstract
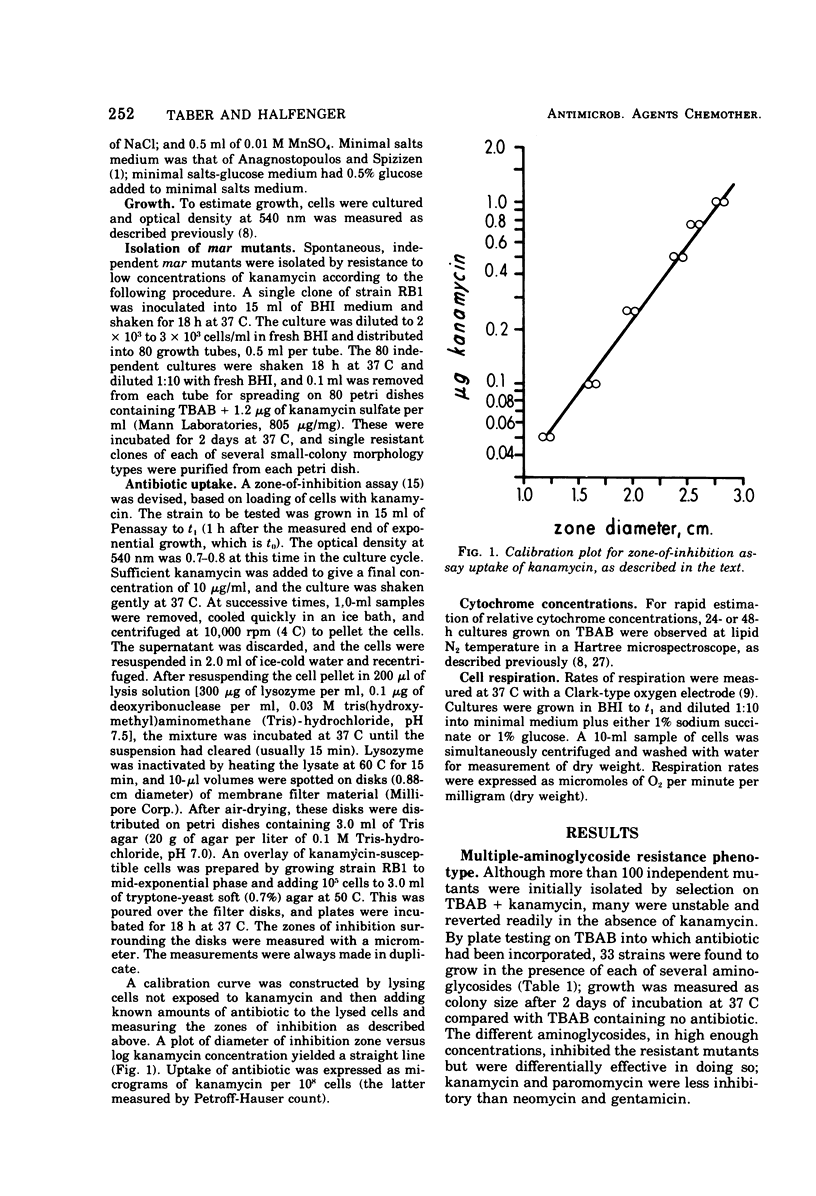
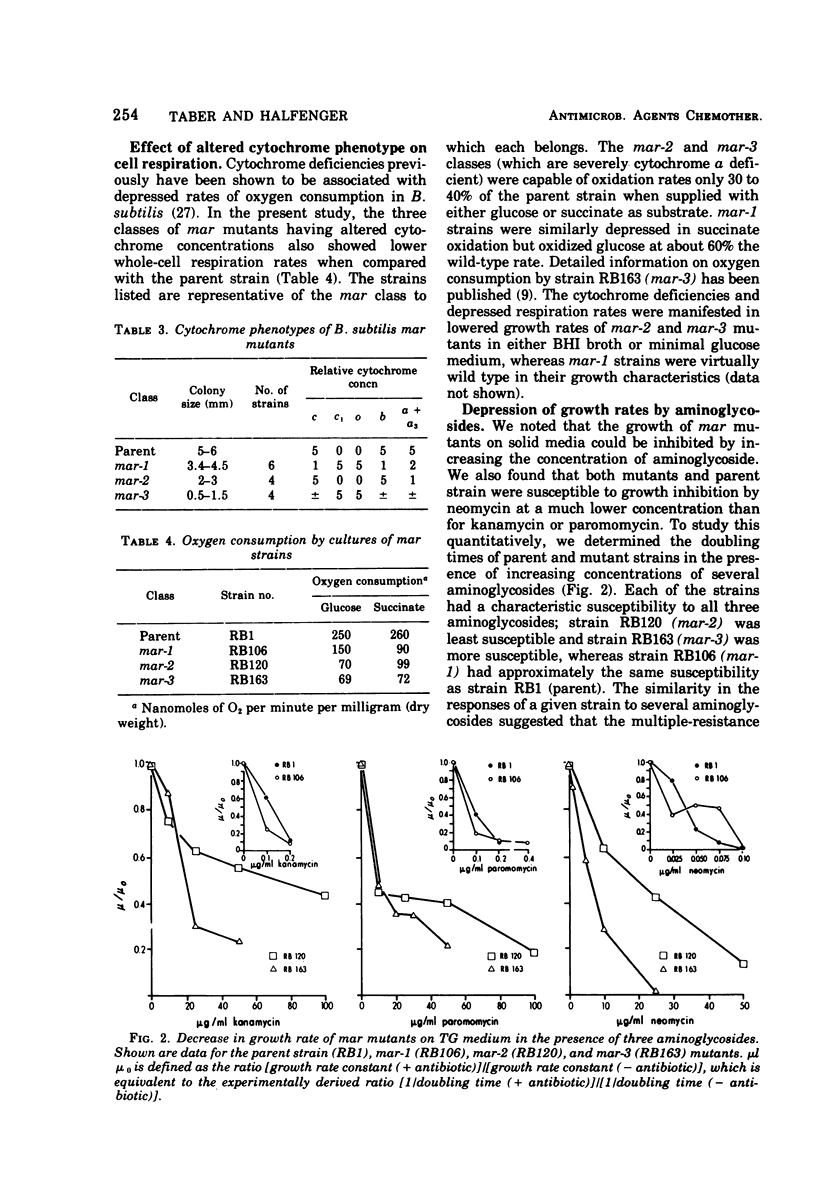
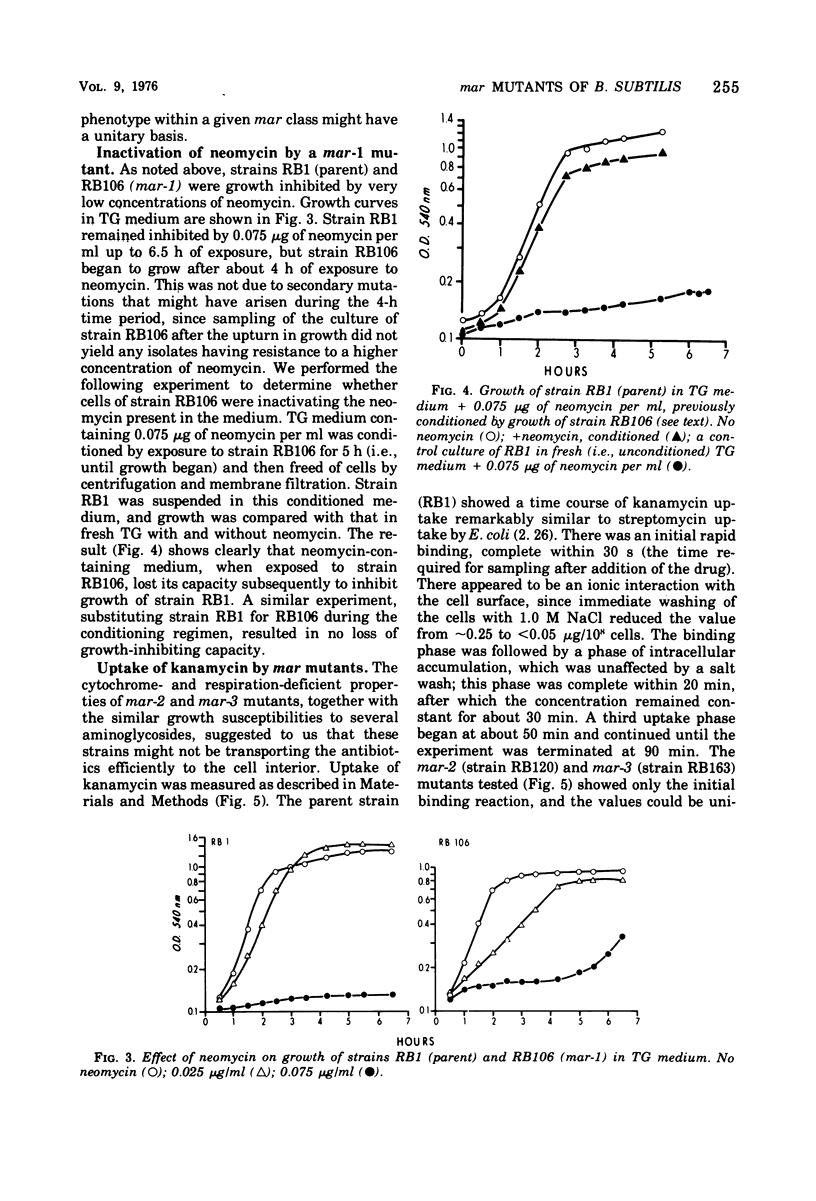
Three classes of spontaneous multiple-aminoglycoside-resistant (mar) mutants of Bacillus subtilis were isolated by plating on a low (1.2 μg/ml) concentration of kanamycin sulfate and were found to be resistant also to low concentrations of paromomycin, neomycin and gentamicin. The three classes could be distinguished one from another by their degree of cytochrome deficiency, respiration deficiency, and susceptibility to kanamycin lethality. A fluctuation test showed that the mutations were spontaneous and not induced by the conditions of selection. Representative strains from two classes of mutants (mar-2 and mar-3) accumulated aminoglycoside very poorly in comparison with the parent strain, whereas a strain of the third class (mar-1) inactivated aminoglycoside present in the growth medium. The mar-3 strain studied (aroD163) had previously been shown to be a menaquinone auxotroph (Farrand and Taber, 1973) and to be deficient in amino acid uptake (Bisschop et al., 1975). Such mutants, which are resistant to low concentrations of aminoglycosides, may be of use in elucidating the biochemical and genetic bases of certain bacterial transport systems.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANAND N., DAVIS B. D., ARMITAGE A. K. Uptake of streptomycin by Escherichia coli. Nature. 1960 Jan 2;185:23–24. doi: 10.1038/185023a0. [DOI] [PubMed] [Google Scholar]
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R., Davies J. Mechanisms of antibiotic resistance in bacteria. Annu Rev Biochem. 1973;42:471–506. doi: 10.1146/annurev.bi.42.070173.002351. [DOI] [PubMed] [Google Scholar]
- Bisschop A., de Jong L., Lima Costa M. E., Konings W. N. Relation between reduced nicotinamide adenine dinucleotide oxidation and amino acid transport in membrane vesicles from Bacillus subtilis. J Bacteriol. 1975 Mar;121(3):807–813. doi: 10.1128/jb.121.3.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. E., Rownd R. Transmissible multiple drug resistance in Enterobacteriaceae. Science. 1972 May 19;176(4036):758–768. doi: 10.1126/science.176.4036.758. [DOI] [PubMed] [Google Scholar]
- Farrand S. K., Taber H. W. Physiological effects of menaquinone deficiency in Bacillus subtilis. J Bacteriol. 1973 Sep;115(3):1035–1044. doi: 10.1128/jb.115.3.1035-1044.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrand S. K., Taber H. W. Pleiotropic menaquinone-deficient mutant of Bacillus subtilis. J Bacteriol. 1973 Sep;115(3):1021–1034. doi: 10.1128/jb.115.3.1021-1034.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T. J. Resistance of Escherichia coli to tetracyclines. Changes in permeability to tetracyclines in Escherichia coli bearing transferable resistance factors. Biochem J. 1967 Oct;105(1):371–378. doi: 10.1042/bj1050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funatsu G., Wittmann H. G. Ribosomal proteins. 33. Location of amino-acid replacements in protein S12 isolated from Escherichia coli mutants resistant to streptomycin. J Mol Biol. 1972 Jul 28;68(3):547–550. doi: 10.1016/0022-2836(72)90108-8. [DOI] [PubMed] [Google Scholar]
- HANCOCK R. Uptake of 14C-streptomycin by some microorganisms and its relation to their streptomycin sensitivity. J Gen Microbiol. 1962 Jul;28:493–501. doi: 10.1099/00221287-28-3-493. [DOI] [PubMed] [Google Scholar]
- HUMPHREY J. H., LIGHTBOWN J. W. A general theory for plate assay of antibiotics with some practical applications. J Gen Microbiol. 1952 Aug;7(1-2):129–143. doi: 10.1099/00221287-7-1-2-129. [DOI] [PubMed] [Google Scholar]
- Izaki K., Kiuchi K., Arima K. Specificity and mechanism of tetracycline resistance in a multiple drug resistant strain of Escherichia coli. J Bacteriol. 1966 Feb;91(2):628–633. doi: 10.1128/jb.91.2.628-633.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOGUT M., LIGHTBROWN J. W., ISAACSON P. STREPTOMYCIN ACTION AND ANAEROBIOSIS. J Gen Microbiol. 1965 May;39:155–164. doi: 10.1099/00221287-39-2-155. [DOI] [PubMed] [Google Scholar]
- Levy S. B., McMurry L. Detection of an inducible membrane protein associated with R-factor-mediated tetracycline resistance. Biochem Biophys Res Commun. 1974 Feb 27;56(4):1060–1068. doi: 10.1016/s0006-291x(74)80296-2. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjai E., Kiss I., Ivánovics G. Auxotrophic mutation associated with low streptomycin resistance and slow growth in Bacillus subtilis. Acta Microbiol Acad Sci Hung. 1970;17(2):133–145. [PubMed] [Google Scholar]
- Okamoto S., Suzuki Y. Chloramphenicol-, dihydrostreptomycin-, and kanamycin-inactivating enzymes from multiple drug-resistant Escherichia coli carrying episome 'R'. Nature. 1965 Dec 25;208(5017):1301–1303. doi: 10.1038/2081301a0. [DOI] [PubMed] [Google Scholar]
- Price K. E., Godfrey J. C. Effect of structural modifications on the biological properties of aminoglycoside antibiotics containing 2-deoxystreptamine. Adv Appl Microbiol. 1974;18(0):191–307. doi: 10.1016/s0065-2164(08)70572-0. [DOI] [PubMed] [Google Scholar]
- Sakai T. T., Cohen S. S. Interrelation between guanosine tetraphosphate accumulation, ribonucleic acid synthesis, and streptomycin lethality in Escherichia coli CP78. Antimicrob Agents Chemother. 1975 Jun;7(6):730–735. doi: 10.1128/aac.7.6.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompolinsky D., Krawitz T., Zaidenzaig Y., Abramova N. Inducible resistance to tetracycline in Staphylococcus aureus. J Gen Microbiol. 1970 Aug;62(3):341–349. doi: 10.1099/00221287-62-3-341. [DOI] [PubMed] [Google Scholar]
- Staal S. P., Hoch J. A. Conditional dihydrostreptomycin resistance in Bacillus subtilis. J Bacteriol. 1972 Apr;110(1):202–207. doi: 10.1128/jb.110.1.202-207.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szögyi M., Tamás G., Tarján I. Uptake of streptomycin by Escherichia coli B. Acta Biochim Biophys Acad Sci Hung. 1969;4(4):415–419. [PubMed] [Google Scholar]
- Taber H., Freese E. Sporulation properties of cytochrome a-deficient mutants of Bacillus subtilis. J Bacteriol. 1974 Dec;120(3):1004–1011. doi: 10.1128/jb.120.3.1004-1011.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber H. Isolation and properties of cytochrome a deficient mutants of Bacillus subtilis. J Gen Microbiol. 1974 Apr;81(2):435–444. doi: 10.1099/00221287-81-2-435. [DOI] [PubMed] [Google Scholar]
- Tien W., White D. C. Linear sequential arrangement of genes for the biosynthetic pathway of protoheme in Staphylococcus aureus. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1392–1398. doi: 10.1073/pnas.61.4.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa H., Okanishi M., Kondo S., Hamana K., Utahara R., Maeda K., Mitsuhashi S. Phosphorylative inactivation of aminoglycosidic antibiotics by Escherichia coli carrying R factor. Science. 1967 Sep 29;157(3796):1559–1561. [PubMed] [Google Scholar]



