Abstract
Glioblastoma multiforme (GBM) is the most lethal and aggressive astrocytoma of primary brain tumors in adults. Although there are many clinical trials to induce the cell death of glioblastoma cells, most glioblastoma cells have been reported to be resistant to TRAIL-induced apoptosis. Here, we showed that gingerol as a major component of ginger can induce TRAIL-mediated apoptosis of glioblastoma. Gingerol increased death receptor (DR) 5 levels in a p53-dependent manner. Furthermore, gingerol decreased the expression level of anti-apoptotic proteins (survivin, c-FLIP, Bcl-2, and XIAP) and increased pro-apoptotic protein, Bax and truncate Bid, by generating reactive oxygen species (ROS).We also found that the sensitizing effects of gingerol in TRAIL-induced cell death were blocked by scavenging ROS or overexpressing anti-apoptotic protein (Bcl-2). Therefore, we showed the functions of gingerol as a sensitizing agent to induce cell death of TRAIL-resistant glioblastoma cells. This study gives rise to the possibility of applying gingerol as an anti-tumor agent that can be used for the purpose of combination treatment with TRAIL in TRAIL-resistant glioblastoma tumor therapy.
Keywords: Gingerol, Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), Glioblastoma, Apoptosis, Reactive oxygen species (ROS), p53
Introduction
Glioblastoma multiforme (GBM) is classified as a grade IV astrocytoma by the World Health Organization (WHO) and is a very aggressive malignant astrocytoma that makes up approximately 50% of all astrocytomas (Fuller, 2008; Ohgaki and Kleihues, 2005). As it is known from its name, GBM has morphologically multiple heterogeneous populations (Krakstad and Chekenya, 2010). Although there have been many radiotherapeutic and chemotherapeutic clinical trials to treat glioblastoma, prognosis of glioblastoma patients is very poor and the median survival rate is about 14.6 months (Stupp et al., 2005). Recently, combination therapies such as cocktail treatments that use more than 2 different anti-cancer drugs have been tried to increase the efficacy and survival rate (Doherty et al., 2006; Goudar et al., 2005; Rao et al., 2005; Reardon et al., 2006). For example, drugs that target both survival pathway and apoptotic pathway have simultaneously been used to improve the survival rate for GBM patients (Hawkins, 2004; Krakstad and Chekenya, 2010).
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) has been well known to mediate cellular apoptosis in a wide-range of tumor cell types (Aggarwal, 2003; Pitti et al., 1996). TRAIL binds to its receptor (death receptor (DR) 4/5) to induce receptor trimerization that can recruit downstream molecules such as Fas-associated protein with death domain (FADD) and eventually activate caspase cascade (caspases-8, 10, 9, and 3) to transmit cell death signaling (Aggarwal, 2003; Bellail et al., 2010). However, it has been reported that most glioblastoma cells showed resistance to apoptosis mediated by the TRAIL signaling pathway (Krakstad and Chekenya, 2010).
Gingerol, as a major pungent element of ginger, has been reported to exhibit anti-oxidant, analgesic, anti-pyretic, anti-inflammatory, and anti-tumorigenic activities (Oyagbemi et al., 2010; Shukla and Singh, 2007). Gingerol has also been known to show anti-inflammatory potential by decreasing the expression level of inducible nitric oxide synthase (iNOS) and TNF-α (D.H. Lee et al., 2009; T.Y. Lee et al., 2009). Furthermore, the anti-tumorigenic effects of gingerol have been known to be exerted by the induction of apoptosis of tumor cells (Bode et al., 2001; Chakraborty et al., 2012; Lee and Surh, 1998). However, the detailed molecular mechanism of gingerol-induced apoptosis is still not clear.
Here, we identified that gingerol functions as a sensitizing agent to induce TRAIL-mediated apoptosis of glioblastoma cells which were resistant to apoptosis by TRAIL signaling. Especially, in non-cytotoxic concentrations gingerol efficiently induced cell death by TRAIL in glioblastoma cell lines. Furthermore, we revealed that the sensitizing function of gingerol was performed by elevating the expression level of death receptor (DR) 5, by decreasing the expression of anti-apoptotic proteins (survivin, c-FLIP, Bcl-2, and XIAP) and by inducing the levels of pro-apoptotic proteins (Bax and truncate Bid) in a p53- and reactive oxygen species (ROS)-dependent manner. Our effort in identifying gingerol as the agent that sensitizes TRAIL-mediated apoptosis in glioblastoma and understanding the molecular mechanisms of gingerol-sensitization provides us which an opportunity to make more effective drug combination therapies which are non-toxic to GBM patients.
Materials and methods
Cell culture
Human glioma U87, U343, and T98G, human prostate carcinoma LNCaP cells, human breast carcinoma MCF-7 cells, human liver carcinoma HepG2 cells and human cervical carcinoma HeLa cells were purchased from American Tissue Type Culture Collection (Manassas, VA, USA). Cells were cultured in DMEM and RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) with 10% fetal bovine serum (HyClone, Logan, UT, USA), 1 mM L-glutamine, and 26 mM sodium bicarbonate for monolayer cell culture. Primary cultures of human astrocyte cells (Cryo NHMC) and their corresponding growth medium (CC-3146 MsGM) were purchased from Clonetics (San Diego, California, USA). p53-containing (p53+/+) and p53-deficient (p53−/−) HCT116 human colon carcinoma cell lines were kindly provided by Dr. Bert Vogelstein (Johns Hopkins University, Baltimore, MD, USA). These cell lines were cultured in McCoy's 5A medium (Gibco-BRL, Gaithersburg, MD, USA) containing 10% fetal bovine serum and antibiotics. The dishes containing cells were kept in a 37 °C humidified incubator with 5% CO2.
Reagents and antibodies
6-Gingerol was purchased from LKT Laboratories (St. Louis, MO, USA). Treatments of drugs were accomplished by aspirating the medium and replacing it with medium containing these drugs. Soluble human recombinant SuperKillerTRAIL (referred to as TRAIL in this manuscript) was purchased from Enzo Biochemicals (Enzo Life Sciences), diluted, and stored in KillerTRAIL storage and dilution buffer (Enzo Life Sciences). 6-Carboxy-2′,7′-dichlorofluorescein diacetate (H2DCF-DA) and dihydroethidium (DHE) were from Molecular Probe. N-acetylcysteine was from Sigma. Anti-c-FLIP, anti-Bax, anti-Bcl-2, and anti-p53 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-XIAP, anti-survivin, anti-Bid, anti-phospho ERK and anti-ERK, anti-phospho-p38, anti-p38, anti-cleaved caspase-3, anti-caspase-8, and anti-PARP-1 were purchased from Cell Signaling (Beverly, MA, USA). Anti-phospho JNK and anti-JNK were purchased from Upstate Biotechnology (Lake Placid, NY, USA). Anti-Flag M2 were purchased from Sigma (USA). Anti-DR4 and anti-DR5 were purchased from R&D Systems (Plymouth Metting, PA, USA). Anti-actin antibody was purchased from MP Biomedicals (Solon, OH, USA). For the secondary antibodies, anti-mouse-IgG-HRP and anti-rabbit-IgG-HRP were purchased from Santa Cruz Biotechnology.
Determination of cell viability and cytotoxicity assay
To determine the effect of gingerol on cell death, the trypan blue dye exclusion assay was used, as described previously (D.H. Lee et al., 2009; T.Y. Lee et al., 2009). Briefly, one or two days prior to the experiment, cells were plated into 60-mm dishes at a density of 1 × 105 cells/plate in 5 ml tissue culture medium in triplicate. For the trypan blue exclusion assay, trypsinized cells were pelleted and resuspended in 0.2 ml of medium, 0.5 ml of 0.4% trypan blue solution and 0.3 ml of phosphate-buffered saline solution (PBS). The samples were mixed thoroughly, incubated at room temperature for 15 min, and examined under a light microscope. At least 300 cells were counted for each survival determination. The LDH assay and DNA fragmentation were performed using the Cytoxicity Detection kit and DNA fragmentation ELISA kit (Roche, Mannheim, Germany) according to the manufacturer's instructions.
Measurement of reactive oxygen species
ROS generation was measured after staining the cells with 5-(and-6)-carboxy-2′,7′ dichlorodihydro-fluorescein diacetate (H2DCFDA) and dihydroethidium (DHE). U87 cells were plated at a density of 5 × 105 in 60-mm dishes, allowed to attach overnight, and exposed to 10 mM N-acetylcysteine (Lluis et al., 2010) alone, 25 μM gingerol alone, or NAC plus gingerol for specified time intervals. Samples were kept on ice at the end of the incubation period. The cells were stained with 10 μM H2DCFDA and dihydroethidium (DHE) for 40 min at 37 °C and then observed under a fluorescence microscope (Axiovert 200M; Carl Zeiss).
Bcl-2 constructs and stable transfection
The human Bcl-2 cDNA fragment was digested from pCMV-Flag-Bcl-2 (Addgene) and the myc-DR5 expression plasmid (pcDNA3-myc-DR5), which was kindly provided by Dr. Y.J.Lee (University of Pittsburgh, PA, USA). The Bcl-2 cDNA fragment was digested with KpnI and XhoI and subcloned into the pcDNA 3.1 vector (Invitrogen, Carlsbad, CA, USA) and termed pcDNA 3.1-Bcl-2. The U87 cells were transfected in a stable manner with the pcDNA 3.1-Bcl-2 plasmid or the control plasmid pcDNA 3.1 using Lipofectamine as prescribed by the manufacturer (Invitrogen). After48 h of incubation, transfected cells were selected in primary cell culture medium containing 800 μg/ml G418 (Invitrogen). After 2 or 3 weeks, to rule out the possibility of clonal differences between the generated stable cell lines, the pooled U87/pcDNA 3.1 and U87/Bcl-2 clones were tested for Bcl-2 expression by immunoblotting and were used in this study.
Small interfering RNA (siRNA)
Bcl-2 siRNA (Cat. No. SC-29214) and negative control siRNA (Cat. No. SC-37007) were obtained from Santa Cruz Biotechnology. Cells were transfected with siRNA oligonucleotides using Lipofectamine RNAi Max reagents (Invitrogen) according to the manufacturer's recommendations.
Analysis of cell surface DR4 and DR5
Indirect staining with primary rabbit anti-human DR4 or DR5 followed by FITC-conjugated IgG was used to analyze cells for the surface expression of DR4 and DR5. In brief, cells were detached with Trypsin-EDTA and washed three times with PBS wash buffer supplemented with 0.5% BSA. Cells were resuspended in 200 μl PBS, stained with primary antibody (1 μg/ml), and incubated for 30 min at 4 °C. Unreacted antibody was removed by washing the cells twice with PBS buffer. Cells were stained with secondary antibody conjugated with fluorescein isothiocyanate (FITC) and incubated for 30 min at 4 °C. Unbound FITC-conjugated antibody was washed twice with PBS. Cells were resuspended in 200 μl PBS. Surface expression of DR4 and DR5 were determined by flow cytometry. Fluorescence intensity of the cells was directly proportional to the density of receptor.
Immunofluorescent staining
U87 cells were either treated or not with 25 μM gingerol for 24 h, stained with antibodies against DR5 and processed for fluorescence microscopy. In brief, cells were fixed with 4% paraformaldehyde and incubated with monoclonal antibodies recognizing DR5 (Alexis Biochemicals, San Diego, CA, USA) 1 : 200 in PBS containing 0.1% Tween 20 and 5 mg/ml BSA (PBST/BSA) followed by Alexa Fluor 488-conjugated anti-mouse IgG (Invitrogen, Carlsbad, CA, USA; 1 : 200 in PBST/BSA). Visualization was performed with Alexa fluorophore-conjugated secondary antibodies (1:1,000; Molecular Probes, Inc., Eugene, OR, USA).
Staining with AnnexinV-FITC analysis
Induction of apoptosis was assessed by the binding of Annexin V to phosphatidylserine, which is externalized to the outer leaflet of the plasma membrane early on during induction of apoptosis. Briefly, U87 cells untreated or treated with gingerol, TRAIL, or a combination of the two agents were resuspended for 24 h in the binding buffer provided in the Annexin V-FITC Detection Kit II (BD Biosciences Pharmingen, San Diego, CA, USA). Cells were mixed with 5 μL Annexin V-FITC reagent and incubated for 30 min at room temperature in the dark. Stained cells were analyzed by fluorescent-activated cell sorting on a FACS can flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA).
Immunoblot analysis
Proteins were separated by SDS-PAGE and electrophoretically transferred to a nitrocellulose membrane. The nitrocellulose membrane was blocked with 5% nonfat dry milk in PBS–Tween-20 (0.1%, v/v) at 4 °C overnight. The membrane was incubated with primary antibody (diluted according to the manufacturer's instructions) for 2 h. Horseradish peroxidase conjugated anti-rabbit or anti-mouse IgG was used as the secondary antibody. Immunoreactive proteins were visualized by the chemiluminescence protocol (ECL, Amersham, Arlington Heights, IL, USA). ImageJ software (NIH) was used for quantification of intensities of western blot bands.
Caspase-3/7 assay
Caspase 3/7 activities were measured on untreated and drug-treated cells using the caspase Glo-3/7 assay kit (Promega). Briefly, 5 × 103 cells were plated in a white-walled 96-well plate, and the Z-DEVD reagent, the luminogenic caspase 3/7 substrate, containing a tetrapeptide Asp–Glu–Val–Asp, was added in a 1:1 ratio of reagent to sample. After 60 min at room temperature, the substrate cleavage by activated caspases-3 and -7, and the intensity of a luminescent signal, was measured using a Fusion-α plate reader (PerkinElmer). Differences in caspase-3/7 activity in drug-treated cells compared with untreated cells are expressed as fold-change in luminescence.
Statistical analysis
Statistical analysis was carried out using GraphPad InStat 5 software (GraphPad Software, Inc., San Diego, CA, USA). The results were expressed as the mean of arbitrary values ± SEM. All results were evaluated using an unpaired Student's t test, where a p-value of less than 0.05 was considered significant.
Results
Gingerol can sensitize TRAIL-induced apoptosis in U87 glioblastoma cells
Previously, gingerol has been reported to induce apoptosis of several cell types such as human cervical cancer cells (HeLa), human promyelocytic leukemia cells (HL-60), and human colorectal carcinoma cells (HCT-116, SW480, and LoVo) (Chakraborty et al., 2012; Lee and Surh, 1998; Lee et al., 2008). However, these studies have used high doses of gingerol (200–500 μM) to induce cell death of each cell type and do not show the significant effects in cell growth arrest and apoptosis which are produced by using low doses of gingerol (20–25 μM) (Chakraborty et al., 2012; Lee and Surh, 1998; Lee et al., 2008). Prior to investigating the effect of combined treatment with gingerol and TRAIL on cell viability in U87 cells, we examined whether gingerol alone induces cytotoxicity. Cells were treated with various concentrations (10–100 μM) of gingerol for 24 h. As shown in Figs. 1A and B, gingerol induced cytotoxicity in a dose-dependent manner. Next, we examined the effect of gingerol (25 μM) in combination with 50 ng/ml TRAIL on cell viability (Figs. 1C, D, E). Single treatment of TRAIL and gingerol did not affect cell viability and did not induce apoptosis (annexin V (+) and PI (+)) in U87 glioblastoma cells (Figs. 1A, E, G). Also, they were no morphological changes of U87 cells compared to the control treatment (Fig. 1F). However, co-treatment of TRAIL and gingerol reduced cell viability and significantly increased apoptosis in U87 glioblastoma cells. Furthermore, these results were confirmed by intracellular apoptosis indicators (cleaved caspases-3 and -8, and PARP-1) (Fig. 1H). Therefore, these results indicate that in non-cytotoxic doses (25 μM), gingerol can increase TRAIL-induced apoptosis in TRAIL-resistant U87 glioblastoma cells.
Fig. 1.
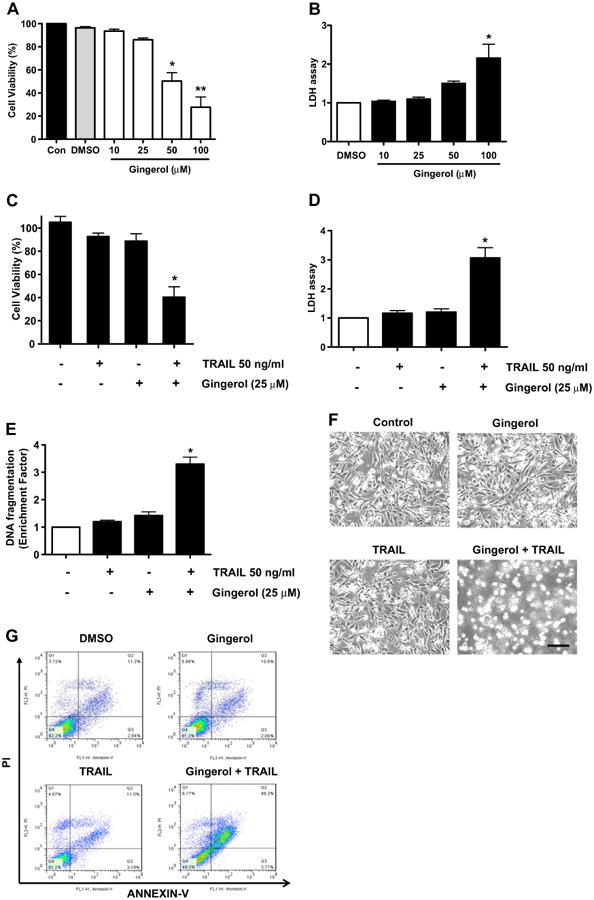
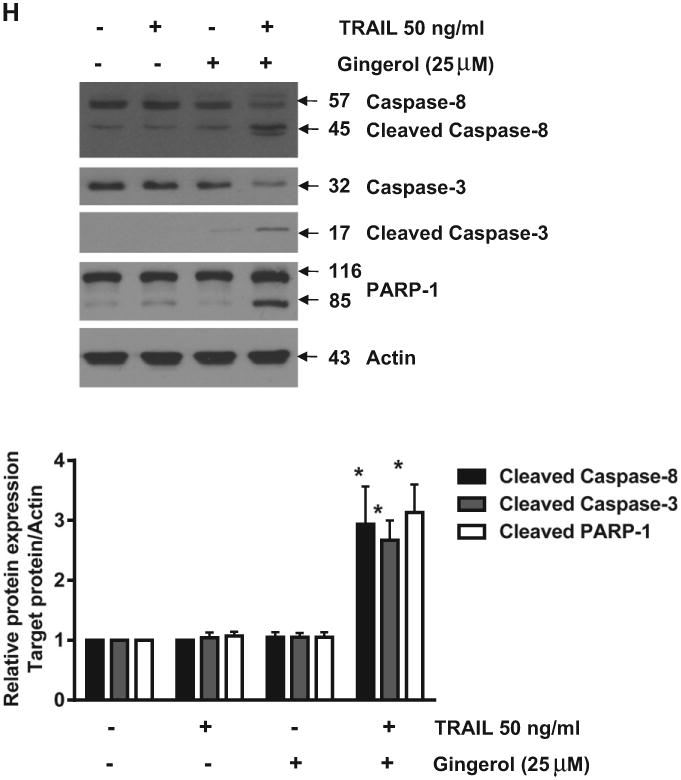
Sensitizing effect of gingerol in TRAIL-induced apoptosis of U87 glioblastoma cells. (A and B) Cells were treated with DMSO (sham control) or various concentrations (10–100μM) of gingerol for 24 h. (C, D, and E) Cells were incubated in the presence or absence of TRAIL (50 ng/ml) and/or gingerol (25 μM) for 24 h. (F) Microscopic cell morphologies. Scale bar: 100 μm. (G) The cells were stained with annexin V and propidium iodide (PI), followed by FACS analysis. (H) The cell lysates were analyzed by western blotting using indicated antibodies. Data is presented as arbitrary values and results are expressed as the mean ± SEM. The effects of nicotine were determined using a Student's unpaired t-test. Asterisk * represents statistically significantly difference between control and gingerol-treated cells at p < 0.05.
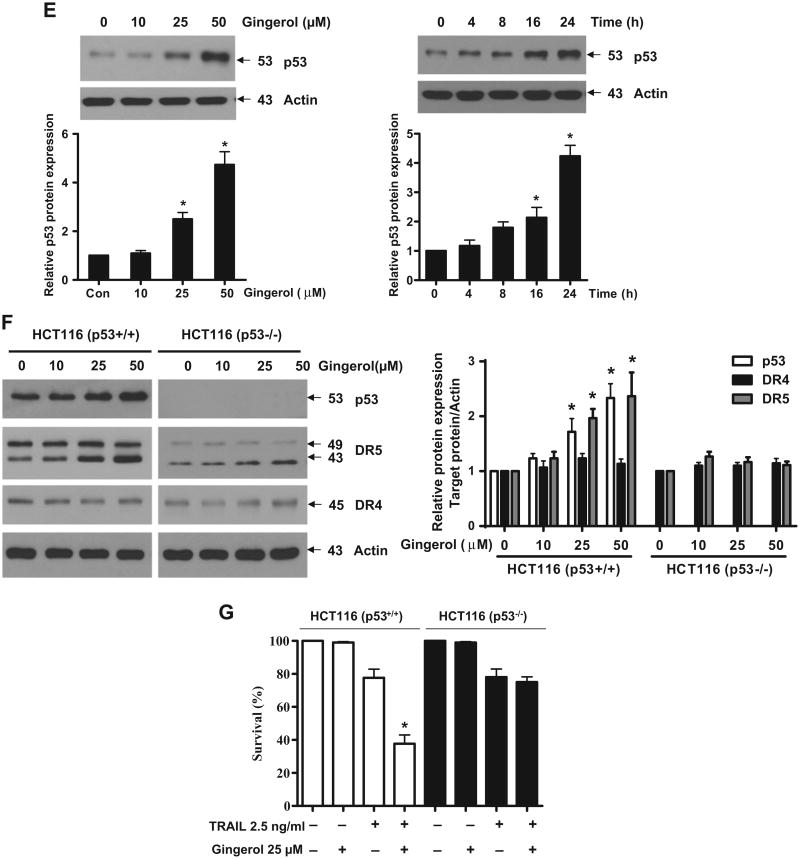
Gingerol can increase the expression of death receptors (DR) 5 in a p53-dependent manner
Next, we investigated how gingerol can sensitize the effect of TRAIL in U87 cells. Death receptors (DR) 4 and 5 are well known as TRAIL receptors (de Wilt et al., 2013; Wang and El-Deiry, 2003). First, we checked the expression level of DR4 and DR5. As shown in Fig. 2A, gingerol elevated the expression of DR5 and not DR4 in a dose- and time-dependent manner. FACS and immunocytochemical analyses also showed the increase of DR5 but not DR4 by treatment of gingerol in U87 glioblastoma cells (Fig. 2B). The same effect (elevation of DR5) of gingerol is also shown in the tumor cell lines of prostate cancer, breast cancer, liver cancer, and cervical cancer (Fig. 2C). To characterize the functional significance of DR5 upregulation in TRAIL-induced apoptosis, we employed a myc-DR5 expression vector (Myc-DR5) to induce ectopic expression of DR5 in U87 cells. As shown in Fig. 2D, Myc-DR5-induced overexpression of DR5 in U87 cells enhanced TRAIL-induced apoptosis compared with cells transfected with pcDNA. Recently, it has been reported that p53 can regulate DR5 expression but not DR4 in myeloma cells (Surget et al., 2012). Thus, we checked the p53 dependency in DR5 expression by gingerol in U87 cells. As shown in Fig. 2E, gingerol increased p53 expression in a dose- and time-dependent manner. To figure out the p53 dependency, we investigated the level of DR5 in colorectal cancer cell lines (HCT116 (p53+/+) and (p53−/−)) (Bunz et al., 1998; Waldman et al., 1995). Although DR4 expression did not change in both cell types, gingerol induced DR5 expression in HCT116 (p53+/+) cells but not in HCT116 (p53−/−) cells (Fig. 2F). We also found that gingerol synergized with TRAIL to increase cytotoxicity in HCT116 p53+/+, but not in HCT116 p53−/− cells (Fig. 2G). Therefore, these data suggest that the sensitizing effect of gingerol could be exerted by elevation of DR5 in a p53 dependent manner and gingerol might sensitize the effect of TRAIL-induced apoptosis in other cancer cell types as well.
Fig. 2.
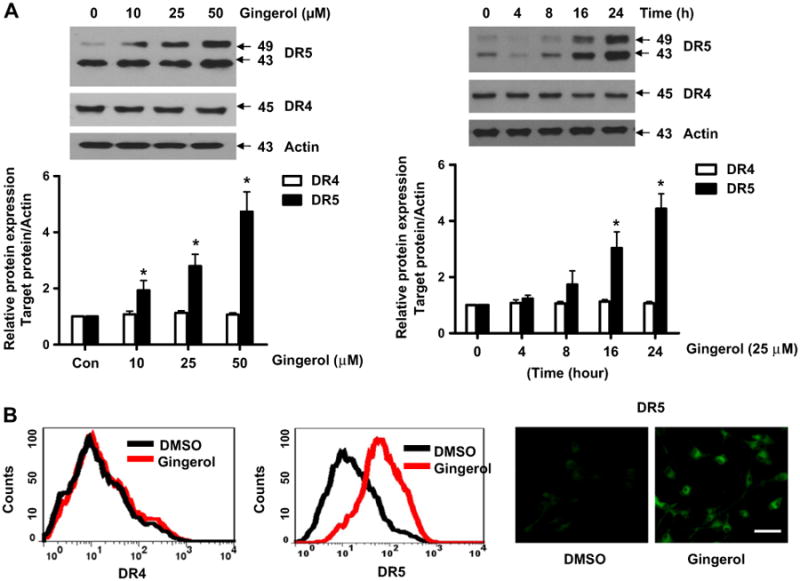
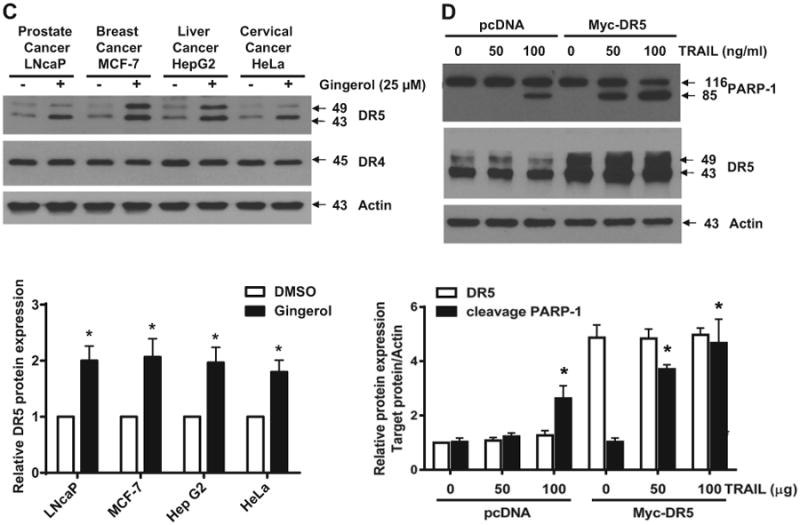
Gingerol can regulate the expression of death receptors (DR) 5, but not DR4. (A) U87 cells were treated with indicated gingerol doses (0, 10, 25, 50 μM) for 24 h or 25 μM gingerol at an indicated time (0, 4, 8, 16, 24 h). Western blotting analysis was performed with DR4 and DR5 antibodies. (B) U87 cells were incubated with DMSO or gingerol (25 μM) for 24 h and stained with DR4 and DR5 antibodies, followed by FACS analysis (left panels) and immunocytochemistry (right panels). Scale bar: 100 μm. (C) The indicated cell types were incubated with DMSO or gingerol (25 μM) for 24 h. The cell lysates were analyzed by western blotting using DR4 and DR5 antibodies. (D) U87 cells were transfected with Myc-DR5 plasmid or pcDNA (control). After treatment with TRAIL for 4 h, western blot analysis demonstrated that Myc-DR5-induced DR5 overexpression increased apoptosis compared with pcDNA. (E) U87 cells were incubated with indicated gingerol doses (0, 10, 25, 50 μM) for 24 h or 25 μM gingerol at an indicated time (0, 4, 8, 16, 24 h) and then analyzed by western blotting using p53 antibody. (F) HCT116 (p53+/+) and HCT116 (p53−/−) cells were incubated with indicated gingerol (0, 10, 25, 50 μM) for 24 h. The cell lysates were analyzed by western blotting using p53, DR4, and DR5 antibodies. Data is presented as arbitrary values and results are expressed as the mean ± SEM. The effects of nicotine were determined using a Student's unpaired t-test. *p < 0.05 vs control. (G) Cell viability was determined using the trypan blue dye exclusion assay. Error bars represent the mean ± SE from six separate experiments. *Significant difference between TRAIL and TRAIL + gingerol-treated cells at p < 0.05. These results are representative of data obtained from at least five independent experiments.
Gingerol can regulate pro-apoptotic and anti-apoptotic signaling pathways in glioblastoma cells
Binding of TRAIL to death receptors has been known to lead to the activation of the apoptotic signaling pathway through cleavage of caspases and activation of pro-apoptotic proteins (Bax and Bak) (Manzo et al., 2009; Wu, 2009). Therefore, we investigated whether gingerol could affect the apoptotic pathway to show the sensitizing effect for apoptosis in glioblastoma cells. As shown in Fig. 3A, as the concentration of gingerol increased, the concentration of pro-apoptotic protein, Bax, and Bid dramatically decreased and levels of anti-apoptotic proteins such as XIAP, survivin, c-FLIP, and Bcl-2 were significantly reduced in U87 glioblastoma cells. In addition, phosphorylation of p38 and JNK, which are known to mediate apoptosis, was also increased by gingerol on U87 glioblastoma cells (Fig. 3B). Furthermore, when we checked the effect of gingerol in other glioblastoma cell lines, we found that gingerol can reduce anti-apoptotic protein Bcl-2 in U343 and T98G (Fig. 3C). These results indicate that the sensitizing effect of gingerol could be exerted by regulating pro-and the anti-apoptotic signaling pathways in TRAIL-resistant glioblastoma cells.
Fig. 3.
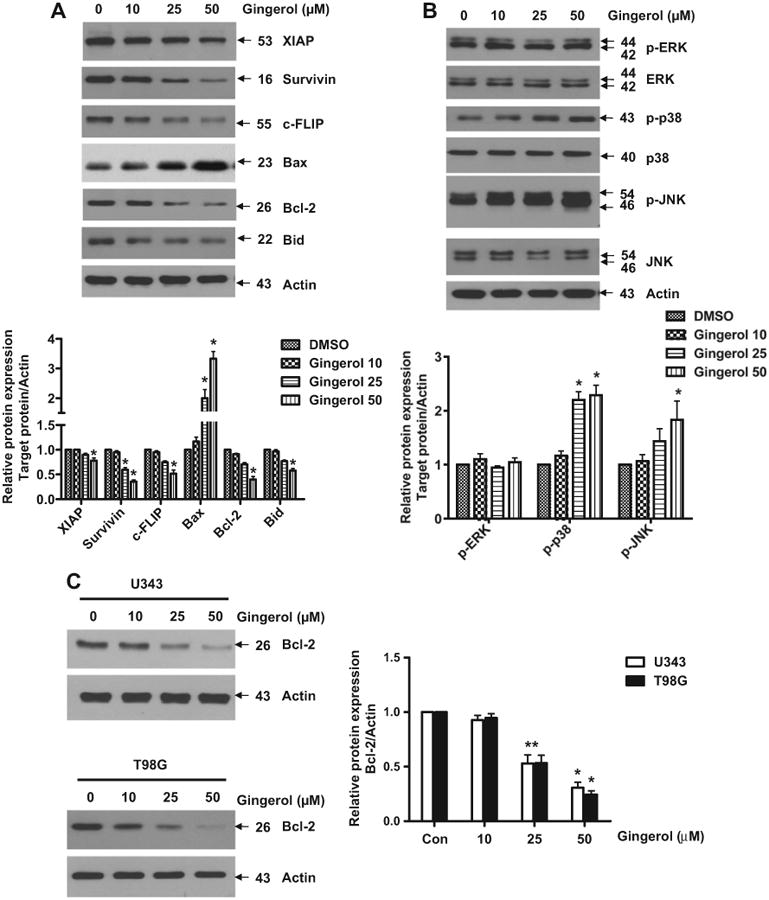
Modulation of gingerol in the pro-apoptotic and anti-apoptotic signaling pathways in glioblastoma cells. U87 cells were treated with indicated gingerol doses (0, 10, 25, 50 μM) for 24 h. (A) Western blotting analysis was done by using indicated antibodies (XIAP, Survivin, c-FLIP, Bax, Bcl-2, and Bid). (B) Phosphorylation of MAP kinases was analyzed by western blotting using phosphorylation-specific antibodies (p-ERK, p-p38, and p-JNK). (C) U343 and T98G cells were treated with indicated gingerol doses (0, 10, 25, 50 μM) for 24 h, followed by western blotting using Bcl-2 antibody. Data is presented as arbitrary values and results are expressed as the mean ± SEM. The effects of nicotine were determined using a Student's unpaired t-test. *p < 0.05 vs control. These results are representative of data obtained from at least five independent experiments.
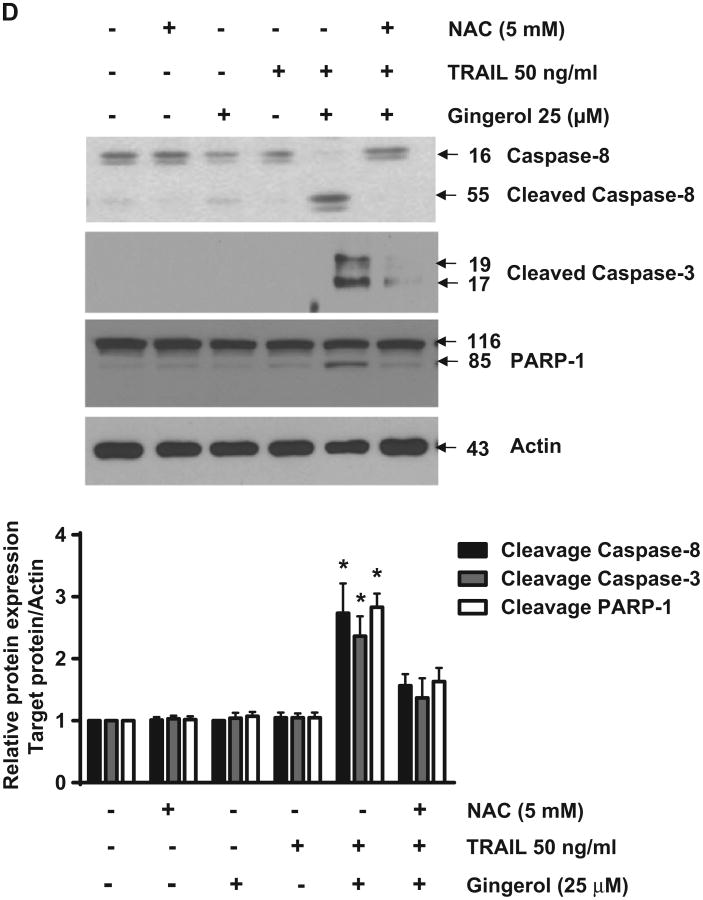
Reactive oxygen species (ROS) generation by gingerol can sensitize the pro-apoptotic signaling of TRAIL in U87 glioblastoma cells
Recently, gingerol has been reported to increase intracellular reactive oxygen species (ROS) in HepG2 cells (Yang et al., 2012). Therefore, we investigated the relationship between gingerol-mediated ROS generation and the sensitizing of gingerol effect in TRAIL-induced cell death of U87 glioblastoma cells. Through measurement of intracellular ROS and immunocytochemical analysis, we found that gingerol treatment led to intracellular ROS generation, and gingerol-mediated ROS generation was blocked by a ROS scavenger, N-acetylcysteine (NAC) (Lluis et al., 2010) (Figs. 4A, B). In addition, regulation of pro- and anti-apoptotic proteins by gingerol was inhibited by NAC treatment (Fig. 4C). NAC treatment caused inhibition of the sensitizing effect of gingerol in TRAIL-induced cleavage of caspases-3 and -8 and PARP-1 of U87 glioblastoma cells (Fig. 4D). Therefore, these results suggest that the potentiating effect of gingerol in TRAIL-mediated apoptosis could be exerted by ROS generation in U87 glioblastoma cells.
Fig. 4.
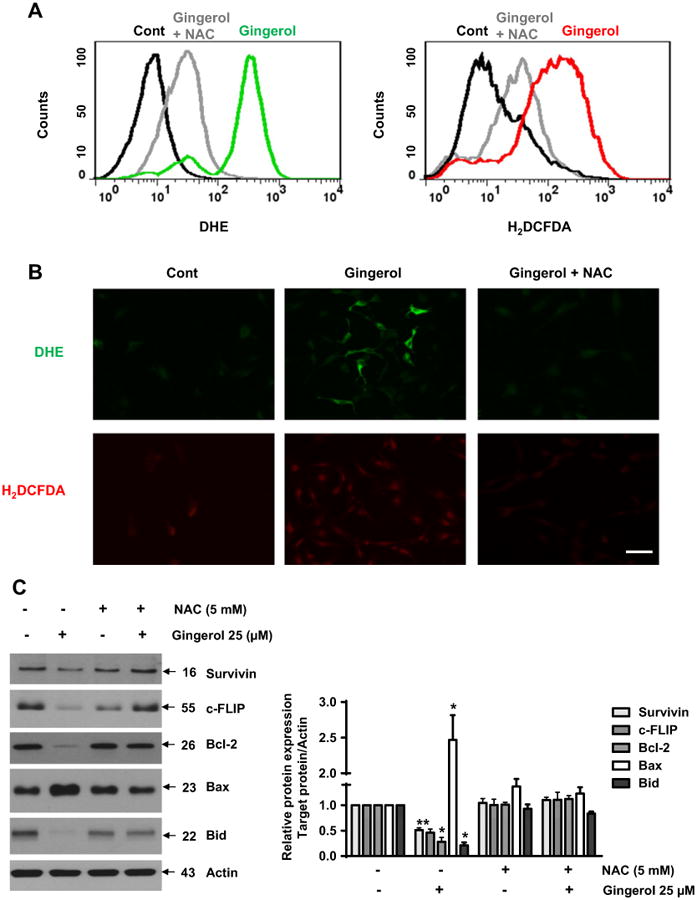
Gingerol can regulate the pro-apoptotic signaling of TRAIL through ROS generation. U87 cells were treated with gingerol (25 μM) in the presence or absence of N-acetylcysteine (Lluis et al., 2010) for 24 h, and then incubated with dihydroethidium (DHE) or 2′,7′-dichlorofluorescein (H2DCF), followed by (A) FACS analysis and (B) immunocytochemical analysis. Scale bar: 100 μm. (C) The cells were incubated with indicated drugs (25 μM gingerol and 5 mM NAC) for 24 h, and then western blotting was performed by using the indicated antibodies (Survivin, c-FLIP, BAX, Bcl-2, and Bid). (D) The cells were treated with indicated reagents (TRAIL, gingerol and NAC) for 24 h. The cell lysates were analyzed by western blotting using indicated antibodies (caspase-3,-8 and PARP-1). Data is presented as arbitrary values and results are expressed as the mean ±SEM. The effects of nicotine were determined using a Student's unpaired t-test. *p < 0.05 vs control. These results are representative of data obtained from at least five independent experiments.
Anti-apoptotic protein, Bcl-2 is important for the sensitizing effect of gingerol in TRAIL-induced apoptosis of U87 glioblastoma cells
Next, we checked the effect of anti-apoptotic protein, Bcl-2, in the sensitizing effect of gingerol for TRAIL-induced apoptosis of U87 glioblastoma cells. We generated a stable cell line overexpressing Bcl-2 and investigated the effect of gingerol. As shown in Fig. 5A, gingerol potentiated TRAIL-mediated cell death in control cells, but Bcl-2 overexpressing cell line blocked the sensitizing function of gingerol for TRAIL-induced apoptosis. Also, silencing of Bcl-2 by siRNA increased TRAIL-induced apoptosis (Fig. 5B). These data indicate that regulation of anti-apoptotic protein, Bcl-2, by gingerol could be required for the sensitizing effect of gingerol in TRAIL-induced apoptosis of U87 glioblastoma cells.
Fig. 5.
Effect of Bcl-2 in the sensitizing function of gingerol. (A) Vector or Bcl-2-overexpressing U87 stable cell lines (upper panel) were treated with TRAIL and gingerol for 24 h. The cell lysates were analyzed by western blotting of Flag antibody. Actin was used to confirm the equal amount of proteins loaded in each lane. All results are representative of the data obtained by five independent experiments. The cells were then analyzed by MTT assay. (B) Bcl-2 was silenced by si-RNA in U87 cells (upper panel). The cells were then treated with TRAIL for 24 h, followed by MTT analysis. Results shown are representative of six independent experiments.
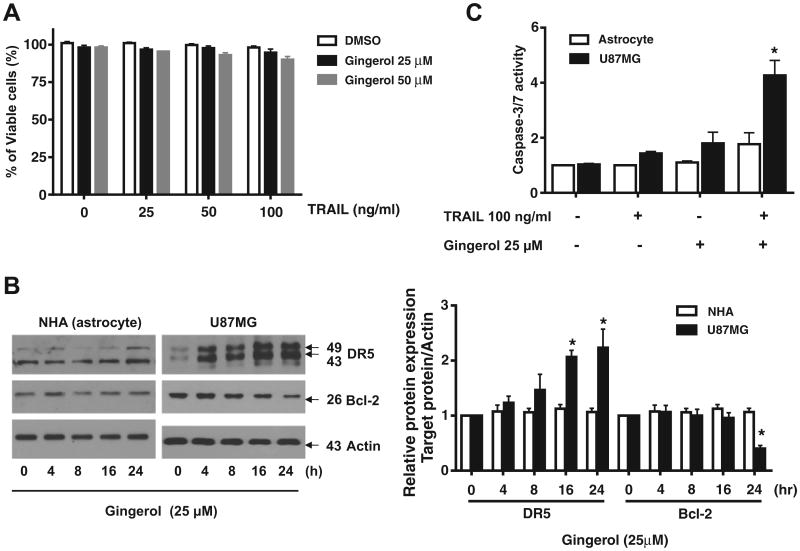
Combined treatment with gingerol and TRAIL does not induce cell death in normal astrocytes
Finally, we investigated the effects of cotreatment with gingerol and TRAIL on the viability of normal astrocytes. Astrocytes were very resistant to TRAIL and gingerol, either alone or in combination (Fig. 6A), indicating that the sensitizing regimen of gingerol plus TRAIL may be preferentially toxic to glioma cells. We then examined whether the resistance of astrocytes to the combined treatment regimen was associated with differential regulation of DR5 and Bcl-2. In contrast to its effects in U87MG cells, gingerol treatment did not affect DR5 protein levels in normal astrocytes. Bcl-2 protein levels, which were significantly down-regulated by gingerol in U87MG cells, were markedly low in normal astrocytes and were not affected by gingerol (Fig. 6B). Furthermore, procaspase-3 processing, which was partially induced in glioma cells by TRAIL alone and was completed in cells cotreated with TRAIL and gingerol, was not observed in astrocytes (Fig. 6C).
Fig. 6.
Astrocytes are resistant to the combined treatment with gingerol and TRAIL. (A) Human astrocytes were treated with or without gingerol for 30 min and further treated with TRAIL for 16 h at the indicated concentrations. Cellular viability was assessed using trypan blue exclusion assay. (B) Following treatment of astrocytes or U87MG cells with 25 μM gingerol for the indicated times, western blotting of DR5 and Bcl-2 antibodies. Actin was used to confirm the equal amount of proteins loaded in each lane. (C) Astrocytes or U87MG cells were treated with 25 μM gingerol alone, 100 ng/ml TRAIL alone or a combination with gingerol and TRAIL for 16 h. Caspase-3/7 activity is shown. Data is presented as arbitrary values and results are expressed as the mean ± SEM. The effects of nicotine were determined using a Student's unpaired t-test. *p < 0.05 vs control. Results shown are representative of six independent experiments.
Discussion
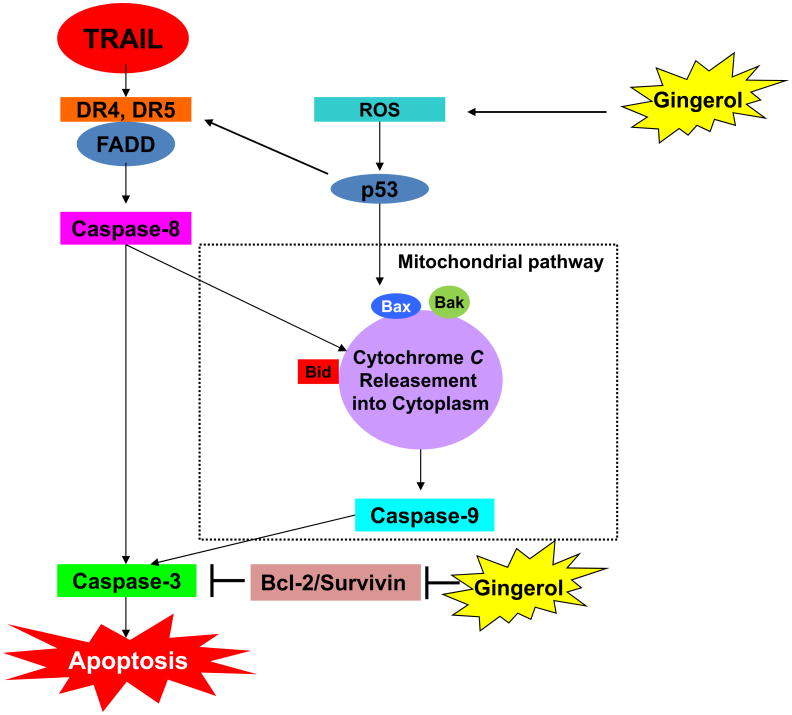
Glioblastoma multiforme (GBM) is a major primary brain tumor in adults that shows high lethality and aggressiveness (Karsy et al., 2012). It has been considered to be very difficult to treat GBM (Bonavia et al., 2011; Burger and Vollmer, 1980; Galli et al., 2004). Furthermore, most glioblastoma cells have been known to show resistance to cell death by TRAIL (Hawkins, 2004; Krakstad and Chekenya, 2010). Although there have been many combinational clinical trials for GBM, there remain many challenges in effective treatment. Here, we showed that gingerol can sensitize cell death by TRAIL in TRAIL-resistant glioblastoma cells. Elevated DR5 can transmit stronger apoptotic signaling such as the caspase cascade. Furthermore, gingerol can decrease the expression of anti-apoptotic proteins (Bcl-2 and survivin) and induce an increase in the level of pro-apoptotic protein (Bax) through ROS generation. Eventually, gingerol can sensitize the cell death of TRAIL-resistant glioblastoma by modulating TRAIL-mediated apoptotic signaling through DR5 and pro-apoptotic protein, and anti-apoptotic proteins. Therefore, this study suggests the possibility that gingerol at non-cytotoxic concentrations can be used as an anti-tumor agent that can serve as one of the combination therapies with TRAIL in TRAIL-resistant glioblastoma patients.
GBM patients that show the median survival rate of about 14.6 months have heterogeneous phenotypes (Boerman et al., 1996; Stupp et al., 2005, 2009). These heterogeneous properties of GBM are one of the reasons why treatment is more difficult than for other tumors (Bonavia et al., 2011; Burger and Vollmer, 1980; Galli et al., 2004). In addition, most GBM is resistant to apoptotic cell death by TRAIL (Hawkins, 2004; Krakstad and Chekenya, 2010). This issue gives rise to the limitations and difficulty of glioblastoma treatment. There are many clinical trials with various drugs targeting survival and anti-apoptotic signaling pathways in GBM patients (erlotinib and gefitinib targeting EGFR, perifosine targeting Akt, temsirolimus targeting mTOR, and gossypol targeting Bcl-2) (Chang et al., 2005; Krakstad and Chekenya, 2010; Rich et al., 2004; van den Bent et al., 2009). Recently, the effectiveness of human TRAIL (hTRAIL) as an anti-tumor drug was demonstrated by showing that hTRAIL can inhibit the growth of glioma in a xenograft mouse model (Roth et al., 1999; Saito et al., 2004). Furthermore, combination treatment with more than 2 anti-tumor drugs has been challenged to overcome the drug resistance of glioblastoma. In the use of TRAIL in TRAIL-resistant glioblastoma, preclinical study has shown that the combination treatment of TRAIL and temozolomide prolonged survival of glioblastoma xenografted mice (Panner et al., 2006; Saito et al., 2004). Although more extensive preclinical studies are required to determine the effects of gingerol in glioblastoma, we expect to see the use of gingerol in combination with TRAIL in future treatments.
Gingerol is a major component of ginger (the rhizome of Zingiber officinalis) that is used as a common spice in foods and for medical purposes (Shukla and Singh, 2007). Gingerol has been known to regulate a variety of cell functions such as inflammation and apoptosis (Oyagbemi et al., 2010; Shukla and Singh, 2007). Specifically, it has been reported that gingerol showed anti-tumor effects by the inducing apoptosis of various tumor cells including human cervical cancer cells, human promyelocytic leukemia cells, and human colorectal carcinoma cells (Chakraborty et al., 2012; Lee and Surh, 1998; Lee et al., 2008). However, these studies have used high concentrations of gingerol (200–500 μM) to induced growth arrest and apoptosis of tumor cells and do not show the detailed molecular mechanism by which gingerol is able to induce apoptosis in tumor cells. We revealed the molecular mechanisms and demonstrated that a non-cytotoxic dose of gingerol (25 μM) can regulate multiple survival and apoptotic proteins (DR5, Bax, Bcl-2, survivin, c-FLIP, and Bid) through ROS and p53 dependent pathways (Figs. 2, 3, and 4). Although gingerol has recently been shown to promote TRAIL-induced apoptosis on gastric cancer cells (Ishiguro et al., 2007), we report here that gingerol sensitizes glioma cells to TRAIL-induced caspase activation and apoptosis at least in part by a novel mechanism involving the antiapoptotic protein Bcl-2. Several studies have reported that gingerol can induce apoptosis in a p53-dependent or independent manner (Lin et al., 2012; Nigam et al., 2010). In the present study, we show for the first time that subtoxic doses of gingerol effectively sensitize different glioma cell lines to TRAIL-induced apoptosis. Moreover, we showed that the molecular mechanisms that gingerol affects contributed to sensitizing apoptotic cell death by TRAIL in glioblastoma (Fig. 7). Understanding these mechanisms underlying the sensitizing of apoptosis in tumors will give us a chance to create more effective combination treatment for anti-tumor therapies. These results may or may not benefit the patient, but we are hoping that our results would improve treatment regiment of the patient. Each type of cancer cells has different traits, so we want to use novel approaches. For future therapeutic application of our experimental strategy, we suggest a dietary intervention together with the targeted delivery of a protected TRAIL protein to tumors to avoid dilution in the body or inactivity due to the short half-life. In this regard, we are currently evaluating the suitability of the transduction of natural killer cells with TRAIL-expressing oncolytic adenoviruses.
Fig. 7.
Schematic diagram of working model of gingerol for sensitizing TRAIL-induced apoptosis.
Acknowledgments
This work was supported by the National Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2012R1A5A1048236).
Footnotes
Conflict of interest statement: The authors declare that they have no competing interests.
References
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- Bellail AC, Tse MC, Song JH, Phuphanich S, Olson JJ, Sun SY, Hao C. DR5-mediated DISC controls caspase-8 cleavage and initiation of apoptosis in human glioblastomas. J Cell Mol Med. 2010;14:1303–1317. doi: 10.1111/j.1582-4934.2009.00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode AM, Ma WY, Surh YJ, Dong Z. Inhibition of epidermal growth factor-induced cell transformation and activator protein 1 activation by [6]-gingerol. Cancer Res. 2001;61:850–853. [PubMed] [Google Scholar]
- Boerman RH, Anderl K, Herath J, Borell T, Johnson N, Schaeffer-Klein J, Kirchhof A, Raap AK, Scheithauer BW, Jenkins RB. The glial and mesenchymal elements of gliosarcomas share similar genetic alterations. J Neuropathol Exp Neurol. 1996;55:973–981. doi: 10.1097/00005072-199609000-00004. [DOI] [PubMed] [Google Scholar]
- Bonavia R, Inda MM, Cavenee WK, Furnari FB. Heterogeneity maintenance in glioblastoma: a social network. Cancer Res. 2011;71:4055–4060. doi: 10.1158/0008-5472.CAN-11-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- Burger PC, Vollmer RT. Histologic factors of prognostic significance in the glioblastoma multiforme. Cancer. 1980;46:1179–1186. doi: 10.1002/1097-0142(19800901)46:5<1179::aid-cncr2820460517>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Chakraborty D, Bishayee K, Ghosh S, Biswas R, Mandal SK, Khuda-Bukhsh AR. [6]-Gingerol induces caspase 3 dependent apoptosis and autophagy in cancer cells: drug-DNA interaction and expression of certain signal genes in HeLa cells. Eur J Pharmacol. 2012;694:20–29. doi: 10.1016/j.ejphar.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Chang SM, Wen P, Cloughesy T, Greenberg H, Schiff D, Conrad C, Fink K, Robins HI, De Angelis L, Raizer J, Hess K, Aldape K, Lamborn KR, Kuhn J, Dancey J, Prados MD. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005;23:357–361. doi: 10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- de Wilt LH, Kroon J, Jansen G, deJong S, Peters GJ, Kruyt FA. Bortezomib and TRAIL: a perfect match for apoptotic elimination of tumour cells? Crit Rev Oncol Hematol. 2013;85:363–372. doi: 10.1016/j.critrevonc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Doherty L, Gigas DC, Kesari S, Drappatz J, Kim R, Zimmerman J, Ostrowsky L, Wen PY. Pilot study of the combination of EGFR and mTOR inhibitors in recurrent malignant gliomas. Neurology. 2006;67:156–158. doi: 10.1212/01.wnl.0000223844.77636.29. [DOI] [PubMed] [Google Scholar]
- Fuller GN. The WHO Classification of Tumours of the Central Nervous System, 4th Edition. Arch Pathol Lab Med. 2008;132:906. doi: 10.5858/2008-132-906-TWCOTO. [DOI] [PubMed] [Google Scholar]
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stemlike neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- Goudar RK, Shi Q, Hjelmeland MD, Keir ST, McLendon RE, Wikstrand CJ, Reese ED, Conrad CA, Traxler P, Lane HA, Reardon DA, Cavenee WK, Wang XF, Bigner DD, Friedman HS, Rich JN. Combination therapy of inhibitors of epidermal growth factor receptor/vascular endothelial growth factor receptor 2 (AEE788) and the mammalian target of rapamycin (RAD001) offers improved glioblastoma tumor growth inhibition. Mol Cancer Ther. 2005;4:101–112. [PubMed] [Google Scholar]
- Hawkins CJ. TRAIL and malignant glioma. Vitam Horm. 2004;67:427–452. doi: 10.1016/S0083-6729(04)67022-1. [DOI] [PubMed] [Google Scholar]
- Ishiguro K, Ando T, Maeda O, Ohmiya N, Niwa Y, Kadomatsu K, et al. Ginger ingredients reduce viability of gastric cancer cells via distinct mechanisms. Biochem Biophys Res Commun. 2007;362:218–223. doi: 10.1016/j.bbrc.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Karsy M, Gelbman M, Shah P, Balumbu O, Moy F, Arslan E. Established and emerging variants of glioblastoma multiforme: review of morphological and molecular features. Folia Neuropathol. 2012;50:301–321. doi: 10.5114/fn.2012.32361. [DOI] [PubMed] [Google Scholar]
- Krakstad C, Chekenya M. Survival signalling and apoptosis resistance in glioblastomas: opportunities for targeted therapeutics. Mol Cancer. 2010;9:135. doi: 10.1186/1476-4598-9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Surh YJ. Induction of apoptosis in HL-60 cells by pungent vanilloids, [6]-gingerol and [6]-paradol. Cancer Lett. 1998;134:163–168. doi: 10.1016/s0304-3835(98)00253-5. [DOI] [PubMed] [Google Scholar]
- Lee SH, Cekanova M, Baek SJ. Multiple mechanisms are involved in 6-gingerol-induced cell growth arrest and apoptosis in human colorectal cancer cells. Mol Carcinog. 2008;47:197–208. doi: 10.1002/mc.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Rhee JG, Lee YJ. Reactive oxygen species up-regulate p53 and puma; a possible mechanism for apoptosis during combined treatment with TRAIL and wogonin. Brit J Pharm. 2009a;157:1189–1202. doi: 10.1111/j.1476-5381.2009.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TY, Lee KC, Chen SY, Chang HH. 6-Gingerol inhibits ROS and iNOS through the suppression of PKC-alpha and NF-kappaB pathways in lipopolysaccharide-stimulated mouse macrophages. Biochem Biophys Res Commun. 2009b;382:134–139. doi: 10.1016/j.bbrc.2009.02.160. [DOI] [PubMed] [Google Scholar]
- Lin CB, Lin CC, Tsay GJ. 6-Gingerol inhibits growth of colon cancer cell LoVo via induction of G2/M arrest Evid. Based Complement Alternat Med. 2012;326096 doi: 10.1155/2012/326096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lluis JM, Nachbur U, Cook WD, Gentle IE, Moujalled D, Moulin M, Wong WW, Khan N, Chau D, Callus BA, Vince JE, Silke J, Vaux DL. TAK1 is required for survival of mouse fibroblasts treated with TRAIL, and does so by NF-kappaB dependent induction of cFLIPL. PLoS One. 2010;5:e8620. doi: 10.1371/journal.pone.0008620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzo F, Nebbioso A, Miceli M, Conte M, De Bellis F, Carafa V, Franci G, Tambaro FP, Altucci L. TNF-related apoptosis-inducing ligand: signalling of a ‘smart’ molecule. Int J Biochem Cell Biol. 2009;41:460–466. doi: 10.1016/j.biocel.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Nigam N, George J, Srivastava S, Roy P, Bhui K, et al. Induction of apoptosis by [6]-gingerol associated with the modulation of p53 and involvement of mitochondrial signaling pathway in B[a]P-induced mouse skin tumorigenesis. Cancer Chemother Pharmacol. 2010;65:687–696. doi: 10.1007/s00280-009-1074-x. [DOI] [PubMed] [Google Scholar]
- Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64:479–489. doi: 10.1093/jnen/64.6.479. [DOI] [PubMed] [Google Scholar]
- Oyagbemi AA, Saba AB, Azeez OI. Molecular targets of [6]-gingerol: its potential roles in cancer chemoprevention. Biofactors. 2010;36:169–178. doi: 10.1002/biof.78. [DOI] [PubMed] [Google Scholar]
- Panner A, Parsa AT, Pieper RO. Use of APO2L/TRAIL with mTOR inhibitors in the treatment of glioblastoma multiforme. Expert Rev Anticancer Ther. 2006;6:1313–1322. doi: 10.1586/14737140.6.9.1313. [DOI] [PubMed] [Google Scholar]
- Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- Rao RD, Mladek AC, Lamont JD, Goble JM, Erlichman C, James CD, Sarkaria JN. Disruption of parallel and converging signaling pathways contributes to the synergistic antitumor effects of simultaneous mTOR and EGFR inhibition in GBM cells. Neoplasia. 2005;7:921–929. doi: 10.1593/neo.05361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon DA, Quinn JA, Vredenburgh JJ, Gururangan S, Friedman AH, Desjardins A, Sathornsumetee S, Herndon JE, II, Dowell JM, McLendon RE, Provenzale JM, Sampson JH, Smith RP, Swaisland AJ, Ochs JS, Lyons P, Tourt-Uhlig S, Bigner DD, Friedman HS, Rich JN. Phase 1 trial of gefitinib plus sirolimus in adults with recurrent malignant glioma. Clin Cancer Res. 2006;12:860–868. doi: 10.1158/1078-0432.CCR-05-2215. [DOI] [PubMed] [Google Scholar]
- Rich JN, Reardon DA, Peery T, Dowell JM, Quinn JA, Penne KL, Wikstrand CJ, Van Duyn LB, Dancey JE, McLendon RE, Kao JC, Stenzel TT, Ahmed Rasheed BK, Tourt-Uhlig SE, Herndon JE, II, Vredenburgh JJ, Sampson JH, Friedman AH, Bigner DD, Friedman HS. Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol. 2004;22:133–142. doi: 10.1200/JCO.2004.08.110. [DOI] [PubMed] [Google Scholar]
- Roth W, Isenmann S, Naumann U, Kugler S, Bahr M, Dichgans J, Ashkenazi A, Weller M. Locoregional Apo2L/TRAIL eradicates intracranial human malignant glioma xenografts in athymic mice in the absence of neurotoxicity. Biochem Biophys Res Commun. 1999;265:479–483. doi: 10.1006/bbrc.1999.1693. [DOI] [PubMed] [Google Scholar]
- Saito R, Bringas JR, Panner A, Tamas M, Pieper RO, Berger MS, Bankiewicz KS. Convection-enhanced delivery of tumor necrosis factor-related apoptosis-inducing ligand with systemic administration of temozolomide prolongs survival in an intracranial glioblastoma xenograft model. Cancer Res. 2004;64:6858–6862. doi: 10.1158/0008-5472.CAN-04-1683. [DOI] [PubMed] [Google Scholar]
- Shukla Y, Singh M. Cancer preventive properties of ginger: a brief review. Food Chem Toxicol. 2007;45:683–690. doi: 10.1016/j.fct.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- Surget S, Chiron D, Gomez-Bougie P, Descamps G, Menoret E, Bataille R, Moreau P, Le Gouill S, Amiot M, Pellat-Deceunynck C. Cell death via DR5, but not DR4, is regulated by p53 in myeloma cells. Cancer Res. 2012;72:4562–4573. doi: 10.1158/0008-5472.CAN-12-0487. [DOI] [PubMed] [Google Scholar]
- van den Bent MJ, Brandes AA, Rampling R, Kouwenhoven MC, Kros JM, Carpentier AF, Clement PM, Frenay M, Campone M, Baurain JF, Armand JP, Taphoorn MJ, Tosoni A, Kletzl H, Klughammer B, Lacombe D, Gorlia T. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol. 2009;27:1268–1274. doi: 10.1200/JCO.2008.17.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman T, Kinzler KW, Vogelstein B. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 1995;55:5187–5190. [PubMed] [Google Scholar]
- Wang S, El-Deiry WS. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene. 2003;22:8628–8633. doi: 10.1038/sj.onc.1207232. [DOI] [PubMed] [Google Scholar]
- Wu GS. TRAIL as a target in anti-cancer therapy. Cancer Lett. 2009;285:1–5. doi: 10.1016/j.canlet.2009.02.029. [DOI] [PubMed] [Google Scholar]
- Yang G, Wang S, Zhong L, Dong X, Zhang W, Jiang L, Geng C, Sun X, Liu X, Chen M, Ma Y. 6-Gingerol induces apoptosis through lysosomal-mitochondrial axis in human hepatoma G2 cells. Phytother Res. 2012;26:1667–1673. doi: 10.1002/ptr.4632. [DOI] [PubMed] [Google Scholar]