Abstract
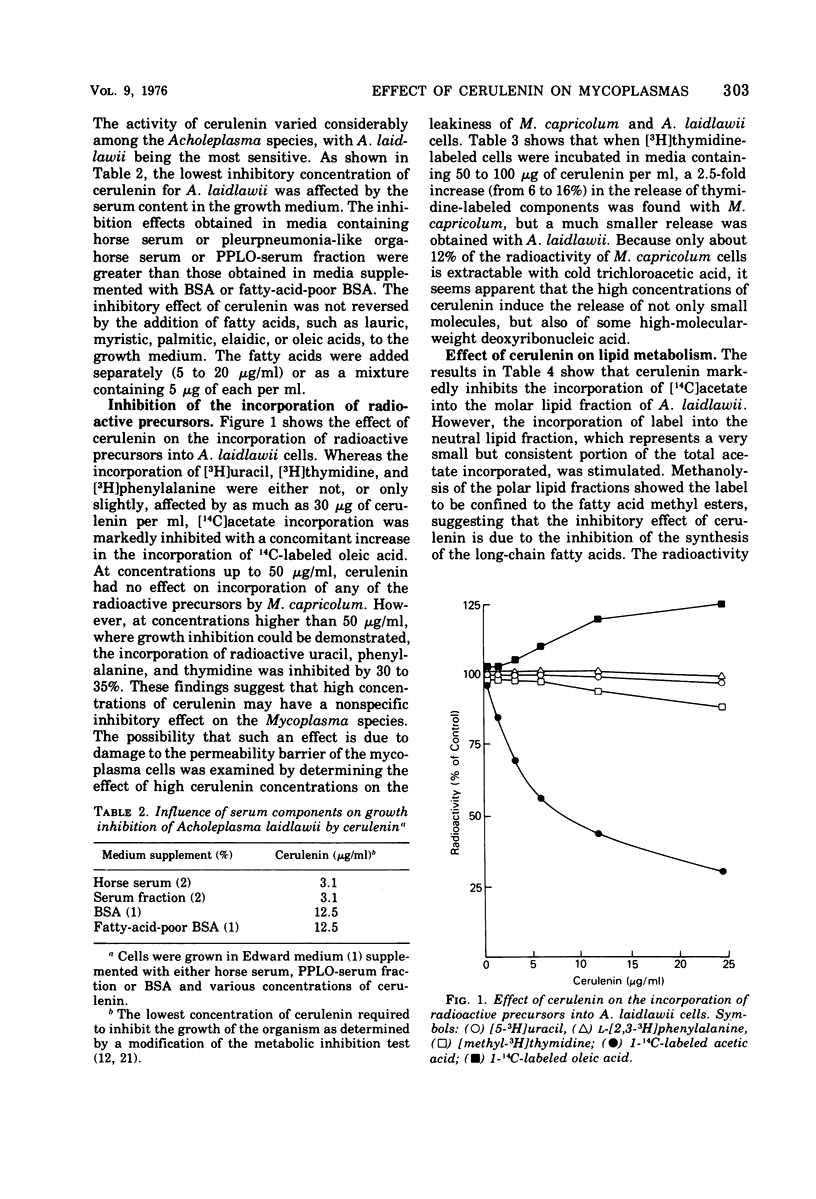
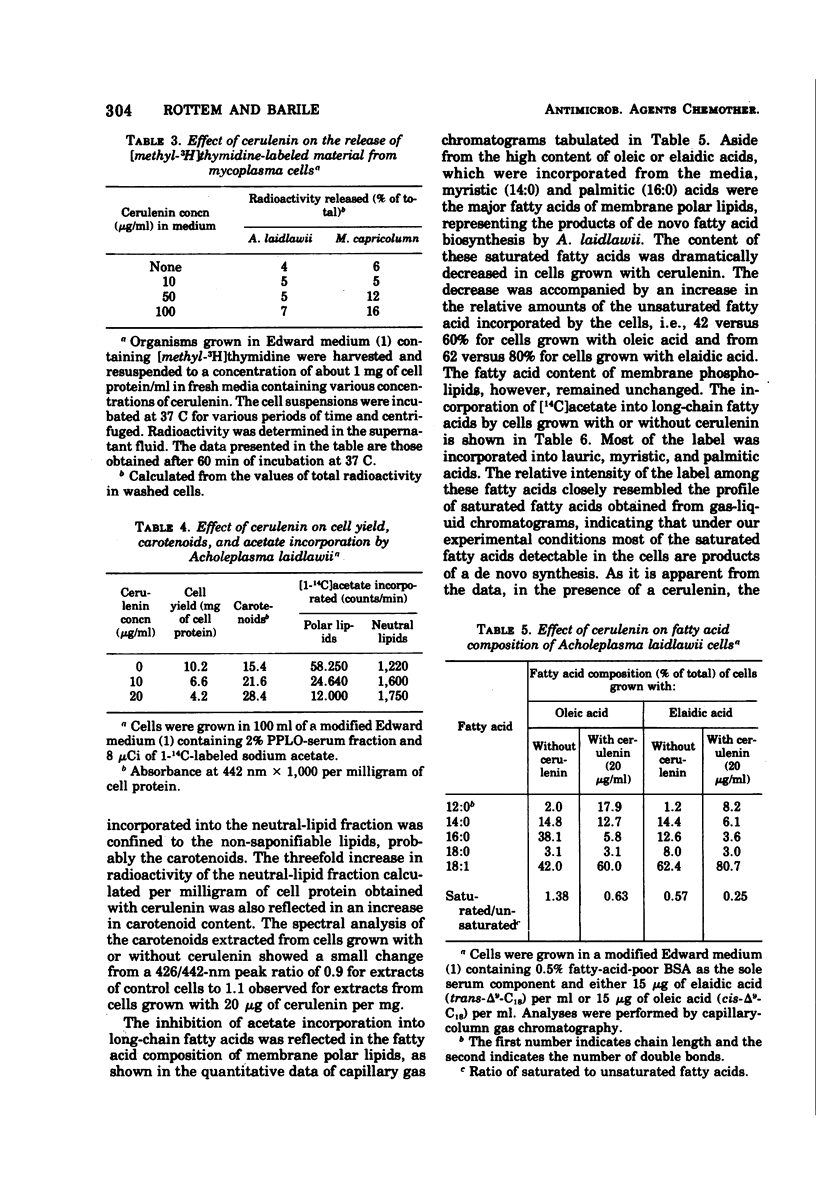
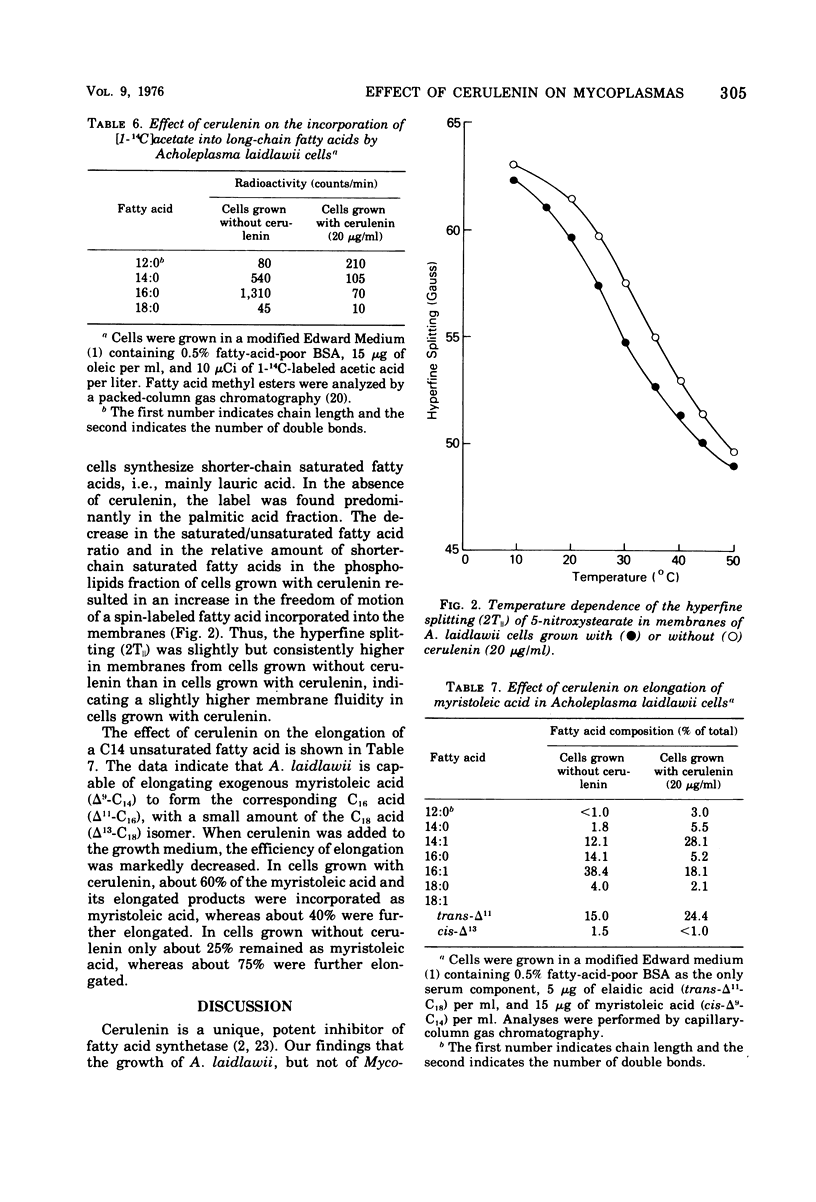
Cerulenin markedly inhibited the growth of Acholeplasma laidlawii. A. axanthum and A. granularum were less susceptible, whereas the sterol-requiring Mycoplasma species examined showed very little susceptibility. The inhibition was not reversed by the addition of long-chain fatty acids to the medium. At a concentration of 20 μg/ml, cerulenin inhibited the incorporation of [14C]acetate into A. laidlawii membrane lipids, but it had no effect on either protein or nucleic acid biosynthesis. Cerulenin inhibited both the de novo synthesis of long-chain fatty acids and the elongation of medium-chain fatty acids. As a result, carotenoid biosynthesis was stimulated, and increased amounts of oleic and elaidic acids were incorporated into membrane polar lipids. Our studies support the concept that cerulenin can serve as a useful tool for obtaining better control of fatty acid composition of A. laidlawii membranes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- D'Agnolo G., Rosenfeld I. S., Awaya J., Omura S., Vagelos P. R. Inhibition of fatty acid synthesis by the antibiotic cerulenin. Specific inactivation of beta-ketoacyl-acyl carrier protein synthetase. Biochim Biophys Acta. 1973 Nov 29;326(2):155–156. doi: 10.1016/0005-2760(73)90241-5. [DOI] [PubMed] [Google Scholar]
- Goldberg I., Walker J. R., Bloch K. Inhibition of lipid synthesis in Escherichia coli cells by the antibiotic cerulenin. Antimicrob Agents Chemother. 1973 May;3(5):549–554. doi: 10.1128/aac.3.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Haug A. Regulation of membrane lipid fluidity in Acholeplasma laidlawii: effect of carotenoid pigment content. Biochim Biophys Acta. 1974 Jun 29;352(3):361–370. doi: 10.1016/0005-2736(74)90228-4. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Orientation and motion of amphiphilic spin labels in membranes. Proc Natl Acad Sci U S A. 1969 Sep;64(1):20–27. doi: 10.1073/pnas.64.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MATSUMAE A., KAMIO Y., HATA T. STUDIES ON CERULENIN. I. STUDIES ON CERULENIN PRODUCING STRAIN. J Antibiot (Tokyo) 1963 Nov;16:236–238. [PubMed] [Google Scholar]
- MATSUMAE A., NOMURA S., HATA T. STUDIES ON CERULENIN. IV. BIOLOGICAL CHARACTERISTICS OF CERULENIN. J Antibiot (Tokyo) 1964 Jan;17:1–7. [PubMed] [Google Scholar]
- McElhaney R. N., Tourtellotte M. E. Mycoplasma membrane lipids: variations in fatty acid composition. Science. 1969 Apr 25;164(3878):433–434. doi: 10.1126/science.164.3878.433. [DOI] [PubMed] [Google Scholar]
- Nomura S., Horiuchi T., Omura S., Hata T. The action mechanism of cerulenin. I. Effect of cerulenin on sterol and fatty acid biosynthesis in yeast. J Biochem. 1972 May;71(5):783–796. doi: 10.1093/oxfordjournals.jbchem.a129827. [DOI] [PubMed] [Google Scholar]
- Panos C., Rottem S. Incorporation and elongation of fatty acid isomers by Mycoplasma laidlawii A. Biochemistry. 1970 Jan 20;9(2):407–412. doi: 10.1021/bi00804a030. [DOI] [PubMed] [Google Scholar]
- Purcell R. H., Taylor-Robinson D., Wong D., Chanock R. M. Color test for the measurement of antibody to T-strain mycoplasmas. J Bacteriol. 1966 Jul;92(1):6–12. doi: 10.1128/jb.92.1.6-12.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAZIN S. OSMOTIC LYSIS OF MYCOPLASMA. J Gen Microbiol. 1963 Dec;33:471–475. doi: 10.1099/00221287-33-3-471. [DOI] [PubMed] [Google Scholar]
- Razin S. Physiology of mycoplasmas. Adv Microb Physiol. 1973;10:1–80. doi: 10.1016/s0065-2911(08)60086-7. [DOI] [PubMed] [Google Scholar]
- Razin S. Structure and function in mycoplasma. Annu Rev Microbiol. 1969;23:317–356. doi: 10.1146/annurev.mi.23.100169.001533. [DOI] [PubMed] [Google Scholar]
- Razin S., Tourtellotte M. E., McElhaney R. N., Pollack J. D. Influence of lipid components of Mycoplasma laidlawii membranes on osmotic fragility of cells. J Bacteriol. 1966 Feb;91(2):609–616. doi: 10.1128/jb.91.2.609-616.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Hubbell W. L., Hayflick L., McConnell H. M. Motion of fatty acid spin labels in the plasma membrane of mycoplasma. Biochim Biophys Acta. 1970;219(1):104–113. doi: 10.1016/0005-2736(70)90065-9. [DOI] [PubMed] [Google Scholar]
- Rottem S., Panos C. The synthesis of long-chain fatty acids by a cell-free system from Mycoplasma laidlawii A. Biochemistry. 1970 Jan 6;9(1):57–63. doi: 10.1021/bi00803a008. [DOI] [PubMed] [Google Scholar]
- Rottem S., Razin S. Membrane lipids of Mycoplasma hominis. J Bacteriol. 1973 Feb;113(2):565–571. doi: 10.1128/jb.113.2.565-571.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Purcell R. H., Wong D. C., Chanock R. M. A colour test for the measurement of antibody to certain mycoplasma species based upon the inhibition of acid production. J Hyg (Lond) 1966 Mar;64(1):91–104. doi: 10.1017/s0022172400040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully J. G., Razin S. Characteristics of a new sterol-nonrequiring Mycoplasma. J Bacteriol. 1969 Jun;98(3):970–978. doi: 10.1128/jb.98.3.970-978.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance D., Goldberg I., Mitsuhashi O., Bloch K. Inhibition of fatty acid synthetases by the antibiotic cerulenin. Biochem Biophys Res Commun. 1972 Aug 7;48(3):649–656. doi: 10.1016/0006-291x(72)90397-x. [DOI] [PubMed] [Google Scholar]


