Abstract
Achalasia is a primary motor disorder of the esophagus diagnosed manometrically in the clinical setting of dysphagia to both solids and liquids. Currently established treatment options include pneumatic dilation, laparoscopic Heller myotomy, botulinum toxin injection performed endoscopically, oral agents that relax the lower esophageal sphincter and esophagectomy for refractory, end-stage disease. Despite their effectiveness, a significant proportion of patients eventually relapses and needs retreatment. In this setting, several new techniques are under investigation promising future enrichment of our therapeutic armamentarium for achalasic patients. Among them, peroral endoscopic myotomy and self-expandable metal stents placed across the gastro-esophageal junction represent the most encouraging modalities, as initial studies assessing their efficacy and safety indicate. This review highlights the role of self-expandable metal stents in the management of patients with achalasia. Their possible position in the therapeutic algorithm of achalasia along with established and novel techniques is also assessed. Finally, the need for large prospective randomized trials is underlined in order to elucidate the numerous relevant issues.
Keywords: Achalasia, Self-expandable metal stents, Dysphagia, Endoscopy, Treatment
Core tip: Recommended treatment of patients with achalasia are associated with significant clinical relapse over subsequent months or years. Therefore, numerous innovative techniques are under evaluation. Self-expandable metal stents may represent a promising alternative according to initial studies. They may gain a place in the therapeutic algorithm of achalasia in the view of its different types and stages, patients’ characteristics and other emerging modalities.
INTRODUCTION
Achalasia is a primary esophageal motility disorder characterized by aperistalsis in the distal portion of the esophageal body and incomplete or absent relaxation of the lower esophageal sphincter (LES). It is a disease of unknown cause; it pathophysiologically results primarily from the degeneration of ganglion cells in the myenteric plexus of the esophageal wall[1,2].
Achalasia is a rare clinical entity with annual incidence and prevalence of approximately 1.6 and 10 cases per 100000 individuals, respectively. Both sexes are affected equally, there is no racial predilection and the age of diagnosis ranges between 25 and 60 years[3,4]. Onset is rather insidious and disease progression gradual accounting for high rates of delayed diagnosis. The predominant symptom of achalasia is dysphagia to solids and liquids. Other symptoms include regurgitation of undigested food or saliva occasionally leading to aspiration and pneumonia, sub sternal chest pain, weight loss and heartburn[5].
The diagnosis of achalasia when clinically suspected is suggested by barium esophagram and established by manometry. On barium swallow supporting findings include aperistalsis, dilation of the esophagus, bird-beak appearance of the gastro-esophageal junction and delayed contrast medium emptying[6]. Manometry typically reveals incomplete or absent LES relaxation in response to a swallow and aperistalsis in the distal 2/3 of the esophagus[7]. Recently high resolution manometry classifies achalasia in 3 subtypes namely I (classic), II (with panesophageal pressurization) and III (spastic or vigorous)[8]. This classification possibly correlates with the final outcome of treatment[9,10]. Endoscopy may be normal or reveals a dilated esophagus with retained saliva and undigested food particles along with difficulty in passing the gastro-esophageal junction. Of importance, endoscopic examination and, when indicated, imaging studies are mandatory to exclude focal malignancy mimicking primary achalasia[11,12].
CURRENT TREATMENT OPTIONS AND THEIR LIMITATIONS
Treatment modalities for achalasia aim at reducing LES resting pressure thus relieving dysphagia and regurgitation and preventing the long-term development or mega-esophagus. This goal is accomplished by either mechanical disruption of the LES muscular fibers (e.g., pneumatic dilation, myotomy either laparoscopic or peroral endoscopic) or by pharmacological decrease in LES pressure (e.g., botulinum toxin injection, oral nitrates and calcium-channel blockers)[13,14].
Pneumatic dilation (PD) represents a highly-accepted first-line therapy for primary achalasia due to its cost-effectiveness and low complication rates. PD is performed in a gradual fashion by experienced endoscopists using standard-diameter balloons. Initial success rates are high and up to 90% of patients report symptomatic relief. Favorable predictors include older age (> 45 years), female gender, narrow esophageal lumen, post-dilation pressure < 10 mmHg and type II pattern on high-resolution manometry[10,15,16]. However, improvement is often not sustainable in the medium - to long-term period, since prospective studies suggest that approximately two thirds of patients eventually relapse and need additional dilations and possibly surgery[17]. Moreover, subsequent dilations seem less effective and patients referred for myotomy are at increased risk for intra-operative complications. Mostly feared complication is esophageal perforation with an overall median rate of 1.9% (range 0%-16%)[18]. Additionally gastroesophageal reflux disease occurs in 15%-35% of patients necessitating antisecretory medications[19].
Laparoscopic Heller myotomy (LHM) coupled with Dor fundoplication is the primary alternative to PD for achalasia. Initial clinical remission is achieved to nearly 90% of patients but this excellent outcome seems to wane over time[18,20]. Long-term studies show that 18% of patients require PD and 5%-10% of them repeat myotomy or esophagectomy 5-11 years post-operatively[21,22]. Nevertheless, a meta-analysis published in 2013 favored LHM over PD in terms of both short- and long-term efficacy[23]. Being more invasive, surgery is associated with a protracted recovery period and numerous complications including gastro-esophageal reflux disease (GERD), dysphagia associated with the fundoplication that may require dilations, perforation, bleeding, leaks and infections which affect negatively its cost-effectiveness[20]. Despite these imperfections, LHM is preferred over PD for patients younger than 40 years as they frequently need more re-dilations than older subjects[5]. To note, very recently, Nau et al[24] suggested that LHM should be used as a benchmark against which other treatments for achalasia are judged, given its outstanding results[24].
Developed by Inoue in Japan peroral endoscopic myotomy (POEM) is the most fascinating new treatment option for achalasia currently being extensively studied in the United States and in Europe. This approach involves endoscopic dissection of the esophageal submucosal space and the creation of a tunnel eventually allowing LES circular muscle bundles dissection[25,26]. Initial studies in a total of 1000 procedures with a mean follow-up from 3 to 12 mo report excellent short term results (clinical success 82%-100%) and only minor self-limited adverse events (mainly tense capnoperitoneum) in less than 10% of patients[27]. The most serious complication is mediastinitis due to esophageal leak, although its incidence seems remarkably low. On the other hand, recent studies show that objectively-measured gastroesophageal reflux disease prevalence after POEM varies from 20% to 46%, higher than that in early reports and similar to those following LHM with Dor fundoplication[28,29]. No procedure-related death has been reported. In all circumstances, further studies with long-term follow-up, as well as randomized trials comparing POEM with LHM and PD are warranted before POEM can be recommended[25] as the procedure of choice.
Intrasphincteric botulinum toxin injection (BTI) can be easily performed during routine endoscopy in poor surgical candidates. Using a sclerotherapy needle, 100 units of the toxin are administered just above the squamocolumnar junction in at least 4 quadrants. Its initial efficacy reaches those of PD and LHM. Unfortunately, symptoms relapse in more than 50% of patients necessitating additional injections at 6-24-mo intervals[30]. Main complications are post-procedural chest pain, heartburn and allergic reactions[19]. In addition, BTI may increase the technical difficulty of subsequent myotomy either surgical or endoscopic[31].
Oral pharmacologic agents indicated for primary achalasia include calcium-channel blockers and nitrates. They represent the least effective means of treatment[32]. Traditionally, they are administered 30 to 60 min prior to meals and act by decreasing basal LES pressure and tone. Their efficacy is variable and their use is limited to those who are not suitable to receive invasive therapies. Moreover, side effects such as headache, hypotension and peripheral edema, as well as tachyphylaxis, diminish their application[19].
Finally, for patients with end-stage achalasia (megaesophagus, or sigmoid-esophagus) who have failed PD and/or LHM, esophagectomy should be considered[33]. Esophageal resection results in symptomatic improvement in more than 80% of patients; however, it is associated with significant mortality reaching 5.4% in uncontrolled studies and recurrence of dysphagia in up to 50% of patients[34].
As shown, all currently available therapeutic modalities for primary achalasia remain of palliative nature, given that the underlying mechanism cannot be reversed. Moreover, a good proportion of patients will experience symptom recurrence and require retreatment. In this context, several new endoscopic treatments are under evaluation over the last years. This review aims to highlight the role of self-expandable metal stents (SEMS) in the management of patients with achalasia.
USED MATERIALS AND METHODS
Using PubMed we carried out a thorough review of the literature to identify all articles published between January 1995 and July 2014 referring to the use of SEMS in achalasia. The search was initially performed using the term “achalasia and stents” as a free text. A total of 43 studies were retrieved and one additional was identified by a manual search of the references cited in the key articles. Each manuscript was subsequently cross-checked by two authors (AS, CM) to achieve a maximum completeness of the reports chosen for inclusion. In case of disagreement, a third senior author (KT) made the final decision. Eventually, 14 studies were considered suitable for review. The article selection process is presented in Figure 1.
Figure 1.
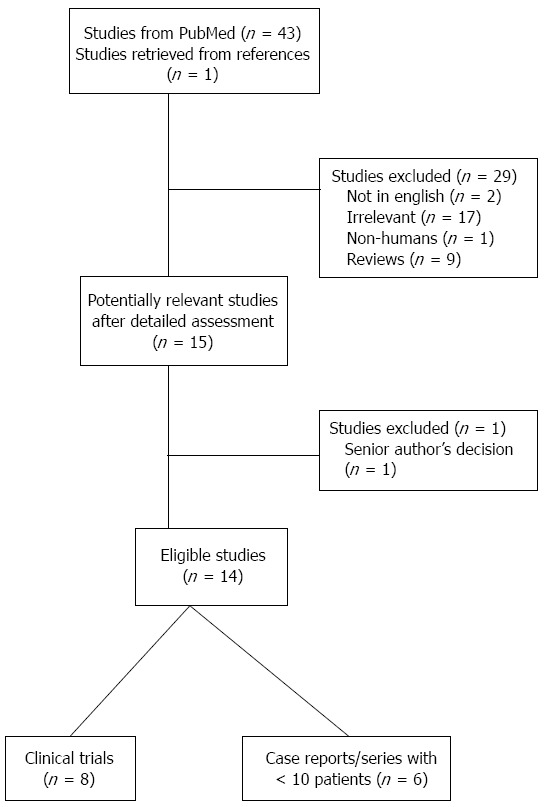
Flow diagram of the literature search strategy and valuation of studies identified for review.
SEMS FOR ACHALASIA TREATMENT
Early reports regarding the use of SEMS in the treatment of achalasia were published in 1998 by De Palma et al[35] A nitinol coil stent (InStent Inc., Eden, Praire, United States), 10 cm long, was placed in 4 patients with long-standing disease who were unresponsive to conventional treatment such as LHM, PD and BTI. Stent placement was successful in all cases and the patients achieved clinical remission during the follow-up period up to 12 mo. One of them developed reflux esophagitis treated medically[35].
Three years later the same authors presented their extended experience in 8 patients followed for a period ranging from 29 to 44 mo. Nitinol coil and Ultraflex (Microvasive, Boston Scientific, Natick, MA, United States) stents were placed across the gastro-esophageal junction. Although stent implantation was technically successful and all patients experienced complete remission of dysphagia, a significant complication rate was noted both in the early (within 30 d) and in the late (after 30 d) phase. In particular, 62.5% of patients had early complications (mainly stent migration, 37.5%) and 57.1% late complications (mainly chest pain, 28.5%). As a result the investigators concluded that the use of SEMS in achalasia should not be generalized but reserved only for carefully selected cases[36].
Unlike the rather promising experience of De Palma et al[35], a case series published in 2000 announced extremely disappointing results. Three different SEMS types, namely Gianturco Rosch Z stent (Wilson Cook Medical, Winston Salem, NC, United States) and Wallstent I and II (Schneider USA, Plymouth, MN, United States) were inserted in 4 achalasic patients. Placement was technically feasible and uneventful. Symptomatic remission before further intervention varied between 2 wk and 10 mo. However, complications such as stent migration and dysphagia recurrence secondary to either food bolus impaction or inflammatory stricture occurred in all cases. Most serious, one patient died from bleeding due to an aorta-enteric fistula developed from a penetrating gastro-esophageal junction ulcer adjacent to the stent. The authors recommended that alternative to SEMS options should be preferred in the management of patient with refractory achalasia[37].
Thereafter, a center from the United States and one from Spain reported few cases of achalasic patients treated with SEMS insertion. The former used metal coil stents (Esophacoil, InStent Inc., MN, United States) in 2 patients with complicated refractory achalasia. Technical and clinical success was achieved; nevertheless, hematemesis secondary to severe erosive esophagitis and small bowel obstruction due to stent migration were encountered a few months after stent placement[38]. The Spanish center announced the use of SEMS (Hanarostent, MI Tech, IZASA, Seoul, South Korea) as an effective short-term bridging therapy in 2 achalasic patients, one pregnant and one with newly diagnosed pituitary tumor[39].
In 2009 Zhao et al[40] reported the results of a prospective study assessing the long term efficacy and safety of a specifically designed partially-covered SEMS, 30 mm in diameter, placed for 3-7 d in 75 achalasic patients. Both technical and post-procedural clinical success was 100%. During the long follow-up period (up to 13 years) the overall remission rates remained extremely high reaching 100% and 83.3% at > 5 and > 10 years, respectively. These excellent results, as well as the low rates of complications including stent migration and perforation (5.3% and 0%, respectively) were attributed to the large-diameter stent that had been used. On the other hand, the same factor was possibly responsible for the relative high rates of chest pain (38.7%), gastro-esophageal reflux (20%) and bleeding (12%). It was therefore suggested that temporary SEMS placement is effective and safe and could serve as an alternative or complementary method in the management of esophageal achalasia[40].
The importance of stent diameter in terms of clinical efficacy was evaluated in a prospective study with long-term follow-up conducted by Cheng et al[41] As the results indicate, the overall cumulative clinical remission rate was significantly higher for patients who underwent a 30 mm stent placement as compared with those who received a 25 mm and 20 mm one (87% vs 73% vs 47%, respectively). Similarly, the wider the stent, the lower the migration rate (6.6% vs 13.3% vs 26.7%) and the higher the chest pain rate (40% vs 33% vs 17%, respectively)[41].
A recent study by Zeng et al[42] assessed for the first time the efficacy of fully-covered SEMS, 20-25 mm in diameter, in achalasia (Z-stent, Sigma, Huaian, China). Fifty-nine patients with no prior treatment were enrolled and underwent stent placement for a 1 mo period. The cumulative remission rates after 6, 12, 18, 24, 30 and 36 mo were 90.9%, 81.8%, 76.4%, 69.1%, 65.%% and 49.1%, respectively. Sub sternal chest pain was the most common complication (25.5%), followed by heartburn (10.6%) and stent migration (8.5%)[42].
Apart from Eastern countries, a study from Italy published a few months ago evaluated the safety and efficacy of SEMS as a temporary treatment in patients with achalasia. Seven patients underwent a 30 mm partially-covered stent (Micro-Tech, Nanjin, China) placement for 6 d and were followed thereafter for a mean period of 19 mo. Beneficial effects on dysphagia were excellent in 5 and good in 2 patients during the follow-up. No serious complication was observed. The authors concluded that large stent placement may permanently disrupt the muscular fibers of the cardia and possibly represents a safe and effective option for patients not fit for more invasive interventions[43]. A stent similar to the one used in this study as well as, a nitinol-covered stent are illustrated in Figure 2. Major points of the above mentioned studies are presented in Table 1.
Figure 2.

Large-diameter self-expandable metallic stent for achalasia. A: Self-expandable metal stents similar to that used by Coppola et al[43]. Picture is provided by courtesy of Mr. Kuhn D, Micro-Tech Europe GmbH, Dusseldorf, Germany; B: Niti-S stent. Picture is provided by courtesy of Mr. Bekzat M, TaeWoong Medical, Seoul, South Korea.
Table 1.
Published series using self-expandable metallic stents for achalasia treatment
| Ref. | Coppola et al[43] (2014) | Zeng et al[42] (2014) | Cheng et al[41] (2010) | Zhao et al[40] (2009) | De Palma et al[36] (2001) | Mukherjee et al[37] (2000) | De Palma et al[35] (1998) |
| Baseline characteristics and effectiveness | |||||||
| Patients, n | 7 | 59 | 90 | 75 | 8 | 4 | 4 |
| SEMS diameter, mm | 30 | 20/25 | 20/25/30 | 30 | 18 | 18/20 | 18 |
| Time to removal, d | 6 | 30 | 4-5 | 3-7 | ? | ? | ? |
| Technical success, % | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Initial remission, % | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Major complications | |||||||
| Stent migration, n | 0 | 4 | 14 | 4 | 4 | 1 | 0 |
| Perforation, n | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Bleeding, n | 0 | 0 | 14 | 9 | 0 | 1 | 0 |
| 30-d mortality, n | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
SEMS VS PD AND BTI IN THE TREATMENT OF ACHALASIA
Several studies compare SEMS vs established treatment options such as PD and BTI in the management of patients with achalasia, as presented in Table 2. Of note, no randomized trials are currently available.
Table 2.
Published comparison studies
| Ref. | Li et al[47] (2010) | Li et al[46] (2010) | Zhu et al[45] (2010) | Cheng et al[44] (2003) | Cai et al[48] (2013) |
| Compared methods | PD vs SEMS (20, 25, 30 mm) | PD vs SEMS (30 mm) | PD vs SEMS (30 mm) | PD vs SEMS (permanent, temporary) | BTI vs SEMS (25 mm) |
| Patients, n | 30/30/30/30 | 80/75 | 38/63 | 60/8/65 | 51/59 |
| Technical success, % | 100/100/100/100 | 100/100 | 100 | 100/100/100 | 100/100 |
| Initial remission, % | 100/100/100/100 | 100/100 | 100 | 100/100/100 | 94.1/100 |
| Remission at maximum follow-up, % | 0/0/28.6/83.3 | 0/83.3 | 42.1/88.9 | 10/33.3/85.5 | 4.17/49.1 |
| Major complications | |||||
| Migration, n | NA/8/4/2 | NA/4 | NA/2 | NA/0/0 | NA/4 |
| Perforation, n | 0/0/0/0 | 0/0 | 0/0 | 0/0/0 | 0/0 |
| Bleeding, n | 2/3/5/6 | 4/9 | 3/10 | 6/3/8 | 0/0 |
| 30-d mortality, n | 0/0/0/0 | 0/0 | 0/0 | 0/0/0 | 0/0 |
PD: Pneumatic dilation; SEMS: Self-expandable metallic stent; NA: Not applicable.
In 2003 Cheng et al[44] compared PD with permanent uncovered or ant reflux covered SEMS and temporary partially-covered SEMS. The latter stents, sized 20-30 mm in diameter, were inserted and withdrawn successfully after 3-7 d. According to the results, temporary partially-covered SEMS exhibited significantly superior long-term therapeutic efficacy as compared to the rest interventions, although immediate symptomatic relief was equally excellent. Interestingly, permanently uncovered metal stent dilation proved to be unsuitable for patients with achalasia due to high rates of gastro-esophageal reflux and stent occlusion secondary to hyperplasia of granulation tissue[44].
To overcome the limitations of their previous study (e.g., relatively short follow-up and great variety in stent diameters) the same investigators reported the results of a retrospective trial comparing PD and temporary partially-covered SEMS (Zhiye Medical Instruments, Guangzhou, China and Youyan Yijin Advanced Materials, Beijing, China). The diameter of the balloon or stent used was 30 mm. The stent was removed within 7 d after placement and the patients were followed both clinically and manometrically for more than 10 years. The results showed that both interventions are very efficacious in the immediate post-procedural period. However, the total symptom scores in patients treated with SEMS were statistically better than those treated with PD throughout the follow-up period (P < 0.05). LES pressure did not exhibit significant differences apart from one time frame (after 8-10 years). As expected, complications such as pain and bleeding occurred more frequently in the stent group compared to the balloon one (42.9% vs 23.6% and 15.9% vs 8%, respectively)[45].
Similar results were obtained by an uncontrolled prospective study with a long-term follow-up comparing SEMS and PD of the same diameter (30 mm). Temporary (3-7 d) SEMS placement was associated with significantly higher clinical remission rates in all follow-up periods (up to > 10 years). Notably, the long-term efficacy of SEMS seems to be comparable with that of LHM. Although of no statistical significance, complications like chest pain and bleeding were more common in the SEMS group, whereas stent migration occurred in 5.3% of patients[46]. Additionally, the same medical group showed prospectively that temporary SEMS with a diameter of 30 mm achieved significantly higher clinical remission rates after more than 10 years of follow-up as compared to patients treated with PD with a 30 mm balloon or SEMS with diameters of 20 or 25 mm (83.3% vs 0%, 0% and 28.6%, respectively). Surprisingly, the clinical remission rate with PD in the long-term was extremely poor suggesting a possible study limitation[47].
The only study that compares BTI and SEMS for the treatment of achalasia has been published by Cai and colleagues in 2013. A partially-covered SEMS 25 mm in width was applied and retrieved after 4 wk. The mean duration of follow-up was 28 mo (range 10-36 mo). Based on the results, the patients in the SEMS group achieved significantly better improvements regarding global symptoms scores, dysphagia and LES pressure. Moreover, differences in remission rates after 12 mo gained statistical significance favoring SEMS placement. No adverse events were observed in the BTI group, whereas 13 episodes of chest pain, 9 cases of regurgitation and 4 stent migrations were captured in the SEMS group[48].
SEMS IN THE NEW ERA
Achalasia treatment should be individualized taking into account both patients characteristics and available expertise. Although current established treatments are effective, emerging techniques such as SEMS placement are being developed, as presented. Nevertheless, what could be the exact position of SEMS in the therapeutic plan of achalasia, especially in the era of very promising interventions like POEM?
As shown in Figure 3, temporal placement of wide, partially covered SEMS could potentially serve as an alternative first-line treatment in both low and high surgical risk patients. This could be of great value mainly for the latter ones, given that the unique currently recommended treatment option (e.g., BTI) exhibits short-term, only, efficacy. Temporal wide partially covered SEMS may also be preferred for all cases of treatment failures, irrespective of the initial therapy, offering an efficacious, well-tolerated choice. It may be hypothesized, that SEMS could possibly serve on a short-term basis as a bridging therapy until surgery is performed.
Figure 3.
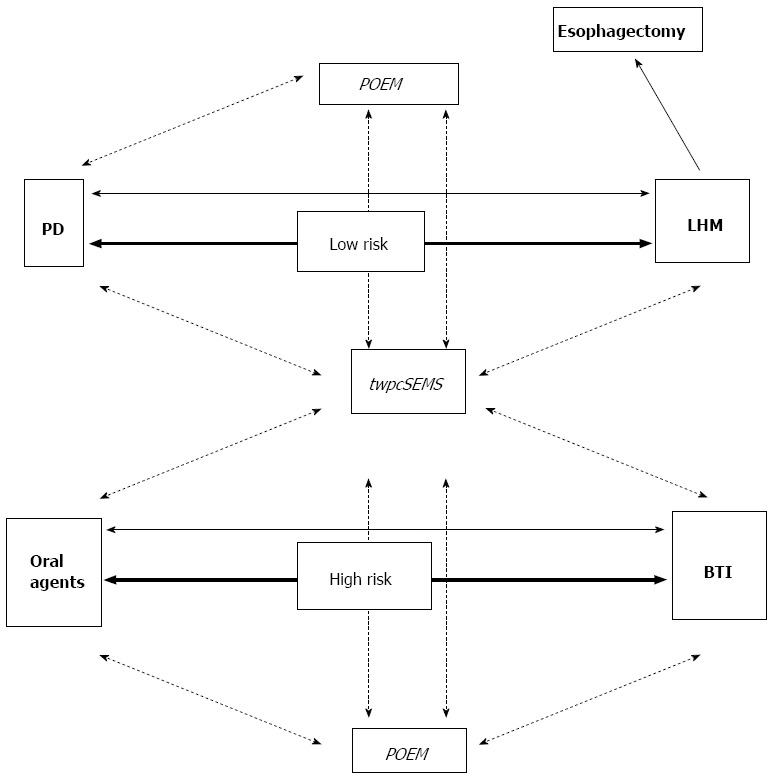
Proposed therapeutic algorithm for achalasia based on surgical risk. Currently established treatments are in bold. Investigational ones are in italics. Arrows indicate current first-line treatments. Lines binding different treatments indicate management of failures. Dotted lines indicate assumed steps in management, while solid lines the to-date recommended[11]. PD: Pneumatic dilation; LHM: Laparoscopic Heller myotomy; BTI: Botulinum toxin injection; twpcSEMS: Temporal, wide, partially covered, self-expandable metallic stent; POEM: Peroral endoscopic myotomy.
One could argue that POEM will eventually predominate in achalasia treatment due to its efficacy and safety profile according to initial studies. However, POEM is still a quite invasive procedure compared to SEMS placement. Additionally, it is by far more technically demanding, requires specific training and can be feasible only in centers of excellence worldwide[49,50]. General anesthesia requirements, time consumption and cost should be undoubtedly considered. Long-term results and adverse events in patients who have undergone POEM are still pending. Given the above, temporal placement of wide, partially covered SEMS seems able to maintain a role in the management of achalasic patients, even in the advent of POEM. Comparative randomized trials are surely appreciated before achalasia therapeutic algorithm takes its definite form.
CONCLUSION
Treatment remains palliative since its neuronal defect seems to be irreversible. In this setting, temporal, wide, partially covered SEMS placement may represent a safe and effective alternative therapy for carefully selected patients. Several technical issues including stent type, stent diameter and length, optimal time for removal and prevention of complications are still open for discussion. Small size of treated population, low quality of studies’ design and the majority of studies performed in Asia also preclude the generalizability of the reviewed evidence.
Additionally, the advent of self-expandable biodegradable stents used in the management of refractory benign esophageal strictures, as well as drug-eluting stents could provide an area for further innovation, in the field of stents in achalasia. Large, multicenter, randomized trials are warranted - while not always feasible - to elucidate the exact position of stent placement in the therapeutic armamentarium for the different profiles of achalasic patients.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 22, 2014
First decision: September 16, 2014
Article in press: November 10, 2014
P- Reviewer: Herbella FAM, Syam AF S- Editor: Ji FF L- Editor: A E- Editor: Zhang DN
References
- 1.Vaezi MF, Richter JE. Diagnosis and management of achalasia. American College of Gastroenterology Practice Parameter Committee. Am J Gastroenterol. 1999;94:3406–3412. doi: 10.1111/j.1572-0241.1999.01639.x. [DOI] [PubMed] [Google Scholar]
- 2.Francis DL, Katzka DA. Achalasia: update on the disease and its treatment. Gastroenterology. 2010;139:369–374. doi: 10.1053/j.gastro.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 3.Sadowski DC, Ackah F, Jiang B, Svenson LW. Achalasia: incidence, prevalence and survival. A population-based study. Neurogastroenterol Motil. 2010;22:e256–e261. doi: 10.1111/j.1365-2982.2010.01511.x. [DOI] [PubMed] [Google Scholar]
- 4.O’Neill OM, Johnston BT, Coleman HG. Achalasia: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2013;19:5806–5812. doi: 10.3748/wjg.v19.i35.5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boeckxstaens GE, Zaninotto G, Richter JE. Achalasia. Lancet. 2014;383:83–93. doi: 10.1016/S0140-6736(13)60651-0. [DOI] [PubMed] [Google Scholar]
- 6.Pandolfino JE, Kahrilas PJ. Presentation, diagnosis, and management of achalasia. Clin Gastroenterol Hepatol. 2013;11:887–897. doi: 10.1016/j.cgh.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 7.Pandolfino JE, Kahrilas PJ. AGA technical review on the clinical use of esophageal manometry. Gastroenterology. 2005;128:209–224. doi: 10.1053/j.gastro.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Pandolfino JE, Kwiatek MA, Nealis T, Bulsiewicz W, Post J, Kahrilas PJ. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology. 2008;135:1526–1533. doi: 10.1053/j.gastro.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahrilas PJ, Boeckxstaens G. The spectrum of achalasia: lessons from studies of pathophysiology and high-resolution manometry. Gastroenterology. 2013;145:954–965. doi: 10.1053/j.gastro.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pratap N, Kalapala R, Darisetty S, Joshi N, Ramchandani M, Banerjee R, Lakhtakia S, Gupta R, Tandan M, Rao GV, et al. Achalasia cardia subtyping by high-resolution manometry predicts the therapeutic outcome of pneumatic balloon dilatation. J Neurogastroenterol Motil. 2011;17:48–53. doi: 10.5056/jnm.2011.17.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol. 2013;108:1238–1249; quiz 1250. doi: 10.1038/ajg.2013.196. [DOI] [PubMed] [Google Scholar]
- 12.Richter JE. The diagnosis and misdiagnosis of Achalasia: it does not have to be so difficult. Clin Gastroenterol Hepatol. 2011;9:1010–1011. doi: 10.1016/j.cgh.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Müller M, Eckardt AJ, Wehrmann T. Endoscopic approach to achalasia. World J Gastrointest Endosc. 2013;5:379–390. doi: 10.4253/wjge.v5.i8.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckardt AJ, Eckardt VF. Treatment and surveillance strategies in achalasia: an update. Nat Rev Gastroenterol Hepatol. 2011;8:311–319. doi: 10.1038/nrgastro.2011.68. [DOI] [PubMed] [Google Scholar]
- 15.Farhoomand K, Connor JT, Richter JE, Achkar E, Vaezi MF. Predictors of outcome of pneumatic dilation in achalasia. Clin Gastroenterol Hepatol. 2004;2:389–394. doi: 10.1016/s1542-3565(04)00123-5. [DOI] [PubMed] [Google Scholar]
- 16.Eckardt VF, Aignherr C, Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103:1732–1738. doi: 10.1016/0016-5085(92)91428-7. [DOI] [PubMed] [Google Scholar]
- 17.Hulselmans M, Vanuytsel T, Degreef T, Sifrim D, Coosemans W, Lerut T, Tack J. Long-term outcome of pneumatic dilation in the treatment of achalasia. Clin Gastroenterol Hepatol. 2010;8:30–35. doi: 10.1016/j.cgh.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Vela MF, Richter JE, Khandwala F, Blackstone EH, Wachsberger D, Baker ME, Rice TW. The long-term efficacy of pneumatic dilatation and Heller myotomy for the treatment of achalasia. Clin Gastroenterol Hepatol. 2006;4:580–587. doi: 10.1016/s1542-3565(05)00986-9. [DOI] [PubMed] [Google Scholar]
- 19.Cheatham JG, Wong RK. Current approach to the treatment of achalasia. Curr Gastroenterol Rep. 2011;13:219–225. doi: 10.1007/s11894-011-0190-z. [DOI] [PubMed] [Google Scholar]
- 20.Campos GM, Vittinghoff E, Rabl C, Takata M, Gadenstätter M, Lin F, Ciovica R. Endoscopic and surgical treatments for achalasia: a systematic review and meta-analysis. Ann Surg. 2009;249:45–57. doi: 10.1097/SLA.0b013e31818e43ab. [DOI] [PubMed] [Google Scholar]
- 21.Richter JE, Boeckxstaens GE. Management of achalasia: surgery or pneumatic dilation. Gut. 2011;60:869–876. doi: 10.1136/gut.2010.212423. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Li YM. Recurrent achalasia treated with Heller myotomy: a review of the literature. World J Gastroenterol. 2008;14:7122–7126. doi: 10.3748/wjg.14.7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoenberg MB, Marx S, Kersten JF, Rösch T, Belle S, Kähler G, Vassiliou MC, Lüth S, von Renteln D. Laparoscopic Heller myotomy versus endoscopic balloon dilatation for the treatment of achalasia: a network meta-analysis. Ann Surg. 2013;258:943–952. doi: 10.1097/SLA.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 24.Nau P, Rattner D. Laparoscopic Heller myotomy as the gold standard for treatment of achalasia. J Gastrointest Surg. 2014;18:2201–2207. doi: 10.1007/s11605-014-2655-5. [DOI] [PubMed] [Google Scholar]
- 25.Inoue H, Tianle KM, Ikeda H, Hosoya T, Onimaru M, Yoshida A, Minami H, Kudo SE. Peroral endoscopic myotomy for esophageal achalasia: technique, indication, and outcomes. Thorac Surg Clin. 2011;21:519–525. doi: 10.1016/j.thorsurg.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Friedel D, Modayil R, Iqbal S, Grendell JH, Stavropoulos SN. Per-oral endoscopic myotomy for achalasia: An American perspective. World J Gastrointest Endosc. 2013;5:420–427. doi: 10.4253/wjge.v5.i9.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stavropoulos SN, Desilets DJ, Fuchs KH, Gostout CJ, Haber G, Inoue H, Kochman ML, Modayil R, Savides T, Scott DJ, et al. Per-oral endoscopic myotomy white paper summary. Gastrointest Endosc. 2014;80:1–15. doi: 10.1016/j.gie.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 28.Verlaan T, Rohof WO, Bredenoord AJ, Eberl S, Rösch T, Fockens P. Effect of peroral endoscopic myotomy on esophagogastric junction physiology in patients with achalasia. Gastrointest Endosc. 2013;78:39–44. doi: 10.1016/j.gie.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Von Renteln D, Fuchs KH, Fockens P, Bauerfeind P, Vassiliou MC, Werner YB, Fried G, Breithaupt W, Heinrich H, Bredenoord AJ, et al. Peroral endoscopic myotomy for the treatment of achalasia: an international prospective multicenter study. Gastroenterology. 2013;145:309–311.e1-3. doi: 10.1053/j.gastro.2013.04.057. [DOI] [PubMed] [Google Scholar]
- 30.Annese V, Bassotti G, Coccia G, Dinelli M, D’Onofrio V, Gatto G, Leandro G, Repici A, Testoni PA, Andriulli A. A multicentre randomised study of intrasphincteric botulinum toxin in patients with oesophageal achalasia. GISMAD Achalasia Study Group. Gut. 2000;46:597–600. doi: 10.1136/gut.46.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith CD, Stival A, Howell DL, Swafford V. Endoscopic therapy for achalasia before Heller myotomy results in worse outcomes than heller myotomy alone. Ann Surg. 2006;243:579–584; discussion 584-586. doi: 10.1097/01.sla.0000217524.75529.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaezi MF, Richter JE. Current therapies for achalasia: comparison and efficacy. J Clin Gastroenterol. 1998;27:21–35. doi: 10.1097/00004836-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Glatz SM, Richardson JD. Esophagectomy for end stage achalasia. J Gastrointest Surg. 2007;11:1134–1137. doi: 10.1007/s11605-007-0226-8. [DOI] [PubMed] [Google Scholar]
- 34.Molena D, Yang SC. Surgical management of end-stage achalasia. Semin Thorac Cardiovasc Surg. 2012;24:19–26. doi: 10.1053/j.semtcvs.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 35.De Palma GD, Catanzano C. Removable self-expanding metal stents: a pilot study for treatment of achalasia of the esophagus. Endoscopy. 1998;30:S95–S96. doi: 10.1055/s-2007-1001412. [DOI] [PubMed] [Google Scholar]
- 36.De Palma GD, lovino P, Masone S, Persico M, Persico G. Self-expanding metal stents for endoscopic treatment of esophageal achalasia unresponsive to conventional treatments. Long-term results in eight patients. Endoscopy. 2001;33:1027–1030. doi: 10.1055/s-2001-18933. [DOI] [PubMed] [Google Scholar]
- 37.Mukherjee S, Kaplan DS, Parasher G, Sipple MS. Expandable metal stents in achalasia--is there a role? Am J Gastroenterol. 2000;95:2185–2188. doi: 10.1111/j.1572-0241.2000.02301.x. [DOI] [PubMed] [Google Scholar]
- 38.Lee JG, Hsu R, Leung JW. Are self-expanding metal mesh stents useful in the treatment of benign esophageal stenoses and fistulas? An experience of four cases. Am J Gastroenterol. 2000;95:1920–1925. doi: 10.1111/j.1572-0241.2000.02246.x. [DOI] [PubMed] [Google Scholar]
- 39.Díaz Roca AB, Sampascual SB, Calderón AJ, Menéndez F, Varela JI, Baranda A, Ruíz P, de Zarate JO, Bravo M, Hijona L, et al. Self-expanding esophageal prostheses as an alternative temporary treatment for achalasia. Gastrointest Endosc. 2009;69:980. doi: 10.1016/j.gie.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 40.Zhao JG, Li YD, Cheng YS, Li MH, Chen NW, Chen WX, Shang KZ. Long-term safety and outcome of a temporary self-expanding metallic stent for achalasia: a prospective study with a 13-year single-center experience. Eur Radiol. 2009;19:1973–1980. doi: 10.1007/s00330-009-1373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng YS, Ma F, Li YD, Chen NW, Chen WX, Zhao JG, Wu CG. Temporary self-expanding metallic stents for achalasia: a prospective study with a long-term follow-up. World J Gastroenterol. 2010;16:5111–5117. doi: 10.3748/wjg.v16.i40.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng Y, Dai YM, Wan XJ. Clinical remission following endoscopic placement of retrievable, fully covered metal stents in patients with esophageal achalasia. Dis Esophagus. 2014;27:103–108. doi: 10.1111/dote.12083. [DOI] [PubMed] [Google Scholar]
- 43.Coppola F, Gaia S, Rolle E, Recchia S. Temporary endoscopic metallic stent for idiopathic esophageal achalasia. Surg Innov. 2014;21:11–14. doi: 10.1177/1553350613492024. [DOI] [PubMed] [Google Scholar]
- 44.Cheng YS, Li MH, Chen WX, Chen NW, Zhuang QX, Shang KZ. Selection and evaluation of three interventional procedures for achalasia based on long-term follow-up. World J Gastroenterol. 2003;9:2370–2373. doi: 10.3748/wjg.v9.i10.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu YQ, Cheng YS, Tang GY, Li MH, Zhao JG, Li F. Comparison of temporary stent insertion with pneumatic dilation of the same diameter in the treatment of achalasia patients: a retrospective study. J Gastroenterol Hepatol. 2010;25:499–505. doi: 10.1111/j.1440-1746.2009.06107.x. [DOI] [PubMed] [Google Scholar]
- 46.Li YD, Cheng YS, Li MH, Chen NW, Chen WX, Zhao JG. Temporary self-expanding metallic stents and pneumatic dilation for the treatment of achalasia: a prospective study with a long-term follow-up. Dis Esophagus. 2010;23:361–367. doi: 10.1111/j.1442-2050.2010.01048.x. [DOI] [PubMed] [Google Scholar]
- 47.Li YD, Tang GY, Cheng YS, Chen NW, Chen WX, Zhao JG. 13-year follow-up of a prospective comparison of the long-term clinical efficacy of temporary self-expanding metallic stents and pneumatic dilatation for the treatment of achalasia in 120 patients. AJR Am J Roentgenol. 2010;195:1429–1437. doi: 10.2214/AJR.10.4407. [DOI] [PubMed] [Google Scholar]
- 48.Cai XB, Dai YM, Wan XJ, Zeng Y, Liu F, Wang D, Zhou H. Comparison between botulinum injection and removable covered self-expanding metal stents for the treatment of achalasia. Dig Dis Sci. 2013;58:1960–1966. doi: 10.1007/s10620-013-2564-6. [DOI] [PubMed] [Google Scholar]
- 49.Kurian AA, Dunst CM, Sharata A, Bhayani NH, Reavis KM, Swanström LL. Peroral endoscopic esophageal myotomy: defining the learning curve. Gastrointest Endosc. 2013;77:719–725. doi: 10.1016/j.gie.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 50.Teitelbaum EN, Soper NJ, Arafat FO, Santos BF, Kahrilas PJ, Pandolfino JE, Hungness ES. Analysis of a learning curve and predictors of intraoperative difficulty for peroral esophageal myotomy (POEM) J Gastrointest Surg. 2014;18:92–98; discussion 98-99. doi: 10.1007/s11605-013-2332-0. [DOI] [PubMed] [Google Scholar]


